Abstract
This revision of Cantharellus in Madagascar deals with species that are associated with strictly endemic host trees and shrubs. Based on morphological differences and molecular sequence data of the tef-1 gene, five new species are proposed (C. albidolutescens, C. ambohitantelyensis, C. ibityensis, C. paucifurcatus and C. sebosus), as well as one new subspecies, C. subincarnatus ssp. rubrosalmoneus, whereas C. decolorans and C. platyphyllus ssp. bojeriensis are epitypified. A key is provided to all Cantharellus that grow with native vegetation in Madagascar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cantharellus Adans.:Fr., a genus of edible mushrooms reputed for its high culinary and commercial value, is one of the prominent fungal genera in Madagascar with several species being sold along roads and in local markets in large quantities (Buyck 2008). The majority of these marketed species, however, are associated with introduced eucalypts and will not be discussed here as this paper only deals with the Cantharellus associated with endemic vegetation (gray shaded boxes in Fig. 1), and not with the species associated with introduced eucalypts (unshaded boxes in Fig. 1), nor with those found under the amphipacific Intsia bijuga (Caesalpiniaceae, not shown) such as on Madagascar’s East coast for example. Introduced pines (Pinus kesiya, P. patula) appear not to be associated with any Cantharellus in Madagascar.
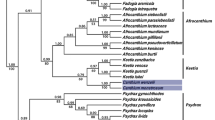
Maximum likelihood tree inferred from the analysis of tef-1 locus for 85 taxa. Branches that received bootstrap support ≥70 and BPP ≥95 % are in bold (MLbs/BPP). Only BS values are reported along the branches except for the branch in bold grey which is supported only by Bayesian analysis. The Malagasy chanterelles are in bold font, with gray shaded boxes indicating the species associated with native vegetation, the subject of this paper. Malagasy collections associated with exotic trees are indicated by unshaded boxes
Until recently, very little was known about the diversity and ecology of chanterelles in Madagascar. The earliest, original descriptions of Malagasy chanterelles (Patouillard 1924; Heim 1936; Heinemann 1958) were very brief and did not mention whether these species had been collected in native vegetation or under the introduced exotic trees that were already widely planted on the island at that time. More recently, Eyssartier and Buyck (1999a), Buyck et al. (2012) and Buyck and Randrianjohany (2013) described six new taxa of Cantharellus that were all exclusively associated with endemic Malagasy trees: three species as well as three additional taxa that were so similar to African species that they were provisionally described at the infraspecific level.
This study is based on tef-1 sequence data for 14 of the 19 different species of chanterelles that have been collected in Madagascar by the senior author and collaborators. In our experience, tef-1 is an excellent gene for the characterization of chanterelle species (Buyck et al. 2011, 2013; Buyck and Hofstetter 2011). Amplification of the ITS region (the standard bar code region for fungi) can either produce several bands, typically one short (0.5–0.6 K bases) and one long fragment, or a single band of a length corresponding to 1.2–1.8 K bases that cannot be sequenced without the use of internal primers in addition to the two general primers used for amplification., whereas tef-1is only 800 bases long and, with very few exceptions, very easy to amplify and sequence (at least from fresh collections or from tissues stored in CTAB buffer). Moreover, it offers sufficient variation to discriminate between closely related taxa and has the alignment advantages of a coding gene (the four introns have to be excluded from the aligment as there is no evolutionary model that is adapted for sequences that are only 50 bp).
Materials and methods
Morphological data
Nearly all sequenced collections were gathered by the senior author over the past 25 years. All cited specimens are deposited at the mycological herbarium of the Paris Natural History Museum (PC). Color notations follow Kornerup and Wanscher (1978). Microscopic features were examined and sketched by B. Buyck with the aid of a camera lucida. Original drawings for all elements of the hymenium or pellis were made at a magnification of 2400×. All microscopic observations and measurements were made in ammoniacal Congo red after a short aqueous KOH pretreatment to improve tissue dissociation and matrix dissolution. Measurements of basidiospores cite length, width and length/ width ratio (Q) in this format: (minimum–)mean minus standard deviation– mean value–mean plus standard deviation (− maximum measured); spore measurements are based on “n” spores. References to infrageneric placements follow the most recent molecular-based phylogeny of the genus by Buyck et al. (2014). Paratype designations were only accepted for sequenced collections, the other collections were identified based on morphological features.
Taxon sampling, molecular data and phylogenetic analyses
The taxon sampling includes 85 collections (Table 1) comprising 83 Cantharellus and two outgroups (Craterellus and Hydnum). This dataset is representative of all major clades that were recognized in the most recent classifications of the genus Cantharellus (Eyssartier and Buyck 2001b, Buyck and Hofstetter 2014). DNA was isolated from fresh material stored in CTAB buffer or from dried basidiocarps following the protocol described in Hofstetter et al. (2002). Tef-1 amplification used the primers and conditions of Morehouse et al. (2003). When amplification of tef-1 gene generated multiple bands, PCR products were cloned with pSTBlue-1 AccepTor VectorTM Kit (Novagen). Amplification of four clones was sufficient to recover at least one PCR product of the expected 850–900 bp length for the partial tef-1 gene, secondary bands being much shorter (500–650 bp). Sequencing used the same primers as for amplification. All tef-1 sequences were produced by the authors and deposited in GenBank for recent papers discussing new American and African chanterelles (Buyck et al. 2011, 2013; Buyck and Hofstetter 2011). Sequences were assembled and edited with the software package Sequencher 3.0 (Gene Codes Corp., USA). The alignment of the tef-1 sequences for 85 taxa has been submitted to the TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S14662). A maximum likelihood (ML) analysis was conducted on our tef-1, 85-specimen dataset in RAxML v. 7.0.3 (Stamatakis 2006), using a GTRmodel with a gamma distribution. Bootstrap proportions (MLbs) (Felsenstein 1985; Stamatakis et al. 2008) were estimated with 1000 replicates, and frequencies >70 % were considered significant. We also conducted Bayesian analyses in MrBayes v3.1.1 (Huelsenbeck and Ronquist 2001), sampling every 500th tree for 10,000,000 generations, and implementing a GTR model of nucleotide substitution with an estimated proportion of invariable sites and a gamma distribution approximated by four categories. We conducted three independent runs to verify that all runs converged to the same log-likelihood stationary level. After discarding the burn-in, the last 10 000 trees of each run were pooled, and the resulting 30 000 trees were used to calculate a 50 %-majority rule consensus tree in order to obtain Bayesian posterior probabilities (PP).
Results
Molecular analyses
The full alignment includes 1025 characters. After exclusion of ambiguous regions, including four spliceosomal introns, the dataset accounts for 633 bp in which 227 characters are parsimony-informative. Data partitioning that maximized likelihood was the one with three partitions (tef-1 1st, 2nd and 3rd codon position). The ML tree with the highest likelihood value (lnL −5353.986075) based on 500 RAxML searches is shown in Fig. 1. The tef-1 locus provided low support for basal relationships except for the monophyly of genus Cantharellus that received significant support (MLbs = 76 %). The partial tef-1 gene sequence did, however, better resolve terminal relationships for the species newly described here (Fig. 1).
