Abstract
Leptosphaeriaceae is a family in the order Pleosporales comprising economically important plant pathogens. Species may also be endophytes or saprobes on various host plants. In recent classifications Alternariaster, Leptosphaeria, Neophaeosphaeria, Paraleptosphaeria, Heterospora, Subplenodomus and Plenodomus were included in the family. The taxonomy of genera and species in Leptosphaeriaceae has been problematic due to the lack of understanding of the importance of morphological characters used to distinguish taxa, as well as the lack of reference strains. In order to establish evolutionary relationships and to provide a backbone tree for Leptosphaeria and allied genera, we sequenced the 18S nrDNA, 28S nrDNA, ITS, RPB2, TEF and ACT gene regions of Leptosphaeriaceae species and analysed this data. Multi-locus phylogenies together with morphology robustly support the monophyletic nature of Leptosphaeriaceae among the other families in Pleosporales, and the inclusion of the genera Alternariaster, Heterospora, Leptosphaeria, Paraleptosphaeria, Sphaerellopsis, Subplenodomus, Plenodomus and three novel genera Alloleptosphaeria, Neoleptosphaeria and Pseudoleptosphaeria. Five new species, Alternariaster centaureae-diffusae, Leptosphaeria cichorium, Paraleptosphaeria rubi, Plenodomus guttulatus and P. salviae are introduced. An account of sexual morph of Alternariaster centaureae-diffusae is provided, and the sexual morph of Leptosphaeria doliolum is re-described and illustrated using modern concepts from fresh collections. A novel family Neophaeosphaeriaceae is established to accommodate the genus Neophaeosphaeria and its species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Leptosphaeriaceae was established by Barr (1987) in the order Pleosporales and is typified by Leptosphaeria. Leptosphaeriaceae was separated from the family Pleosporaceae because of its coelomycetous, rather than hyphomycetous asexual morph, as well as the ascal walls which are thinner and narrower. Species of Leptosphaeriaceae can be saprobic, hemibiotropic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats (Hyde et al. 2013). Members of this family usually possess single, papillate, immersed or erumpent, perithecial ascomata, with relatively thick peridia, bitunicate cylindrical asci and hyaline to brown, transversely septate ascospores in their sexual morph (Hyde et al. 2013). The asexual morphs of the family Leptosphaeriaceae can be coelomycetous or hyphomycetous (Alves et al. 2013; De Gruyter et al. 2013; Hyde et al. 2013; Zhang et al. 2012) and are illustrated later in this paper.
Historic outline of Leptosphaeriaceae
When Barr (1987) introduced the family, she included Curreya, Didymolepta, Heptamaeria, Leptosphaeria and Ophiobolus. Eriksson and Hawksworth (1991) included only Ophiobolus and Leptosphaeria in this family (Dong et al. 1998), while Zhang et al. (2012) accepted two genera, i.e. Leptosphaeria and Neophaeosphaeria.
Combined analysis of LSU, SSU, RPB2 and TEF1 gene data has shown that members of Leptosphaeriaceae form a paraphyletic clade with moderate bootstrap support (Schoch et al. 2009; Zhang et al. 2012). Therefore based on molecular data, Leptosphaeriaceae is accommodated in Pleosporineae, which is a phylogenetically well-established suborder of Pleosporales (Schoch et al. 2009; Zhang et al. 2012; Hyde et al. 2013; Wijayawardene et al. 2014). In the same study Ophiobolus and Shiraia clustered in this family with minor support, but Zhang et al. (2012) suggested that these genera may be more closely related to Phaeosphaeriaceae. Coniothyrium was considered an asexual morph of Leptosphaeria (Muthumeenakshi et al. 2001; De Gruyter et al. 2009; Zhang et al. 2012). De Gruyter et al. (2013) however, showed that C. palmarum Corda generic type of Coniothyrium has distant phylogenetic relationships with Leptosphaeriaceae. Therefore they treated Coniothyriaceae as a separate family in Pleosporales.
De Gruyter et al. (2013) introduced Paraleptosphaeria to this family based on the phylogeny determined by analysis of 28S nrDNA (LSU) and ITS sequence data. This genus is characterized by immersed, subglobose, thick-walled ascomata containing interascal filamentous pseudoparaphyses, with bitunicate, broad asci bearing fusiform, transversely 3–5-septate, hyaline to yellow-brown ascospores. Paraleptosphaeria is typified by Paraleptosphaeria nitschkei (Rehm ex G. Winter) Gruyter et al.
De Gruyter et al. (2013) introduced Subplenodomus typified by S. violicola (P. Syd.) Gruyter et al. to accommodate some phoma-like species clustering within Leptosphaeriaceae. Plenodomus and Subplenodomus are necrotrophs and plant pathogens (De Gruyter et al. 2013). Ascospores in Plenodomus are 3–7-septate, whereas no sexual morph has thus far been recorded in Subplenodomus (De Gruyter et al. 2013). The scleroplectenchymatous conidiomatal cell wall considered as the main character of Plenodomus, whereas in Subplenodomus the conidiomatal cell wall is pseudoparenchymatous (De Gruyter et al. 2013).
Literature reviews coupled with molecular data have shown that Plenodomus lingam (Tode) Höhn and Leptosphaeria doliolum (Pers.) Ces. & De Not., the generic types of Plenodomus and Leptosphaeria respectively, are genetically distant (Dong et al. 1998; Câmara et al. 2002; De Gruyter et al. 2013). Species of Leptosphaeria produce dark brown, 3-septa ascospores, which were believed the primitive character, as compared to more recently evolved species producing ascospores that are paler, longer and narrower, and have more than 3 septa (Wehmeyer 1946). Recent studies have shown that the taxonomy of the generic type, Leptosphaeria doliolum and its Phoma asexual morph is complex with a number of subspecies and varieties described (Câmara et al. 2002; Eriksson and Hawksworth 2003; Wunsch and Bergstrom 2011; De Gruyter et al. 2013). Leptosphaeria doliolum subsp. Doliolum and L. doliolum subsp. Errabunda and their asexual morphs Ph. acuta subsp. Errabunda (Desm.) Boerema et al. and Ph. acuta subsp. Acuta Fuckel are morphologically very similar (De Gruyter et al. 2012). De Gruyter et al. (2013) suggested that both subspecies of L. doliolum are closely related in LSU and ITS data and that L. doliolum represents a species complex. A detailed multi-locus phylogenetic analysis based on ITS, ACT, TUB and CHS genes, revealed that their subspecies could clearly be resolved, and represent two subclades in the L. doliolum species complex (De Gruyter et al. 2013).
The genus Alternariaster, which differs from Alternaria in conidial characters (Alves et al. 2013), was introduced to accommodate Alternaria helianthi (Hansf.) E.G. Simmons, by Simmons (2007). Alternariaster helianthi causes leaf spots on Helianthus annuus (sunflower). Molecular data coupled with morphology confirmed Alternariaster to be a well-delimited genus in Leptosphaeriaceae, rather than Pleosporaceae, to which Alternaria belongs (Alves et al. 2013).
Based on morphology coupled with DNA sequence data, Trakunyingcharoen et al. (2014) placed Sphaerellopsis in the family Leptosphaeriaceae. Species of Sphaerellopsis are well known mycoparasites on a wide range of rusts (Trakunyingcharoen et al. 2014). Trakunyingcharoen et al. (2014) designated a neotype for Sphaerellopsis filum (Biv.) B. Sutton, and introduced the new species Sph. macroconidialis Crous & Trakun. and Sph. paraphysata Crous & Alfenas. They treated Eudarluca as the sexual morph of Sphaerellopsis and proposed to conserve Sphaerellopsis over Eudarluca on the basis that Sphaerellopsis is more frequently used in the literature, is the older generic name, and is the morph commonly seen in the field. Phookamsak et al. (2014) classified Eudarluca in Phaeosphaeriaceae based only on morphology. Furthermore they suggested that Eudarluca may not be related to Sphaerellopsis based on its morphological characters and is typical of Phaeosphaeriaceae. This aspect needs further careful study.
Major circumscription changes of the genera in Leptosphaeriaceae are given in Table 1.
Asexual morphs of Leptosphaeriaceae
Zhang et al. (2012) listed the asexual morphs of Leptosphaeriaceae as Camarosporium, Coniothyrium, Phoma, Plenodomus and Pyrenochaeta. Schoch et al. (2009) however, showed that phylogenetically Camarosporium quaternatum Schulzer and Pyrenochaeta nobilis De Not., the generic types of their respective genera, grouped in Leptosphaeriaceae. De Gruyter et al. (2009, 2010) and Aveskamp et al. (2010) showed that Coniothyrium and some species of Phoma also grouped in Leptosphaeriaceae. However, De Gruyter et al. (2013) restricted Phoma sensu stricto to Didymellaceae, where the generic type P. herbarum Westend., is located. In their analysis they showed that Phoma species grouped in four different clades, with the other species moved to the new genera Heterospora, Paraleptosphaeria, Plenodomus and Subplenodomus. They also transferred some Phoma species to Leptosphaeria, and these species grouped in a different clade to Leptosphaeria doliolum, the generic type of Leptosphaeria. De Gruyter et al. (2013) also concluded that Coniothyrium palmarum, the generic type of Coniothyrium, clustered in a separate clade from Leptosphaeriaceae. They reinstated the family Coniothyriaceae which was previously synonymised with Leptosphaeriaceae (Kirk et al. 2008). Hyde et al. (2013) accepted Heterospora, Plenodomus, and Subplenodomus as the asexual morphs of Leptosphaeriaceae.
We have been working on the families of Pleosporales based on both morphology and molecular phylogeny, in order to provide a natural classification of this order (Ariyawansa et al. 2013a, b, c; Ariyawansa et al. 2014a, b, c, d, e, f; Ariyawansa et al., 2015; Zhang et al. 2012). The present study was initiated to outline the phylogenetic lineages within Leptosphaeriaceae, and to build a robust taxonomy to act as a backbone tree for the family based on the analysis of ITS, LSU, SSU RPB2 and TEF1 sequence data. Another objective was to evaluate further the phylogenetic significance of ascomatal characteristics, as well as asexual morph and host. The significance of asexual morph characteristics has not been evaluated fully and the asexual morphs of species in Leptosphaeria and allied genera have been placed in several different genera (Rossman et al. 1987; Crane and Shearer 1991; De Gruyter et al. 2013). A further aim of this study was to establish a stable taxonomy and phylogeny for Leptosphaeria species complex by conducting multi-locus analysis of LSU, ITS and ACT sequence data.
Material and methods
Specimen examination
Fresh material of species of Leptosphaeriaceae were collected in Germany, Italy, Russia and Thailand during 2011–2014. Specimens were taken to the laboratory in Ziplock plastic bags. The samples were processed and examined following the method described in Ariyawansa et al. (2015). Fresh and dried herbarium material were examined using a Motic SMZ 168 dissecting microscope to locate and isolate ascomata fruiting bodies. Hand sections of the fruiting structures were mounted in water for microscopic studies and photomicrography. The taxa were examined using a Nikon ECLIPSE 80i compound microscope and photographed with a Canon 450D digital camera fitted to the microscope. Measurements were made with the Tarosoft (R) Image Frame Work program and images used for figures processed with Adobe Photoshop CS3 Extended version 10.0 software (Adobe Systems, USA). Isolations were made from single ascospores, following a modified method of Chomnunti et al. (2014). Contents of the sectioned fruiting bodies were transferred to a drop of sterile water on a flame-sterilized slide. Drops of the spore suspension were pipetted and spread on a Petri dish containing 2 % water agar (WA) and incubated at 25 °C. Germinated ascospores were transferred singly to MEA media (Alves et al. 2006).
Herbarium specimens were obtained on loan from the Swedish Museum of Natural History (S) and the New York Botanical Garden (NY). Voucher specimens are deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand, Kunming Institute of Botany (KIB) and New Zealand Fungal Herbarium (PDD), New Zealand. Living cultures are deposited at the Mae Fah Luang University Culture Collection (MFLUCC), International Collection of Microorganisms from Plants (ICMP) and Queensland Plant Pathology Herbarium (BRIP), the latter under Material Transfer Agreement No. 4/2010 (MTA). Each genus is listed along with a description of the type species, except in cases where there is only a single species in the genus. Faces of fungi numbers were obtained as in Jayasiri et al. (2015) and IF numbers are in Index Fungorum (2015).
DNA extraction, PCR amplification and sequencing
Isolates originating from single ascospore were grown on malt extract agar (MEA) or potato dextrose agar (PDA) for 28 days at 25 °C in the dark. Genomic DNA was extracted from the growing mycelium using the Biospin Fungus Genomic DNA Extraction Kit (BioFlux®) following the manufacturer’s protocol (Hangzhou, P.R. China). Otherwise DNA was extracted directly from ascomata using a DNA extraction kit (E.Z.N.A.® Forensic DNA kit, D3591- 01,Omega Bio-Tek) following Telle and Thines (2008).
The amplification procedure was performed in a 50 μl reaction volume containing 5–10 ng DNA, 0.8 units Taq polymerase, 1X PCR buffer, 0.2 mM d’NTP, 0.3 μm of each primer with 1.5 mM MgCl2 (Cai et al. 2009). The PCR reactions for amplification of the recently ratified universal fungal barcode ITS1-5.8S-ITS2 of the nuclear ribosomal DNA operon (Schoch et al. 2012), were performed under standard conditions (White et al. 1990; Stielow et al. 2010). PCR conditions for amplifying the partial SSU and LSU r-DNA followed the protocol of Phillips et al. (2008). Amplification of ACT followed the protocol of De Gruyter et al. (2012). The PCR products were observed on 1 % agarose electrophoresis gels stained with ethidium bromide. Purification and sequencing of PCR products were carried at Shanghai Sangon Biological Engineering Technology and Services Co. (China).
