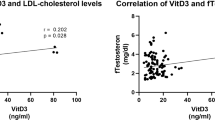
Abstract
Background
Vitamin D receptor (VDR) regulates insulin sensitivity, lipid metabolism and hormone balance in women. There are contrasting opinions on influence of VDR gene polymorphism for PCOS predisposition. Therefore, the present study investigated associations between VDR polymorphism (FOK1 and APA1) and PCOS phenotype as well as related biochemical and clinical parameters.
Methods
A total 240 female participants were enrolled in the study which consists of 120 women with PCOS and 120 control in group. We collected 5 ml of blood from each participant from which 3 ml was used for biochemical analysis and 2 ml was used to investigate genetic polymorphism in VDR genes via PCR–RFLP. Chi-square test, one-way ANOVA and Kruskal–Wallis test were used for the data analysis.
Results
We found that polymorphism in FOK1 and APA1 genotypes were significantly associated with the body mass index (BMI), high-density lipoprotein (HDL), prolactin level and fasting blood sample (FBS), total cholesterol, triglyceride, HDL level respectively among PCOS patients (p-value < 0.05). Similarly, APA1 polymorphism was also associated with the hip-waist ratio, FBS, HDL, LDL and LDL/HDL ratio, estradiol and prolactin level in control group (p < 0.05). However, we didn’t find any difference in the genotypic distribution of the FOK1 and APA1 gene polymorphs among the PCOS and controls group (p > 0.05).
Conclusion
The polymorphism in FOK1 and APA1 genes can indirectly alter the biochemical parameter and hormonal balance in body which might contribute to the development of PCOS. Dyslipidemia and hormonal imbalance must be ruled out in relation to VDR gene polymorphism in PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine disorders among the women of reproductive age (15–49 years), and the leading cause of anovulatory infertility. Globally, the prevalence of PCOS ranges from 6 to 20%, whereas recently it has been reported that 8.2% to 22.5% women in India are diagnosed with PCOS [1, 2]. It is often characterized by oligo/anovulation (OA), hyperandrogenism (HA) and polycystic ovary morphology (PCOM). Generally, there are four phenotypes of PCOS women: phenotype A (HA, OA and PCOM), phenotype B (HA and OA), phenotype C (HA and PCOM) and phenotype D (OA and PCOM) [3]. The underlying etiopathogenesis of PCOS is unclear and still under active research. However, few studies have unraveled a complex interplay of neurological, endocrine and genetics factors for predisposition of PCOS [4]. About 67% to 87% of PCOS women experience a higher prevalence of vitamin D deficiency, and it has been identified as major contributor for insulin resistance, ovulatory dysfunction and hyperandrogenemia [5]. Moreover, vitamin D is involved in a wide range of biological processes including hormonal and metabolic disorders such as PCOS. Most of the biological processes of vitamin D are mediated through vitamin D receptor (VDR), which is widely expressed in reproductive organs including ovaries, uterus and testes. When calcitriol (1α,25-(OH)2 D) binds with free floating VDR and retinoic acid receptor, a heterodimer complex is formed that translocate into the nucleus. This complex transcribes the VDR gene located on chromosome 12q13.11, which leads to cellular response and systemic effects such as insulin sensitization, lipid metabolism and anti-inflammatory effects [6]. Recently, a wide range of genetic alterations responsible for PCOS have come into spotlight, they can be either polymorphic or single-nucleotide variations (SNP). Single-nucleotide polymorphisms are the most prevalent genetic variations implicated in various diseases including PCOS. There are four most widely studied single-nucleotide polymorphisms in VDR gene, namely FOK1 (rs10735810), BSM1 (rs1544410), APA1 (rs7975232) and TAQ1 (rs731236). These polymorphisms have also been associated with the common presentation of PCOS such as obesity, insulin resistance, increased serum levels of testosterone and luteinizing hormone and low levels of vitamin D [7]. In the past decade, various endeavors have been made to establish association between VDR gene polymorphism and PCOS predisposition. However, the results are inconsistent, and epigenetic factors may play a role in the variations among different races. Moreover, the evidence in Indian population is scarce [8]. Therefore, we aimed to investigate the possible associations between VDR polymorphism (FOK1 and APA1) and PCOS phenotype as well as related biochemical and clinical parameters.