All our Malagasy collections on the tree are placed within boxes (Fig. 1) that are shaded for those associated with endemic hosts and transparent for those associated with exotic trees (the latter are not further discussed here). Reading the tree from top to bottom we have first a highly supported clade (MLbs = 100 %) in which C. platyphyllus is monophyletic with a subclade (MLbs = 71 %) composed of the Malagasy collection C. aff symoensii. (see discussion under C. platyphyllus), sister to the African collections of C. symoensii. There is no clear support to consider African and Malagasy collections of C. platyphyllus as distinct taxa in our dataset: the branch leading to both African collections is weakly supported (MLbs = 68 %), whereas our MLbs analysis obtained threshold bootstrap support (MLbs = 70 %) for only two of the three Malagasy collections as a distinct subsp. bojeriensis. The above taxa represent subgenus Afrocantharellus in our dataset. The Malagasy C. subcyanoxanthus clusters with high support (MLbs = 92 %) with the Tanzanian C. aff. subcyanoxanthus. The eucalypt-associated collections for the Malagasy C. cf. decolorans clustered with high support (MLbs = 100 %) with C. decolorans from endemic vegetation, but were supported to be distinct from the latter (MLbs = 88 %). There is no support to resolve the relationships between these different subclades. The clade only supported by Bayesian analysis (MLbs = 65 %, PP = 99 %) contains no chanterelles from endemic vegetation, but the eucalypt-associated C. cf. congolensis was monophyletic (MLbs = 100 %) and sister (MLbs = 84 %) to African collections of C. congolensis.
The next two subclades in our phylogeny are sister to each other but without support. The first of these two subclades obtains significant support (MLbs = 72 %) and is entirely composed of European and American species, whereas the well supported second clade (MLbs = 81 %) contained three endemic Malagasy chanterelles (C. ibityensis, C. ambohitantelyensis and C. sebosus) and two recently described African species, C. humidicolus and C. gracilis. Cantharellus sebosus was the most basal taxon within that clade, followed by C. ambohitantelyensis, which was sister to the subclade uniting the remaining three other species (MLbs = 81 %). The species pair C. ibityensis and C. humidicolus were sister with maximum support (MLbs = 100 %) with the African C. gracilis.
The next large subclade received only weak support (MLbs = 64 %) and is again exclusively composed of African and Malagasy species, some of which are associated with exotic eucalypts (C. sp. ined. in Madagascar and the African C. cf. tanzanicus, both not further discussed here). Cantharellus albidolutescens clustered with C. densifolius, the eucalypt-associated C. sp. ined., and with another, still undescribed Malagasy species with high support (MLbs = 99 %). With the exception of the highly supported monophyly of the eucalypt-associated C. cf. tanzanicus and the African C. tanzanicus (both MLbs = 100 %), the relationships between the other species included in this subclade remained unresolved. The endemic Malagasy C. paucifurcatus was part of a clade otherwise entirely composed of African species but its placement within that clade was not significantly supported. Finally, the highly supported basal clade in our genus phylogeny is comprised of a single Malagasy taxon, here proposed as C. subincarnatus subsp. rubrosalmoneus on the basis of morphological and ecological differences.
Taxonomy
Cantharellus albidolutescens Buyck, Eyssart. & V. Hofstetter, spec. nov. — MycoBank MB 803639; Figs. 2–4, 20a
Pileus up to 45 mm diam., first plane or slightly convex with distinctly involute margin, then gradually concave and becoming often very irregular with strongly lobed to straight margin, dull, dry, when very young nearly smooth and pale ochre, then quickly fribillose-squamulose from small, concentrically arranged, concolorous or darker squamae on a paler to off-white background. Hymenophore composed of decurrent, more or less densely arranged but never crowded gill-folds, forked, sometimes anastomosed-veined in between, pure white to off-white, immediately and intensely yellowing upon injury or on handling. Stipe 10–20 × 2–3 mm, subcylindrical or narrowing toward the base, often bent, having a dull, dry, off-white surface bearing similar squamulae as on the pileus, strongly yellowing upon handling or with age, particularly at the base which gradually develops an intense, golden yellow to chrome yellow color. Flesh moderately thick to thin, often quickly attacked by insect larvae, first white but soon strongly yellowing. Taste mild. Smell a typical, although weak apricot smell. Spore print not obtained, probably white.
Spores ellipsoid to slightly reniform, 6.5 − 7 − 7.5 (−8) × 4– 4.42 − 5 μm, Q = 1.4 − 1.61 − 1.7, smooth, hyaline. Basidia measuring mostly 44–56 × 6.5 − 8 μm, 4 − 5(−6) spored, clavate. Cystidia absent. Subhymenium not filamentous and mainly composed of cells that are broader than those of the hymenium, measuring up to 15 μm diam. Pileipellis composed of mostly horizontal, intermixed, more or less densely septate hyphal extremities, 4 − 5(−7) μm in diam., composed for the larger part of regularly cylindrical cells, occasionally slightly inflated, at the surface mostly distinctly thick-walled, at least the last 2–3 terminal cells, then quickly thin-walled underneath and broader, up to 10(−15) μm diam. toward the context; some hyphae in the lower layers with distinct zebroid incrustation from pigment; the terminal cell not particularly differentiated and quite short, 20 − 40(−55) μm. Clamp connections absent from all tissues.
Etymology: from albidus, ‘off-white’, and lutescens, ‘yellowing’, referring to the yellowing context of the basidiocarps.
Holotype: Madagascar. Central Highlands: Special Reserve of Ambohitantely, on soil along botanical track in dense forest with Uapaca densifolia, Leptolaena and Sarcolaena spp., 19 Jan 2008, Buyck & Hofstetter 08.070 (HOLOTYPUS, PC0084751)
Additional material examined: MADAGASCAR. Central Highlands. Special Reserve of Ambohitantely, along botanical track in dense evergreen rain forest with Uapaca densifolia, Leptolaena and Sarcolaena spp., alt. ca 1500 m, 15 Feb 2000, Buyck et al. 00.1512 (PC0084841); ibid., 24 Jan 2000, Buyck et al. 00.0882 (PC0084843), 00.0798 (PC0084842), ibid., 19 Jan 2008, Buyck & Hofstetter 08.057 (PC0084750); ibid., 5 Feb 2008, Buyck & Hofstetter 08.337 (PC0084840). Andasibe, dense forest with U. densifolia, 22 Jan 2001, Buyck 01.517 (PC0124237)
Commentary: Cantharellus albidolutescens is strongly reminiscent of C. densifolius, sharing the squamulose surface of pileus and stipe, the similar overall habit and colors as well as very similar microscopical features. It is a typical member of subgenus Rubrinus Eyssart. & Buyck emend. Buyck & V. Hofstetter, section Isabellinus as defined by Eyssartier and Buyck (2001b). Cantharellus albidolutescens fruits in very small groups or even with single basidiocarps, whereas C. densifolius usually forms large groups with sometimes up to many dozens of basidiocarps and is a commonly collected edible mushroom on the African continent that is offered for sale in huge quantities (Buyck 1994). In the field, the less crowded gill folds, the paler overall color and the intensely yellowing context, particularly near the stipe base, distinguish our species easily from the mainland African C. densifolius. Cantharellus albidolutescens also lacks the pale yellow color of the gill folds that gradually develops in mature C. densifolius and which is completely different from the intense yellow stains that develop upon handling in C. albidolutescens. In the littoral forest of the east coast we have observed completely white basidiocarps that are otherwise identical.
