Abstract
The objective of the present study was to investigate lactic acid bacteria (LAB) isolated from kimchi for their potential probiotic use. Ten preselected LAB strains were evaluated for their functionality and safety. Examined characteristics included acid and bile tolerance, cell adhesion, antimicrobial activity against pathogens, hemolytic activity, undesirable biochemical characteristics, and antibiotic resistance. Results indicated that consumption of these 10 strains does not pose any health risk, as they were not hemolytic and exhibited no undesirable biochemical activity or antibiotic resistance. In particular, three strains, Lactobacillus plantarum NO1, Pediococcus pentosaceus MP1, and Lactobacillus plantarum AF1, showed high degrees of acid and bile tolerance, adherence to Caco-2 and HT-29 cells, and antimicrobial activity against four pathogens (Staphylococcus aureus, Escherichia coli O157:H7, Salmonella typhi, and Listeria monocytogenes). These results suggest that LAB strains from kimchi may have potential use as novel probiotics.
Similar content being viewed by others
Introduction
The growing demand for healthier foods is stimulating innovation and new product development in the food industry worldwide (Saarela et al. 2000). For example, the health-promoting effects of probiotics have led to their increased use in fermented dairy foods (Guglielmotti et al. 2007; Maragkoudakis et al. 2006; Bertazzoni et al. 2004). Among these microorganisms, lactic acid bacteria (LAB), especially Lactobacillus and Bifidobacterium spp., are the most commonly used probiotics in food for human consumption (Foligné et al. 2010). LAB are generally regarded as safe (GRAS), as they have a long history of safe use as starter culture bacteria (Carr et al. 2002). However, it has been frequently reported that some members of the genera Lactobacillus, Leuconostoc, Pediococcus, Enterococcus, and Bifidobacterium cause infections that in some patients has led to clinical conditions such as endocarditis and septicemia (Liong 2008). There are many sources of exposure to these bacteria, including probiotics, fermented foods, and the host’s own microbiota (Borriello et al. 2003), and it was recently speculated that bacteria in food may act as reservoirs of antibiotic resistance genes (Franz et al. 2005; Ammor et al. 2007; Clementi and Aquilanti 2011; Mathur and Singh 2005). Indeed, although LAB have been accepted as safe, this assessment was not until recently based on any real scientific criteria (Donohue 2006).
It is now recognized that probiotic products exhibit specific properties such as gastric acid and bile tolerance, adherence to epithelial surfaces, and antagonist activity against pathogens (Saarela et al. 2000). They also lack undesirable properties such as expression of virulence factors, harmful biochemical activity, and antibiotic resistance (Donohue 2006; Ammor et al. 2007; Clementi and Aquilanti 2011). These activities offer opportunities for the development of beneficial products for humans and animals. Accordingly, new species and more specific bacterial strains are continuously being sought as novel probiotic candidates. At the same time, the efficacy of these new strains should be carefully assessed. And an evaluation of the new candidates should be applied to all strains of bacteria, including those traditionally used in food fermentation, to confirm their safety status.
Kimchi is a traditional Korean food and has a long history of safe production and consumption (Chang and Chang 2010). Kimchi is consumed every day as a side dish in Korea, with Korean people consuming an average of 91.9 g of kimchi per day (World Institute of Kimchi 2011). Kimchi fermentation is a natural process that is initiated by a variety of microorganisms originally present in the raw kimchi materials. Although there are about 200 species of microorganisms involved in kimchi fermentation, the microorganisms primarily responsible are LAB such as Leuconostoc spp. and Lactobacillus spp. (Chang and Chang 2010; Nam et al. 2009). Consequently, kimchi is a good source of potentially beneficial LAB.
The objective of the present study was to investigate LAB isolated from kimchi for their potential probiotic use. Ten preselected LAB isolates from kimchi, including Lactobacillus spp., Leuconostoc spp., and Pediococcus spp., were evaluated for their functionality and safety. Examined characteristics included acid and bile tolerance, cell adhesion, antimicrobial activity against pathogens, hemolytic activity, undesirable biochemical characteristics, and antibiotic resistance.
