Abstract
Obesity, insulin resistance, and hyperandrogenism are considered crucial parameters of polycystic ovary syndrome (PCOS) which might be related to vitamin D metabolism. The aim of this study was to investigate the associations between polymorphisms (TaqI and ApaI) in the vitamin D receptor gene (VDR) and PCOS among Egyptian women. We aimed also to elucidate the impact of these polymorphisms on vitamin D level, hormonal and metabolic parameters of PCOS. One hundred and fifty Egyptian women with PCOS and 150 unrelated controls were enrolled in this study. Polymorphisms of VDR Taq-I T/C (rs731236) and Apa-I A/C (rs7975232) gene were genotyped using polymerase chain reaction restriction fragment length polymorphism (PCR–RFLP). Serum 25 hydroxy vitamin D [25(OH) D] levels were measured by high-performance liquid chromatography. PCOS women had significantly lower levels of 25(OH) D compared to healthy women. Our results revealed that Taq-I CC genotype and C allele were associated with increased risk of PCOS, while the Apa-I polymorphism was not. Haplotype Taq-I C/ Apa-I C was associated with a higher PCOS risk more than controls. Moreover, there was a significant decrease of 25(OH) D levels in carriers of haplotype Taq-I C/ Apa-I C (with variant alleles) compared to the non-carriers. Results showed also that there was an obesity- VDR Taq-I genotypes interactions. These results suggested that, VDR Taq-I gene polymorphism is associated with increased risk of PCOS in Egyptian women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder, which has a strong genetic component with a prevalence of 5–10 % in women of reproductive age [1]. Clinical manifestations of PCOS include menstrual irregularity, hirsutism, acne, infertility, and/or alopecia [2]. Its endocrinal features include hyperandrogenism, high levels of luteinizing hormone (LH) with normal levels of follicle stimulating hormone (FSH), and higher values of the LH/FSH ratio. PCOS has significant psychological features also including increased anxiety, depression and worsened quality of life [2]. Moreover, women with PCOS suffer from metabolic abnormalities including insulin resistance (IR), compensatory hyperinsulinemia, central obesity [3], and type 2 diabetes mellitus [4].
IR stimulates the accumulastion of more weight and induces hyperandrogenism and other symptoms of PCOS. The strong association between IR and ovarian hyperandrogenism suggests that insulin directly influences ovarian function [5]. Interestingly, IR increases with increasing body mass index (BMI) and waist circumference as a clinical sign of central obesity [6]. Obesity is a common feature of PCOS that worsens the phenotype of this disease [7]. IR is most commonly found in obese PCOS patients (65 %), but can also occur in about 20 % of lean PCOS patients [8].
Accumulating evidences suggest that vitamin D deficiency might be a causal factor in the pathogenesis of IR and the metabolic syndrome in PCOS [4]. On the other hand, obesity is also linked with low 25 hydroxy vitamin D [25(OH) D] levels in PCOS cohorts [4, 9] and in others including various groups of obese and normal weight women and men [10]. Hahn et al. [4] suggested that low levels of vitamin D may be a primary factor in the initiation and development of PCOS, and that dietary repletion of this important vitamin could help to restore normal menstrual cycles in women with this condition.
The actions of vitamin D are mediated by the vitamin D receptor (VDR), a nuclear receptor with a DNA-binding domain that acts through vitamin D response elements located near the start site of target genes [4]. VDR gene is located on the long arm of chromosome 12 (12q14) [11]. It encompasses two promoter regions, eight protein-coding exons (namely 2–9) and six untranslated exons (1a–1f) [12]. Interestingly, VDR gene regulates about 3 % of the human genome, including genes that are crucial for glucose, lipid metabolism and blood pressure regulation [13]. It has been suggested that single nucleotide polymorphisms (SNPs) within the VDR gene may influence the stability, quantity, and activity of VDR protein and the rate of VDR gene transcription [14]. The presence of the VDR in pancreatic β-cells supports the findings of earlier study reported that vitamin D affected insulin secretion [15] and the discovery of a vitamin D response element on the insulin receptor gene suggests a mechanism by which vitamin D deficiency could affect insulin sensitivity [4]. Moreover, previous study has revealed significant associations between VDR gene polymorphisms and insulin sensitivity [16].