There remains, however, a caveat as to the correct interpretation of C. densifolius, which is a species originally described from the rain forest area, but later mainly reported from the Zambezian woodlands (Buyck 1994; Buyck et al. 2000; Heinemann 1966). As explained in some recent papers on African chanterelles (Buyck 2012; Buyck et al. 2013), the very different climate and ecology of the rain forest versus the open savannah woodlands, is a strong argument against the presence of the same species in both vegetation types. In this particular case, however, we think that woodland and rain forest collections may indeed represent the same taxon because our woodland collections of C. densifolius were nearly always made in humid places (swampy places with abundant Zingiberaceae, forest galleries along streams, etc.). Nevertheless, recollection in its original habitat and more sequencing are needed to confirm this.
Cantharellus ambohitantelyensis Buyck & V. Hofstetter spec. nov. — MycoBank MB 803640; Figs. 5–7, 20b
Pileus very small, 10–15 mm diam., convex with inrolled, irregularly lobed or sinuate margin that remains so at maturity, a bright orange to reddish orange (7A7 − 8), minutely pruinose to smooth, dull. Hymenophore decurrent, formed of low veins, up to 1 mm high, rather spaced for the small size (3–5/5 mm), sparsely forking or with few interstitial veins or anastomoses, distinctly paler than the rest of the fruit body and cream to off-white. Stipe small, 10–12 × 1.5 − 3 mm, subcylindrical, either slightly widening downward or near the hymenophore, dull, smooth, reddish orange, more yellowish at the extreme base, sometimes hollowing in its lower, central part at maturity. Context relatively thick for the very small size, orange-yellow (4A5−6), almost whitsh in the stipe base. Taste none. Smell weak, fruity. Spore print not obtained.
Spores ellipsoid to ovoid, (6.9)7.0 − 7.3 − 7.5(7.7) × (4.4)4.5 − 4.8 − 5.1(5.2) μm. Q = (1.3)1.4 − 1.5 − 1.6(1.7), smooth. Basidia slender, subclavate, mostly 60–70 × 6–8 μm and 5 − 6-spored. Cystidia absent. Lamellar trama composed of slender, entangled hyphae, 2.5 − 3(−4) μm wide, often more or less inflated near the septa, becoming larger and more regular towards the well-differentiated subhymenium which is composed of ramified, 8–10 μm wide hyphae. Pileipellis composed of mostly regularly and narrowly cylindrical, 4 − 7 μm wide, some widening upward, hyphal extremities with thin- to sometimes slightly thickened, more or less yellowish wall, some covered by zebroid pigment incrustations; the terminal cell quite short, rarely exceeding 35 μm long, with rounded, sometimes nearly subcapitate tip. Clamp connections absent.
Etymology: named after the type locality.
Holotype: MADAGASCAR. Central Plateau, Natural Forest Reserve of Ambohitantely, on soil along the “botanical track” in dense forest with Uapaca densifolia, Leptolaena and Sarcolaena spp., 5 Feb. 2008, Buyck & Hofstetter 08.336 (HOLOTYPUS, PC0084754).
Commentary: Our species resembles the somewhat larger, orange C. madagascariensis Pat., but differs in nearly all other features. It is also very similar to C. ibityensis (see below) from which it differs in the more reddish colors of the pileus and particularly of the stipe, in the distinctly paler hymenophore which is less well-developed, and also in the differences in size and form of the spores and the locally thickened terminal cell walls in the pellis. Together with two other species that are here newly described, C. ibityensis and C. sebosus, this species is part of a well-supported clade, sister without support to the core of the genus (C. cibarius clade). The absence of both clamps and squamulae on the pileus, as well as the orange-red overall color make it a typical representative of subgen. Rubrinus section Heinemannianus Eyssart. & Buyck.
Cantharellus decolorans Eyssart. & Buyck, Mycotaxon 70:204–206. 1999.
Epitype: On a mountain crest with Uapaca ferruginea, along the road bordering the Ranomafana Nat. Park next to the Setam Lodge 5 km before arriving at Ranomafana village, in rich wood humus, 30 Jan 2008, Buyck & Hofstetter 08.278, (EPITYPUS, hic designatus, PC0084098)
Additional material examined: MADAGASCAR. Tamatave Prov. Forest station of “La Mandraka”, in dense mountain forest, along mountain crest in monospecific Uapaca densifolia population, growing on the inside of a rotten log, 3 Feb 1997, Buyck, Eyssartier & Moreau leg., in Buyck 97.227 (HOLOTYPUS, PC0084784), ibid., 14 Feb 1997, in Buyck 97.494 (PC0084785), ibid., 20 Feb 1997, in Buyck 97.585 (PC0084996); Andasibe, forest station near the Andasibe national Park, in dense mountain forest with U. densifolia, growing inside a rotten Pandanus, 13 Feb. 1997, Buyck, Eyssartier & Moreau leg., in Buyck 97.463 (PC0084997); Fianarantsoa Prov. On a mountain crest with U. ferruginea, along the road bordering the Ranomafana Nat. Park next to the Setam Lodge 5 km before Ranomafana village, in rich woody humus, 3 Feb 1999, Buyck & Eyssartier 99.414 (PC0084995), ibid., Buyck & Hofstetter 08.280 (PC0084999).
Commentary: This species was described and discussed in detail by Eyssartier and Buyck (1999b) as an endemic species typically associated with Uapaca-dominated crests of humid mountain forests on Madagascar’s Central Highlands, where it is always found in or near completely rotten wood debris. All here cited specimens are from this same type of habitat and agree completely with the original description. As we were unable to get sequences out of the type material, we have epitypified the species name here.
A similar chanterelle with identical microscopic features and similar habit, but much more variable in color, occurs in exotic eucalypt plantations all over Madagascar (labeled as Cantharellus “cf. decolorans” in Fig. 1) (Buyck 2008). Although our phylogenetic analysis supports the recognition of C. decolorans as a distinct species, more research and sequencing is needed to exclude a possible co-identity between the taxon occurring under eucalypts in Madagascar and the Australian, eucalypt-associated Cantharellus concinnus Berk., which may have been imported together with its host in Madagascar.