Materials and methods
Bacterial strains and media
A total of 10 LAB strains preselected among 409 LAB cultures isolated from kimchi were used. The preselected isolates have been identified and genotipically/phenotipically typed in previous works (see Table 1 for references). The selection of strains was carried out previously based on distinct characteristics, including antimicrobial activity and metabolic characteristics [e.g., production of γ-aminobutyric acid (GABA), exopolysaccharide (EPS), or mannitol]. Bacterial strains and media used in the present work are summarized in Table 1 along with relevant references. Lactobacillus rhamnosus GG ATCC 53103 and Bacillus cereus ATCC 14579 were used as reference strains for the examination of cell adhesion and hemolysis, respectively. Pathogens were cultured for 12 h at 37 °C in Luria-Bertani (LB) broth (Difco, Sparks, MD, USA) or Brain Heart Infusion (BHI) broth (Difco). LAB were propagated at 30 °C for 24 h in de Man Rogosa and Sharpe (MRS; Difco) and Muller Hinton (MH; Difco) broth without shaking. For EPS production, Leuconostoc kimchii GJ2 was cultivated in sucrose medium (1 % tryptone, 0.5 % yeast extract, 0.5 % dipotassium phosphate, 0.5 % diammonium citrate, 5 % sucrose, pH 7.0). ATCC strains were purchased from the American Type Culture Collection (Manassas, VA, USA).
Acid and bile tolerance
Tolerances levels of LAB to acid and bile salt were assessed as described previously with modification (Santini et al. 2010; Lian et al. 2003). LAB were first cultivated in 5 ml of MRS broth at 30 °C for 24 h. Cultures were then harvested (9,950 g, 5 min), after which approximately 8.2–9.6 log CFU/ml of cells were resuspended in 1 ml of phosphate-buffered saline (PBS, pH 2.5; Hyclone, Logan, UT, USA) or simulated gastric juice (SGJ; pepsin 3 mg dissolved in 1 ml of 0.5 % saline buffer, pH 2.5) and/or bile salt (0.3 % oxgall dissolved in PBS, pH 8.0). Suspensions were incubated at 37 °C for 1 h in PBS (pH 2.5) or SGJ, or for 3 h in bile salt. Thereafter, the suspensions were harvested (9,950 g, 5 min) and resuspended in MRS broth, after which viable cell numbers were counted on MRS agar after incubation at 30 °C for 48 h. In parallel, controls were set up in which LAB were suspended in MRS broth without acid or bile salt. To investigate the effects of EPS on acid and bile tolerance, Leuconostoc kimchii GJ2 was cultivated in both MRS and sucrose broth media.
In vitro adhesion assay
Adhesion of LAB to Caco-2 and HT-29 cells was assayed according to the method of Fernández et al. (2003) with modification. In brief, monolayers of Caco-2 (American Type Culture Collection, Manassas, VA, USA) and HT-29 cells (American Type Culture Collection) were prepared by inoculating 5.7 log CFU/ml into 24-well tissue culture plates (Corning Costar, Cambridge, MA, USA) containing Dulbecco’s Modified Eagle Medium (DMEM; Hyclone) or Rosewell Park Memorial Institute 1640 medium (RPMI; Hyclone), respectively. Both media were supplemented with 10 % (v/v) fetal bovine serum (FBS; Hyclone). Once cells had formed a monolayer, approximately 7.2–9.6 log CFU/ml of viable LAB was added to each well and incubated at 37 °C for 1 h in a 5 % CO2 incubator (Sci 165D; Astec, Tokyo, Japan). After incubation, monolayers were washed three times with PBS to release unattached bacteria. Total numbers of adherent bacteria in each well were then counted by lysing cells; 1 ml of 0.05 % (v/v) Triton X-100 was added to wells, after which the plate was shaken (Green SSeriker Vison, Gyeonggi-Do, Korea) for 10 min at 160 rpm at room temperature. Counts of viable bacteria were then made on MRS agar after incubation at 30 °C for 24–48 h. Lactobacillus rhamnosus GG was used as a positive control for the adhesion assay.
Antimicrobial activity
Antimicrobial activities against four pathogens were assessed using the agar well diffusion method with modification (Magnusson and Schnürer 2001). BHI or LB plates were spread with each pathogen at a concentration of 6.0 log CFU/ml. A well with a diameter of 5.0 mm was then punched out from each agar plate, after which LAB (9.0 log CFU/ml) in 70 μl of MRS soft agar were deposited in each well. After incubation at 37 °C for 24 h, the diameter of the clear zone around the well was measured.
