Abstract
Medical genetics has evolved over a decade, and hence, all investigations are available for clinical practice. Many diseases are diagnosed accurately today because of new investigations. These advanced investigations are affordable, accessible and available in day-to-day practice. Hence, there is a need and it is a time for us to understand these advanced technologies. Karyotyping and rapid aneuploidy tests are basic tests, while chromosomal microarray and next-generation sequencing are advanced technologies. It is time to update the knowledge and utilize them in day-to-day practice. These tests are utilized both in prenatal diagnosis and in some clinical scenarios, which are elaborated in detail. Karyotyping is the basic tool to detect both numerical and structural abnormalities. It is advantageous in that it is accurate with error of 0.001% but has a resolution of up to 5 MB. Rapid aneuploidy detection tests are equally accurate and detect as good as 99%. They are FISH, QF-PCR and MLPA. They have high sensitivity and specificity, and results are available within 3 days of time. Hence, these tests are apt for Indian scenarios, where late detection of anomalies (18–20 weeks) is common. Chromosomal microarray is the hybridization technique which detects aneuploidy of all chromosomes. This is useful for detection of deletion and duplication in chromosomes. This is not available for prenatal diagnosis in India now, whereas this is available for prenatal diagnosis in developed countries. Whole-exome sequencing and whole-genome sequencing are advanced techniques which have been described and discussed at length.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been tremendous developments in the field of medical genetics in the past decade, especially in deciphering the genome and identifying the phenotypic effects of genomic variations. Diagnosis and management of genetic disorders, namely monogenic and chromosomal disorders, are affected by genomic techniques like next-generation sequencing and microarray.
Here, basic investigations, interpretation of these tests and common clinical conditions needing genetic investigations are discussed at length.
Karyotyping in Clinical Practice
As early as 1882, Walther Flemming, an Austrian cytologist and professor of Anatomy, published the first illustration of human chromosomes. In 1959, Lejeune described extra chromosome in Down syndrome in each cell. The word chromosome is derived from the Greek word “colored body.” Human cells contain 46 chromosomes comprising 22 pairs. The numbers are assigned in descending order of length, size and centromere position of each chromosome pair, with long arm as “q” and short arm as “p.” Karyotype is done by g banding technique with resolution of up to 350–550 MB [1, 2].
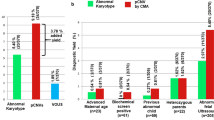
Chromosome abnormalities can be either numerical or structural abnormality. Most common abnormalities are aneuploidies, i.e., Down syndrome, Trisomy 21, Trisomy 18, Trisomy 13 and monosomy X (Figs. 1, 2).
Common clinical conditions to order karyotype are:
- 1.
Suspected/known chromosomal abnormality like Down syndrome, trisomy 18, turner syndrome and Klinefelter syndrome.
- 2.
Unexplained intellectual disability (developmental delay with dysmorphic features) (yield of 4–8%). Chromosomal microarray increases the yield.
- 3.
Disorders of sex development: Individuals with ambiguous genitalia, delayed or incomplete pubertal development need a karyotype. Often turner syndrome and Klinefelter syndrome are diagnosed.
- 4.
Pregnancy loss and infertility: Chromosomal structural rearrangements can often lead to recurrent spontaneous abortions (5.5% is the yield with three or more first trimester losses).
- 5.
Parents of the child with structural chromosomal abnormality like balanced translocation in mother need karyotyping.
Advantages of karyotyping are:
Time-tested study with vast clinical experience.
Widely available.
Can be done in peripheral blood, abortal tissues, amniotic fluid and in chorionic villous sampling.
It is accurate with an error rate of 0.001% which is primarily related to maternal cell contamination, sample mix-up and typographical errors.
Well-established cytogenetic technique which has been extensively used as a diagnostic tool for pregnant women undergoing prenatal invasive procedures.
Whole chromosome is analyzed.
Karyotyping using amniotic fluid is considered the “gold standard” in fetal aneuploidy testing due to high sensitivity and relatively low risks.
Limitations/Disadvantages of Karyotyping
-
Small submicroscopic alterations below 4–5 MB size are usually not picked up by routine karyotype.
-
It is important to remember that most mendelian disorders have mutations involving only one or very few nucleotides and are not diagnosed by karyotype. It is also worthwhile to note that commonly used genetic test with fair diagnostic yield has limited resolution, which can be explained by large size and complexity of the human genome.
-
Long culture days.
-
Possibility of culture failure.
-
Requires actively growing cells.
-
Maternal cell contamination (0.1–0.2%).
-
Inability to detect mosaicism (prevalence of 0.1–0.2%).
Prenatal Diagnosis and Genetics
The various indications for fetal cytogenetic testing include:
- 1.
Abnormal ultrasound scan.
- 2.
Abnormal maternal serum biochemical results.
- 3.
Advanced maternal age (≥ 35 years of age at the expected time of confinement).
- 4.
One of the parents, being a carrier of a chromosomal rearrangement.
- 5.
History of previous offspring with chromosomal disorder.