VDR gene is highly polymorphic and allele frequencies are highly variable among different races and ethnic groups [17]. Furthermore, data on the role of gene variants involved in vitamin D metabolism in PCOS are inconclusive or inconsistent. Therefore the aim of our study was to investigate the possible associations of VDR [ Apa-I (in intron8), and Taq-I (in exon 9)] gene polymorphisms with susceptibility to PCOS. Also, we aimed to clarify the impact of these polymorphisms on vitamin D level, hormonal and metabolic parameters.
Subjects and methods
Subjects
This case–control study included 300 unrelated women. 150 women with PCOS and 150 healthy control women, who had regular ovulatory cycles, and they were matched to cases by age, BMI, ethnic origin (Caucasian), and sun exposure habits. All patients were recruited from outpatient clinics of Obstetrics and Gynecology Department of Benha University Hospitals; and Endocrinology Unit of Internal Medicine Department of Zagazig University Hospitals in the period from August 2010 to October 2011. The diagnosis of PCOS was based on the revised Rotterdam criteria [18]. At least two of the following three features needed to be present: oligo-ovulation or anovulation (<6 menstrual periods per year); (2) clinical and/or biochemical signs of hyperandrogenism, including hirsutism (Ferriman-Gallwey score >8), severe persistent acne, and/or total testosterone level >0.8 ng/ml); and (3) sonographic evidence of PCOS. None of the PCOS patients had other cause of oligomenorrhea.
All participants were subjected to thorough medical history taking and complete clinical assessment including; blood pressure and anthropometric variables. None of the patients and the controls had history of hyperandrogenic states (such as nonclassic congenital adrenal hyperplasia, androgen secreting tumours, Cushing’s syndrome, or hyperprolactinaemia). Also all study participants had no history of diabetes mellitus, hypertension, liver, kidney, thyroid diseases, hormonal medications, vitamin D supplementation, or calcium for the last 6 months. Patients and controls were then stratified according to the degree of obesity into two groups [lean, BMI <25 kg/m2; and obese, BMI ≥25 kg/m2 [19]. The ethical committee of Faculty of Medicine, Zagazig University approved our study protocol, and written informed consent assigned by all participants.
Blood sampling
Blood samples were drawn from all subjects during the early follicular phase of the menstrual cycles after an overnight fast. We divided blood sample into three portions: 1 ml of whole blood was collected into evacuated tubes containing EDTA for genomic DNA extraction and HbA1c. Secondly; 1 ml of whole blood was collected into evacuated tubes containing fluoride for fasting blood glucose. Sera were separated immediately from the remaining third portion and stored at −20 °C until analysis.
Biochemical and hormonal assays
We determined fasting blood glucose by the glucose oxidase method (Spinreact, Girona, Spain). Total cholesterol and triglycerides were assessed by routine enzymatic methods (Spinreact, Girona, Spain). HDL cholesterol was determined after precipitation of the apoB-containing lipoproteins. LDL cholesterol was calculated using the Friedewald formula [20]. Fasting serum insulin, FSH, LH, total testosterone, free testosterone, sex hormone-binding globulin (SHBG) concentrations were measured using high-sensitivity enzyme-linked immunosorbent assay kit provided by (DRG International, IRC, USA). Serum 25(OH) D levels were measured using a high-performance liquid chromatography (HPLC) according to [21]. Homeostasis model assessments of IR were estimated including HOMA-IR and HOMA-β; an index of β-cell function [22]. Insulin sensitivity was calculated by the quantitative insulin sensitivity check index (QUICKI) according to the following formula: 1/{log [fasting insulin (μU/ml)] + log [fasting glucose (mg/dl)]} [23].
Genomic DNA extraction
Genomic DNA was isolated and purified from whole blood using QIAamp-spin-columns according to the protocol provided by the manufacturer (QIAamp Blood Kit; Qiagen GmbH, Hilden, Germany). DNA was stored at −20 °C till the time of use.
Analysis of the VDR Taq-I (rs731236) and VDR Apa-I (rs7975232)
VDR Taq-I and VDR Apa-I polymorphisms were analyzed by means of PCR restriction fragment length polymorphism (RFLP) method according to [24].