Enzymatic activities
Enzymatic activities were assayed using an API-ZYM kit (BioMérieux, Lyon, France) according to the manufacturer’s instructions. LAB cultures were harvested and resuspended in sterile distilled water, after which 65 μl of suspension (Mcfarland standard 1) was deposited into each well, and the plate was incubated at 37 °C for 4 h. Then, one drop of ZYM-A and ZYM-B reagent was added to each well, and enzyme activity was read after allowing the reaction to run for 5 min.
Hemolysis
Hemolysis was detected by streaking bacterial cells on blood agar containing 7 % horse blood (Oxoid, Hampshire, UK). The plate was then incubated at 30 °C for 24–48 h, after which the clear zone around the colony was observed.
Antibiotic susceptibility
LAB were evaluated for their susceptibility to antibiotics according to the technical guidelines of the European Food Safety Authority (EFSA 2008). The minimal inhibitory concentrations (MIC) of nine antibiotics, including ampicillin, vancomycin, gentamycin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol (Sigma, St. Louis, MO, USA), were determined. After culturing LAB in MRS broth for 24 h, cells were centrifuged (9,950 g, 5 min) and resuspended in MH broth containing 0.5 % dextrose. Resultant cell suspensions were then further diluted in the same medium to a density of 5.0 log CFU/ml. Each antibiotic was added to aliquots of the diluted cell suspension, which were incubated at 30 °C for 24–48 h without shaking. Cell growth was observed visually and measured based on the turbidity of the suspensions at 600 nm (Ultrospec 2100 pro; Amersham Biosciences, Uppsala, Sweden). MIC values were determined using the serial antibiotic dilution procedure in MH broth containing 0.5 % dextrose.
Statistical analysis
Data are presented as the means and standard deviations (means ± SD) of three independent experiments performed in triplicate. All statistical analyses on the data were performed using SPSS v.18.0 for Windows (SPSS, Chicago, IL, USA) with statistical significance determined at P < 0.05.
Results and discussion
Effects of acid and bile on cell survival
We initially tested the abilities of the 10 selected LAB strains to survive acid or bile stress. We found that treatment of LAB with acid or bile reduced viable cell numbers (Table 2). Following acid treatment, counts of viable Lactobacillus plantarum strains (AF1, NO1) and Pediococcus pentosaceus MP1, a homofermentative LAB, were clearly higher than counts of other strains (heterofermentative LAB). It has been previously shown that Leuconostoc kimchii GJ2 can produce 21.49 ± 0.46 mg/ml and 0.14 ± 0.09 mg/ml of EPS (in crude form) in sucrose and MRS media, respectively (Kim and Chang 2006). In this study, to investigate the effect of EPS production on bacaterial cell viability following acid and bile treatment, Leuconostoc kimchii GJ2 was cultivated in sucrose media for EPS production as well as in MRS media as a control. Acid tolerance level of Leuconostoc kimchii GJ2 producing EPS in sucrose media was twice that in MRS media. Bile salt had a smaller effect on LAB viability than did acid. Bile treatment resulted in a 1–2 log reduction in viable cell numbers whereas acid reduced viable cell numbers by 1–6 log. EPS production further reduced the effect of bile on bacterial cell viability. These findings are consistent with earlier investigations, which reported that EPS production reduces the effects of low pH and bile on the cell viability of various strains (Sabir et al. 2010; Yuksekdag and Aslim 2010).
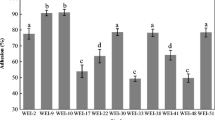
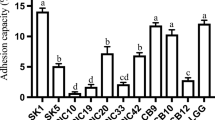
Adhesion properties to human cell lines
As shown in Fig. 1, Lactobacillus plantarum NO1, Pediococcus pentosaceus MP1, and EPS-producing Leuconostoc kimchii GJ2 all showed a higher percentage of adhesion to Caco-2 cells than did Lactobacillus rhamnosus GG. Based on these data, we selected P. pentosaceus MP1 and EPS-producing Leuconostoc kimchii GJ2, which showed the highest adhesion property to Caco-2 cells in Fig. 1, and examined the efficiency of their adhesion to HT-29 cells (Fig. 2). Both P. pentosaceus MP1 and EPS-producing Leuconostoc kimchii GJ2 showed greater adhesion to HT-29 cells than did Lactobacillus rhamnosus GG, regardless of bacterial cell density. Adhesion of all LAB isolates to both Caco-2 and HT-29 cells (inoculated bacterial cells into cell lines) was concentration-dependent, with adhesion to HT-29 cells (average adhesion rate of 0.1–1.0 %) being clearly lower than adhesion to Caco-2 cells (average rate of 0.2–2.3 %) (Figs. 1, 2). Previous results have similarly indicated that adhesion of bacterial cells to HT-29 cells is markedly lower than to Caco-2 cells (Laparra and Sanz 2009; Gopal et al. 2001).