DNA sequence data was obtained from the internal transcribe spacer (ITS), small and large subunits of the nuclear ribosomal RNA genes (SSU, LSU). Primer sets used for these genes were as follows: ITS: ITS5/ITS4 SSU: NS1/NS4; LSU: LR0R/LR5 (Liu et al. 1999; Sung et al. 2007). The ACT region was amplified using the primer pairs ACT-512F/ACT-783R (Carbone and Kohn 1999) and the amplification reactions were performed and analysed as described by De Gruyter et al. (2009). The TEF1 region with EF1-728F and EF1-986R (Carbone and Kohn 1999) and RPB2 gene with the primers fRPB2-SF and fRPB2-7cR. Primer sequences are available at the WASABI database at the AFTOL website (aftol.org). Sequences are deposited at NCBI GenBank under the accession numbers provided in Supplementary Table 1. Alignments are deposited in TreeBASE.
Sequence alignment and phylogenetic analysis
Multiple sequence alignments were generated with MAFFT v. 6.864b (http://mafft.cbrc.jp/alignment/server/index.html). The alignments were checked visually and improved manually where necessary. Three different datasets were used to estimate three phylogenies; a Pleosporineae family tree, backbone tree for Leptosphaeriaceae and a tree dealing with Leptosphaeria doliolum species complex phylogeny. The first tree focuses on phylogenetic placement of Leptosphaeriaceae and the new family Neophaeosphaeriaceae proposed in this study, in the suborder Pleosporineae, the second to show the placement of Leptosphaeria and allied genera in family Leptosphaeriaceae, and the final tree to show the placement of newly reported Leptosphaeria species and taxa of the Leptosphaeria doliolum species complex. All introns and exons were aligned separately. Regions containing many leading or trailing gaps were removed from the ITS, SSU, LSU, RPB2, TEF and ACT alignments prior to tree building. All sequences obtained from GenBank and used by Alves et al. (2013), Ariyawansa et al. (2014a), De Gruyter et al. (2013), Hyde et al. (2013), Liu et al. (2015), Phookamsak et al. (2014) Schoch et al. (2009), and Zhang et al. (2012) are listed in supplementary Table 1.
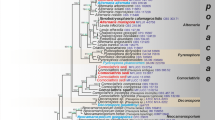
Maximum likelihood analyses including 1000 bootstrap replicates were run using RAxML v. 7.2.6 (Stamatakis 2006; Stamatakis et al. 2008). The online tool Findmodel (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html) was used to determine the best nucleotide substitution model for each partition. The best scoring tree for Pleosporineae family tree, Leptosphaeriaceae backbone tree and Leptosphaeria species complex tree were selected with final likelihood values of −20,128.721105, −4990.604099 and −9859.396412 respectively. The resulting replicates were plotted on the best scoring trees previously obtained. Maximum Likelihood bootstrap values (ML) equal or greater than 50 % are given below or above each node (Fig. 1).
RAxML tree based on a combined dataset of ITS, SSU, LSU, RPB2 and TEF sequence data from 49 strains. Bootstrap support values for maximum likelihood greater than 50 % and Bayesian posterior probabilities greater than 0.90 are given below and above the nodes. Halojulella avicenniae is the out group taxon. The original isolate numbers are noted after the species names. Ex-type culture numbers are in bold. The type species of each genus is indicated in blue
The model of evolution was performed by using MrModeltest 2.2 (Nylander 2004) for each partition. Posterior probabilities (BP) (Rannala and Yang 1996; Zhaxybayeva and Gogarten 2002) were determined by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist 2001). The Bayesian inference was conducted under different models for each partition of the matrix as evaluated by MrModeltest 2.2 (Nylander 2004). Six simultaneous Markov chains were run for 30 × 106 generations and every 1000th generation a tree was sampled. MCMC heated chain was set with a “temperature” value of 0.15. The distribution of log-likelihood scores was examined to determine stationary phase for each search and to decide if extra runs were required to achieve convergence, using the program Tracer 1.5 (Rambaut and Drummond 2007). All sampled topologies beneath the asymptote (20 %) were discarded as part of a burn-in procedure, the remaining trees were used for calculating posterior probabilities in the majority rule consensus tree. Bayesian Posterior Probabilities (BP) equal or greater than 0.90 is given below or above each node (Fig. 1).
In order to determine the species limits in the Leptosphaeria doliolum species complex, we applied the criteria of Genealogical Concordance Phylogenetic Species Recognition (GCPSR) (Taylor et al. 2000; Dettman et al. 2003). Dettman et al. (2003) emphasised that species should be recognised if they satisfy one of two criteria: genealogical concordance or genealogical non-discordance. Clades were genealogically concordant if they were present in at least some of the gene trees and genealogically non-discordant if they were strongly supported (MP ≥ 70 %; ML ≥ 70 %) in a single gene and not contradicted at or above this level of support in any other single gene tree. This criterion prohibited poorly supported non-monophyly at one locus from undermining well-supported monophyly at another locus. Phylogenetic trees and data files were viewed in MEGA v. 5 (Tamura et al. 2011), TreeView v. 1.6.6 (Page 1996) and FigTree v. 1.4 (Rambaut and Drummond 2008).
Results
Phylogeny
The data for the aligned sequence matrices for the trees obtained in the different analyses are provided below. In the case that alignments of multi-gene were involved, the topologies of the obtained trees for each gene were compared manually, to confirm that the overall tree topology of the individual datasets, were similar to each other and to that of the tree obtained from the combined alignment. The ML analyses showed similar tree topologies and were congruent to those obtained in the Bayesian analyses. The results of the molecular phylogenetic analyses are supplied below (Figs 1, 2, 3).
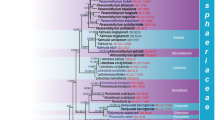
RAxML tree based on a combined dataset of ITS, SSU and LSU sequence data of 108 strains. Bootstrap support values for maximum likelihood greater than 50 % and Bayesian posterior probabilities greater than 0.90 are given below and above the nodes. Didymella exigua is the out group taxon. The original isolate numbers are noted after the species names. Newly generated strains in this study are indicated in red. The type species of each genus is indicated in blue
RAxML tree based on a combined dataset of ITS, ACT and LSU sequence data of 35 strains. Bootstrap support values for maximum likelihood greater than 70 % and Bayesian posterior probabilities greater than 0.90 are given below and above the nodes. Alternariaster helianthi CBS 327.69 is the out group taxon. The original isolate numbers are noted after the species names. Newly generated strains in this study are indicated in red. The type species of each genus is indicated in blue
Phylogeny of Leptosphaeriaceae and Neophaeosphaeriaceae in Pleosporineae
The final Pleosporineae alignment included 49 strains, representing nine families including the new family Neophaeosphaeriaceae and the new genera proposed in the present study. The data set consisted of 3978 characters (SSU 946, LSU 938, ITS 634, RPB2 833, and TEF 794). In the SSU alignment there was a large insertion at position 440–788 in the isolates Heterospora dimorphospora (Speg.) Gruyter et al. (CBS 165.78) and Ophiosphaerella herpotricha (Fr.) J. Walker (CBS 620.86) and they were excluded from the analyses. The Bayesian analysis resulted in 30,000 trees after 30,000,000 generations. The first 6000 trees, representing the burn-in phase of the analyses, were discarded, while the remaining trees were used for calculating posterior probabilities in the majority rule consensus tree.
A best scoring RAxML tree is shown in Fig. 1, with the value of −20,128.721105. Phylogenetic trees obtained from Maximum Likelihood and Bayesian analysis yielded trees with similar overall topology at subclass and family level relationships in agreement with previous work based on Maximum Likelihood analysis and Bayesian analysis (Alves et al. 2013; Ariyawansa et al. 2014a; De Gruyter et al. 2013; Hyde et al. 2013; Liu et al. 2015; Schoch et al. 2009; Wijayawardene et al. 2014; Zhang et al. 2012). The support values for the different phylogenetic methods vary, with the Bayesian posterior probabilities being higher than the RAxML bootstrap support values.
The genus Neophaeosphaeria, N. filamentosa (Ellis & Everh.) M.P.S. Câmara et al. Ramaley (CBS 102203, CBS102202, BPI 802755, BPI 12537) together with the strains of N. agaves Crous & Yáñez-Moral (CPC 21264) and N. quadriseptata (M.E. Barr) M.P.S. Câmara et al. (BPI 1111149) cluster outside Leptosphaeriaceae and formed a distinct clade sister to the familial clades of Cucurbitariaceae and Coniothyriaceae. Therefore, we introduce the new family Neophaeosphaeriaceae based on evidence from molecular phylogeny as well as morphological distinction.
Putative strains of Leptosphaeria proteicola (CPC 18289) clustered within the Camarosporium clade, thus we tentatively treated these as a Camarosporium sp. in this study (Fig. 2). We also observed that the putatively named strains of PHY 06, PHY 30 and PHY 54 clustered in different genera of Leptosphaeriaceae. i.e. Leptosphaeria sp. (PHY 06) and Leptosphaeria sp. (PHY 54) clustered with the genus Paraleptosphaeria, while Leptosphaeria sp. (PHY 30) forms a distinct clade in the Plenodomus clade. Thus we tentatively label them with the genus where they have clustered in phylogenetic tree (Fig. 2), specific name is not given because the morphology and identification of these putative strains in GenBank as far as we can ascertain, cannot be checked, as they are not linked to any herbarium material.
Phylogeny of Leptosphaeriaceae and allied genera
The final Leptosphaeriaceae and allied genera alignment included 108 strains, representing three families and consisted of 2331 characters (SSU 948, LSU 880, ITS 655). In the SSU alignment a large insertion at position 440–788 in the isolate of Heterospora dimorphospora (Speg.) Gruyter et al. (CBS 165.78) was excluded from the phylogenetic analyses. The Bayesian analysis resulted in 800,000 trees after 80,000,000 generations. The first 16,000 trees, representing the burn-in phase of the analyses, were discarded while the remaining trees were used for calculating posterior probabilities in the majority rule consensus tree.
In the multi-locus phylogeny inferred from the combined dataset of LSU, SSU and ITS (Fig. 2), several well supported sub-clades can be recognised in the family Leptosphaeriaceae, which are used for the delimitation of genera, i.e. Alternariaster, Leptosphaeria, Paraleptosphaeria, Plenodomus, Sphaerellopsis and Subplenodomus.
The Plenodomus clade comprises 30 strains including the type species, Plenodomus lingam (Tode: Fr.) Höhn., and forms a well-supported clade within the family Leptosphaeriaceae (Fig. 2). The recently revised genus Alternariaster forms a well supported clade sister to the Plenodomus clade and comprises strains of A. helianthi the type species of Alternariaster, along with A. bidentis J.L. Alves & R.W. Barreto and the new species Alternariaster centaureae-diffusae. Another well supported clade was formed by the genus Sphaerellopsis comprising the type strain, Sphaerellopsis filum (Biv.) B. Sutton (, together with two strains of S. paraphysata Crous & Alfenas and three strains of S. macroconidiale Crous & Trakun.
Another relatively well supported clade forming the major part of the ingroup of the tree comprises the strains of Heterospora chenopodii (Westend.) Gruyter et al., the type species of Heterospora, and H. dimorphospora (Speg.) Gruyter et al. The genus Leptosphaeria sensu stricto forms a well supported clade in the family Leptosphaeriaceae comprising L. doliolum strains, the type species of the genus Leptosphaeria, along with strains of nine other species. The Paraleptosphaeria clade comprises six species and forms a sister clade to the Paraleptosphaeria nitschkei clade. Another well supported clade (Fig. 2) was formed by the four Subplenodomus species.
Strains of the three novel genera proposed in this study, Alloleptosphaeria italica, Neoleptosphaeria rubefaciens and Pseudoleptosphaeria etheridgei, form well-supported clades basal to the Leptosphaeria sensu stricto clade.
Phylogeny of Leptosphaeria species complex
The final Leptosphaeria species complex alignment (Fig. 3) included 35 strains, representing 16 species and consisted of 2130 characters (LSU 873, ITS 546, ACT 240). The Bayesian analysis resulted in 40,000 trees after 40,000,000 generations. The first 8000 trees, representing the burn-in phase of the analyses were discarded, while the remaining trees were used for calculating posterior probabilities in the majority rule consensus tree. Alternariaster helianthi (CBS 199.86) is the out group taxon.
A best scoring RAxML tree is shown (Fig. 3), with the value of Likelihood: - 9859.396412. Phylogenetic trees obtained from Maximum Likelihood and Bayesian analysis yielded trees with similar overall topology at the species level in agreement with previous studies based on Maximum Likelihood analysis and Bayesian analysis (Alves et al. 2013; De Gruyter et al. 2013).
In the Leptosphaeria sensu stricto clade there are 12 monotypic lineages, each of which is treated as appropriate for the delimitation of species, although positions vary between the different gene regions or combinations used and are similar with Alves et al. (2013) and De Gruyter et al. (2013). All the species limits were determined by applying the criteria of Genealogical Concordance Phylogenetic Species Recognition (GCPSR) (Taylor et al. 2000; Dettman et al. 2003). The support values for the different phylogenetic methods vary, with the Bayesian posterior probabilities being higher than the RAxML bootstrap support values (Fig. 3).
Clade A comprises seven strains of Leptosphaeria doliolum. The taxonomy of the generic type species L. doliolum and Phoma asexual morph is complex with several subspecies and varieties (De Gruyter et al. 2013). Boerema et al. (1994) suggested that L. doliolum subsp. Doliolum and L. doliolum subsp. Errabunda are morphologically very similar, as well as the asexual morphs P. acuta subsp. Errabunda and P. acuta subsp. Acuta (De Gruyter et al. 2013). We observed that both subspecies of L. doliolum are closely related in a phylogenetic analysis based on LSU and ITS sequence data. However, our phylogenetic analysis based on ITS, LSU and ACT genes, demonstrated that both subspecies could be clearly differentiated, and represent two subclades (A and C) in the L. doliolum species complex and are thus distinct species. Leptosphaeria veronicae which is a pathogen specifically occurring on Veronica spp. (Scrophulariaceae), forms a sister clade (Clade B) to the L. doliolum species complex. It is related to L. doliolum based on both morphology and phylogeny, but is a distinct species.