Materials and Methods
Study Population
In this case–control prospective study, a total of 120 PCOS patients of age group 18–40 years diagnosed based on Rotterdam criteria (Smet and McLennan, 2018), i.e., oligo and /or amenorrhea or anovulation, clinical and /or biochemical signs of hyperandrogenism and polycystic appearance of ovary on ultrasonography in case group. Patients with similar symptoms but diagnosed with other metabolic disorders such as hyperprolactinemia, androgen-secreting tumors, Cushing’s syndrome, and nonclassical congenital adrenal hyperplasia, patients on supplementation of vitamin D, estrogen, progesterone, combined oral contraceptive pills (OCPs), gonadotropins and antiepileptic drugs were excluded from the study. Similarly, 120 women between the age group of 18–49 years with regular menstrual cycles (26–34 days) and normal ovarian morphology were included as controls. The study was initiated after the approval of the study from the Central Ethics Committee (CEC) on human subjects (Ref. No.: NU/CEC/2018/0205 dated on 12.10.2018), and informed consent was obtained from each participant. Further, the study was performed according to the Declarations of Helsinki.
Clinical Data Collection
Patient demographic details and anthropometric measurements such as weight, height, BMI (body mass index) and waist-to-hip ratio (WHR) were measured.
Biochemical Tests
Five milliliter of peripheral blood was collected, of which 3 ml was used for biochemical analysis and remaining for genotyping. The hormonal levels, such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), prolactin (PRL), total testosterone (T) and estradiol (E2), were done on 2nd or 3rd day of menstrual cycle using an automated ELISA reader. Additionally, vitamin D levels and fasting insulin levels were performed by commercially available ELISA kits, and absorbance was read by (Spark Tecan) ELISA reader. Fasting blood glucose (FBS) was measured using a semi autoanalyzer. The homeostatic model assessment of insulin resistance (HOMA-IR) formula was used to calculate HOMA-IR: HOMA-IR = fasting insulin (IU/mL) *[fasting glucose (mg/dL)]/405.
Genomic DNA Extraction and Detection of VDR Polymorphisms
The remaining 2 ml blood was collected in a EDTA tube. A commercially available kit was used to isolate DNA from blood collected in EDTA (Qiagen) following the manufacturer’s instructions. A 2% ethidium bromide-stained agarose gel was used to determine the integrity of the DNA using a NanoDrop spectrophotometer (NanoDropTM2000/2000c, Thermo-Fisher Scientific, CA, USA). Molecular genotyping for the VDR Fok1 (rs2228570) and APA1 (rs7975232) was performed by PCR–RFLP (polymerase chain reaction-restriction fragment length polymorphism). The PCR was carried out in a MJ-Mini Thermal cycler (BioRad, Japan) with cycling parameters of initial denaturation at 95 °C for 5 min, amplification step consisting of 35 cycles at 95 °C for 30 s, 30 s of annealing at 66 °C, treatment at 72 °C for 30 s and a final extension step at 72 °C for 5 min. The primer detail’s restriction enzyme, incubation temperature and product size are provided in Table 1.
Statistical Analysis
All the analysis was performed using SPSS statistical software, version 22, IBM SPSS, version 22. We employed Chi-square goodness of fit to compare if the genotypic frequencies among cases and control follow Hardy–Weinberg equilibrium (HWE). The association between the genotype and covariates of interest (clinical and biochemical parameters) were assessed using one-way ANOVA and Kruskal–Wallis test for parametric and non-parametric data, respectively.
Results
Distribution of Genotype
The genotypic frequency distribution remained consistent between PCOS and control group (p > 0.05). The heterozygote recessive (CA) was more prevalent than the homozygotes in the case of APA1, whereas, in case of Fok 1, the homozygote dominant (CC) was very common. The Hardy–Weinberg equilibrium was not significantly violated by any of the genotype frequency distributions (p > 0.05). The distribution of VDR Fok1 and APA1 genotypes among PCOS and control group is depicted in Table 2.