In India, many ultrasound abnormalities become apparent during 18–20 weeks of scan; hence, accurate rapid aneuploidy detection techniques are important.
Rapid aneuploidy detection methods
These are primarily targeted for the diagnosis of common autosomal trisomies (13, 18, 21) and sex chromosomal aneuploidies [3,4,5].
Three methods of diagnostic techniques validated for prenatal diagnosis are:
- 1.
FISH: Fluorescent in situ hybridization.
- 2.
QF-PCR: Quantitative fluorescent in situ hybridization.
- 3.
MLPA: Multiplex ligation-dependent probe amplification.
FISH: Fluorescent In situ Hybridization
It is usually performed on uncultured interphase cells with probes designed specifically for chromosomes 13, 18, 21, X and Y. The number of fluorescent signals per cell gives the number of copies of the targeted chromosome. The technique is known to be almost 100% accurate with an added advantage of ability to detect triploidy also. However, FISH technique is non-automated and time-consuming and necessitates a skilled technician.
QF-PCR: Quantitative Fluorescent In situ Hybridization
This assay has been widely used for the past 2 decades, which uses fluorescent-labeled primers to amplify specific DNA markers which are polymorphic and unique for chromosomes 13, 18, 21, X and Y. Detection of maternal cell contamination, triploidy and mosaicism as low as 15% is an important advantage of these techniques.
MLPA: Multiplex Ligation-Dependent Probe Amplification
It is also a PCR-based method, which is cheaper and less labor-intensive than FISH technique. The free ends of the ligated probes are complementary to the primer which enables the amplification of target sites. The technique has a capacity to quantify up to 40–50 different target sequences in one reaction. One of the major drawbacks of this technique is the failure to detect triploidies, especially in a female fetus. It is a completely automated method and is being increasingly used as a method for RAD, especially where large-scale testing of samples is required. Table 1 summarises advantages and disadvantages of RAD techniques.
New Methods of Genetic Testing for Prenatal Diagnosis: Chromosomal Microarray
This is based on the principle of hybridization, and the strength of signals from these probes is interpreted in an automated manner to provide information regarding the number of copies of that region of the genome. In contrast to the commonly used RAD techniques, chromosomal microarray can detect aneuploidies of all 23 chromosomes as well as submicroscopic copy number abnormalities in a genome-wide manner [6, 7].
Common Clinical Conditions and Application of Genetic Testing
Recurrent pregnancy loss and genetics
Recurrent pregnancy losses, also known as recurrent spontaneous abortions, are traditionally defined as 3 or more consecutive pregnancy losses of less than 20 weeks of gestation. A total of 3–5% of couples experience RPL. The cause of RPL is difficult to assess, and in fact, no cause can be determined in half of the cases in spite of a battery of investigations. This suggests the presence of unidentified genetic causes.
In 3–5% of couples, with RPL, one partner is found to carry a balanced chromosomal rearrangement, 50% are balanced reciprocal translocations, and 12% are sex chromosomal mosaicism. Although carriers of balanced translocation are phenotypically normal, their pregnancies are at increased risk miscarriages or live births with congenital abnormalities or intellectual disability. The remainders are chromosomal inversions and other sporadic abnormalities. In these couples, RPL occurs due to abnormal segregation of gametes at the time of meiosis. In couples, with recurrent miscarriage, chromosomal abnormalities of the embryo account for 30–57% of further miscarriages [8,9,10].
RCOG recommends karyotyping of products of conception of third and subsequent miscarriages and parental peripheral blood karyotyping in couples with unbalanced structural abnormality [11].
Couples with balanced translocations have a low risk (0.8%) of pregnancies with an unbalanced karyotype surviving into second trimester, and chance of having healthy child is 83%.
Preimplantation genetic diagnosis or fetal karyotyping by amniocentesis is an option for these couples to select fetuses with normal chromosomal content. Some chromosomal variations like pericentric inversion of 9, small or large heterochromatin arm of Y chromosome and inversion Y are seen in many normal individuals and are not known to be associated with poor reproductive outcome.
Premature ovarian aging/failure and genetics
Genetic aberrations comprise one-third of women with premature ovarian aging and also ovarian failure. When FSH is elevated above the age-specific cutoffs, premature ovarian aging is considered. A study by Gleicher et al. titled “Do the etiologies of POF and POA same?” concluded that presumed underlying etiologies of POA follow a similar distribution pattern as reported for POF. They proved the hypotheses that POA is a precursor stage of POF and hence requires similar evaluation [12,13,14].
Genetic causes comprised approximately 16% of the total in the study conducted by Gleicher et al. Both autosomes and X chromosomal involvement are documented. They are Turner mosaicism, partial X chromosome deletion, X chromosome mosaicism, X chromosome inactivation and FMR 1 (fragile site mental retardation X gene). X chromosome partial deletions are more common, while balanced X chromosome to autosome translocation of Xq13–q26 is rare, but documented. Autosomes involved are at the following gene loci: 3q, 13q, 14q, 17q, 15q and 11p [15, 16]. We had a rare case of triple X syndrome of premature aging in our clinical practice which is reported and Fig. 3 represents karyotype of the same.