Leptosphaeria sydowii forms a sister clade (Clade E) to L. errabunda. Leptosphaeria sydowii is a pathogen on Asteraceae and Senecio spp., and is closely related to L. errabunda. Leptosphaeria macrocapsa also grouped with the L. errabunda isolates. Leptosphaeria macrocapsa is a host specific pathogen on Mercurialis perennis (Euphorbiaceae) in Europe (Boerema et al. 1994). The species is characterised by large pycnidia (Grove 1935), with a conspicuously broad, long cylindrical neck (Boerema et al. 1994). This is different to the sharply delimited papilla or neck of variable length of the pycnidia of L. errabunda. Our phylogeny (Figs 2, 3) shows that other pathogenic species i.e. L. conoidea, L. slovacica and L. pedicularis, as well as our novel species L. cichorium are related to the L. doliolum species complex, but are distinct species.
Clades M, Nand O are distant from Leptosphaeria sensu stricto. Thus, we introduce three new genera (Alloleptosphaeria, Neoleptosphaeria and Pseudoleptosphaeria) to accommodate taxa in these clades.
Alignments were analysed corresponding to single gene study of ITS, SSU, LSU RPB2, EF and ACT and combined alignments of the three phylogenies. Comparison of the alignment properties and nucleotide substitution models are provided in Tables 2-4.
Taxonomy
Leptosphaeriaceae M.E. Barr, Mycotaxon 29: 503 (1987).
Facesoffungi number : FoF 01151.
Saprobic, fungicolus, hemibiotropic or pathogenic on leaves and wood in terrestrial habitats. Sexual morph: Ascomata immersed, erumpent to superficial, globose, subglobose or obypyriform, black to dark brown, coriaceous, ostiolate, periphysate. Ostiole well developed, broadly or narrowly conical, with a dark brown to black papilla, ostiolar canal filled with tissue of hyaline cells. Peridium composed of large, pigmented, thin-walled, scleroplectenchymatous or plectenchymatous cells, usually arranged in textura angularis. Hamathecium of dense, septate, cellular pseudoparaphyses, usually embedded in a mucilaginous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical to oblong, with a pedicel and ocular chamber. Ascospores uniseriate and partially overlapping, brown, reddish brown or yellowish brown, fusoid, narrowly fusoid, obovoid, oblong or filiform, septate and constricted at the septa, smooth-walled, with or without guttules. Asexual morph: Coelomycetous or hyphomycetous. Conidiomata immersed to nearly superficial, depressed globose with a flattened base and cylindrical neck. Ostiole sometimes papillate or with elongated neck. Conidiomata wall scleroplectenchymatous. Conidia oblong, ellipsoidal to subcylindrical. Sclerotia sometimes produced (Boerema et al. 1994; Hyde et al. 2011). Conidiophores solitary or in small groups, hypophyllous, straight to slightly sinuous, simple, 3–6-septate, pale to chestnut-brown, smooth. Conidiogenous cells tretic, integrated, terminal to intercalary, sympodial, cylindrical, yellowish to pale brown. Conidia dry, solitary, cylindrical to subcylindrical, apex and base rounded, transversally 2–9 septate, often deeply constricted at septa, eguttulate, subhyaline to pale brown, smooth-walled, hilum thickened and darkened (Alves et al. 2013; Zhang et al. 2012).
Family type: Leptosphaeria Ces. & de Not., Comm. Soc. crittog. Ital. 1(4): 234 (1863).
Treatment of genera in Leptosphaeriaceae
Alloleptosphaeria Ariyawansa, Wanasinghe & K.D. Hyde, gen. Nov.
Index Fungorum number: IF 551460, Facesoffungi number: FoF 01152.
Etymology: In reference to the morphological resemblance to Leptosphaeria.
Type species: Alloleptosphaeria italica Wanasinghe, Camporesi, Ariyawansa & K.D. Hyde.
Saprobic on dead herbaceous branches in terrestrial habitats. Sexual morph: Ascomata solitary, scattered, immersed to semi-erumpent, globose or subglobose, dark brown to black, coriaceous, fused to the host tissue, ostiolate. Ostiole papillate, black, smooth, ostiolar canal filled with sparse periphyses, upper covering of ostiole comprising short, light brown setae-like structures. Peridium thin-walled, composed of a few layers of thin-walled, brown to dark brown, or reddish brown, pseudoparanchymatous cells, forming a textura angularis. Hamathecium comprising 1.5–2.5 μm wide, filamentous, branched septate, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindric-clavate to clavate, pedicellate, rounded and thick-walled at the apex, with an ocular chamber. Ascospores overlapping 1–2-seriate, initially hyaline, becoming yellowish brown at maturity, fusiform to clavate, 3-septate, constricted at the septa, cell above central septum widest, narrowly rounded at both ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph: undetermined.
Notes: Alloleptosphaeria is introduced in the family Leptosphaeriaceae to accommodate A. italica, based on both morphology and phylogeny. Alloleptosphaeria shares similarities with Leptosphaeria in having conical ascomata, a well-developed papilla, cellular pseudoparaphyses, and cylindrical asci with an ocular chamber, bearing narrowly fusoid, reddish to yellowish brown, 3-septate ascospores. Alloleptosphaeria however, differs from Leptosphaeria in having immersed ascomata, with a thin-walled peridium, composed of a few layers of thin-walled, brown to dark brown or reddish brown, pseudoparanchymatous cells, forming a textura angularis and fusing outwardly with the host cells, while Leptosphaeria has superficial ascomata with thick-walled, scleroplectenchymatous cells, arranged in a textura angularis-globulosa, with an outer black amorphous layer not fusing with the host tissues. This is also supported phylogenetically as Alloleptosphaeria italica forms a distant clade, outside the Leptosphaeria sensu stricto clade with high bootstrap support (Figs 2, 3).
Alloleptosphaeria italica Wanasinghe, Camporesi, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF 551461, Facesoffungi number: FoF 01153.
Etymology: in reference to its occurrence in Italy.
Holotype: MFLU 15-1517.
Saprobic on dead branches in terrestrial habitats. Sexual morph: Ascomata 250–200 high × 150–200 μm diam. (x̅ = 217.9 × 166.8 μm, n = 10), solitary, scattered, immersed to semi-erumpent, globose or subglobose, dark brown to black, coriaceous, outwardly fusing with the host tissue, ostiolate. Ostiole 30–70 μm diam (x̅ = 43.8 μm, n = 10), papillate, black, smooth, ostiolar canal filled with sparse periphyses, upper covering of ostiole comprising short, light brown setae-like structures. Peridium 10–15 μm wide at the base, 10–20 μm wide at the sides, composed with brown to dark brown, or reddish brown, pseudoparanchymatous cells of textura angularis. Hamathecium comprising numerous, 1.5–2.5 μm (x̅ = 2, n = 30) wide, filamentous, branched septate, pseudoparaphyses. Asci 60–75 × 7–10 μm, (x̅ = 66.4 × 8.3 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindric-clavate to clavate, with a bulbous pedicel, thick-walled at the apex, with ocular chamber. Ascospores 13–19 × 4–6 μm (x̅ = 17.2 × 4.8 μm, n = 50), overlapping 1–2-seriate, initially hyaline, becoming yellowish brown at maturity, narrowly ovoid to clavate, 3-septate, constricted at the septa, cell above central septum widest, narrowly rounded at both ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph: undetermined.
Material examined: Italy, Forlì-Cesena Province, San Piero in Bagno, Valbonella, dead and hanging branches of Clematis vitalba (Ranunculaceae), 27 Sep. 2013, E. Camporesi (MFLU 15-1517, holotype); ex-type living culture, MFLUCC 14-0934 (Fig. 4).
Alternariaster E.G. Simmons, CBS Diversity Ser. (Utrecht) 6: 667 (2007).
Facesoffungi number : FoF 01154.
Pathogenic or saprobic on stems and leaves in terrestrial habitats. Sexual morph: Ascomata solitary or in groups of 2–10, erumpent through host tissue, semi-immersed or nearly superficial, uniloculate, globose to subglobose, coriaceous, black, ostiolate. Ostiole periphysate, papillate, black. Periphyses cellular, aseptate, wide, with a blunt apex, hyaline. Peridium composed of 5–10 rows of scleroplectenchymatous cells, outer part thin, amorphous and black, central part widest, comprising thick-walled cells of textura globularis, inner layer composed of flattened cells of textura angularis. Hamathecium comprising 3–4 μm wide, hyaline, distinctly septate, branched, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-subclavate, with a short bulbous pedicel, rounded at the apex. Ascospores fasciculate, filiform, multi-septate, constricted at the apical septum, apical cell swollen, conical, yellowish brown, smooth-walled, with a mucilaginous cap. Asexual morph: Hyphomycetous. Conidiophores solitary or in small groups, hypophyllous, straight to slightly sinuous, simple, 3–6-septate, pale to chestnut-brown, smooth. Conidiogenous cells tretic, integrated, terminal to intercalary, sympodial, cylindrical, yellowish to pale brown. Conidia dry, solitary, cylindrical to subcylindrical, apex and base rounded, transversally 2–9 septate, often deeply constricted at septa, eguttulate, subhyaline to pale brown, smooth-walled, hilum thickened and darkened (from Alves et al. 2013).
Type species: Alternariaster helianthi (Hansf.) E.G. Simmons, CBS Diversity Ser. (Utrecht) 6: 667 (2007).
≡ Helminthosporium helianthi Hansf., Proc. Linn. Soc. London: 49 (1943) [1942–43]
Pathogenic on leaves of Helianthus annuus L. Sexual morph: undetermined. Asexual morph: Lesions on living leaves starting as dispersed punctiform spots, occurring throughout the leaf blade, becoming subcircular to irregular in shape, yellowish, 3–11 × 2–9 mm, surrounded by a halo of dark green tissue, at the later stages lesions coalesce, resulting in leaf blight and premature plant death. Conidiophores 100–225 × 7.5–10 μm, solitary or in small groups, hypophyllous, straight to slightly sinuous, simple, 3–6-septate, pale to chestnut-brown, smooth. Conidiogenous cells 25–100 × 5–7.5 μm, yellowish to pale brown, tretic, integrated, terminal to intercalary, sympodial, cylindrical. Conidiogenous cells with conspicuous scars, 1–2 per cell, protuberant, up to 5 μm diam, thickened and darkened. Conidia 60–115 × 11–29 μm, subhyaline to pale brown, dry, solitary, cylindrical to subcylindrical, occasionally with cells of different sizes, apex and base rounded, transversally 5–9-septate (1–2 longitudinal or oblique septa), often deeply constricted at septa, eguttulate, smooth-walled, hilum thickened and darkened. Germ tubes orientated perpendicularly to the main axis of the conidium, and also polar (from Alves et al. 2013) (Fig. 5).
Alternariaster helianthi (redrawn from Alternariaster helianthi in Ellis 1971, Fig. 319.). Scale bars: 650 μm
Notes: The genus Alternariaster was introduced by Simmons (2007) to accommodate Alternaria helianthi. Alternariaster differs from Alternaria in having cylindrical, ellipsoid or broadly ovoid, subhyaline to greyish brown conidia, rarely forming longitudinal or oblique septa and not formed in chains (Alves et al. 2013). Alves et al. (2013) introduced Alternariaster bidentis which forms a separate clade in the family Leptosphaeriaceae together with the strains of A. helianthi. The collection of a second species in the genus Alternariaster and the multi-gene phylogenetic analysis of these two species, confirmed Alternariaster to be a well delimited genus in the Leptosphaeriaceae rather than the Pleosporaceae, to which Alternaria belongs (Alves et al. 2013). In this study we illustrate the sexual morph of this genus based on our new species Alternariaster centaureae-diffusae collected from Russia.
In our phylogeny, Alternariaster forms a robust clade sister to Plenodomus, Sphaerellopsis and Heterospora. Therefore, we accept Alternariaster as a well defined genus in Leptosphaeriaceae based on morphological and molecualr evidence.
Moreover we introduce a novel species, Alternariaster centaureae-diffusae, which is the first report of the sexual morph of this genus.
Alternariaster centaureae-diffusae R.H. Perera, Bulgakov, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF 551462, Facesoffungi number: FoF 01155.
Etymology: The specific epithet centaureae-diffusae is based on the host plant species from which the fungus was isolated.
Holotype: MFLU 15-1521.
Saprobic on dead stems of Centaurea diffusa Lam. Sexual morph: Ascomata 170–360 μm high, 146–290 μm diam., solitary or in groups of 2–10, erumpent through host tissue, semi-immersed or nearly superficial, uniloculate, globose to subglobose, coriaceous, black, ostiolate. Ostiole periphysate, papillate, black. Periphyses aseptate, wide, with a blunt apex, hyaline. Peridium 40–70 μm (x̄ = 50 μm, n = 10) wide, composed of 5–10 rows of scleroplectenchymatous cells, outer part thin, amorphous and black, central part widest, comprising thick-walled cells of textura globularis, inner layer composed of flattened cells of textura angularis. Hamathecium comprising 3–4 μm wide, dense, hyaline, distinctly septate, branched, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 130–170 × 11–13 μm (x̄ = 150 × 12 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-subclavate, with a short bulbous pedicel, rounded at the apex. Ascospores 76–132 × 2.2–4 μm (x̄ = 104 × 3.2 μm, n = 40), fasciculate, filiform, multi-septate, constricted at the apical septum, apical cell swollen, conical, yellowish brown, smooth-walled, with a mucilaginous cap. Asexual morph: undetermined.