VDR Taq-I was amplified by using the sense primer 5′-CAGAGCATGGACAGGGAGCAAG-3′ and antisense primer 5′-GCAACTCCTCATGGCTGAGGTCTCA-3′ and VDR Apa-I amplification was done using the sense primer 5′-CAGAGCATGGACAGGGAGCAAG-3′ and antisense primer 5′-GCAACTCCTCATGGCTGAGGTCTCA-3′. PCR was performed in a final volume of 25 μL containing 5.5 μL of H2O, 5 μL of genomic DNA, 1 μL of each primer (1 μM), and a 2×Super Hot PCR Master Mix (12.5 μL) (Bioron, Ludwigshafen am Rhein, Germany). PCR protocol was performed at 94 °C for 5 min, followed by 35 cycles at 93 °C for 45 s, at 66 °C for 30 s, and at 72 °C for 45 s. A final extension step was carried out at 72 °C for 7 min. The PCR products were digested overnight at 65 °C with Taq-I restriction enzyme (MBI-Fermentas, United Kingdom) for VDR Taq-I polymorphism. The digestion of DNA resulted in three fragments of 290, 245 and 205 bp (C allele) in the presence of the polymorphic site and two fragments of 245 and 495 bp (T allele) in its absence, due to an additional monomorphic Taq-I site (Fig. 1). While for VDR Apa-I variants, the final products were digested overnight at 37 °C with Apa-I restriction enzyme (MBI-Fermentas). The digestion of DNA in the presence of the restriction sites resulted in 2 fragments of 210 and 530-bp fragments for (C allele), and one fragment of 740 bp (A allele) (Fig. 2). Both undigested and digested PCR products were visualized in 2.5 % agarose (Serva) stained with ethydium bromide.
Statistical analysis
The results for continuous variables are expressed as the mean ± SD. The means of the three genotype groups were compared in a one-way analysis of variance. Genotype frequencies in cases and controls were tested for Hardy–Weinberg equilibrium, and any deviation between the observed and expected frequencies was tested for significance using the Chi square (χ2) test. The correlation coefficients were calculated using Spearman correlation. The statistical significances of differences in the frequencies of variants between the groups were tested using the χ2 test. In addition, the odds ratios (ORs) and 95 % confidence intervals (95 % CIs) were calculated as a measure of the association of both VDR Taq-I T/C and Apa-I A/C genotypes with PCOS. Haplotypes were determined based on Bayesian alogorithm using the Phase program. Linear regression analysis was performed to test possible association between PCOS and other parameters. A difference was considered significant at P < 0.05. All data were evaluated using statistical package for social sciences (SPSS) for windows version 17.
Results
Clinical and laboratory characteristics of the study subjects
Clinical and laboratory characteristics of the study subjects are shown in Table 1. PCOS women had significantly higher values of hirsuitism score, total cholesterol, triglycerides, LDL cholesterol, fasting blood glucose, fasting insulin, HOMA-IR, and HOMA-B when compared to controls. Moreover, PCOS women had higher total and free testosterone, LH, Dehydroepiandrosterone sulfate (DHEA-S) and androstenedione levels more than healthy controls. Women with PCOS had a higher antral follicle count (AFC) per ovary than controls.
On the contrary, PCOS women had significantly lower levels of HDL cholesterol, QUICKI, SHBG, FSH, and 25(OH) D when compared to controls. By linear regression analysis, BMI, hirsuitism score, DHEA-S, SHBG, HOMA and QUICKI were independent risk factors for PCOS (P < 0.001 for each).
Distribution of genotype and allele frequencies of VDR Taq-I and Apa-I polymorphisms in healthy controls and PCOS women
Genotype frequencies of the VDR Taq-I and Apa-I polymorphisms were in agreement with Hardy–Weinberg equilibrium in both groups. There was a significant difference between PCOS patient group and controls regarding the genotype and allele distributions of VDR Taq-I polymorphism. Prevalence of CC genotype and C allele were significantly higher in PCOS women as compared to controls (P = 0.001, <0.001 respectively). The odds ratio and 95 % CI for the C allele of Taq-I was 1.9 (1.4–2.6). Concerning VDR Apa-I, there was no significant difference in genotype and allele frequencies between PCOS and control women (P = 0.8, 0.5 respectively) and the odds ratio and 95 % CI for the C allele of Apa-I was 0.9 (0.7–1.3) (Table 2). By linear regression analysis, Taq-I was independent risk factor for PCOS (P < 0.001) while Apa-I was not (P = 0.73).