Clinical and Biochemical Features in PCOS Women and Its Association with FOK1 and APA1 Gene Polymorphism
The overall data for fasting insulin level (18.23 ± 16.25), HOMA-IR (4.30 ± 4.79), LDL/HDL ratio (1.67 ± 0.81), FSH (4.70 ± 2.56), LH (11.52 ± 9.25), Testosterone (3.40 ± 2.11), estradiol (0.62 ± 0.45) and prolactin (581.96 ± 316.69) showed skewed distribution for PCOS patients, whereas fasting insulin level (9.02 ± 10.04), HOMA-IR (2.04 ± 2.33), FSH (4.96 ± 2.91), LH (8.53 ± 6.32), testosterone (2.44 ± 1.82) and estradiol (0.66 ± 0.38) showed skewed distribution for control group in our data. The remaining covariates were normally distributed for both the groups. We found that FOK1 genotypes were significantly associated with the BMI, HDL and prolactin level among PCOS patients (p-value < 0.05), whereas we didn’t observe any association between the FOK1 genotype and any of the covariates among control group (p-value > 0.05). Among the PCOS women, CC genotype showed highest BMI (26.78 ± 6) followed by CT genotype (23.81 ± 5) and TT genotype (23.36 ± 5). Further, PCOS women with CC genotype (50.8 ± 7.9) had lowest HDL levels when compared to CT (56.71 ± 13.52) and TT genotype (52.6 ± 7.1). Additionally, the highest prolactin level was observed in TT genotype (850.59 ± 189.85) followed by CT (628.27 ± 383.26) and CC (548.88 ± 287.59) (Table 3).
Similarly, we observed significant association of APA1 genotype with the FBS, total cholesterol, triglyceride and HDL level in PCOS group and with hip-waist ratio, FBS, HDL, LDL and LDL/HDL ratio, estradiol and prolactin level in control group (p < 0.05). The CC genotype had the highest FBS level and lowest HDL level in both the PCOS and control group. The total cholesterol and triglyceride levels were significantly increased in homozygous dominant (CC) genotype in compared to the in homozygous (TT) and heterozygous (CT) recessive genotype. Additionally, LDL and LDL/HDL ratio was greater in CC genotype as compared to the CA and AA genotype in control group. Further, the serum prolactin levels were highest in homozygous dominant (CC) and heterozygous (CA) genotype than the homozygous recessive (AA) in control group. Furthermore, the high hip-waist ratio and estradiol levels among control group are strongly associated with homozygous dominant (CC) and homozygous recessive (AA) genotype (Table 4).
Discussion
PCOS is a metabolic havoc and is often accompanied by multiple complications such as dyslipidemia, hypertension, cardiovascular risk and diabetes mellitus [9]. Nearly, 241 potential gene variants have been identified in PCOS aetiology with predominant involvement in steroidogenesis, ovarian theca cell functioning, insulin secretion, fat metabolism, etc. [10]. Vitamin D receptor is a ligand-dependent transcription factor of the nuclear receptor superfamily. Although regulation of VDR gene expression by vitamin D receptor is not completely deciphered, few studies have suggested that vitamin D receptor controls gene transcription through coactivator or corepressor, histone acetylation and chromatin remodeling. Calcitriol, the active form of vitamin D, promotes insulin sensitivity, lipid metabolism as well as production of testosterone, progesterone, estrone, and estradiol [10, 11]. Generally, vitamin D signaling pathway enhances insulin synthesis and release. Moreover, insulin resistance in PCOS due to vitamin D polymorphism may reduce hepatic production of sex hormone-binding globulin which results in hyperandrogenism. Additionally, vitamin D stimulates microsomal triglyceride protein (MTP) via calcium influx further turning down circulating serum cholesterol level. However, this mechanism is disrupted in PCOS, contributing dyslipidemia in PCOS women (depicted in Fig. 1) [12]. Previous studies have demonstrated that VDR gene polymorphisms are linked to insulin resistance, dyslipidemia, and hyperandrogenism in PCOS [13]. We evaluated the association of PCOS phenotype with two VDR gene polymorphism, FOK1 (C > T rs2228570) and APA1 (C > A rs7975232).