Cantharellus ibityensis Buyck, Randrianjohany & V. Hofstetter, spec. nov. — Mycobank MB803641; Figs. 8–10, 20c
Basidiomes in groups (mostly close to a dozen) and dispersed. Pileus very small, 15–25 mm diam., rather fragile and thin, orange to yellowish orange (3−4A7−8), slightly veined to smooth, dull. Hymenophore strongly decurrent, of low gill-folds, 1(−2) mm high, rather spaced (5 − 7/cm), sparsely forking, with rare interstitial venation or anastomoses, slightly paler (egg-yolk yellow) to concolorous with stipe and pileus. Stipe small, 12–15 × 1.5 − 2 mm, concolorous with pileus. Context relatively thick for the very small size, often whitish in the stipe center, distinctly yellow (2A7−8) to orange-yellow elsewhere. Taste none. Smell weak, fruity. Spore print not obtained, but probably white judging from deposits on hymenophore.
Spores ellipsoid to elongate and strangulate (narrower in the middle), (7.9−)8.4 − 8.9 − 9.4(−9.8) × (3.9−)4.6 − 5.1 − 5.5(−5.8) μm. Q = (1.4−)1.6 − 1.8 − 1.9(−2.4), smooth. Basidia narrowly clavate, measuring mostly 52–67 × 7–9 μm, 5(−6)-spored with stout sterigmata. Subhymenium of subcylindrical cells, equal to or less in diam. than hymenium-cells. Cystidia absent. Pileipellis composed of interwoven, thin-walled, long, cylindrical hyphal extremities, 3–6 μm diam., frequently branching and with terminations that are relatively difficult to observe; septa distant, except for the terminal and sometimes subterminal cell which are both often much shorter (terminal cell mostly 15–45 μm long), with cell walls that are slightly refringent from a minutely granular pigment deposition; the underlying hyphae very strongly intermixed and intricately branched. Clamp connections absent.
Etymology : referring to the hill of Ibity, the type locality.
Holotypus: Madagascar. Central Highlands, Ibity, near Antsirabe, on soil in primary Uapaca bojeri woodland, on naked soil and between rocks of a vertical slope bordering a small riverbed, 27 Jan 2008, Buyck & Randrianjohany 08.196 (HOLOTYPUS, PC0084109)
Additional material examined: Madagascar. Central Highlands, Ibity, near Antsirabe, in primary Uapaca bojeri-Sarcolaenaceae woodland, on naked soil and between rocks on a vertical, muddy slope bordering a small riverbed, S20.06586 E046.99884, 1616 m alt., 27 Jan 2008, Buyck & Randrianjohany 08.203 (PARATYPUS, PC0084722), 08.209 (PC s.n.), ibid., on a vertical part with bare soil bordering a narrow walking trail, S20.06373 E047.00076, 1655 m alt., 14 Dec 2012, Buyck & Randrianjohany 12.103.
Commentary: Our species possesses the same general habit and coloration as well as similar spores as C. madagascariensis but differs from the latter by the absence of clamp connections, a feature that makes it a typical member of subgenus Rubrinus section Heinemannianus. Two very similar, small, equally afibulate chanterelles, C. pseudofriesii Heinem. and C. tenuis Heinem., were described from the African rain forest, and differ not only by their general ecology and their more slender habit, but also in the size of their spores.
This species is only known from the type locality where it is constantly found on bare, very humid soil that is more or less vertically oriented, such as near river beds. It typically grows in groups composed of at least a dozen, dispersed specimens.
Cantharellus paucifurcatus Buyck, Randrianjohany & V. Hofstetter, spec. nov. — MycoBank MB 803642; Figs. 11–13, 20d
Basidiomes solitary or in small groups. Pileus small but relatively firm and fleshy (almost triangular in section), up to 20 mm diam., when young convex-flattened, with strongly involute margin, sitting on the very tip of an often already well-developed stipe, strongly velvety-velutinous and strongly brown where handled, with age becoming rapidly less involute, depressed in the center, but with the margin never entirely plane or uplifted, paler brown and distinctly fibrillose-squamulose (inocyboid). Hymenophore decurrent, composed of well-differentiated, relatively thick gill-folds, mostly 2–3 mm high, unequal from mostly two different lengths of lamellulae, very sparsely forked (at the most a few gills forked once in a single pileus), without interstitial venation or anastomoses, creamy with a pinkish hue. Stipe 20–30 × 3–7 mm, subcylindrical or slightly narrowing downward, off-white to pale yellowish brown, paler near the extremities and even chalk-white and hairy-woolly from mycelial development at its very base, more pruinose near the hymenophore, minutely fibrillose on the rest of its surface. Context white, firm, unchanging or very slightly developing weak yellowish hues from handling. Smell hardly perceptible. Taste mild. Spore print not obtained, but obviously very pale (pinkish ?).
Spores ellipsoid, (7.9−)8.3 − 8.8 − 9.3(−9.6) × (5.0−)5.2 − 5.7 − 6.2(−6.7) μm, Q = (1.3−)1.4 − 1.5 − 1.6(−1.8), smooth, hyaline. Basidia narrowly clavate and relatively short, mostly 45–60 × 7–9 μm, regularly 4-spored with stout sterigmata. Cystidia absent. Subhymenium of locally inflated cells. Pileipellis of slender, distinctly thick-walled hyphal extremities, measuring 4–6 μm diam., with relatively distant septa; the terminal cell variable in length, mostly 40–100 μm long, often undulate or repeatedly constricted in outline. Clamp connections absent.
Etymology: from ‘paucus’ meaning little or few, and ‘furcatus’ meaning forked, referring to the hymenophore composed of sparsely forked gills, a quite unexpected feature in this genus.
Holotypus: Madagascar. Central Highlands, Ibity, near Antsirabe, on bare soil in primary Uapaca bojeri woodland, ca 1500 m alt., 3 Feb 2008, Buyck & Randrianjohany 08.320 (HOLOTYPUS, PC0084729)
Additional material examined: MADAGASCAR. Central Highlands, Ibity, in primary U. bojeri woodland, ca 1500 m alt., 27 Jan 2008, Buyck & Hofstetter 08.201 (PARATYPUS, PC0085063)
Commentary: This species is difficult to recognize as a chanterelle because of the absence of vivid colors, the very sparsely forked and nearly regularly unequal, well-developed gills, the brown, fibrillose pileus surface and regularly four-spored basidia. We know of no other chanterelle that can be confused with it. The recently described C. eyssartieri Buyck & Randrianjohany comes probably closest, but differs in nearly all microscopical features: thin-walled hyphal endings, larger spores and mostly 5-spored basidia (Buyck and Randrianjohany 2013). The African C. tomentosus Buyck & Eyssart. differs from our species in the much smaller spores, shorter basidia and distinctly shorter terminal cells at the pileus surface. All of these clampless species are member of subgenus Rubrinus section Isabellinus.