Haplotype frequencies of VDR Taq-I and Apa-I polymorphisms in PCOS and controls
Concerning haplotype analysis, haplotype CC (with all the variant alleles) was found to be associated with a higher risk of PCOS patients than controls [the odds ratio and 95 % CI was 1.8 (1.2–2.6) and P = 0.01]. On the other hand, frequency of haplotype TA was significantly higher in control group as compared to PCOS patients [the odds ratio and 95 % CI was 1.7 (1.2–2.3) and P = 0.002] (Table 3).
Genotype, allele and haplotype frequencies of VDR Taq-I and Apa-I polymorphisms in PCOS and control women when stratified into obese and lean subjects
We stratified both PCOS patients and controls according to their BMI into lean and obese groups. When comparing between obese and lean women within each group, we found that significant differences between lean group and obese women regarding the genotype and allele distributions of VDR Taq-I in PCOS patients. The C allele was more frequent in the obese group as compared to lean group in PCOS patients (54 vs. 22 %, P < 0.001) while there was no significant difference between lean and obese groups of controls regarding genotypes and allele frequencies. These results showed that there was an obesity-VDR Taq-I genotypes interactions (Table 4).
On the other hand, there were no significant differences in allele or genotype frequencies between lean and obese for VDR Apa Apa Apa-I polymorphism (Table 4). Interestingly, when we compared between the obese women in both groups; PCOS patients and controls, we found significant differences in allele and genotype frequencies of VDR Taq-I (P < 0.001 for each). The same results were found concerning the lean women in both groups (P < 0.001 for each).
When we studied haplotypes frequencies between obese and lean subjects as a whole, we found no significant differences of the haplotypes frequencies between both groups (Table 5). Again when we compared between the haplotype frequencies between obese and lean patients in PCOS group or in control group, we found no statistically significant difference between them (data not shown).
Impact of VDR Taq-I and Apa-I gene polymorphisms and haplotypes on clinical, biochemical, and hormonal characteristics of the groups studied
The characteristics of the PCOS patients according to the VDR Taq-I and Apa-I genotypes are shown in Table 6. Individuals carrying CC and TC genotypes of the VDR Taq-I had significantly higher BMI, AFC, fasting insulin, HOMA-IR, HOMA-B, total testosterone, free testosterone, DHEA-S and androstenedione levels and significantly lower levels of 25(OH)D and QUICKI than women homozygous for T alleles. However, there was no association of Apa-I polymorphism with the metabolic parameters or the 25(OH) D levels. The same results have been shown when studying the relation between VDR Taq-I or Apa-I genotypes and hormonal, clinical and morphological states in controls (data not shown). When studying these parameters among different Taq-I Apa-I haplotypes, we found that there was a significant decrease of 25(OH) D levels (P < 0.001) and significant increase of total testosterone, free testosterone, DHEA-S and androstenedione levels (P = 0.006, 0.001, 0.02 and <0.001 respectively) in carriers of Taq-I C/ Apa-I C compared to the non carriers.
Surprisingly, we found no effect of Taq-I and Apa-I genotypes and haplotypes on the clinical parameters, 25(OH) D levels or PCOS morphology when stratifying PCOS or control groups into obese and lean.
Correlation of vitamin D with other parameters in PCOS and control groups
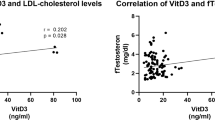
Correlation between 25(OH) D levels and other parameters in PCOS and control groups is shown in Table 7. 25 hydroxy vitamin D levels were negatively correlated with BMI, hirsuitism score, total cholesterol, triglycerides, LDL cholesterol, fasting blood glucose, total testosterone, free testosterone, LH, DHEA-S, androstenedione and AFC. On the contrary, 25(OH) D levels were positively correlated with HDL cholesterol, FSH and SHBG.
When we stratified the participants in this study into obese and lean, we found that 25(OH) D levels were negatively correlated with BMI, AFC, total cholesterol, triglycerides and LDL cholesterol in both groups.
Discussion
To the best of our knowledge, this is the first study to investigate the association of VDR gene polymorphisms, VDR haplotypes, and vitamin D level with susceptibility to PCOS among Egyptian women. PCOS is one of the most common etiologies of infertility and menstrual irregularity in women [25]. Also, vitamin D has an important proven role in reproduction [26]. However, the exact patho-physiology of PCOS is complex and remains largely unclear.