Calcitriol enters the cell through blood vessel. It binds to free floating vitamin D receptor (VDR) and retinoid x receptor (RXR) in cytoplasm forming a heterodimer. This complex translocates into the nucleus and binds to vitamin D receptor elements. A polymorphism in VDR such as Fok1 (C > T rs2228570) and APA1 (C > A rs7975232) can result in expression of hyperandrogenism, dyslipidemia and insulin resistance in polycystic ovarian syndrome through abnormal activation of genomic pathway
The variant distributions between our PCOS patients and controls were identical. Similarly, an Iranian study found that VDR FOK1 allelic frequencies did not present any significant association among PCOS and control subjects [14]. In contrast, Albahlol et al. observed a significant association of T allele of rs2228570 (Fok1) and C allele of rs7975232 (APA1) with PCOS group. Additionally, this association was prominent among phenotype C (hyperandrogenemia with polycystic ovary morphology) and phenotype D (oligo/anovulation with polycystic ovary morphology) [15]. Another study by Mahmoudi et al. found that VDR APA1 CC was associated with increased risk of PCOS, whereas AC genotype was related to decrease PCOS susceptibility and none of the FOK1 alleles were associated with PCOS [16]. Previously, it has been reported that distribution of APA1 AA genotype and FOK1 FF genotype in PCOS were considerably greater than controls and were related with decreased levels of vitamin D [17]. In our study, the biochemical and clinical parameters were associated with the genotypic distribution of FOK1 and APA1 alleles. We observed that PCOS women with FOK1 CC variants and TT variants posed abnormal BMI, HDL and prolactin levels. Correspondingly, an Iranian study discovered statistically significant relationship with BMI for women carrying the FOK1 CC genotype when compared to the CT genotype within the PCOS group and suggesting obesity to be positively linked with FOK1 gene polymorphism [18]. According to an Iraqi study, serum prolactin levels were substantially on a higher side in the carriers of Fok1 CC variants among PCOS patients [19]. Further, we also found APA1 polymorphism in CC genotype to be associated with low levels of HDL in both PCOS and control subjects, whereas total cholesterol and triglyceride were was significantly associated with APA1 CC genotype in only PCOS patients. Similarly, Santos et al. reported CC genotype of APA1 SNP to be associated with higher total cholesterol, triglyceride levels and lower HDL levels [20]. The present study observed significant association between FBS and APA1 CC genotype in both PCOS and control group although no significant associations were found with fasting insulin and HOMA-IR. Contrastingly, a South Indian study reported that fasting insulin levels and HOMA-IR scores were significantly elevated in APA1 GG genotypes of PCOS patients and fasting glucose did not present any statistically significant relation with APA1 SNP. This indicated hyperinsulinemia and insulin resistance to be linked with APA1 polymorphisms in PCOS [21]. On the other hand, studies conducted to investigate the effect of APA1 gene polymorphisms on metabolic variables in PCOS women have not found any connections with insulin resistance [22, 23]. Moreover, we observed higher prolactin and lower estradiol levels to be associated with CA genotype of APA1 polymorphism in control group. Previous studies have linked prolactin to APA1 polymorphism; however, estradiol did not show any statistically significant associations with APA1 polymorphism. [19, 24] Although we could not establish any significant association with other biochemical/clinical parameters such as fasting insulin, HOMA-IR, LDL, LDL/HDL ratio, FSH, LH, testosterone and vitamin D, various studies have reported positive relationship of Fok1 and APA1 polymorphism with these parameters. Moreover, conflicting results may be due to variation in the allelic frequencies, genotypic markers, statistical methods and sometimes false-positive results [25,26,27].
Overall, our study suggests that BMI, HDL, prolactin, FBS, cholesterol and triglyceride have significance association with the VDR gene polymorphism (FOK1 and APA1 gene). Thus, the polymorphism in genes like FOK1 and APA1 might contribute to the hormonal imbalance and dyslipidemia which in turn leads to development of PCOS.