Cantharellus platyphyllus subsp. bojeriensis Eyssart. & Buyck, Mycotaxon 70:208. 1999
Epitype: Madagascar. Central Highlands, bought along the RN7 between Antananarivo and Antsirabe, under Uapaca bojeri, 25 Jan 2008, Buyck & Hofstetter 08.160 (EPITYPUS, hic designatus, PC0084740); Central Highlands, near Arivonimamo, 60 km from Antananarivo, under Uapaca bojeri, 30 Jan 1997, Buyck, Eyssartier & Moreau 97.055 (HOLOTYPUS PC).
Commentary: We refer to the illustrated original description for more details of this very common representative of subgenus Afrocantharellus in the Uapaca bojeri woodlands of the Central Highlands. We have tried without success to sequence the type specimen and designate here an epitype allowing for the molecular characterization of this taxon. Our tef-1 data does not allow to decide whether the Malagasy specimens present a distinct species. Bootstrap support is near limit (based on only one base pair difference, even when considering all four spliceosomal introns) and not enough collections have been sequenced. Moreover, there appear to exist additional, undescribed Malagasy taxa in subgenus Afrocantharellus as indicated here by one specimen (C. aff. symoensii in Fig. 1) that we also collected as C. platyphyllus subsp. bojeriensis but which had a quite different tef-1 sequence. Unfortunately, this collection consisted of a single, very immature specimen that can hardly serve for a good description and here also future collections are necessary to elucidate its correct status.
Cantharelllus sebosus Buyck, Randrianjohany & V. Hofstetter spec. nov. — MycoBank MB 803644; Figs. 14–16, 20e
Basidiomes never abundant, solitary or often with few fused together at the stipe base. Pileus up to 45(−60) mm diam., fleshy and firm, usually very irregularly, undulate-lobed near the margin, with the margin strongly involute and remaining so at maturity, glabrous, more or less greasy (slightly at the touch but certainly so in general aspect), dull, smooth, not pruinose although the whitish, subcutaneous frost-like areas certainly suggest so, often with white areas when young, then mostly becoming yellow to orange yellow. Hymenophore strongly decurrent, most often formed of low, poorly differentiated veins that fork and anastomose frequently, mostly up to 1(−2) mm high, rarely resembling gill-folds, sometimes also completely smooth in part or most of its surface, lemon yellow to finally almost orange-yellow (egg yolk yellow), strongly contrasting with the pale, off-white stipe below. Stipe very firm and fleshy, 30–40 × (10−)15 − 25(−30) mm, irregular, usually narrowing downward, sometimes curved, white, at the base white from woolly-hairy mycelium development. Context thick, firm, white, only somewhat yellowish underneath the pileus surface, unchanging upon injury or from handling (but the lateritic soil easily leaves reddish stains on the fruit bodies). Taste completely mild. Smell typical apricot-smell of chanterelles. Spore print not obtained, possibly yellowish.
Spores ellipsoid, 6.9 − 7.3 − 7.6(−7.9) × (4.6−)4.7 − 5.2 − 5.7(−6.3), Q = (1.2−)1.3 − 1.4 − 1.5(−1.6), smooth, hyaline. Basidia very long and slender, mostly 80–100 × 7–9 μm, (4−)5-spored with stout sterigmata. Subhymenium composed of very long and slender cells, 3–4 μm diam. Pileipellis rather densely composed of thin-walled, ramifying hyphal extremities of very regular diam., 3–5 μm wide; the terminal cell often tortuous – undulated or repeatedly but weakly constricted, mostly (20−)30 − 50(−100) μm long, obtuse at the tip, thin-walled to slightly thickened and without evident pigment incrustations but some terminal cells with distinct granular-refringent contents. Clamp connections absent in all parts.
Etymology: ‘sebosus’ means greasy, referring to the aspect of the pileus surface.
Holotypus: MADAGASCAR. Central Highlands, Col des Tapias, along RN7 between Ambositra and Antsirabe, ca 1500 m alt., on lateritic soil in secondary Uapaca bojeri woodland with Pinus kesiya ingression, S20 14 18.4 E47 06 03.5, 29 Jan 2008, Buyck & Hofstetter 08.234 (HOLOTYPUS, PC0084736).
Additional material examined: Madagascar. Central Highlands, bought from villagers along RN7 between Antananarivo and Antsirabe, under Uapaca bojeri, 25 Jan 2008, Buyck & Hofstetter 08.162 (PARATYPUS, PC0084079), ibid., Buyck & Hofstetter 08.165 (PC0084990); Central Highlands, near Arivonimamo, bought along road side from local pickers, U. bojeri woodland on lateritic soil, 5 Feb 1997, Buyck, Moreau & Eyssartier 97.267 (PC0084822), 97.268 (PC0084826); bought from villagers at Tsaramandroso, 3 km SW of Arivonimamo, 11 Feb 2011, Randrianjohany 11.137 (PC0085601).
Commentary: A morphologically well-characterized species with branched hyphal extremities of very regular diameter ending in terminal cells that are frequently tortuous in outline. The hyphal diameter shows almost no difference between surface and adjacent pileus trama where hyphae are hardly wider or slightly more variable in diameter (in strong contrast with species such as C. eyssartieri for example). The description above is mainly based on the type collection, but examination of other collections shows that the mean length of the terminal cell in the pileipellis is variable among collections, sometimes longer (with mean length closer to 100 μm), sometimes shorter, and that the cell walls at the pileus surface can be distinctly refringent so as to appear slightly thickened in microscopic preparations, but the thickness of the wall is never comparable to typical species of subgenus Cantharellus, from which our species is well separated by the lack of clamp connections. Notwithstanding its yellow coloration, Cantharellus sebosus is a member of section Heinemannianus in subgen. Rubrinus as defined in Buyck et al. (2014) and has been considered a “smooth chanterelle” in the sense that the hymenophore ranges from the development of distinct veins or ridges to an almost completely smooth surface (Buyck 2014).
This species appears to be rather widespread (although never abundant) on the Central Plateau, and may be typical of Uapaca bojeri woodland on lateritic soil. It is consumed and sold mixed with other chanterelles in the area of Arivonimamo, near Antananarivo, under the name “holabona”. The African C. defibulatus (Heinem.) Eyssart. & Buyck has very similar microscopic characters, but is a larger species from the Zambezian woodlands with distinct gill-folds and more elongated spores (7–7.77−9 × 4–4.47 − 5 μm, Q = 1.4 − 1.73 − 2).