Material examined: Russia, Rostov region, Shakhty city, 20th anniversary of Red Army urban microdistrict, Solyonaya Balka hollow, steppe on stony slope, on dead stems of Centaurea diffusa Lam. (Asteraceae), 17 June 2014, T. Bulgakov RU-83 (MFLU 15-1521, holotype); ibid., (GZAAS, isotype); ex-type living cultures MFLUCC 14-0992, ICMP. Russia, Rostov region, Oktyabrsky district, Persianovsky rural settlement, ruderal plant community on railroad embankment, on dead stems of Centaurea diffusa Lam., 04 June 2014, T. Bulgakov RU-111 (MFLU 15-0009, paratype), ex-type living culture MFLUCC 14-1158 (Fig. 6).
Alternariaster centaureae-diffusae (MFLU 15-1521) a, b Appearance of ascomata on host substrate. c. Vertical section through ascoma. d. Close-up of the peridium. e. Cellular pseudoparaphyses f–h. Immature and mature asci. i. Ascospore. j, k Ascospores in Indian ink. Scale bars: b = 500 μm, c = 200 μm, d–I = 50 μm, j, k = 20 μm
Notes: Alternariaster centaureae-diffusae is the first report of a sexual morph in the genus Alternariaster and is introduced as a new species based on both morphology and phylogeny. Alternariaster centaureae-diffusae forms a robust clade sister to A. bidentis and A. helianthi with high bootstrap support (Figs 1, 2). Alternariaster centaureae-diffusae shares similarities with the family type of Leptosphaeriaceae, Leptosphaeria doliolum in having ascomata with a peridium of scleroplectenchymatous cells. Alternariaster centaureae-diffusae differs from Leptosphaeria and other genera in the family in having long filiform, multi-septate ascospores.
Heterospora (Boerema et al.) Gruyter et al., Stud. Mycol. 75: 18 (2012).
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata 100–250 μm wide, pycnidial, immersed to semi-immersed, globose to subglobose, black with one or two distinct ostiole. Ostiole sometimes papillate or with elongated neck. Conidiomata wall composed of several layers of cells of textura angularis. Conidiogenous cells phialidic, subglobose to short conical. Conidia hyaline, aseptate, oblong to ellipsoidal. Sclerotia sometimes produced (van der and Kesteren 1979).
Type species: Heterospora chenopodii (Westend.) Gruyter et al., Stud. Mycol. 75: 18 (2012).
Basionym: Phyllosticta chenopodii Westend., Bull. Acad. Roy. Sci. Belgique Ser. 2, 2: 567. 1857; not Phyllosticta chenopodii Sacc., Syll. Fung. 3: 55. 1884
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata 100–550 μm wide, pycnidial, immersed to semi-immersed, globose to subglobose, black with one or two distinct ostiole. Ostiole sometimes papillate or with elongated neck. Conidiomata wall 15–60 μm wide, composed of 3–5 layers of cells of textura angularis, pale yellowish brown. Conidiogenous cells 4–8 × 4–6 μm phialidic, subglobose to short conical. Conidia 4–7 × 1–3 μm, hyaline, aseptate, oblong to ellipsoidal with two to many guttules (van der Aa and van Kesteren 1979).
Notes: Initially Heterospora was treated as a section of Phoma by Boerema (1997), and was raised to generic rank by De Gruyter et al. (2013) to accommodate two species of Phoma sect. Heterospora that cluster in the Leptosphaeriaceae, i.e. H. chenopodii and H. dimorphospora. All other species of Phoma sect. Heterospora clustered in the family Didymellaceae (Aveskamp et al. 2010). Heterospora is closely related to Subplenodomus and no sexual morph is known for this genus (De Gruyter et al. 2013).
In our phylogeny Heterospora chenopodii and H. dimorphospora formed a separate clade basal to the Alternariaster and Sphaerellopsis clades (Fig. 2). Therefore, we treat Heterospora as a separate genus in Leptosphaeriaceae (Fig. 7).
Heterospora chenopodii (redrawn from Phoma chenopodiicola in De Gruyter et al. 1993, Fig. 25.). Scale bars: 10 μm
Leptosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(4): 234 (1863).
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata solitary, scattered or in small groups, erumpent to superficial, globose to subglobose, broadly conical, small- to medium-sized, smooth, easily removed from the host substrate, with a flattened base, black, coriaceous, ostiolate. Ostiole apex with a well developed, conical, shiny, papilla, ostiolar canal filled with periphyses, dark brown to black. Peridium composed of two strata, outer stratum composed of small, thick-walled, scleroplectenchymatous cells of textura angularis, surface heavily pigmented, thinner at the apex, wide at the sides, inner layer composed of subhyaline or light brown, scleroplectenchymatous cells of textura angularis, cells near the base comparatively larger. Hamathecium comprising 1.5–3 μm wide, dense, long, septate, cellular pseudoparaphyses, branching and anastomosing above the asci, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical, pedicel furcate, rounded at the apex, with an ocular chamber. Ascospores uni- or bi-seriate, partially overlapping, reddish to yellowish brown, narrowly fusoid with sharp to narrowly rounded ends, 3-septate, constricted at each septum, cell above central septum widest, smooth-walled, lacking a mucilaginous sheath. Asexual morph: Coelomycetous. Conidiomata depressed globose with a flattened base and cylindrical neck, wall scleroplectenchymatous. Conidia ellipsoidal to subcylindrical (Boerema et al. 1994; Hyde et al. 2011).
Type species: Leptosphaeria doliolum (Pers.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(4): 234 (1863).
≡ Sphaeria doliolum Pers., Icon. Desc. Fung. Min. Cognit. (Leipzig) 2: 39 (1800)
Saprobic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata 340–460 μm high × 360–500 μm diam. (x̅ = 390 × 440 μm, n = 10), solitary, scattered or in small groups, erumpent to superficial, globose to subglobose, smooth, easily removed from the host substrate, with a flattened base, black, coriaceous, usually with 2–4 ring-like ridges surrounding the ascomata surface, ostiolate. Ostiole apex with a conical, shiny, well developed papilla, ostiolar canal filled with periphyses, dark brown to black. Peridium 85–110 μm wide at sides, thinner at the apex, comprising two types of cells, outer layer composed of small thick-walled cells of textura angularis, cell wall up to 8 μm thick, surface heavily pigmented and inner lightly pigmented, apex cells smaller, walls thicker, and cells more heavily pigmented, inner layer composed of subhyaline relatively thinner-walled cells of textura angularis, 3–6 μm diam., wall up to 5 μm, cells near the base larger and wall thinner and paler. Hamathecium of 1.5–3 μm (x̅ = 2.5 μm, n = 10) wide, septate, and cellular pseudoparaphyses, branching and anastomosing, embedded in a gelatinous matrix. Asci 105–150 × 7–10 μm (x̅ = 130 × 8 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with short, bulbous pedicel, rounded at the apex, with a distinct ocular chamber. Ascospores 25–30 × 4–6 μm (x̅ = 27 × 5 μm, n = 30), uni- or bi-seriate, partially overlapping, reddish to yellowish brown, narrowly fusoid with sharp to narrowly rounded edges, 3-septate, constricted at each septum, cell above central septum widest, smooth-walled, lacking a mucilaginous sheath, guttulate. Asexual morph: undetermined.
Material examined: England, Kellie Castle, on dead stem, 25 June 2012, J.E. Taylor, MFLU 15-1875 (Fig. 8).
Notes: Leptosphaeria was introduced by Cesati and de Notaris (1863) with 26 species and L. doliolum was treated as the lectotype species for the genus (Shearer et al. 1990). Material observed by Persoon (1800), based on collection of 12 specimens of Sphaeria doliolum present in Nationaal Herbarium Netherlands, Leiden University (L) was selected to lectotypify S. doliolum and thereby L. doliolum (Shearer et al. 1990). Leptosphaeria was described with ascospores being ellipsoid or fusoid, with one to many septa, and hyaline to dark brown (Crane and Shearer 1991). Höhnel (1909) divided the genus based on centrum structure into three genera, viz. Leptosphaeria, Scleropleella and Nodulosphaeria (Zhang et al. 2012). By giving priority to the ascospore characters as well as pseudothecial and centrum structure, Müller (1950) subdivided Leptosphaeria into four sections and this treatment was modified by Munk (1957), who named these four sections as section I (Eu-Leptosphaeria), section II (Para-Leptosphaeria), section III (Scleropleella) and section IV (Nodulosphaeria). The genus has been included in Leptosphaeriaceae (Barr 1987; Eriksson and Hawksworth 1991) or Phaeosphaeriaceae (Eriksson and Hawksworth 1986). Even though Leptosphaeria shares some similar morphological characters with Amarenomyces, Bricookea, Diapleella, Entodesmium, Melanomma, Nodulosphaeria, Paraphaeosphaeria, Passeriniella, Phaeosphaeria and Trematosphaeria, it differs in producing ascomata on dicotyledonous hosts, in having cylindrical asci with short bulbous pedicels and smooth-walled, fusoid, multi-septate ascospores. Recent studies based on multi-gene analysis showed that Leptosphaeria clustered within the order Pleosporales, in the family Leptosphaeriaceae. Species of Leptosphaeria (including the type of Leptosphaeriaceae) and Neophaeosphaeria formed a paraphyletic clade with moderate bootstrap support (Schoch et al. 2009; Zhang et al. 2012; Hyde et al. 2013).
Neophaeosphaeria forms a distinct clade outside the family Leptosphaeriaceae in our phylogeny. Therefore we exclude it from the family.
We carried out a separate phylogenetic study for the Leptosphaeria doliolum species complex to show the placement of some newly introduced species and to show the placement of our fresh collections of L. doliolum and to resolve the complexity of the genus and its allied species. Our study concluded that the fresh collection L. doliolum (MFLU 15-1875) is morphologically and phylogenetically 100 % similar to the type strain of L. doliolum (CBS 541.66), thus representing additional collection for L. doliolum. Moreover in our phylogeny we observed that clades B–L (Fig. 3) representing L. veronicae, L. sydowii, L. errabunda, L. macrocapsa, L. italiensis, L. slovacica, L. pedicularis, L. conoidea, L. sclerotioides, and L. cichorium are closely related to L. doliolum species complex, thus we confined them to Leptosphaeria sensu stricto.
During the study we found a novel sexual species of Leptosphaeria from Italy which formed the sexual morph in culture and is described below.
Leptosphaeria cichorium Phukhamsakda, Camporesi, Ariyawansa & K.D. Hyde, sp. nov. ,
Index Fungorum number: IF551463, Facesoffungi number: FoF 01156.
Etymology: The specific epithet cichorium is based on the host genus from which the taxon was isolated.
Holotype: MFLU 15-1406.
Saprobic on dead stem of Cichorium intybus L. Sexual morph: Ascomata 206–240 μm high × 251–363 μm diam. (x̅ = 190 × 328 μm, n = 10), solitary, scattered or in small groups, erumpent to superficial, globose to subglobose, small- to medium-sized, smooth, easily removed from the host substrate, with a flattened base, black, coriaceous, ostiolate. Ostiole apex dark brown to black, conical, shiny, with a well-developed papilla, ostiolar canal filled with periphyses. Peridium 2.5–7.5 μm wide at sides, composed of two layers, outer layer composed of small thick-walled cells of textura angularis, thinner at the apex, wide at sides, inner layer composed of subhyaline or light brown relatively thin-walled cells of textura angularis, cells near the base comparatively larger. Hamathecium of 1–2 μm (x̅ = 1.5 μm, n = 20) wide, long, septate, and cellular pseudoparaphyses, branching and anastomosing above the asci, embedded in a gelatinous matrix. Asci 71–115 × 5–8 μm (x̅ = 95 × 7 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, rounded at the apex, with an ocular chamber. Ascospores 11–20 × 3–6 μm (x̅ = 16 × 5 μm, n = 50), uni- or bi-seriate, partially overlapping, reddish to yellowish brown, fusoid with sharp to narrowly rounded edges, 3-septate, slightly constricted at each septum, cell above central septum slightly wider, smooth-walled, lacking a sheath. Asexual morph: Produced on sterilized bamboo pieces on water agar. Coelomycetous. Conidiomata 189–200 × 196–220 μm (x̅ = 190 × 205.9 μm, n = 10), pycnidial, superficial, immersed in media, globose to subglobose, black, without an ostiole. Conidiomata wall 15–25 μm wide, composed of muti-cell layer of textura angularis, pale yellowish brown. Conidiogenous cells 2–5 × 2–4 μm (x̅ = 3.4 × 2.6 μm, n = 30), enteroblastic, phialidic, determinate, discrete, subglobose to short conical. Conidia 3–6 × 1–3 μm (x̅ = 3.7 × 1.3 μm, n = 50), hyaline, aseptate, oblong to ellipsoidal, thin-walled, smooth, guttulate.
Material examined: Italy, Province of Forlì-Cesena [FC], Fiumicello - Premilcuore, on dead stem of Cichorium intybus, 29 August 2014, E. Camporesi IT 2067, (MFLU 15-1406, holotype); ibid. (HKAS88970, isotype); − ex-type living culture, MFLUCC 14–1063, BRIP (Fig. 9).
Leptosphaeria cichorium a, b. Appearance of ascomata on host surface. c. Section of ascoma. d. Ostiole with periphyses. e. Close-up of the peridium. f. Pseudoparaphyses g–i. Immature and mature asci with short bulbous pedicels. j–l. Ascospores. m. germinating ascospore n. Conidiomata on host substrate. o. Close-up of the conidioma wall. p. Section of conidioma. q–s. Conidiogenous cells. t. Conidia Scale bars: a = 200 μm, b = 100 μm, c–d = 50 μm, e–h = 20 μm, i–l = 10 μm, o– t = 5 μm
Notes: Leptosphaeria cichorium is introduced here based on both morphology and phylogeny. Leptosphaeria cichorium is typical of Leptosphaeria in having a peridium of schleroplectenchymatous cells and reddish to yellowish brown, fusoid, 3-septate ascospores.
Phylogenetically Leptosphaeria cichorium forms a robust clade sister to L. slovacica, but can be separated from this and other species in the genus with high bootstrap supports (Fig. 2). Morphologically L. cichorium is similar to L. slovacica, but differs in its comparatively smaller ascospores (11–20 versus 18–22) and clear ascomatal ostiole with periphyses.