Primary amenorrhea and genetics
Clinical features with Turner syndrome like webbed neck, short stature, cubitus valgus with absent menstruation and absent secondary sexual characteristics by the age of 15 years are definite indications for karyotyping. There may be Y element in karyotype, and this is an indication for gonadectomy to prevent gonadoblastoma.
Recent Advances and Technologies in Medical Genetics
Whole-exome sequencing (WES) and whole-genome sequencing (WGS) [17] are two important new technologies which have become affordable, accessible and available in India. This has enhanced the diagnostic yield in medical genetics. This is not yet used routinely for prenatal diagnosis because of the following reasons:
- 1.
Variants of unknown significance (VUS) Since few genetic abnormalities have to be interpreted and correlated with clinical data, these genetic abnormalities cannot be interpreted correctly.
- 2.
If these genetic data are not interpreted correctly, this may lead to misjudgement and hence may lead to decisions like termination of pregnancy.
Whole-Exome Sequencing
This is a genomic technique for sequencing of all protein coding genes in genome. This consists of two steps. First step consists of separating the subset of DNA that encodes the proteins called exons, which constitute about 1% of the total genome or approximately 30 million base pairs. The second step is to sequence the exonic DNA using any high-throughput DNA sequencing technology. After sequencing, three kinds of interpretation are obtained:
- 1.
Known pathogenic.
- 2.
Benign.
- 3.
Variants of unknown significance.
Whole-Genome Sequencing
This is the process of determining the complete DNA sequence of an organism’s genome at a single time. This entails sequencing all of an organism’s chromosomal DNA as well as DNA contained in the mitochondria.
As of now, these diagnostic technologies are used for diagnosis of the following:
- 1.
Children with intellectual disability.
- 2.
Children with neurodevelopmental delay and white matter disorders.
- 3.
Complete diagnostic workup of inborn errors of metabolism.
Hence, prenatal diagnosis with whole-exome sequencing is now confined to the clinical scenarios wherein there is positive previous targeted gene testing.
References
Kingston HM. ABC of clinical genetics. BMJ. 1989;298:813–6.
Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–2.
Allingham-Hawkins DJ, Chitayat D, Cirigliano V, et al. Prospective validation of quantitative fluorescent polymerase chain reaction for rapid detection of common aneuploidies. Genet Med. 2011;13:140–7.
Armengol L, Nevado J, Serra-Juhé C, et al. Clinical utility of chromosomal microarray analysis in invasive prenatal diagnosis. Hum Genet. 2012;131:513–23.
Mann K, Donaghue C, Fox SP, et al. Strategies for the rapid prenatal diagnosis of chromosome aneuploidy. Eur J Hum Genet. 2004;12:907–15.
Van Opstal D, Boter M, de Daneille J, et al. Rapid aneuploidy detection with multiplex ligation-dependent probe amplification: a prospective study of 4000 amniotic fluid samples. Eur J Hum Genet. 2009;17:112–21.
Wright D, Carey L, Battersby S, et al. Validation of a chromosomal microarray for prenatal diagnosis using a prospective cohort of pregnancies with increased risk for chromosome abnormalities. Genet Test Mol Biomark. 2016;20:791–8.
Rajshekar M, Sreelakshmi K, Gopinath PM, et al. Cytogenetics study in recurrent pregnancy loss. An experience from tertiary care centre. Genet Couns. 2013;24(3):347–9.
Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11.
Dutta UR, Rajitha ARP, Pidugu VK, et al. Cytogenetic abnormalities in 1162 couples with recurrent miscarriages in Southern region of India: report and review. J Assist Reprod Genet. 2011;28:145–9.
Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81:171–6.
Kodandapani S, Pai MV, Nambiar J, et al. Premature ovarian aging in primary infertility: triple X syndrome. J Hum Reprod Sci. 2011;4(3):153–4.
Barad DH, Weghofer A, Gleicher N. Age-specific levels for basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109:1404–10.
Gleicher N, Weghofer A, Oktay K, et al. Do etiologies of premature ovarian ageing (POA) mimic those of premature ovarian failure (POF). Hum Reprod. 2009;24:2395–400.
Goswami D, Conway SG. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410.
Otter M, Constance TR, Schrander S, et al. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010;18:265–71.
Di Resta C, Ferrari M. Next generation sequencing: from research area to clinical practice. EJIFCC. 2018;29(3):215–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Human and Animal Rights
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sreelakshmi K. N., MS, DNB, FICOG is an Associate Professor of KMC, Manipal, India. Professor and Head of Subbaiah Medical College, Shimoga, Karnataka, India. Fellowship from SIAMG (Society of Indian Academy of Medical Genetics), KMC, Manipal, India.
Rights and permissions
About this article
Cite this article
Sreelakshmi, K.N. Medical Genetics for Practicing Obstetrician. J Obstet Gynecol India 70, 6–11 (2020). https://doi.org/10.1007/s13224-019-01257-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-019-01257-9