Cantharellus subincarnatus subsp. rubrosalmoneus Buyck & V. Hofstetter subsp. nov. — MycoBank MB803645; Figs. 17–19, 20f
Fresh basidiocarps of new taxa. A. Cantharellus albidolutescens (BB&VH 08.057). B. C. ambohitantelyensis (holotype). C. C. ibityensis (paratype). D. C. paucifurcatus (paratype). E. C. sebosus (holotype). F. C. subincarnatus subsp. rubrosalmoneus (BB&VH 11.183). One square on the white paper in the background equals 5 × 5 mm (all photos B. Buyck)
Basidiomes in small groups or as single individuals. Pileus mostly 30 − 45(60) mm diam., first convex-plane, then uplifted and sometimes irregularly lobed to infundibuliform, with the margin strongly involute when young, when mature sometimes with short radial striations; surface dull, finely pruinose-pubescent, sometimes locally wrinkled, in particular near the margin, of an intense reddish orange to carmine red (near 7A6-8), quickly paler when drying out and pale pinkish (7A3-5). Hymenophore strongly decurrent, of well-differentiated gill-folds, 2–3 mm high, sometimes wrinkled or undulating radially because of the interstitial veins and anastomoses that are decurrent on the gill sides, at other times without interstitial venation or only so near the extreme margin, most gills once or twice forked, sometimes mixed with some shorter lamellulae, paler than the pileus or stipe surface and of a soft salmon pink (near 7A2-3), yellowing when touched and then more orange. Stipe 20–40 × 9–15 mm, subcylindrical, rarely narrowing downward (sometimes also slightly wider below), the upper stipe when young with a minute pinkish pubescence (7A2–4), distinctly yellowing on the surface where handled (particularly in its lower portion) and then sometimes turning brownish (5B4–5), compact. Context white except underneath the cap and stipe surface where there exist pinkish-pale yellowish hues, firm, moderately yellowing when injured in the lower part of the stipe and on the whole surface when manipulated, probably also with age, when cut even leaving ferruginous traces. Taste mild. Smell typical of Cantharellus. Spore print not obtained.
Spores short ellipsoid to ovoid with often a distinct larger basal part, (6.9−)7.0 − 7.31 − 7.7(−8.1) × (4.2 −)4.7 − 4.92 − 5.2(−5.4) μm, Q = (1.3 −)1.4 − 1.49 − 1.6(−1.7), smooth, hyaline. Basidia irregularly clavulate, mostly 55–80 × 7–10 μm, bearing 5(−6) stout sterigmata. Subhymenium filamentous, composed of very long and slender cells, 3–5 μm diam. Pileipellis composed of thin-walled, cylindrical and regular hyphae, 5 − 7(−9) μm diam.; the terminal cell mostly (20 −)35 − 90 μm long, regularly cylindrical, obtuse. Clamp connections abundant in all tissues.
Holotype: MADAGASCAR. Tamatave Prov., Forest Station of Tampolo, near Fénérive Est, in deep sandy soil of the littoral forest, with Uapaca and Sarcolaenaceae div. spp., 25 Jan 2006, Buyck & Hofstetter 06.080 (HOLOTYPUS, PC0084727)
Additional examined material: MADAGASCAR. Tamatave Prov., Forest Station of Tampolo, near Fénérive Est, in deep sandy soil of the littoral forest, with Uapaca and Sarcolaenaceae div. spp., 24–25 Jan 2006, Buyck & Hofstetter 06.096 (PARATYPUS, PC0084755).
Commentary: Our Malagasy collections are in all respects extremely close to the African C. subincarnatus Eyssart. & Buyck, but the smaller spores, which are more ovoid because of their inflated lower halve, and the brighter orange-red coloration of the pileus, together with the particular habitat and endemic host trees, justify in our opinion at least the recognition of a distinct, Malagasy subspecies. It occupies an isolated phylogenetic position although it is morphologically speaking a completely normal chanterelle. (see Buyck et al. 2014).
Once again, there is an urgent need for sequence data for the Central African chanterelles. Both macro- and microscopically, C. rhodophyllus Heinem. is extremely similar, if not identical, to C. subincarnatus, although the original descriptions do not mention this (both are illustrated side by side). Heinemann (1959) does not comment on the resemblance between both taxa, but suggests instead that C. rhodophyllus is more similar to C. longisporus, from which it would differ mainly in the somewhat less elongate spores. Eyssartier (2001), who has re-examined the type-specimens for both taxa, found quasi identical values for the spores of both type-specimens (spores rhodophyllus: 6.5 − 7.39 − 9 × 3.5 − 4.21 − 5 μm, Q = 1.62 − 1.76 − 1.87; spores subincarnatus: 6–7.5 − 9 × 4–4.5 − 5 μm, Q = 1.4 − 1.66 − 2.0) as well as for all other microscopic structures. We adopt here nevertheless the name of C. subincarnatus because of the even shorter spores of our Malagasy specimens; also the general size of the fruit bodies is closer (C. rhodophyllus being described with a pileus up to 120 mm diam.). Eyi Ndong et al. (2011) reported recent collections of C. subincarnatus from the rain forest in Gabon, but reversed the situation, attributing and illustrating longer spores for the latter compared to more ellipsoid spores for C. rhodophyllus. According to Eyi Ndong et al. (2011), C. subincarnatus produces an ochraceous spore print.
Also the Central African C. ruber Heinem., a rare woodland species, has nearly identical microscopic features but possesses a less well-developed hymenophore (compared to our Malagasy collections) and produces a pink spore print (Buyck 1994).
Key to the Cantharellus species associated with endemic vegetation
-
1.
Basidiocarp lacking reddish, orange, vinaceous, pinkish, purplish or violaceous-lilac tints 2
-
1.
Pileus or stipe at least partly with reddish, orange, vinaceous, pinkish, purplish or violaceous-lilac tints. 8
-
2.
Basidiocarp (at least the pileus) principally brownish or grayish. Some species with clamps. 3
-
2.
Basidiocarp principally whitish to yellow. Clamps always absent. 6
-
3.
Context strongly yellowing, but not becoming ferruginous. Pileus squamulose, grayish to whitish or pale yellowish. Lacking clamps. Growing with various Uapaca species or Sarcolaenaceae in dense forest. Rather common. Cantharellus albidolutescens
-
3.
Context not strongly yellowing. Pileus not squamulose, pale brown to brownish grey. 4
-
4.
Hymenophore darker than pileus and stipe, without blackening context (as opposed to the eucalypt-associated C. cf. congolensis), with elongate spores. Clamps abundant everywhere. Insufficiently known taxon. Ecology unknown. Cantharellus avellaneus
-
4.
Hymenophore much paler than pileus surface. Clamps always absent. 5
-
5.
Terminal hyphae at the pileus surface distinctly thick-walled. Growing with Uapaca bojeri on the Central Plateau. Rare. Cantharellus paucifurcatus
-
5.
Terminal hyphae at the pileus surface thin-walled. Growing with other Uapaca spp. in dense forest. Rare. Cantharellus eyssartieri
-
6.
Yellowish species that reminds of the European C. cibarius (fleshy, short-stiped, yellow). Hymenophore generally composed of low veins that fork and anastomose frequently. Stipe usually short and thick. Growing with Uapaca bojeri on the Central Plateau. Widespread but not common. Cantharellus sebosus
-
6.