Neoleptosphaeria Ariyawansa & K.D. Hyde, gen. Nov.
Index Fungorum number: IF551464, Facesoffungi number: FoF 01157.
Etymology: The generic epithet, neo (Lat., new), refers to the similarity to Leptosphaeria.
Pathogenic on wood, bark and fruits of herbaceous or woody plants in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata (350–)400–500 μm wide, semi-immersed to immersed, globose, papillate, or with a short cylindrical neck. Conidiomata wall composed of randomly arranged, polygonal, scleroplectenchyma cells. Conidiogenous cells well differentiated, cone-shaped. Conidia ovoid-oval to subcylindrical, eguttulate or with 1–2 min guttules.
Notes: Neoleptosphaeria is introduced to accommodate Leptosphaeria rubefaciens (Togliani) Gruyter et al., in Leptosphaeriaceae, and it is monotypic. Phylogenetically Neoleptosphaeria is close to Pseudoleptosphaeria, introduced in this study, and both genera form distant clades from the Leptosphaeria sensu stricto clade. This was also shown by Alves et al. (2013) and De Gruyter et al. (2013). Neoleptosphaeria differs from Pseudoleptosphaeria in the structure of the conidiomata wall (scleroplectenchyma versus pseudoparenchymatous), nature of the spore wall (guttules versus smooth) and the host (usually on Malus spp. versus Populus tremuloides). Therefore, to provide a stable taxonomy we introduce Neoleptosphaeria.
Type species: Neoleptosphaeria rubefaciens (Togliani) Ariyawansa & K.D. Hyde, comb. Nov.
Index Fungorum number: IF551465, Facesoffungi number: FoF 01158.
Basionym: Phoma rubefaciens Togliani, Annali Sper. agr., N.S. 7: 1626 (1953).
≡ Leptosphaeria rubefaciens (Togliani) Gruyter et al., in Gruyter et al., Stud. Mycol. 75: 19 (2012).
Pathogenic on wood, bark and fruits of herbaceous or woody plants in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata (350–)400–500 μm wide, semi-immersed to immersed, globose, papillate or with a short cylindrical neck. Conidiomata wall composed of randomly arranged polygonal scleroplectenchyma cells. Conidiogenous cells well differentiated, cone-shaped. Conidia (3.5–)4–5 × 2–2.5(−3) μm, ovoid-oval to subcylindrical, eguttulate or with 1–2 min guttules (from Boerema et al. 1994) (Fig. 10).
Neoleptosphaeria rubefaciens (redrawn from Phoma rubefaciens in Boerema et al., 1994, Fig. 275.). a. Conidiomata. b. Conidia. Scale bars: a, b = 10 μm
Paraleptosphaeria Gruyter et al., in Gruyter et al., Stud. Mycol. 75: 20 (2012).
Saprobic, fungicolous or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata immersed, subglobose, solitary or aggregated, thick-walled, ostiolate, unilocular. Peridium thick-walled, comprising several scleroplectenchymatous cell layers, the outer layer heavily encrusted with pigment and often longitudinally striate on the surface, inner layer with flattened, light brown to dark brown cells. Hamathecium of dense 0.5–2 μm wide, long, septate, cellular pseudoparaphyses. Asci 8-spored, bitunicate, cylindric-clavate to broadly ellipsoidal, with bulbous pedicel, apically rounded, with an ocular chamber. Ascospores biseriate, hyaline to yellow brownish, fusiform and tapering to the ends, transversally 1–5-septate, slightly constricted at the central spetum, cell above central septum swollen, with or lacking a mucilaginous sheath. Asexual morph: Conidiomata pycnidial, globose to subglobose, scleroplectenchymatous, with papillate pore, unilocular. Conidiogenous cells phialidic, ampulliform to doliiform. Conidia hyaline, aseptate, oblong to ellipsoidal. Sclerotia sometimes produced (De Gruyter et al. 2013).
Type species: Paraleptosphaeria nitschkei (Rehm ex G. Winter) Gruyter et al., in Gruyter et al., Stud. Mycol. 75: 20 (2012).
≡ Leptosphaeria nitschkei Rehm ex G. Winter, Flora, Regensburg 55: 510 (1872)
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata (296–)328–362 μm high × (406–)420–423 μm diam. (x = 329 × 416 μm, n = 5), superficial, solitary or scattered, subglobose to obpyriform, dark brown, without subiculum covering the host. Ostiole with protruding papilla. Peridium 38–)45–56(−64) μm wide, comprising several scleroplectenchymatous cell layers, the outer layer heavily encrusted with pigment and often longitudinally striate on the surface, inner layer with flattened, light brown to dark brown cells. Hamathecium comprising 0.5–1 μm wide, cylindrical to filiform, pseudoparaphyses. Asci (80–)97–102(−107) × 9–10 μm (x̅ = 98 × 9 μm, n = 10), 8-spored, bitunicate, cylindric-clavate, slightly curved, with short, bulbous pedicel, apically rounded, with an ocular chamber (ca. 1–1.5 μm wide). Ascospores 27–29(−34) × 4.5–6 μm (x̅ = 29 × 5 μm, n = 10), 1–2-seriate, overlapping, hyaline, fusiform and tapering to the ends, 3-euseptate, slightly constricted at the central septum, cell above central septum swollen, straight or slightly curved, thick and smooth-walled, lacking a mucilaginous sheath. Asexual morph: Coelomycetous. Conidiomata 435–455(−475) × (276–)300–330 μm (x̅ = 455 × 305 μm, n = 5), pycnidial, globose to subglobose, solitary, peridium 30–35(−38) μm, thick-walled, scleroplectenchymatous. Conidiogenous cells 3–4 × 5–6 μm, phialidic. Conidia 1.5–2 × (−12)15–20 μm, hyaline, aseptate, cylindrical to filiform.
Material examined: Italy, Province of Forlì-Cesena [FC], Passo la Calla, Santa Sofia, on stems of Petasites sp. (Asteraceae), 14 July 2012, E. Camporesi IT563 (MFLU 14–0021, reference specimen), living culture, MFLUCC 13-0688. Italy, Province of Forlì-Cesena (FC), Monte Falco, on decaying grass stems of Petasites sp., 21 May 2013, E. Camporesi IT648 (MFLU 13–0644), DNA was extracted from the fruiting body (Fig. 11).
Notes: De Gruyter et al. (2013) introduced Paraleptosphaeria to accommodate Leptosphaeria nitschkei and its allied species in the family Leptosphaeriaceae. Munk (1957) treated Leptosphaeria as four sections as section I (Eu-Leptosphaeria), section II (Para-Leptosphaeria), section III (Scleropleella) and section IV (Nodulosphaeria), but section Para-Leptosphaeria is an invalid taxon and it is also a heterogenous group. The section was separated from Eu-Leptosphaeria, which comprised the generic type species L. doliolum (De Gruyter et al. 2013). Leptosphaeria nitschkei was considered as typical of section Eu-Leptosphaeria (Müller and von Arx 1950). Molecular studies, including the present study show that L. nitschkei is only distantly related to L. doliolum. The seperation of Leptosphaeria in sections Eu-Leptosphaeria and Para-Leptosphaeria cannot be upheld from a evolutionary point of view. Section Eu-Leptosphaeria, with L. agnita and L. maculans (Munk 1957), group in Plenodomus. Liu et al. (2015) designated a reference specimen for Paraleptosphaeria nitschkei to provide a stable taxonomy and phylogeny. In the present study we include another collection of Para. nitschkei from Petasites sp., whch we illustrate. Thus we recognise Paraleptosphaeria as a well-supported genus in the family Leptosphaeriaceae based on both morphology and phylogeny.
In this study we also introduce a novel species, Paraleptosphaeria rubi, based on both morphology and phylogeny.
Paraleptosphaeria rubi Mapook, Camporesi, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF551466, Facesoffungi number: FoF 01159.
Etymology: The specific epithet rubi is based on the host genus from which the fungus was isolated.
Holotype: MFLU 15-1127.
Fungicolous or saprobic on dead branch of Rubus sp. Sexual morph: Ascomata 150–200 μm high × 200–250 μm diam. (x̅ = 180 × 215 μm, n = 5), immersed or erumpent, solitary or scattered, globose to subglobose, brown to dark brown, smooth. Ostiole with protruding papilla with periphyses. Peridium 10–20 μm wide, consisting of a single stratum, comprising brown to dark brown, scleroplectenchymatous cells of textura angularis. Hamathecium comprising 1.5–2 μm wide, cylindrical to filiform, septate, branched, cellular pseudoparaphyses. Asci 60–80 × 10–15 μm (x̅ = 75 × 12 μm, n = 10), 8-spored, bitunicate, cylindric-clavate, slightly curved, with a short, bulbous pedicel, apically rounded, with an ocular chamber. Ascospores 20–30 × (5–)9–15 μm (x̅ = 25 × 10 μm, n = 20), 1–2-seriate, overlapping, hyaline, ellipsoidal, 1-septate, deeply constricted at the septum, upper cell widest and tapering toward rounded ends, straight or slightly curved, guttulate, surrounded by hyaline, mucilaginous sheath. Asexual morph: undetermined.
Material examined: Italy, Province of Forlì-Cesena [FC], Dovadola, on dead branch of Rubus sp., 17 January 2014, E. Camporesi IT 1870 (MFLU 15-1127, holotype); ibid., (GZAAS, isotype); − extype living culture (MFLUCC 14-0211, ICMP).
Notes: Paraleptosphaeria rubi sits well with the generic concept of Paraleptosphaeria in having immersed, subglobose ascomata, thick-walled peridium comprising dark brown cells of textura angularis with cellular pseudoparaphyses, and cylindric-clavate asci bearing fusiform, transversally septate ascospores.
Paraleptosphaeria rubi is phylogenetically closely related to the type species of Paraleptosphaeria, P. nitschkei. Paraleptosphaeria rubi differs from P. nitschkei based on the position of the ascomata on the host (immersed versus superficial), ascospore septation (1-septate versus 1–3-septate) and habit (fungicolous versus saprobic). Furthermore, our new taxa has a mucilaginous sheath surrounding the ascospores, whereas the spores of P. nitschkei lack a sheath (Hyde et al. 2015).
The habit of this species is hard to determine. The ascoma appear to form within a dead ascoma of another ascomycete. This is evident by the inner “neck”, forming within the old ascoma. Whether the species is fungicolous, just using this space or forms a second “neck could not be established from the specimens (Fig. 12).
Paraleptosphaeria rubi a, b. Appearance of the ascomata on substrate. c. Section through ascoma. d. Ostiolar canal filled with periphyses. e. Peridium. f. Pseudoparaphyses. g–j. Asci k-m. Ascospores, N, surrounded by hyaline gelatinous sheath in Indian ink. o. Germinating ascospore. Scale bars: a = 500 μm, b = 200 μm, c, g–j = 50 μm, d = 20 μm, e, k–o = 10 μm, f = 5 μm
Plenodomus Preuss, Linnaea 24: 145 (1851).
≡ Phoma sect. Plenodomus (Preuss) Boerema, Kesteren & Loer., Trans. Brit. Mycol. Soc. 77: 61. 1981.
Saprobic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata solitary, scattered or in small groups, erumpent to superficial, subglobose, broadly or narrowly conical, small- to medium-sized, dark brown to black, smooth, ostiolate. Ostiole apex with a conical, well developed papilla. Peridium composed of two to several layers of scleroplectenchymatous cells. Hamathecium comprising 1–3 μm wide, long, septate, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, rounded at the apex, with an ocular chamber, short pedicel. Ascospores cylindrical to ellipsoidal, yellowish brown, multi-septate, not or slightly constricted at septa, cell above central septum slightly wider, guttulate and lacking a mucilaginous sheath. Asexual morph: Coelomycetous. Conidiomata. Type 1: solitary, scattered or in small groups, erumpent to superficial, subglobose or flask shaped with a broad base, mostly black, ostiolate. Ostiole apex with a long neck and well developed poroid papilla. Type 2: solitary, scattered or in small groups, erumpent to superficial, mostly subglobose, ostiolate. Ostiole slightly papillate with a narrow pore or opening via a rupture. Conidiomata wall composed of several layers with thick-walled cells of textura angularis, surface heavily pigmented. Conidia hyaline, aseptate, ellipsoidal to subcylindrical.
Type species: Plenodomus lingam (Tode: Fr.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 120: 463. 1911
Saprobic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata 200–250 μm high × 300–500 μm diam., solitary, scattered or in small groups, semi-immersed to immersed, subglobose to depressed globose, broadly or narrowly conical, small- to medium-sized, dark brown to black, smooth, ostiolate. Ostiole apex with a conical, well developed dark brown to black papilla, ostiolar canal filled with periphyses. Peridium 8–12 μm wide at sides, composed of two layers, scleroplectenchymatous, outer layer composed of small thick-walled cells of textura angularis, surface heavily pigmented, thinner at the apex, wide at sides, inner layer composed of subhyaline or light brown cells of textura angularis, cells near the base comparatively larger. Hamathecium comprising 1.5–3 μm (x̅ = 2.5 μm, n = 10) wide, long, septate, cellular pseudoparaphyses, branching and anastomosing, embedded in a gelatinous matrix. Asci 90–110 × 10–12 μm, 8-spored, bitunicate, fissitunicate, cylindrical, rounded at the apex, short pedicel with an ocular chamber. Ascospores 35–70 × 5–8 μm, uni-seriate, partially overlapping, yellowish brown, cylindrical to ellipsoidal, 5-septate, not or slightly constricted at each septum, guttulate, lacking a mucilaginous sheath. Asexual morph: Coelomycetous, with two types of Conidiomata. Type 1: 150–350 μm wide, solitary, scattered or in small groups, erumpent to superficial, subglobose or flask-shaped, with a broad base, mostly black, ostiolate. Ostiole apex with a long neck and well developed poroid papilla. Type 2: 300–700 μm wide, solitary, scattered or in small groups, erumpent to superficial, mostly subglobose, ostiolate. Ostiole slightly papillate with a narrow pore or opening via a rupture. Conidiomata wall composed of several layers with thick-walled cells of textura angularis, surface heavily pigmented (often termed scleroplectenchyma). Conidia 3–5 × 1–2 μm, hyaline, aseptate, ellipsoidal to subcylindrical, rarely with two small polar guttules (Sivanesan 1984) (Fig. 13).