Leuconostoc kimchii GJ2 as a control was clearly less adherent to both Caco-2 and HT-29 cells than EPS-producing Leuconostoc kimchii GJ2 (Figs. 1, 2). This is consistent with the findings of Russo et al. (2012), who reported that bacterial adhesion increases with EPS production. Bacterial adhesion to the intestinal epithelial mucosa is a complicated process that is influenced by multiple surface biophysical and biochemical properties of both the bacteria and epithelial mucosa (Servin and Coconnier 2003). It has been suggested that the EPS produced by LAB has an ecological function related to cell adhesion (Ruas-Madiedo et al. 2002).
Antimicrobial activity
The 10 selected LAB strains generally exerted growth inhibitory effects on the four tested pathogens, although Lactobacillus buchneri MS did not inhibit Listeria monocytogenes (Table 3). In particular, Lactobacillus plantarum AF1, Lactobacillus plantarum NO1, Leuconostoc mesenteroides DM1, and Pediococcus pentosaceus MP1 showed strong antimicrobial activities against all four tested pathogens, and all LAB strongly inhibited Staphylococcus aureus. Antimicrobial compounds from LAB include organic acids, CO2, H2O2, and bacteriocins (Ammor et al. 2006). Organic acids, bacteriocins, δ-dodecalactone, and cyclo (Leu-Leu) are all known to be inhibitory substances released by the LAB isolates (Chang et al. 2007; Kim and Chang 2006; Yang and Chang 2008). Production of these substances, which inhibit the growth of undesirable bacteria and pathogens, is a beneficial feature of probiotics (Dunne et al. 2001).
Enzymatic activities
Enzymatic activities of the selected LAB were measured using an API-ZYM kit (Table 4). None of the isolates showed alkaline phosphatase, α-chymotrypsin, β-glucuronidase, or α-fucosidase activity. It has been reported that β-glucuronidase or α-chymotrypsin activity may have negative effects in the colon (Heavey and Rowland 2004; Delgado et al. 2008). Weak-to-moderate N-acetyl-β-glucosaminidase activity was observed with Lactobacillus plantarum NO1, Lactobacillus plantarum AF1, Leuconostoc citreum GJ7, and Pediococcus pentosaceus MP1. Further, only 3 of the LAB isolates (Lactobacillus buchneri MS, L. plantarum AF1, L. plantarum NO1) showed β-galactosidase activity. β-Galactosidase released by probiotics reportedly contributes to the relief of lactose maldigestion symptoms (Leahy et al. 2005; Ouwehand et al. 2002), since β-galactosidase hydrolyzes lactose to glucose and galactose. When we examined lactose fermentation ability using an API 50 CHL kit (BioMérieux), we found that only 3 (Lactobacillus buchneri MS, L. plantarum AF1, L. plantarum NO1) of the 10 isolates were able to ferment lactose (data not shown). This result was surprising as most LAB can ferment lactose (Liu 2003). However, some LAB isolated from kimchi have been previously shown to have lost that ability (Chang 2010), which is consistent with this study. This loss of lactose fermentation ability suggests a lack of a lactose component in kimchi; consequently, kimchi LAB have no need to metabolize lactose. Among LAB in kimchi, the more evolutionally developed strains might have deleted or turned off the expression of lactose metabolic genes in favor of genes enabling the use of other sugars such as glucose, maltose, or sucrose as energy sources. Indeed, all three of these sugars are present in kimchi.
Hemolysis
In this study, none of the tested LAB isolates induced hemolysis on horse blood agar (γ-hemolytic). In contrast, Bacillus cereus ATCC 14579 produced a clear zone around its colony on horse blood agar (β-hemolysis).
Antibiotic resistance
The 10 LAB isolates were evaluated for their resistance to nine antibiotics, including those highlighted by EFSA (2008). All the isolates were susceptible to all the antibiotics tested, except vancomycin (Table 5). Bacteria from the genus Leuconostoc are known to be intrinsically resistant to vancomycin (Ammor et al. 2007; Clementi and Aquilanti 2011). Moreover, no breakpoint for vancomycin is required for Lactobacillus reuteri, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus paracasei, Lactobacillus obligate/facultative heterofermentative, Pediococcus spp., or Leuconostoc spp. according to the technical guidelines of the EFSA (2008). Therefore, it seems reasonable to conclude that consumption of the LAB isolates examined in the present study does not represent a health risk to humans due to antibiotic resistance.