In the present study, we have found a decrease of serum 25(OH) D levels in PCOS women compared with healthy controls. These results confirmed the results of previous studies of [4, 27, 28]. However, Panidis et al. [29] found no significant difference of serum 25(OH) D concentrations between women with PCOS and controls. Interestingly, Thys-Jacobs et al. [30] found that administration of high doses of vitamin D led to the attenuation of hyperandrogenism and menstrual disturbances in women with PCOS. Moreover, Yildizhan et al. [31] added that low concentrations of 25(OH) D should be kept in mind during managing obese women with PCOS.
In our Egyptian control sample, the minor allele frequency (MAF) for VDR Taq-I was 33.6 % and for Apa-I was 33.4 %. They were similar to that reported in Caucasians (MAF > 10 % for each polymorphism) [32]. In the current study, the prevalence of CC genotypes and C alleles of VDR Taq-I polymorphism were significantly higher in PCOS patients compared to controls. Also, we found that Taq-I polymorphism was independent risk factor for PCOS. These results are in agreement with [24] who suggested that VDR TaqI “CC” genotype is involved in pathogenesis of PCOS through its effects on LH levels. However, the same authors could not identify the molecular mechanism through which this polymorphism influences LH levels. Furthermore, it has been suggested a modulating role of 1, 25(OH)2 D in the control of FSH secretion [33]. On the contrary, Mahmoudi [3] and Wehr et al. [28] reported a non significant association between VDR TaqI polymorphism and PCOS among Austrian and Iranian women, respectively.
On the other hand, there were no significant differences in VDR Apa-I genotypes and allele frequencies between PCOS and healthy women, and it was not independent risk factor for PCOS. These results are in agreement with other studies found no significant association between the VDR Apa-I genotypes and PCOS susceptibility [34, 35]. On the contrary, a study by Mahmoudi demonstrated that the VDR Apa-I AC genotype (Aa in his study) was a marker of decreased PCOS susceptibility, whereas the CC (aa in his study) was associated with an increased risk for PCOS [3].
Because obesity is a common feature of PCOS that worsens its phenotype, we also analyzed whether the VDR polymorphisms were associated with obese PCOS women. Our results revealed that more than half of the PCOS patients were obese (73.3 %). These findings confirmed the results of previous studies of [4] and [36]. Moreover, in obese women the reproductive phenotype of PCOS can be reversed by weight loss [37].
We have also found a significant differences between VDR Taq-I genotypes and allele frequencies between lean and obese groups in PCOS women, but not VDR Apa-I polymorphism. Our results were compatible with those reported by Ye et al. [38] who found that VDR Taq-I polmorphisms are associated with susceptibility to obesity in subjects with early-onset type 2 diabetes mellitus. The pathophysiological mechanisms of these associations remain unexplained. A direct effect of vitamin D on adipocyte differentiation and metabolism is a possible mechanism, as VDR is expressed in preadipocytes [39]. Moreover, it has been shown that vitamin D inhibits the differentiation of preadipocytes in vitro [40], and stimulates the terminal differentiation of adipocytes as well as the synthesis and secretion of lipoprotein lipase [41, 42].
Our study demonstrated that there were associations of VDR Taq-I TC and CC genotypes with anthropometric, endocrine, or metabolic disturbances in PCOS. Moreover, women carrying TC and CC genotypes had significantly lower levels of 25(OH) D than women homozygous for T alleles. The association between VDR Taq-I polymorphism and the low 25(OH) D levels may be explained by that signaling through VDR may regulate vitamin D levels but future functional analysis of VDR action may be needed. These results are in agreement with Mahmoudi [3] who found a significant decrease of serum levels of 25(OH) D in women with PCOS carrying the TC and CC genotypes (Tt and tt in his work) compared with those carrying the TT genotypes. On contrast, another study found no significant association of VDR Taq-I polymorphisms with anthropometric, endocrine, or metabolic parameters of PCOS [35].
On other hand, we reported a non significant association of Apa-I polymorphism with metabolic parameters or 25(OH) D levels. This agreed with the results obtained by Mahmoudi [3] who reported a non significant association of Apa-I AC genotype (Aa in his work) with PCOS status. This disagrees with the results found an association of VDR Apa-I genotypes with androgen levels [35]. However, the same authors did not observe an association of Apa-I polymorphism with metabolic parameters or 25(OH) D levels [35].