Conclusion
From this study, we conclude that FOK1 and APA1 gene polymorphism might not be directly associated with the development of PCOS. However, FOK1 gene polymorphism might be involved in alteration BMI, HDL and prolactin level, whereas APA1 gene polymorphism might influence the FBS, cholesterol, triglyceride and HDL level and contribute to the enigmatic pathology of PCOS. Thus, hormonal dysregulation and dyslipidemia must be ruled out during diagnosis of PCOS patients.
Abbreviations
- BMI:
-
Body mass index
- FSH:
-
Follicular-stimulating hormone
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- HWE:
-
Hardy–Weinberg equilibrium
- LDL:
-
Low-density lipoprotein
- LH:
-
Luteinizing hormone
- PCOS:
-
Polycystic ovarian syndrome
- RFLP:
-
Restriction fragment length polymorphism
- SNP:
-
Single-nucleotide polymorphism
- VDR:
-
Vitamin D receptor
- WHR:
-
Waist-to-hip ratio
References
Mehreen TS, Ranjani H, Kamalesh R, Ram U, Anjana RM, Mohan V. Prevalence of polycystic ovarian syndrome among adolescents and young women in India. J Diabetol. 2021;12(3):319–25.
Bharali MD, Rajendran R, Goswami J, Singal K, Rajendran V. Prevalence of polycystic ovarian syndrome in India: a systematic review and meta-analysis. Cureus. 2022;14(12).
Aversa A, La Vignera S, Rago R, Gambineri A, Nappi RE, Calogero AE, Ferlin A. Fundamental concepts and novel aspects of polycystic ovarian syndrome: expert consensus resolutions. Front Endocrinol. 2020;11(11):516.
Siddiqui S, Mateen S, Ahmad R, Moin S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 2022;39(11):2439–73.
Niu YM, Wang YD, Jiang GB, Bai G, Chai HB, Li XF, Hu YY, Shen M. Association between vitamin D receptor gene polymorphisms and polycystic ovary syndrome risk: a meta-analysis. Front Physiol. 2019;10(9):1902.
Kalyanaraman R, Pal L. A narrative review of current understanding of the pathophysiology of polycystic ovary syndrome: focus on plausible relevance of vitamin D. Int J Mol Sci. 2021;22(9):4905.
Shahmoradi A, Aghaei A, Ghaderi K, Rezaei MJ, Azarnezhad A. A meta-analysis of the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the vitamin D receptor gene with the risk of polycystic ovary syndrome in the Eastern Mediterranean Regional Office population. Int J Reprod Biomed. 2022;20(6):433.
Deswal R, Nanda S, Dang AS. Unveiling the association between Vitamin D receptor and poly cystic ovary syndrome–A systematic review and meta-analysis. Int J Vitamin Nutr Res. 2019.
Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;24:249–60.
Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X. 2019;1(3): 100060.
Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, Seto-Young D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. 2010;42(10):754–7.
Guo S, Tal R, Jiang H, Yuan T, Liu Y. Vitamin D supplementation ameliorates metabolic dysfunction in patients with PCOS: a systematicreview of RCTs and insight into the underlying mechanism. Int J Endocrinol. 2020;22(2020):1–8.
Liang F, Ren N, Zhang H, Zhang J, Wu Q, Song R, Shi Z, Zhang Z, Wang K. A meta-analysis of the relationship between vitamin D receptor gene ApaI polymorphisms and polycystic ovary syndrome. Adv Clin Exp Med. 2019;28(2):255–62.
Bagheri M, Rad IA, Jazani NH, Nanbakhsh F. Lack of association of vitamin D receptor FokI (rs10735810)(C/T) and BsmI (rs1544410)(a/G) genetic variations with polycystic ovary syndrome risk: a case-control study from Iranian Azeri Turkish women. Maedica (Buchar). 2012;7:303.