Notes: The genus Plenodomus was introduced by Preuss (1851) and is typified by P. rabenhorstii. The combination P. lingam as published by von Hohnel (1911) was favoured over P. rabenhorstii Preuss (1851) by Boerema & Kesteren (1964)because the type material of P. rabenhorstii had been lost during the World War II. Therefore, P. rabenhorstii was treated as a nomen dubium by De Gruyter et al. (2013). We follow von Hohnel (1911)proposal by treating Plen. lingam as the type species for the genus Plenodomus. The asexual and sexual connection between the type of Plenodomus, P. lingam and its sexual morph Leptosphaeria maculans has been proven by single spore isolation (Boerema and Kesteren, 1964. This was also confirmed by molecular data by De Gruyter et al. (2013) as well as in this study (Fig. 2).
Currently 93 epithets of Plenodomus are listed in Index Fungorum (2015). We introduce two novel species of Plenodomus, P. guttulatus and P. salvia from Germany and Italy, respectively, based on morphology and phylogeny.
Plenodomus guttulatus Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF551467, Facesoffungi number: FoF 01160.
Etymology: The name refers to the nature of the ascospore wall (containing, or appearing to be sprinkled with drops of oil).
Holotype: MFLU 15-1876.
Saprobic on dead stems in terrestrial habitats. Sexual morph: Ascomata 230–325 μm high × 260–310 μm diam (\( \overline{x} \)= 298 × 286 μm, n = 5), solitary, scattered or in small groups, appearing as small, raised black dots on host surface, semi-immersed to immersed, subglobose to depressed globose, broadly or narrowly conical, small- to medium-sized, dark brown to black, smooth, ostiolate. Ostiole with a conical, black, well developed papilla, ostiolar canal filled with periphyses. Peridium 48–88 μm wide at the sides, comprising two layers of scleroplectenchymatous cells, outer layer composed of heavily pigmented, thick-walled cells of textura angularis, inner layer composed of subhyaline or light brown cells of textura angularis, cells near the base comparatively larger. Hamathecium 1–1.5 μm (x̅ = 1.25 μm, n = 10) wide, long, septate, cellular pseudoparaphyses, branching and anastomosing between the asci, embedded in a gelatinous matrix. Asci 70–72 × 5–8 μm (\( \overline{x} \)= 71 × 7 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with a bulbous pedicel, rounded at the apex, with an ocular chamber. Ascospores 22–24 × 2–3 μm (\( \overline{x} \)= 23 × 2.8 μm, n = 40), uni-seriate, partially overlapping, yellowish brown, long fusiform, 5-septate, not or slightly constricted at each septum, cell above central septum slightly wider, guttulate only at the central cells, lacking a mucilaginous sheath. Asexual morph: undetermined.
Material examined: Germany, Frankfurt, on dead stem, 28 November 2013, H.A. Ariyawansa (MFLU 15-1876, holotype); ibid., (GZAAS, isotype);.
Notes: Single spore isolation was not successful and DNA was extracted directly from the fruiting body. Therefore no living culture is available (Fig. 14).
Plenodomus guttulatus (holotype) a. Appearnce of ascomata on host specimen. b. Close-up of ascomata with ostiole. c, d. Sections of ascomata. e. Cellular pseudoparaphyses f. Section of peridium. g-j. Asci with 8 ascospores. k-m Mature and immature ascospores guttulate only at the central cells. Scale bars: c = 75 μm, d = 50 μm, e = 10 μm, f = 20 μm, g-i = 25 μm, j = 35 μm, k–m = 5 μm
Plenodomus salviae Thambugala, Camporesi, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF551468, Facesoffungi number: FoF 01161.
Etymology : The specific epithet salviae is based on the host genus from which the fungus was isolated.
Holotype: MFLU 15–0515.
Sarobic on stems of Salvia glutinosa L. in terrestrial habitats. Sexual morph: Ascomata 130–250 μm high × 150–280 μm diam. (\( \overline{x} \)= 172 × 233 μm, n = 5), solitary, scattered or in small groups, semi-immersed to immersed, subglobose to depressed globose, broadly or narrowly conical, black, coriaceous, ostiolate. Ostiole apex with a well developed, dark brown to black, conical, papilla, ostiolar canal filled with periphyses. Peridium 30–68 μm (\( \overline{x} \)= 50.8 μm, n = 10), composed of two scleroplectenchymatous strata, outer stratum composed of small, thick-walled cells of textura angularis, surface heavily pigmented, inner stratum composed of subhyaline or light brown cells of textura angularis, cells near the base comparatively larger. Hamathecium of 1.5–3 μm (x̅ = 2.5 μm, n = 10) wide, long, septate, cellular pseudoparaphyses, branching and anastomosing, embedded in a gelatinous matrix. Asci 57–88 × 8.6–11.4 μm (\( \overline{x} \)= 72.9 × 10.2 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical, with short bulbous pedicel, rounded at the apex, with a distinct ocular chamber. Ascospores 30–48 × 3.1–4.3 μm (\( \overline{x} \) = 40.6 × 3.8 μm, n = 15), uni- or bi-seriate, partially overlapping, yellowish brown, cylindric-fusiform, 5-septate, not or slightly constricted at each septum, guttulate, lacking a mucilaginous sheath. Asexual morph: undetermined.
Material examined: Italy, Province of Forlì-Cesena [FC], Campigna - Santa Sofia, on Salvia glutinosa (Lamiaceae), 4 October 2012, E. Camporesi IT 773 (MFLU 15–0515, holotype); ibid., (GZAAS, isotype); ex-type living culture, MFLUCC 13–0219, CFTCC (Fig. 15).
Notes: Plenodomus guttulatus and P. salviae are introduced based on morphological differences coupled with phylogeny. Plenodomus guttulatus and P. salviae are typical of Plenodomus in having a scleroplectenchymatous peridium, and cylindric-fusiform yellowish brown, transversely septate ascospores. In the phylogenetic analysis, P. guttulatus forms a distinct clade sister to P. wasabiae and P. hendersoniae, while P. salviae forms a robust clade sister to Plenodomus sp. (PHY 30) with relatively high bootstrap support (71 % and 96 %).
Pseudoleptosphaeria Ariyawansa & K.D. Hyde, gen. Nov.
Index Fungorum number: IF551469, Facesoffungi number: FoF 01162.
Etymology: In reference to its similarity with Leptosphaeria.
Parasitic on bark of gall on trunk of Populus tremuloides Michx. Sexual morph: undetermined. Asexual morph: Conidiomata immersed to superficial, solitary to gregarious, globose to pear-shaped, dark brown to black, pseudoparenchymatous, covered to varying degrees by hyaline to dark coloured hyphae. Conidiogenous cells phialidic, hyaline, globose to flask-shaped. Conidia enteroblastic, ellipsoidal to ovoid to oblong, hyaline, smooth-walled, unicellular.
Notes: During the re-disposition of phoma-like species in Pleosporales, De Gruyter et al. (2013) classified Phoma rubefaciens and Phoma etheridgei in the genus Leptosphaeria and suggested that these species are more distantly related to Leptosphaeria sensu stricto. In Alves et al. (2013) and in the present study the phylogenetic trees are similar (Figs 1-3). We introduce Pseudoleptosphaeria to accommodate Phoma etheridgei in family Leptosphaeriaceae. Phylogenetically Pseudoleptosphaeria is closely related to Neoleptosphaeria. They form a distinct clade sister to Neoleptosphaeria rubefaciens with high bootstrap support. Morphological differences between these two genera were discussed earlier in this paper.
Type species: Pseudoleptosphaeria etheridgei (L.J. Hutchison & Y. Hirats) Ariyawansa & K. D. Hyde, comb. nov.
Index Fungorum number: IF551470, Facesoffungi number: FoF 01163.
Basionym: Phoma etheridgei L.J. Hutchison & Y. Hirats., in Hutchison, Chakravarty et al., Can. J. Bot. 72(10): 1425 (1994).
≡ Leptosphaeria etheridgei (L.J. Hutchison & Y. Hirats.) Gruyter, Aveskamp & Verkley, in Gruyter et al., Stud. Mycol. 75: 19 (2012).
Pathogenic on bark of gall on trunk of Populus tremuloides. Sexual morph: undetermined. Asexual morph: Conidiomata (105–)120–270 μm high × (94–)120–200(−250) μm diam., immersed to superficial, solitary to gregarious, globose to pear-shaped, dark brown to black, pseudoparenchymatous, covered to varying degrees by hyaline to dark coloured hyphae. Conidiogenous cells 6–7 × 4–5 μm, phialidic, hyaline, globose to flask-shaped. Conidia 3–4.5(−5) × 1–1.8(−2) μm, enteroblastic, ellipsoidal to ovoid or oblong, hyaline, smooth-walled, unicellular (from Hutchison et al. 1994).
Sphaerellopsis Cooke, Grevillea 12(no. 61): 23 (1883).
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants or rust in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata eustromatic, pycnidioid, immersed, but becoming erumpent, locules often appearing as separate pycnidia, dark brown to black in vivo, pale brown to brown in vitro, uni- or multi-locular, each locule with a separate simple ostiole. Conidiomata wall basal wall composed of pale brown textura angularis, locular wall of dark brown, thick-walled textura angularis. Pseudoparaphyses when present hyaline, filiform, septate, with ends rounded, sometimes branching. Conidiophores reduced to conidiogenous cells, occasionally with a supporting cell. Conidiogenous cells phialidic, indeterminate, cylindrical to doliiform, hyaline to pale brown, smooth, often with 1–3 percurrent proliferations, or determinate with visible periclinal thickening. Conidia hyaline, becoming pale brown and irregularly verruculose, 0–1(−3)-euseptate, constricted at septa, apex obtuse, base truncate, straight, fusoid-ellipsoidal, occasionally Y-shaped or digitate; ends with mucoid polar appendages (type H sensu Nag Raj 1993). Microconidia subcylindrical to ellipsoid or globose, aseptate, smooth-walled, hyaline (Trakunyingcharoen et al. 2014).
Type species: Sphaerellopsis filum (Biv.) B. Sutton, Mycol. Pap. 141: 196 (1977).
Basionym: Sphaeria filum Biv., Stirp. Rar. Sic. 3: 12 (1815).
= Sphaerellopsis quercuum Cooke, Grevillea 12 (61): 23 (1883).
Saprobic or parasitic ditto in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata 300 μm diam., pycnidial, erumpent, aggregated, globose, dark brown with a central ostiole, exuding copious amounts of creamy orange conidia. Conidiomata wall composed of 3–6 layers of dark brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10 × 3–5 μm, brown, smooth, and thick-walled, ampulliform, to doliiform, with prominent periclinal thickening or several prominent, flaring, percurrent proliferations at the apex. Conidia (11–)14–16(−18) × (3–)4(−5) μm, hyaline, smooth-walled, guttulate, fusoid to fusoid-ellipsoid, 1(−2)-septate, usually constricted at septa, apex subobtuse, tapering at the base with a flattened scar, with funnel-shaped, mucoid appendages at each end (from Trakunyingcharoen et al. 2014) (Fig. 16).
Notes: The genus Sphaerellopsis was introduced by Sutton (1977) and is typified with S. filum (basionym; Sphaeria filum). Species of Sphaerellopsis are well-known mycoparasites occurring on a wide range of rusts and play a potential role as biocontrol agents (Trakunyingcharoen et al. 2014). The asexual and sexual morph connection between Eudarluca caricis, the generic type of Eudarluca and Sphaerellopsis filum was derived from ascospores growing on Puccinia extensicola-oenotherae (Mont.) Arthur on Carex sp. in Pennsylvania, USA (Keener 1951) and confirmed by Yuan et al. (1998). Yuan et al. (1998) grew ascospores of Eudarluca caricis on PDA and derived Sphaerellopsis conidia and conidiomata after 12 days. Zhang et al. (2012) suggested that Eudarluca caricis is compatible with Leptosphaeria species and mentioned that Eudarluca should be treated in Leptosphaeriaceae based on DNA sequence data and also morphological characters. Phookamsak et al. (2014) tentatively placed Eudarluca in Phaeosphaeriaceae based on its morphological characters which are typical of Phaeosphaeria as the peridium is thin-walled, and composed of pseudoparenchymatous cells, asci are cylindrical, subsessile to short pedicellate and ascospores are fusiform and phragmosporous.
In order to provide a stable taxonomy and phylogeny Trakunyingcharoen et al. (2014) designated a neotype for the generic type of Sphaerellopsis, S. filum and placed Sphaerellopsis in the family Leptosphaeriaceae. Moreover Trakunyingcharoen et al. (2014) considered Eudarluca as the sexual morph of Sphaerellopsis and proposed to synonymise Eudarluca under Sphaerellopsis by giving the priority to the oldest name.
During our phylogeny, the ex-neotype strain of S. filum (CBS 317.68) together with another two species, S. paraphysata and S. macroconidiale form a distinct clade sister to Alternariaster and Heterospora clades with high bootstrap support (89 %). Therefore, we accept Sphaerellopsis as a well supported genus in the family Leptosphaeriaceae and we maintain Eudarluca in Phaeosphaeriaceae following Phookamsak et al. (2014), until morphology coupled with molecular data can resolve the correct placement of this unusual genus.
Subplenodomus Gruyter et al., Stud. Mycol. 75: 23 (2012).