Our novel findings concerning haplotype analysis; the frequency of haplotype Taq-I C/ Apa-I C was significantly increased in PCOS women compared to that in the controls. This haplotype was associated with nearly 1.8 fold increase in the risk of PCOS syndrome. On the other hand, the haplotype Taq-I T/ Apa-I A was more prevalent in control and was found to be associated with a low risk of PCOS. Yet when we compared between the haplotype frequencies between obese and lean patients in PCOS group or in control group, we found no significant difference between them. Another important finding that there was a significant decrease of 25(OH) D levels in carriers of haplotype Taq-I C/ Apa-I C (with mutant alleles) compared to the non carriers. This disagreed with Santos et al. [43] who found the association of the haplotype containing the wild alleles of Taq-I, Apa-I and Bsm-I with lower vitamin D levels in Brazilian girls. These differences may be attributed to that the haplotype analysis was performed in two different ethnic groups.
Our results revealed that 25(OH) D levels were negatively correlated BMI, fasting insulin, HOMA-IR, HOMA-B, and QUICKI in PCOS patients. Our findings were consistent with many reports found that increased body weight had a significant negative correlation on 25(OH) D levels in PCOS patients [4, 28, 35, 33].
The exact mechanisms underlying the association of vitamin D and IR are not fully understood. First, vitamin D may have a beneficial effect on insulin action by stimulating the expression of insulin receptor and thereby enhancing insulin responsiveness for glucose transport [13]. The vitamin D-responsive element is present in the promoter of the human insulin gene [16] and the transcription of the human insulin gene is activated by 1, 25(OH) D2 [41]. Secondly, vitamin D regulates extracellular and intracellular calcium that is essential for insulin-mediated intracellular processes in insulin-responsive tissues such as skeletal muscle and adipose tissue [13]. Finally, as vitamin D has a modulating effect on the immune system [44], hypovitaminosis D might induce a higher inflammatory response, which is again associated with IR [45].
Our results reported that 25(OH) D levels were negatively correlated with total cholesterol, triglycerides, LDL cholesterol, while it is positively correlated with HDL cholesterol. This agreed with the results obtained by Hahn et al. [4], Li et al. [27] and Wehr et al. [28].
Of particular interest is the observation that 25(OH) D concentrations were correlated negatively with hirsutism score, which is in line with previous study by Ranjzad et al. [24]. This association might be caused by various mechanisms. First, the cosmetic distress may cause hypovitaminosis D due to the decreased sun exposure of hirsute women. Secondly, the VDR is found in keratinocytes of the outer root sheath as well as in cells of the bulge, indicating an important role of vitamin D in hair follicle cycling [46]. However, the mechanism by which the VDR regulates hair follicle cycling and its potential role in hirsutism remains unclear.
Although our small sample size, this study was well designed and focused on the role of VDR gene variants, VDR haplotypes, and vitamin D levels on metabolic and biochemical parameters of PCOS. Consequently, further researches including larger sample numbers and analyzing the whole VDR gene are necessary to clarify the role of these polymorphisms in PCOS, and its effects on disease phenotypes. Moreover, studies evaluating the impact of vitamin D supplementation on obesity and PCOS are needed.
In conclusion, the present study provided evidence showing the important role of VDR Taq-I gene polymorphisms in the development and phenotypes of PCOS among lean and obese patients. Furthermore, our data demonstrated, for the first time, that VDR haplotype Taq-I C/ Apa-I C was found to be associated with a higher risk of PCOS. Genotyping of VDR gene may be useful in early detection of obese PCOS women.