Hymenophore composed of well-differentiated gill folds. Stipe usually quite slender. 7
-
7.
Pileus smooth, with yellowish-orange-greenish tints. Context not yellowing. Growing with Uapaca bojeri on the Central Highlands. Common. Cantharellus platyphyllus subsp. bojeriensis
-
7.
Pileus squamulose. Context strongly yellowing. Growing with other Uapaca species or Sarcolaenaceae in dense forest. Rather common, but never abundant. Cantharellus albidolutescens
-
8.
Basidiocarp with lilac-violaceous tints, particularly on the pileus, otherwise predominantly yellowish. Clamps always abundant. 9
-
8.
Basidiocarp more pinkish, reddish or orange, lacking lilac-violaceous tints. Clamps absent or present. 10
-
9.
Reminiscent of C. cibarius because of the short stipe and yellow color, except for the presence of dark and sometimes intense, lilac-violaceous stains on the pileus. Growing with Uapaca bojeri on the Central Plateau. Rare. Cantharellus subcyanoxanthus
-
9.
Slender chanterelle with a long, but fleshy stipe and relatively narrow pileus that is entirely dark violaceous at first, but may become much paler to nearly off-white or cream with age. Growing with other Uapaca spp in the sandy soils of the East Coast. Rare. Cantharellus longisporus subsp. littoralis
-
10.
Medium-sized fruit bodies with pileus >30 mm diam. 11
-
10.
Small species with pileus distinctly <30 mm diam. 13
-
11.
Context not yellowing. Clamps absent. Growing with Uapaca bojeri on the Central Plateau. Common. Cantharellus platyphyllus subsp. bojeriensis
-
11.
Context yellowing, especially near the stipe base. Clamps present. 12
-
12.
Pileus and stipe soft salmon pink and hymenophore more yellowish. Growing with Uapaca bojeri on the Central Plateau. Rare. Cantharellus longisporus subsp. isaloensis
-
12.
Basidiocarp entirely reddish orange. Growing with other Uapaca spp in the sandy soils of the East Coast. Rare. Cantharellus subincarnatus subsp. rubrosalmoneus
-
13.
Basidiocarp pink, discoloring yellowish with age. Clamps abundant everywhere. Growing with Uapaca densifolia and other Uapaca spp. in dense forest at higher altitudes. Rare. Cantharellus decolorans
-
13.
Basidiocarp yellowish orange to orange-red. Central Highlands. 14
-
14.
Spores elongate. Clamps abundant everywhere. Ecology unknown. Cantharellus madagascariensis
-
14.
Spores ellipsoid. Clamps absent. 15
-
15.
Basidiocarp entirely yellowish orange. Growing with Uapaca bojeri in open woodlands on the Central Plateau, typically on bare, humid slopes. Rare. Cantharellus ibityensis
-
15.
Basidiocarp reddish orange with paler hymenophore. Growing with other Uapaca species or Sarcolaenaceae in dense forest on the Central Plateau. Rare. Cantharellus ambohitantelyensis
Discussion
Cantharellus avellaneus and C. madagascariensis, the first two chanterelles described from Madagascar (Patouillard 1924) remain unfortunately very problematic. A revision of the type specimens by Eyssartier (2001) revealed features that do not match any of the Cantharellus species that we collected to date in Madagascar. Even though the original description of C. avellaneus did not mention a blackening context, we have long been convinced (Buyck 2008) that this was merely an omission in the original description of C. avellaneus, and that Patouillard’s species most likely represented Malagasy specimens of C. congolensis, a species with blackening context described from the Central African rain forest (Beeli 1928). The latter, or its look-alike, is also found in Madagascar but always associated with exotic eucalypts. However, on second thought, it is impossible not to mention the blackening of the context when present, as the reaction is instantaneous and very strong. Moreover, the spores of the type of C. avellaneus are much more elongate and voluminous than those of C. congolensis and its look-alikes. Therefore, we consider Patouillard’s species to represent a similarly colored (brownish), but non-blackening species, probably unrelated to the African C. congolensis, which has a look-alike in Madagascar that grows exclusively with eucalypts.
A similar spore difference, but especially the abundant clamp connections in all tissues, separate the small, orange C. madagascariensis from the here newly described two other small, orange chanterelles.
Thanks to recent recollection of C. cyanoxanthus sensu Heim, we were fortunately able to solve the taxonomic confusion concerning the interpretation of this third ‘older’ species, invalidly described from Madagascar by Heim (1936) and later validated on different material from Central Africa (Heinemann 1958). Again as a result of differences in spore features, C. cyanoxanthus is now considered absent from Madagascar, but several more or less similarly colored and equally rare taxa have been described from both the woodlands of the Central Plateau (C. subcyanoxanthus. C. longisporus ssp. isaloensis) and the warmer climate of the littoral forest of the East coast (C. longisporus ssp. littoralis, which is C. cyanoxanthus sensu Heim non Heinemann) (see Buyck et al. 2012). As pointed out by Buyck et al. (2012, 2013) there is an urgent need for a modern, sequence based revision of the Central African chanterelles to allow for a correct interpretation and delimitation of these Central African species. For some chanterelles of the surrounding African woodlands, recent publications by Buyck et al. (2013) and Tibuhwa et al. (2012) provided the first sequence data, although the latter paper introduced an supplementary complication by accepting two different genera (see Shao et al. 2014)
Nothing in our dataset hints at an ancient vicariance or separate evolution of the Malagasy chanterelles, and this in spite of a 100 % endemicity of all potential ectomycorrhizal host trees, including species of the endemic families Sarcolaenaceae and Asteropeiaceae, both usually codominant with Uapaca trees (Buyck 2002). Malagasy and African chanterelles are often such close sister-taxa that in several instances we opted for describing them as separate subspecies rather than species. In the case of C. platyphyllus, our data even questions the recognition of a distinct subspecies (bojeriensis), originally described on the basis of clear differences in general color.
Including the taxa described in the present paper, at least 18 different species of Cantharellus (including C. aff. symoensii, but not accounting for the two very different subspecies of C. longisporus) are now known to occur in Madagascar, exceeding by far the number of chanterelles known from Australia (Eyssartier and Buyck 2001a) or South America (Nascimento et al. 2014; Pinheiro and Wartchow 2013; Wartchow et al. 2012a, b; Wilson et al. 2012) for example. We are convinced that many more undescribed taxa of Cantharellus occur on the island, as only very few places have been explored for fungi. Unfortunately, the very rapid degradation of the natural habitats due to the pressure of the rapidly growing population, frequent fires and the extremely aggressive expansion of exotic trees, in particular pines, into the remaining natural forests all pose severe threats to the survival of the native ectomycorrhizal species. In several of the above-mentioned type localities, the original vegetation has already completely vanished and been replaced by construction, agricultural land or exotic trees.