Albahlol IA, Neamatallah M, Serria MS, El-Gilany AH, Setate YA, Alkasaby NM, Mostafa SA, Abdelaziz M, Elazab H, Ammar OA. Vitamin D receptor gene polymorphism and polycystic ovary syndrome susceptibility. BMC Med Genomics. 2023;16(1):1–9.
Mahmoudi T, Majidzadeh-A K, Farahani H, Mirakhorli M, Dabiri R, Nobakht H, Asadi A. Association of vitamin D receptor gene variants with polycystic ovary syndrome: a case control study. Int J Reprod BioMed. 2015;13(12):793.
Humadi EH, Showman HA, Al-azzawie HF. Association of vitamin-D receptor gene polymorphisms (FokI and ApaI) and vitamin D Serum Levels in a sample of Iraqi women with polycystic ovary syndrome. Indian J Public Health Res Dev. 2018;9(10).
Ranjzad F, Mahban A, IraniShemirani A, Mahmoudi T, Vahedi M, Nikzamir A, Zali MR. Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet. 2011;28:225–32.
Kadhim SJ, Abdul-hassan IA. Correlation between vitamin D receptor gene polymorphisms and levels of some hormones in Iraqi infertile women with polycystic ovary syndrome. Iraqi J Biotechnol. 2017;16(4).
Santos BR, Lecke SB, Spritzer PM. Apa-I polymorphism in VDR gene is related to metabolic syndrome in polycystic ovary syndrome: a cross-sectional study. Reprod Biol Endocrinol. 2018;16:1–8.
Thathapudi S, Erukkambattu J, Addepally U, Kodati V, Hasan Q. Association of insulin-like growth factor 2 Apa1 A820G gene (rs680) polymorphism with polycystic ovarian syndrome. Int J Reprod Contracept Obstet Gynecol. 2016;5(8):2618–23.
El-Shal AS, Shalaby SM, Aly NM, Rashad NM, Abdelaziz AM. Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome. Mol Biol Rep. 2013;40:6063–73.
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–9.
Al-Mulhim AA, Abul-Heija AA, Al-Talib AA, Al-Turki HA, Gasim TG. Hormonal, metabolic and clinical profile of Saudi women with polycystic ovary syndrome. Saudi J Med Med Sci. 2013;1(1):30–4.
Dasgupta S, Dutta J, Annamaneni S, Kudugunti N, Battini MR. Association of vitamin D receptor gene polymorphisms with polycystic ovary syndrome among Indian women. Indian J Med Res. 2015;142(3):276.
Al Thomali A, Daghestani MH, Daghestani MH, Kaya N, Warsy A. Polymorphic variations in VDR gene in Saudi women with and without polycystic ovary syndrome (PCOS) and significant influence of seven polymorphic sites on anthropometric and hormonal parameters. J Med Biochem. 2018;37(4):415.
Han FF, Lv YL, Gong LL, Liu H, Wan ZR, Liu LH. VDR gene variation and insulin resistance related diseases. Lipids Health Dis. 2017;16(1):157.
Acknowledgements
We would like to thank Department of Gynaecology and Obstetrics, Justice KS Hegde Charitable Hospital, Mangalore, India.
Funding
No funds, grants or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization, writing—review, revision and editing were presented by Vijayalaxmi BM and Lakshmi Manjeera. Methodology and supervision were performed by Lakshmi Manjeera. Formal analysis, investigation and writing—original draft preparation were provided by Vijayalaxmi BM. All authors critically revised the manuscript and approved the final version. Lakshmi Manjeera holds the final responsibility of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Central Ethics Committee (CEC) on human subjects (Ref. No.: NU/CEC/2018/0205 dated on 12.10.2018), and the study was performed according to the Declarations of Helsinki.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vijayalaxmi Bangra Manjeshwara: Lecturer, Dr Lakshmi Manjeera: HOD and Professor, Dr Prima D’souza: Assistant Professor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bangra Manjeshwara, V., Manjeera, L. & D’souza, P. FOK1 and APA1 Gene Polymorphism Among Polycystic Ovary Syndrome: A Prospective Cohort Study. J Obstet Gynecol India (2024). https://doi.org/10.1007/s13224-024-02024-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13224-024-02024-1