References
Asuncio´n M, Calvo RM, San Milla´n JL, Sancho J, Avila S , Escobar- Morreale HF (2000) A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85(7):2434–2438
Teede H, Deeks A, Moran L (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impact on health across the lifespan. BMC Med 30:8–41
Mahmoudi T (2009) Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril 92(4):1381–1383
Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann, K, Janssen OE (2006) Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 114(10):577–583
Weber RF, Pache TD, Jacobs ML, Docter R, Loriaux DL, Fauser BC, Birkenhäger JC (1993) The relation between clinical manifestations of polycystic ovary syndrome and beta-cell function. Clin Endocrinol (Oxf) 38(3):295–300
Aronne LJ, Segal KR (2002) Adiposity and fat distribution outcome measures: assessment and clinical implications. Obes Res 10(1):14–21
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R (2002) Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 26(7):883–896
Dale PO, Tanbo T, Vaaler S, Abyholm T (1992) Body weight, hyperinsulinemia, and gonadotropin levels in the polycystic ovarian syndrome: evidence of two distinct populations. Fertil Steril 58:487–491
Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z (2010) Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril 93(4):1208–1214
Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R (2008) Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr 47:87–91
Chiu KC, Chuang LM, Yoon C (2001) The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med Genet 2:2
Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325–349
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW (1988) Cloning the expression of full length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA 85:3294–3298
DeLuca HF (2004) Therapeutic potential of the 2-alkyl and 2-alkylidene-19-nor-20S-modified analogs of 1a, 25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 89(90):67–73
Maestro B, Da´vila N, Carranza MC & Calle C (2003) Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84:223–230
Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K et al (2002) Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 51:2294–2300
The Rotterdam ESHRE-ASRM-sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47
National Institutes of Health Consensus Development Panel (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obes Res 6(2):51–209
Frieldewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Turpeinen U, Hohenthal U, Stenman UH (2003) Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin Chem 49(9):1521–1524
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Ranjzad F, Mahban A, Shemirani AI, Mahmoudi T, Vahedi M, Nikzamir A, Zali MR (2011) Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet 28(3):225–232
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53
Rashidi B, Haghollahi F, Shariat M, Zayerii F (2009) The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol 48(2):142–147
Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK (2011) Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 60(10):1475–1481
Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR et al (2009) Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 161(4):575–582
Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, Katsikis I, Zournatzi V, Diamanti-Kandarakis E (2005) Serum parathyroid hormone concentration are increased in women with polycystic ovary syndrome. Clin Chem 51(9):1691–1697
Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP (1999) Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 6:430–435
Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, Kamaci M (2009) Serum 25-hydroxyvitamin D concentrations in obese and non obese women with polycystic ovary syndrome. Arch Gynecol Obstet 280(4):559–563
Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E et al (2004) Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 13:1633–1639
Zofkova I, Scholz G, Starka L (1989) Effect of calcitonin and 1, 25 (OH)2-vitamins D3 on the FSH, LH and testosterone secretion at rest and LHRH stimulated secretion. Horm Metab Res 21:682–685
Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook P N, Eisman JA (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284–287
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B (2011) Vitamin D–associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 164(5):741–749
Muscogiuri G, Sorice GP, Prioletta A, Policola C, Della Casa S, Pontecorvi A, Giaccari A (2010) 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 18:1906–1910
Pasquali R, Casimirri F, Vicennati V (1997) Weight control and its beneficial effect on fertility in women with obesity and polycystic ovary syndrome. Hum Reprod 1:82–87
Ye WZ, Reis AF, Dubois-Laforgue D, Bellanné-Chantelot C, Timsit J, Velho G (2001) Vitamin D receptor gene polymorphisms are associated with obesity in Type 2 diabetic subjects with early age of onset. Eur J Endocrinol 145:181–186
Kamei Y, Kawada T, Kazuki R, Ono T, Kato S ,Sugimoto E (1993) Vitamin D receptor gene expression is up-regulated by 1,25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 193:948–955
Lenoir C, Dace A, Martin C, Bonne J, Teboul M, Planells R et al (1996) Calcitriol down-modulates the 3,5,30 triiodothyronine (T3) receptors and affects, in a biphasic manner, the T3-dependent adipose differentiation of Ob 17 preadipocytes. Endocrinology 137:4268–4276
Dace A, Martin-El Yazidi C, Bonne J, Planells R, Torresani J (1997) Calcitriol is a positive effector of adipose differentiation in the OB 17 cell line: relationship with the adipogenic action of triiodothyronine. Biochem Biophys Res Commun 232:771–776
Kawada T, Kamei Y, Sugimoto E (1996) The possibility of active form of vitamins A and D as suppressors on adipocyte development via ligand-dependent transcriptional regulators. Int J Obes Relat Metab Disord 20(3):52–57
Santos BR, Mascarenhas LP, Satler F, Boguszewski MC, Spritzer PM (2012) Vitamin D deficiency in girls from South Brazil: a cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr 8(12):62
Maestro B, Molero S, Bajo S, Da´vila N, Calle C (2002) Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 20:227–232
Bikle D (2009) Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94:26–34
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180
Acknowledgments
The authors wish to express their sincere thanks to all volunteers and patients.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Shal, A.S., Shalaby, S.M., Aly, N.M. et al. Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome. Mol Biol Rep 40, 6063–6073 (2013). https://doi.org/10.1007/s11033-013-2716-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2716-y