Conclusion
For the development of novel probiotics, new species and more specific strains of bacteria are being sought. For this purpose, the selection and evaluation of new microorganisms from traditional fermented foods could be a means of ensuring safety. Here, we evaluated the functionality and safety of 10 LAB strains isolated from kimchi. By investigating their virulence determinants, undesirable biochemical characteristics, and antibiotic resistance pattern, all the tested isolates were found to be safe for human consumption. In particular, Lactobacillus plantarum NO1, Lactobacillus plantarum AF1, and Pediococcus pentosaceus MP1 appear to meet the functional criteria required to be a beneficial probiotic (in vitro); i.e., acid and bile tolerance, cell adherence, and antagonistic activity against pathogens. We therefore propose that these strains can be considered new probiotic candidates.
References
Ammor S, Tauveron G, Dufour E, Chevallier I (2006) Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility 1–screening and characterization of the antibacterial compounds. Food Control 17:454–461
Ammor MS, Flórez AB, Mayo B (2007) Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24:559–570
Bertazzoni Minelli E, Benini A, Marzotto M, Sbarbati A, Ruzzenente O, Ferrario R, Hendriks H, Dellaglio F (2004) Assessment of novel probiotic Lactobacillus casei strains for the production of functional dairy foods. Int Dairy J 14:723–736
Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V (2003) Safety of probiotics that contain lactobacilli of bifidobacteria. Clin Infect Dis 36:775–780
Carr FJ, Chill D, Maida N (2002) The lactic acid bacteria: a literature survey. Crit Rev Microbiol 28:281–370
Chang HC (2010) Potential of antimicrobial compounds from kimchi lactic acid bacteria. In: Proceedings of 2010 International Symposiuim & Annual Meeting. The Korean Society for Microbiology and Biotechnology, Seoul, p 161
Chang JY, Chang HC (2010) Improvements in the quality and shelf life of kimchi by fermentation with the induced bacteriocin-producing strain, Leuconostoc citreum GJ7 as a starter. J Food Sci 75:103–110
Chang JY, Lee HJ, Chang HC (2007) Identification of the agent from Lactobacillus plantarum KFRI464 that enhances bacteriocin production by Leuconostoc citreum GJ7. J Appl Microbiol 103:2504–2515
Chang HC, Chang JY, Jung JH, Ryu EH (2011) Lactic acid bacterium separated from kimchi and fermented food using the strain. Republic of Korea patent, 1020110037005
Cho YR, Chang JY, Chang HC (2007) Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol 17:104–109
Clementi F, Aquilanti L (2011) Recent investigations and updated criteria for the assessment of antibiotic resistance in food lactic acid bacteria. Anaerobe 17:394–398
Delgado S, O’Sullivan E, Fitzgerald G, Mayo B (2008) In vitro evaluation of the probiotic properties of human intestinal Bifidobacterium species and selection of new probiotic candidates. J Appl Microbiol 104:1119–1127
Donohue DC (2006) Safety of probiotics. Asia Pac J Clin Nutr 15:563–569
Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, Kiely B, O’Sullivan GC, Shanahan F, Collins JK (2001) In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 73:386S–392S
EFSA (2008) Technical guidance–update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance–prepared by the panel on additives and products or substances used in animal feed. EFSA J 732:1–15
Fernández MF, Boris S, Barbés C (2003) Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
Foligné B, Dewulf J, Breton J, Claisse O, Lonvaud-Funel A, Pot B (2010) Probiotic properties of non-conventional lactic acid bacteria: Immunomodulation by Oenococcus oeni. Int J Food Microbiol 140:136–145
Franz CMAP, Hummel A, Holzapfel WH (2005) Problems related to the safety assessment of lactic acid bacteria starter cultures and probiotics. Mitt Lebensm Hyg 96:39–65
Gopal PK, Prasad J, Smart J, Gill HS (2001) In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol 67:207–216
Guglielmotti DM, Marcó MB, Golowczyc M, Reinheimer JA, Quiberoni AD (2007) Probiotic potential of Lactobacillus delbrueckii strains and their phage resistant mutants. Int Dairy J 17:916–925
Heavey PM, Rowland IR (2004) Microbial-gut interactions in health and disease. Gastrointestinal cancer. Best Pract Res Clin Gastroenterol 18:323–336