Saprobic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata pycnidial, globose to papillate, or with an elongate neck, solitary or aggregated, thin-walled pseudoparenchymatous, or thick-walled scleroplectenchymatous, ostiolate, unilocular. Conidiogenous cells phialidic, ampulliform to doliiform. Conidia hyaline, aseptate, ellipsoid to cylindrical (from De Gruyter et al. 2013).
Notes: The genus Subplenodomus was introduced by De Gruyter et al. (2013) to accommodate some phoma-like species in the order Pleosporales and classified in the family Leptosphaeriaceae based on morphology coupled with phylogeny. Although the genus is similar to Plenodomus in the production of thick-walled conidiomata, the conidiomata cell wall of Subplenodomus often remains pseudoparenchymatous, similar to the conidiomata wall of species of Phoma. In Plenodomus the conidiomata wall is scleroplectenchymatous (De Gruyter et al. 2013). Currently no sexual morph has been recorded for this genus.
Subplenodomus apiicola (Kleb.) Gruyter et al., S. drobnjacensis (Bubák) Gruyter et al., S. valerianae and S. violicola (Henn.) Gruyter et al., all have conidiomata with an elongated neck, similar to Plenodomus (De Gruyter et al. 2013). Conidiomata with scleroplectenchymatous walls have been only observed in S. drobnjacensis. Subplenodomus apiicolus, S. drobnjacensis and S. valerianae have relatively small conidia, up to 4.5 × 2 μm (De Gruyter & Noordeloos 1992) in congruence with many of the Plenodomus species.In contrast, S. violicola produces relatively large conidia, up to 11 × 3 μm (Boerema and Coworkers, 1993; De Gruyter et al. 2013).
Type species: Subplenodomus violicola (P. Syd.) Gruyter et al., in Gruyter et al., Stud. Mycol. 75: 23 (2012).
≡ Phoma violicola P. Syd., Hedwigia 38(Beibl.): (137) (1899)
Parasitic on leaves in terrestrial habitats. Sexual morph: undetermined. Asexual morph: Conidiomata 125–250 μm wide, pycnidial, globose to papillate, or with an elongated neck, solitary or aggregated, thin-walled, pseudoparenchymatous, unilocular, ostiolate. Ostiole consists of elongated neck, often covered with hypae. Conidiogenous cells 5–11 μm wide, phialidic, ampulliform to doliiform. Conidia 9–10 × 2–3 μm, hyaline, aseptate, ellipsoid to cylindrical, with two conspicuous guttules (from Boerema and Coworkers, 1993) (Fig. 17).
Excluded genus
Neophaeosphaeria M.P.S. Câmara et al., in Câmara et al., Mycol. Res. 107(5): 519 (2003).
Type species: Neophaeosphaeria filamentosa (Ellis & Everh.) M.P.S. Câmara, et al., in Câmara et al., Mycol. Res. 107(5): 519 (2003).
≡ Leptosphaeria filamentosa Ellis & Everh., J. Mycol. 4(8): 76 (1888)
Neophaeosphaeriaceae Ariyawansa & K.D. Hyde fam. nov.
Index Fungorum number: IF551471, Facesoffungi number: FoF 01164.
Saprobic or pathogenic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: Ascomata scattered or clustered in circular areas, immersed, depressed globose, with a small ostiolar pore slightly penetrating above the surface, under clypeus, coriaceous, papilla not conspicuous. Peridium comprising 3 layers of large, pigmented thin-walled, pseudoparenchymatous cells of textura angularis. Hamathecium of 1.5–2.5 μm wide, dense, septate, cellular pseudoparaphyses embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to oblong, with a short, broad, bulbous, furcate pedicel, apically rounded, with an ocular chamber, best seen in immature asci. Ascospores obliquely uniseriate and partially overlapping, yellowish brown, oblong to broadly fusiform, mostly 3-septate, verruculose. Asexual morph: Coelomycetous, coniothyrium-like. Conidiomata pseudoparenchymatous, sometimes stromatic. Conidiogenous cells lining entire locule, holoblastic, proliferating percurrently, usually resulting in conspicuous annellations. Conidia globose, ovoid or ellipsoid, aseptate, yellowish brown often becoming brown at maturity, verrucose to punctuate (from Camara et al. 2003).
Type genus: Neophaeosphaeria M.P.S. Câmara et al., in Câmara et al., Mycol. Res. 107(5): 519 (2003).
Saprobic or pathogenic in terrestrial habitats, presently only known for Yucca sp. Sexual morph: Ascomata scattered or clustered in circular areas, immersed, depressed globose, with a small ostiolar pore, slightly penetrating above the surface, under clypeus, coriaceous, papilla not conspicuous. Peridium comprising 3-layers of large pigmented thin-walled, pseudoparenchymatous, cells of textura angularis. Hamathecium of 1.5–2.5 μm wide dense, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to oblong, with a short, furcate, bulbous pedicel, apically rounded, with an ocular chamber. Ascospores obliquely uniseriate and partially overlapping, brown, ellipsoidal, 1–3-septate, constricted at the septum, cell above central septum widest, verruculose. Asexual morph: Coniothyrium-like. Conidiomata pseudoparenchymatous, sometimes stromatic. Conidiogenous cells lining entire locule. Conidiogenous cells holoblastic, proliferating percurrently, usually resulting in conspicuous annellations. Conidia globose, ovoid or ellipsoid, aseptate, yellowish brown, often becoming brown at maturity, verrucose to punctuate.
Notes: We introduce Neophaeosphaeriaceae to accommodate Neophaeosphaeria and its allied species in the suborder Pleosporineae, order Pleosporales, Dothideomycetes. Justification for the new family is based on combined gene analysis of the large and small subunits of the nuclear ribosomal RNA genes (LSU, SSU), ITS and two protein coding genes RPB2 and TEF1, as well as morphological characters. The main difference between Neophaeosphaeriaceae and its closest known linage Leptosphaeriaceae is the peridium. In Leptosphaeriaceae the peridium is scleroplectenchymatous, whereas in Neophaeosphaeriaceae it is pseudoparenchymatous.
Neophaeosphaeria has been referred to various families based on both morphology and phylogeny. The four Neophaeosphaeria species form a monophyletic clade based on combine gene analysis of ITS and SSU rDNA sequence data (Câmara et al. 2001; Checa et al. 2002), and they clustered in a clade including members of Phaeosphaeriaceae and Leptosphaeriaceae (Câmara et al. 2003). Neophaeosphaeria filamentosa, the generic type of Neophaeosphaeria, nested in Leptosphaeriaceae with low to moderate bootstrap values (Schoch et al. 2009; Zhang et al. 2009). De Gruyter et al. (2013) suggested that although Neophaeosphaeria is related to Coniothyrium based on the molecular data, Neophaeosphaeria probably belongs in a separate phylogenetic clade. Moreover De Gruyter et al. (2013) concluded that the grouping of N. filamentosa with the Coniothyrium species in their study was poorly supported and N. filamentosa proved to be more distantly related in previous molecular phylogenetic studies (Verkley et al. 2004; De Gruyter et al. 2013). Thus the placement of the Neophaeosphaeria was confused. Currently Neophaeosphaeria comprises five epithets in Index Fungorum (2015).
The type species of Neophaeosphaeria, N. filamentosa along with N. agaves Crous & Yáñez-Mora and N. quadriseptata (M.E. Barr) M.P.S. Câmara et al., form a separate clade sister to the familial clades of Cucurbitariaceae and Phaeosphaeriaceae. We therefore exclude Neophaeosphaeria from Leptosphaeriaceae and introduce a new family to accommodate this distinct lineage.
Type species: Neophaeosphaeria filamentosa (Ellis & Everh.) M.P.S. Câmara et al., Mycol. Res. 107(5): 519 (2003).
≡ Leptosphaeria filamentosa Ellis & Everh., J. Mycol. 4(8): 76 (1888)
Pathogenic on dead parts in living leaves of Yucca filamentosa L. Sexual morph: Ascomata 115–157 μm high × 115–186 μm diam. (x̅ = 140 × 155 μm, n = 10), scattered or clustered in circular areas, immersed, depressed globose, with a small ostiolar pore slightly penetrating the surface through the host epidermis, under clypeus, coriaceous, dark brown to black, papilla not conspicuous. Peridium 18–30 μm (x̅ = 24 μm, n = 10) thick, composed of thin-walled, pseudoparenchymatous, cells of textura angularis. Hamathecium comprising 1.5–2.5 μm wide, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 70–105 × 8–10 μm (x̅ = 85 × 10 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to oblong, with a short, broad pedicel apically rounded, 6–13 μm long, with an ocular chamber. Ascospores 12–15 × 4–5 μm (x̅ = 13 × 5 mm, n = 30), obliquely uniseriate and partially overlapping, yellowish brown, oblong, (1–)3-septate, constricted at the primary septum, cell above central septum widest, verruculose, containing four refractive globules. Asexual morph: Not observed in this collection.
Material examined: USA, New Jersey, Newfield, on dead parts in living leaves of Yucca filamentosa (Asparagaceae), July 1888, Ellis & Everhart (NY, holotype 00,914,294) (Fig 18).
Neophaeosphaeria filamentosa . a Herbarium label. b, c Close-up of ascomata d Close-up of peridium. e Arrangement of cellular pseudoparaphyses in hamathecium f-h Cylindrical asci with an indistinct ocular chamber. i-l Ascospores with verruculose ornamentation. Scale bar: d = 70 μm, e = 10 μm, f-h = 20 μm, m-r = 5 μm
Notes: Neophaeosphaeria was introduced by Câmara et al. (2003) when reassessing the Paraphaeosphaeria species with 3–4-septate ascospores. Asexual morphs produced ovoid to ellipsoid, aseptate, brown, verrucose to punctuate conidia, forming from percurrently proliferating conidiogenous cells. Neophaeosphaeria filamentosa was selected as the generic type. All species in Neophaeosphaeria are presently known from Yucca sp. (Asparagaceae).
Discussion
Molecular data together with morphology has provided the foundation for modern classification of the Kingdom Fungi, but have some limitations in application (Hibbett et al. 2007; Ariyawansa et al. 2014a, f; Boonmee et al. 2014; Hyde et al. 2014; Nilsson et al. 2014; Schoch et al. 2014; Thambugala et al. 2014a). The most significant and confusing problem is that the phylogenetic data gathered from any gene may not reveal the evolution history of the organism (Ariyawansa et al. 2014a, b). Therefore, polyphasic taxonomy, including genotypical and phenotypical characteristics are recommended in all studies (Ariyawansa et al. 2014a, b). Another difficulty is that in early phylogenetic studies focus was on techniques and strains of fungi used that in most cases were not carefully referenced, thus creating confusion as explained in Ariyawansa et al. (2014a, b). Most strains in GenBank are named without link to voucher specimens and it is not practical to verify their characters to ensure accurate naming.
Leptosphaeriaceae is an important family in the order Pleosporales (Zhang et al. 2012; Hyde et al. 2013), including economically important pathogens on various hosts (De Gruyter et al. 2013; Hyde et al. 2014). Host association previously played a vital role in the classification of the species of Leptosphaeriaceae (De Gruyter et al. 2010; Hyde et al. 2014) and there have only been a few molecular studies of Leptosphaeriaceae, as compared to the morphology based studies (Câmara et al. 2003; De Gruyter et al. 2013). Genera with bitunicate asci and hyaline or pigmented ascospores with or without septation viz. Phaeosphaeria and Leptosphaeria have usually been classified under Phaeosphaeriaceae and Leptosphaeriaceae. Leptosphaeriaceae shares similarities with Phaeosphaeriaceae, but can clearly be differentiated by the characters of the peridium, host and the asexual morph. Leptosphaeriaceae species usually occur on dicotyledons, have a peridium of scleroplectenchymatous cells, and asexual morphs are known as coniothyrium-like and phoma-like (Câmara et al. 2002; Hyde et al. 2013; Kirk et al. 2008; Zhang et al. 2009). Phaeosphaeriaceae species are generally associated with monocotyledons and the peridium comprises pseudoparenchymatous cells and produce coelomycetous asexual states (Câmara et al. 2002; Kirk et al. 2008; Zhang et al. 2009). Molecular studies however have shown that these particular characters have evolved in different families such as Didymellaceae and Montagnulaceae (Zhang et al. 2012; Hyde et al. 2013).
Recent studies such as those of Zhang et al. (2012), De Gruyter et al. (2013) and Hyde et al. (2013), did not discuss details of the family Leptosphaeriaceae and related genera. De Gruyter et al. (2013) mainly focused on the phoma-like asexual morphs in the family, while Zhang et al. (2012)gave a detailed account for some of the sexual morphs. Hyde et al. (2013) provided description and phylogenetic placement of the family and provided a key to the genera in Leptosphaeriaceae in their work on Dothideomycetes.
In this study we provide a backbone tree to Leptosphaeriaceae using as much vouchered sequence data as possible. Ten clades of Leptosphaeriaceae are recognized in our analysis based on ITS, LSU and SSU sequence data, which are treated as groups for the delimitation of genera. Even though relationships among these clades may be weakly supported and some may vary in detail, some tentative conclusions can be drawn. Several current taxonomic hypotheses are supported by our molecular data, and this makes it possible to propose some taxonomic hypotheses about the relationships among the genera in Leptosphaeriaceae. By combining multi-locus DNA sequencing with detailed morphological analyses, we were able to define and propose five new species in the family Leptosphaeriaceae and one novel family Neophaeosphaeriaceae in order Pleosporales. We accept seven sexual or asexual genera and exclude one genus from the family Leptosphaeriaceae based on morphology together with molecular data.
In order to provide a clear account for the Leptosphaeria doliolum species complex we provide a separate phylogenetic analysis based on ITS, LSU and ACT sequence data. Leptosphaeria doliolum and L. veronicae proved to be closely related in a phylogenetic analysis utilising LSU and ITS. A detailed multi-locus phylogenetic study including ITS, ACT and LSU sequence data, however, demonstrate that both species could be clearly differentiated, and represent two subclades in Leptosphaeria sensu stricto. We further concluded that Leptosphaeria sensu stricto includes several possibly host-specific, pathogenic species. Providing data with voucher sequences for L. doliolum and related taxa will allow better resolution of the genus.