References
Beeli M (1928) Contribution à l'étude de la Flore Mycologique du Congo. Fungi Goossensiani, VI. Bull Soc R Bot Belg 61:78–100, pl. 3–6
Buyck B (1994) Ubwoba: les champignons comestibles de l’ouest du Burundi. Adm Gén Coop Dév 34:1–123, Publ Agric, Bruxelles
Buyck B (2002, for 2001) Preliminary observations on the diversity and habitats of Russula (Russulales, Basidiomycotina) in Madagascar. Micol Vegetat Medit 16:133–147
Buyck B (2008) Wild edible mushrooms in Madagascar: an evolving enigma. Econ Bot 62:509–520
Buyck B (2012) One neo- and four epitypifications for Cantharellus species from tropical African savannah woodlands. Cryptogam Mycol 33:11–17
Buyck B (2014) Exploring the diversity of “smooth chanterelles” (Cantharellus, Cantharellales). Cryptogam Mycol 35:23–40
Buyck B, Hofstetter V (2011) The contribution of tef-1 sequences for species delimitation in the Cantharellus cibarius complex in the southeastern USA. Fungal Divers 49:5–46
Buyck B, Randrianjohany E (2013) Cantharellus eyssartierii sp. nov. (Cantharellales, Basidiomycota) from monospecific Uapaca ferruginea stands near Ranomafana (eastern escarpment, Madagascar). Cryptogam Mycol 34:29–34
Buyck B, Eyssartier G, Kivaisi A (2000) Addition to the inventory of the genus Cantharellus (Basidiomycotina, Cantharellaceae) in Tanzania. Nova Hedwigia 71:491–502
Buyck B, Lewis DP, Eyssartier G, Hofstetter V (2010) Cantharellus quercophilus sp.nov. and its comparison to other small, yellow or brown American chanterelles. Cryptogam Mycol 31:17–33
Buyck B, Cruaud C, Couloux A, Hofstetter V (2011) Cantharellus texensis sp. nov. from Texas, a southern lookalike of C. cinnabarinus revealed by tef-1 sequence data. Mycologia 103:1037–1046
Buyck B, Randrianjohany E, Eyssartier G (2012) Observations on some enigmatic Cantharellus (Cantharellales, Basidiomycota) with lilac-violaceous tints from Africa and Madagascar. Cryptogam Mycol 33:167–179
Buyck B, Kauff F, Cruaud C, Hofstetter V (2013) Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers 58:281–298
Buyck B, Kauff F, Eyssartier G, Couloux A, Hofstetter V (2014) A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Divers 64:101–121
Eyi Ndong HE, Degreef J, De Kesel A (2011) Champignons comestibles des forêts denses d’Afrique Centrale. Taxonomie et identification. ABC Taxa 10, 253 pp
Eyssartier G (2001) Vers une monographie du genre Cantharellus Adans.:Fr. 259 p. Dissertation, National History Museum Paris
Eyssartier G, Buyck B (1999a) Contribution à la systématique du genre Cantharellus en Afrique tropicale : étude de quelques espèces rouges. Belg J Bot 131:139–149
Eyssartier G, Buyck B (1999b) Contribution à un inventaire mycologique de Madagascar. II. Nouveaux taxons dans le genre Cantharellus. Mycotaxon 70:203–211
Eyssartier G, Buyck B (2001a) Notes on the Australian species described in the genus Cantharellus. Aust Syst Bot 14:587–598
Eyssartier G, Buyck B (2001b) Note nomenclaturale et systématique sur le genre Cantharellus. Doc Mycol 31(121):55–56
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. J Mol Evol 39:783–791
Heim R (1936) Aperçu sur les Champignons toxiques et comestibles des Colonies françaises. Traité Pathol Exot Vét Comp 3:1–31
Heinemann P (1958) Champignons récoltés au Congo Belge par Madame M. Goossens-Fontana, III. Cantharellineae. Bull Jard Bot Brux 28:385–438
Heinemann P (1959) Cantharellineae. Fl Icon Champ Congo 8:153–165, pl. 26–28
Heinemann P (1966) Cantharellineae du Katanga. Bull Jard Bot Brux 36:335–352
Hofstetter V, Clémençon H, Vilgalys R, Moncalvo JM (2002) Phylogenetic analyses of the Lyophylleae (Agaricales, Basidiomycetes) based on nuclear and mitochondrial rDNA sequences. Mycol Res 106:1043–1059
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Methuen & Co. Ltd., London, 252 p, 30 pl
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Nascimento CC, Pinheiro FGB, Wartchow F, Alves MH (2014) Cantharellus squamulipes, a new chanterelle from the Brazilian semi-arid. Cryptog Mycol 35(4):(in print)
Patouillard N (1924) Basidiomycètes nouveaux de Madagascar. Bull Mus Natl Hist Nat (Paris) 30:406–413
Pinheiro FGB, Wartchow F (2013) Cantharellus protectus, a new species from Paraíba, Brazil. Sydowia 65:27–31
Shao S-C, Buyck B, Hofstetter V, Tian X-F, Geng Y-H, Yu F-Q, Liu P-G (2014) Cantharellus hygrophorus, a new species in subgenus Afrocantharellus from tropical southwestern China. Cryptogam Mycol 35:281–289
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Tibuhwa DD, Savić S, Tibell L, Kivaisi AK (2012) Afrocantharellus gen. stat. nov. is part of a rich diversity of African Cantharellaceae. IMA Fungus 3:25–38
Wartchow F, Buyck B, Maia LC (2012a) Cantharellus aurantioconspicuus (Cantharellales), a new species from Pernambuco, Brazil. Nova Hedwigia 94:129–137
Wartchow F, Santos JC, Fonseca MDP (2012b) Cantharellus amazonensis, a new species from Amazon. Mycosphere 3:414–418
Wilson AW, Aime MC, Dierks J, Mueller GM, Henkel TW (2012) Cantharellaceae of Guyana I: new species, combinations and distribution records of Craterellus and a synopsis of known taxa. Mycologia 104:1466–1477
Acknowledgments
We are grateful to the staff members of CNRE in Antananarivo for logistic support and field assistance. The field work in Madagascar was financed in part by the National Geographic Society (grants 6365-98, 7921-05) and in more recent years by the ATM-project “Past and present biodiversity” of the Muséum national d’histoire naturelle (Dirs. Ph. Janvier and S. Peigné). For sequencing, this study receives continuing support from the ATM-project “Barcode of life” of the Muséum national d’histoire naturelle (Dirs. L. Legall and S. Samadi). The authors are grateful to Else Vellinga, Keith Seifert and two anonymous reviewers for improvements of an earlier draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buyck, B., Kauff, F., Randrianjohany, E. et al. Sequence data reveal a high diversity of Cantharellus associated with endemic vegetation in Madagascar. Fungal Diversity 70, 189–208 (2015). https://doi.org/10.1007/s13225-014-0314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-014-0314-5