Hornstra LM, de Vries YP, Wells-Bennik MHJ, deVos WM, Abee T (2006) Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl Environ Microbiol 72:44–53
Jung JH, Chang HC (2011) Probiotic characterization of lactic acid bacteria isolated from kimchi. In: Proceedings of 2011 International Symposiuim & Annual Meeting. The Korean Society for Microbiology and Biotechnology, Gyeongju, p 388
Kim HJ, Chang HC (2006) Isolation and characterization of exopolysaccharide producing lactic acid bacteria from Kimchi. Kor J Microbiol Biotechnol 34:196–203
Laparra JM, Sanz Y (2009) Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett Appl Microbiol 49:695–701
Leahy SC, Higgins DG, Fitzgerald GF, van Sinderen D (2005) Getting better with bifidobacteria. J Appl Microbiol 98:1303–1315
Lebeer S, Verhoeven TLA, Perea Vélez M, Vanderleyden J, De Keersmaecker SCJ (2007) Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73:6768–6775
Lee Y, Chang HC (2008) Isolation and characterization of kimchi lactic acid bacteria showing anti-Helicobacter pylori activity. Kor J Microbiol Biotechnol 36:106–114
Lian WC, Hsiao HC, Chou CC (2003) Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int J Food Microbiol 86:293–301
Liong MT (2008) Safety of probiotics: translocation and infection. Nutr Rev 66:192–202
Liu SQ (2003) Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int J Food Microbiol 83:115–131
Magnusson J, Schnürer J (2001) Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol 67:1–5
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2006) Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J 16:189–199
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria–a review. Int J Food Microbiol 105:281–295
Nam YD, Chang HW, Kim KH, Roh SW, Bae JW (2009) Metatranscriptome analysis of lactic acid bacteria during kimchi fermentation with genome-probing microarrays. Int J Food Microbiol 130:140–146
Ouwehand AC, Salminen S, Isolauri E (2002) Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82:279–289
Park EJ, Alexander E, Taylor GA, Costa R, Kang DH (2009) The decontaminative effects of acidic electrolyzed water for Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on green onions and tomatoes with differing organic demands. Food Microbiol 26:386–390
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Russo P, López P, Capozzi V, de Palencia PF, Dueñas MT, Spano G, Fiocco D (2012) Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int J Mol Sci 13:6026–6039
Saarela M, Mogensen G, Fondén R, Mättö J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–215
Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D (2010) Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp. strains isolated from kefir. J Food Sci 75:568–573
Santini C, Baffoni L, Gaggia F, Granata M, Gasbarri R, Di Gioia D, Biavati B (2010) Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int J Food Microbiol 141:S98–S108
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754
Stasic AJ, Wong AC, Kaspar CW (2012) Osmotic and desiccation tolerance in Escherichia coli O157:H7 requires rpoS (σ 38). Curr Microbiol 65:660–665
Tindall BJ, Grimont PAD, Garrity GM, Euzéby JP (2005) Nomenclature and taxonomy of the genus Salmonella. Int J Syst Evol Microbiol 55:521–524
Trampuz A, Murphy CK, Rothstein DM, Widmer AF, Landmann R, Zimmerli W (2007) Efficacy of a novel rifamycin dericative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob Agents Chemother 51:2540–2545
World Institute of Kimchi, an Annex of Korea Food Research Institute (2011) Trends in kimchi industry. Korean Studies Information, Seoul
Yang EJ, Chang HC (2008) Antifungal activity of Lactobacillus plantarum isolated from kimchi. Kor J Microbiol Biotechnol 36:276–284
Yang EJ, Chang HC (2010) Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int J Food Microbiol 139:56–63
Yuksekdag ZN, Aslim B (2010) Assessment of potential probiotic and starter properties of Pediococcus spp. isolated from Turkish-type fermented sausages (sucuk). J Microbiol Biotechnol 20:161–168
Acknowledgment
This research was supported by the Technology Development Program for Food, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, E.H., Chang, H.C. In vitro study of potentially probiotic lactic acid bacteria strains isolated from kimchi. Ann Microbiol 63, 1387–1395 (2013). https://doi.org/10.1007/s13213-013-0599-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-013-0599-8