We introduce a new family Neophaeosphaeriaceae in the order Pleosporales to accommodate the genus Neophaeosphaeria, thus resolving the confusion surrounding the genus Neophaeosphaeria and confirm its phylogenetic placement.
The family Leptosphaeriaceae is a well supported phylogenetic lineage in multiple analyses and this is also supported by morphology. The significance of accurate DNA sequence data attached with both correct taxonomic names and clearly annotated specimen data is essential. This also results in a more stable and understandable taxonomy and nomenclature. We were unable to borrow some type material, viz. the epitype designated for Paraleptosphaeria nitschkei could not loaned due to the policies of CBS herbarium, thus we represent this species via drawings from the literature. We provide a well resolved back-bone tree for the family Leptosphaeriaceae and allied genera, representing all the sexual and asexual genera, which presently have DNA sequences and provide a baseline platform for resolving species and genera in the family.
References
Alves JL, Woudenberg JHC, Duarte LL, Crous PW, Barreto RW (2013) Reappraisal of the genus Alternariaster (dothideomycetes). Persoonia 31:77
Ariyawansa HA, Jones EBG, Suetrong S, Alias SA, Kang JC, Hyde KD (2013a) Halojulellaceae a new family of the order Pleosporales. Phytotaxa 130:14–24
Ariyawansa HA, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2013b) Towards a natural classification of dothideomycetes 1: the genera Dermatodothella, Dothideopsella, Grandigallia, Hysteropeltella and Gloeodiscus (dothideomycetes incertae sedis). Phytotaxa 147(2):35–47
Ariyawansa HA, Maharachchikumbura SSN, Karunarathne SC, Chukeatirote E, Bahkali AH, Kang JC, Bhat JD, Hyde KD (2013c) Deniquelata barringtoniae from Barringtonia asiatica, associated with leaf spots of Barringtonia asiatica. Phytotaxa 105:11–20
Ariyawansa H, Phookamsak R, Tibpromma S, Kang JC, Hyde KD (2014a) A molecular and morphological reassessment of Diademaceae. Sci World J 2014a:1–11
Ariyawansa HA, Camporesi E, Thambugala KM, Mapook A, Kang JC, Alias SA, Chukeatirote E, Thines M, Mckenzie EHC, Hyde KD (2014b) Confusion surrounding Didymosphaeria — phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa 176(1):102–119
Ariyawansa HA, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2014c) Pyrenophora. Mycosphere 5(2):351–362
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014d) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Thambugala KM, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2014e) Towards a natural classification of dothideomycetes 2: the genera Cucurbidothis, Heterosphaeriopsis, Hyalosphaera, Navicella and Pleiostomellina (dothideomycetes incertae sedis). Phytotaxa 176(1):7–17
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Senanayake IC, Shivas RG, Tian Q, Xu JC (2014f) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69(1):57–91
Ariyawansa HA, Tanaka K, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Phookamsak R, Boonmee S, Wanasinghe DN, Hongsanan S, Singtripop C, Chukeatirote E, Kang JC, Jones EBG, Hyde KD (2015) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71:85–139
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise phoma and related pleosporalean genera. Stud Mycol 65:1–60
Barr ME (1987) New taxa and combinations in the loculoascomycetes. Mycotaxon 29:501–505
Boerema GH (1997) Contributions towards a monograph of phoma (coelomycetes) – V. subdivision of the genus in sections. Mycotaxon 64:321–333
Boerema GH, Coworkers (1993). Check-list for scientific names of common parasitic fungi. Libri botanici; Vol. 10. IHW-Verlag, Eching.
Boerema GH, Kesteren HA v (1964) The nomenclature of two fungi parasitizing brassica. Persoonia 3:17–28
Boerema GH, Gruyter J, Kesteren HA (1994) Contributions towards a monograph of phoma (coelomycetes) – III-1. Section plenodomus: Taxa often with a leptosphaeria teleomorph. Persoonia 15:431–487
Boonmee S, Rossman AY, Lui JK, Li WJ, Dai DQ, Bhat JD, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov. integrating sexual and asexual generic names. Fungal Divers 68(1):239–298
Câmara MPS, Palm ME, van Berkum P, Stewart EL (2001) Systematics of Paraphaeosphaeria: a molecular and morphological approach. Mycol Res 105:41–56
Câmara MPS, Palm ME, van Berkum P, O’Neill NR (2002) Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94:630–640
Câmara MP, Ramaley AW, Castlebury LA, Palm ME (2003) Neophaeosphaeria and Phaeosphaeriopsis, segregates of paraphaeosphaeria. Mycol Res 107:516–522
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Cesati V, De Notaris G (1863) Schema di classificazione degle sferiacei italici aschigeri piu' o meno appartenenti al genere sphaeria nell'antico significato attribuitoglide persono. Comm Soc Crittog Ital 1:177–420
Checa J, Ramaley AW, Palm-Hernandez ME, Câmara MPS (2002) Paraphaeosphaeria barrii, a new species on yucca schidigera from Mexico. Mycol Res 106:375–379
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu J, Liu X, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Crane JL, Shearer CA (1991) A nomenclator of leptosphaeria V. Cesati & G. de notaris (mycota – ascomycotina – loculoascomycetes). Illinois Nat Hist Surv Bull 34:1–355
De Gruyter JD, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
De Gruyter JD, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in phoma section paraphoma, pyrenochaeta and pleurophoma. Mycologia 102:1066–1081
De Gruyter JD, Woudenberg JHC, Aveskamp AA, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in pleosporales. Stud Mycol 75:1–36
Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW (2003) Reproductive isolation and phylogenetic divergence in neurospora: comparing methods of species recognition in a model eukaryote. Evolution 57:2721–2741
Dong JW, Chen WD, Crane JL (1998) Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other loculoascomycetes based on nuclear ribosomal DNA sequences. Mycol Res 102:151–156
Ellis MB (1971) Dematiaceous hyphomycetes. CMI, Kew, England
Eriksson OE, Hawksworth DL (1991) Outline of the Ascomycetes – 1990. Syst Ascomyc Reprint of Volumes 1–4 (1982–1985) 9: 39–271
Eriksson E, Hawksworth DL (2003) Saccharicola, a new genus for two Leptosphaeria species on sugar cane. Mycologia 95:426–433
Grove WB (1935) British stem- and leaf-fungi (coelomycetes) Vol. In: 1 sphaeropsidales. UK. Cambridge University Press, Cambridge
Gruyter J de , Noordeloos ME, Boerema GH (1993). Contributions towards a monograph of phoma (coelomycetes) – I-2. Section phoma: additional taxa with very small conidia and taxa with conidia up to 7 mm long. Persoonia 15: 369–400
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Lumbsch HT, Lutzoni F, Matheny PB, Mclaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Höhnel F v (1909) Fragmente zur mykologie: VI. Mitteilung (Nr. 182 bis 288). Sber Akad Wiss Wien, Math-Naturw Kl, Abt I 118:275–452
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hutchison LJ et al. (1994) Phoma etheridgei sp. nov. from black galls and cankers of trembling aspen (Populus tremuloides) and its potential role as a bioprotectant against the aspen decay pathogen phellinus tremulae. Can J Bot 72(10):1424–1431
Hyde KD, McKenzie EHC, KoKo TW (2011) Towards incorporating anamorphic fungi in a natural classification – checklist and notes for 2010. Mycosphere 2:1–88
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li X, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop: backbones trees for important phytopathogenic 5 genera: I (2014). Fungal Divers 67(1):21–125
Jayasiri SC et al (2015) The Faces of Fungi database: Fungal names linked with morphology, molecular and human activities. Fungal Divers (In press)
Keener PD (1951) An ascigerous stage of darluca filum (Biv.) castagne. Plant Dis Rep 35:86–87
Kirk PM, Cannon PF, Minter DW, Staplers JA (2008) Dictionary of the fungi, 10th edn. CABI Bioscience, UK
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16:1799–1808
Liu JK, Hyde KD, Gareth EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura S, McKenzie EHC, Phookamsak R, Phukhamsakda R, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doliom M, Fan LX, Goonasekara D, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Satinee S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga U, Wijayawardene NN, Wanasinghe D, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Li XH, Liu ZY, Matumura M, Mortimer PE, Rambold R, Randrianjohany E, Sato G, Indrasutdhi VS, Verbeken A, Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Lumbsch HT, Huhndorf SM (2010) Outline of Ascomycota – 2009. Fieldiana Life and Earth Sciences 1:1–60
Müller E (1950) Die schweizerischen arten der gattung leptosphaeria und ihrer verwandten. Sydowia 4:185–319
Munk A (1957) Danish pyrenomycetes. A preliminary flora. Dansk Bot Ark 17:1–491
Muthumeenakshi S, Goldstein AL, Stewart A, Whipps JM (2001) Molecular studies on intraspecific diversity and phylogenetic position of Coniothyrium minitans. Mycol Res 105(09):1065–1074
Nilsson RH, Hyde KD, Pawłowska J, Ryberg M, et al (2014) Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers 67(1):11–19
Nylander JAA (2004) MrModeltest 2.0. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Persoon CH (1800) Icones et description Fugorum-minus cognitorum 2: 27–67 Lispsiae.
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa HA, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Preuss CGT (1851) Ubersicht untersuchter pilze, besonders aus der umgegend Hoyerswerda. In Linnaea 24:99–153
Rambaut, A., & Drummond, A. J. (2007). Tracer v1. 5. htt p. beast. bio. ed. ac. UK/Tracer
Rambaut A, Drummond A (2008) FigTree: Tree figure drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys Å, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW (2009) A class–wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Shearer CA, Crane JL, Chandra Reddy KR (1990) Studies in Leptosphaeria. Lectotypification of Sphaeria Doliolum. Mycologia 82:496–500
Simmons EG (2007) Alternaria: an identification manual. CBS Fungal Biodiversity Center, Utrercht
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J. Cramer, Vaduz
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2269
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–777
Stielow B, Bubner B, Hensel G, Munzenberger B, Hoffmann P, et al (2010) The neglected hypogeous fungus hydnotrya bailii soehner (1959) is a widespread sister taxon of hydnotrya tulasnei (berk.) berk. And broome (1846). Mycol Prog 9:195–203
Sung G-H, Sung J-M, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44:1204–1223
Sutton BC (1977) Coelomycetes VI. Nomenclature of generic names proposed for coelomycetes. Mycol Pape 141:1–253
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Telle S, ThinesM(2008) Amplification of cox2 (620 bp) from 2 mg of up to 129 years old herbarium specimens, comparing 19 extraction methods and 15 polymerases. PLoS One 3:e3584
Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R, Liu ZY, Chukeatirote E, Hyde KD (2014a) Dothideales. Fungal Divers 68(1):105–158
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, To-anun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5(2):391
Verkley GJM, da Silva M, Wicklow DT, Crous PW (2004) Paraconiothyrium, a new genus to accommodate the mycoparasite coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud Mycol 50:323–335
von Hohnel F (1911) Fragmentezur Mykologie(XIII. Mitteilung,Nr. 642bis 718). In Sber. Akad. Wiss. Wien (Math.-naturw. Kl., Abt. I) 120:380–484
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a Guide to Methods and Applications 18:315–322
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Chomnunti P, Dai DQ, D'souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat JD, Gueidan C, De Hoog GS, Knudsen K, McKenzie EHC, Miller AN, Mortimer PE, Wanasinghe DN, Phillips AJL, Raja HA, Slippers B, Shivas RS, Taylor JE, Wang Y, Woudenberg JHC, Piątek M, Cai L, Jaklitsch WM, Hyde KD (2014) Naming and outline of dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69(1):1–55
Wunsch MJ, Bergstrom GC (2011) Genetic and morphological evidence that Phoma sclerotioides, causal agent of brown root rot of alfalfa, is composed of a species complex. Phytopathol 101(5):594–610
Yuan ZW, Pei MH, Hunter T, Royle DJ (1998) Eudarluca caricis, the teleomorph of the mycoparasite sphaerellopsis filum, on blackberry rust phragmidium violaceum. Mycol Res 102:866–868
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Hyde KD (2009) Multi-locus phylogeny of the pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012) Pleosporales. Fungal Divers 52:1–225
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3(1):4
Acknowledgments
The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research group (PRG-1436-09). MFLU grant number 56101020032 is thanked for supporting studies on Dothideomycetes. We are grateful to the Mushroom Research Foundation, Chiang Rai, Thailand for supporting studies on Dothideomycetes. Kevin D. Hyde thanks the Chinese Academy of Sciences, project number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. H.A Ariyawansa and J.C. Kang are grateful to the agricultural science and technology foundation of Guizhou province (Nos. NY[2013]3042 ), the international collaboration plan of Guizhou province (No. G [2012]7006) and the innovation team construction for science and technology of Guizhou province (No. [2012]4007) from the Science and Technology Department of Guizhou province, China. Chayanard Phukhamsakda would like to thank Royal Golden Jubilee Ph. D. Program under Thailand Research Fund, for the award of a scholarship no. PHD/0020/2557 to study towards a PhD. Shaun Pennycook is thanked for checking and suggesting corrections to most of the Latin names. Ausana Mapook would like to thank Research and Researchers for Industries (RRI) PHD57I0012 under the Thailand Research Fund for the award of a to study towards a PhD. Hiran Ariyawansa is grateful to A.D Ariyawansa, D.M.K Ariyawansa, Ruwini Ariyawansa and Amila Gunasekara for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOC 140 kb)
Rights and permissions
About this article
Cite this article
Ariyawansa, H.A., Phukhamsakda, C., Thambugala, K.M. et al. Revision and phylogeny of Leptosphaeriaceae . Fungal Diversity 74, 19–51 (2015). https://doi.org/10.1007/s13225-015-0349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0349-2