Abstract
Liangzhou fumigated vinegar is famous in China for its distinct flavor. How to enhance flavor in traditional fermented foods, such as vinegar, has attracted a large interest in recent years. Acetoin (ACT) is a precursor of tetramethylpyrazine (TTMP), a popular food flavor found in different traditional fermented foods. We obtained 200 acid-producing isolates from the solid fermentative substrate of Liangzhou fumigated vinegar by the method of forming transparent zones on GYC agar, and 28 bacteria producing ACT by Voges–Proskauer colorimetric assay and gas chromatography analysis, including 15 strains of Acetobacter, eight strains of Lactobacillus and five strains of Staphylococcus. Based on ACT level, the isolate C92 with the highest yield was selected for further analysis. It was identified as Acetobacter pasteurianus by physiological and biochemical characteristics, as well as 16S rRNA gene sequence similarities. We examined the ACT level of strain C92 under different conditions, and found that a temperature of 31.31 °C, pH of 6.67, shaking speed of 209 rpm, and inoculation volume of 8.27 % were the best conditions for strain C92 to produce a yield of 19.04 g/L at 40 h post-culture. In a time–ACT curve, ACT reached the maximum value at 40 h and then decreased afterward, suggesting that ACT produced by C92 is a growth-associated primary metabolite from sugar. Therefore, the C92 isolate is regarded as a strain with promising ACT-production ability in vinegar. More studies are needed before C92 is applied in the industrial production of vinegar.
Similar content being viewed by others
Introduction
Liangzhou fumigated vinegar is a naturally fermented product brewed with a mixture of various microbes (i.e., yeasts, molds and bacteria, etc.) in the solid state, which is different from products in Western countries brewed with only one or a few microbes (Zhao and Li 2005). Liangzhou fumigated vinegar produced by traditional Chinese solid brewing technology is famous for its quality and flavor in China (Chen and Zhai 2001). As we know, it possesses its distinct flavor due to the unique solid-state acetic acid fermentation process. Han et al. (2012) reported that tetramethylpyrazine (TTMP) is the characteristic flavor compound of Liangzhou fumigated vinegar, and that the flavor of vinegar product is enhanced significantly with increasing of TTMP. Acetoin (ACT), 3-hydroxy-2-butanone, the precursor of TTMP biosynthesis, is an important metabolite excreted by many microorganisms (Zhu et al. 2010). In recent years, many researches have been focused on methods for flavor enhancement in traditional fermented foods, such as Chinese liquors (Fan et al. 2007). Several studies have shown that TTMP in Chinese liquor is mainly produced from microbial metabolic reactions, but not the Maillard reaction (Zhu and Xu 2010a). Further studies in Bacillus showed that the production of TTMP increased effectively with the addition of proper exogenous threonine and ACT (Besson et al. 1997; Larroche et al. 1999). In addition, a positive correlation between ACT and TTMP has also been reported in a study of determination of ACT and TTMP in traditional vinegars by high-pressure liquid chromatography (Chen et al. 2010). However, excessive exogenous ACT may cause inhibition of TTMP production and cytotoxicity (Schrader 2007), resulting in cell metabolism balance disorder (Larroche et al. 1999). Therefore, isolating a bacterial strain to produce endogenous ACT effectively is more important for the flavor of Liangzhou fumigated vinegar.
In the vinegar industry, acetic acid bacteria play key roles in the biotransformation of different primary alcohols and diols into acetic acid (Romano et al. 2002; Chen et al. 2009; Durán et al. 2010). Moreover, they could enhance the special flavor of Liangzhou fumigated vinegar (Han et al. 2012). Faveri et al. (2001) reported that Acetobacter hansenii could produce ACT at 8.93 g/L. In a study of microbial communities during solid-state acetic acid fermentation, Xu et al. (2011) found that a strain of Acetobacter could produce ACT and TTMP in Zhejiang aromatic vinegar. Moens et al. (2014) examined the metabolites of four cocoa-specific acetic acid bacterial strains, including Acetobacter pasteurianus 386B, during monoculture laboratory fermentations and found that A. pasteurianus 386B has the best ethanol and lactic acid oxidation potential, which plays a key role during cocoa bean fermentation. Due to the limited acetic acid bacteria strains with good ACT production ability, there is a high demand for qualified strains that could be applied in vinegar production.
The aim of our study was to isolate and select an acetic acid bacterium strain with high ACT production ability from Liangzhou fumigated vinegar solid substrate by Voges–Proskauer colorimetric assay and gas chromatography analysis. We also used response surface methodology to obtain the optimal conditions for ACT production.
Materials and methods
Isolation and culture conditions
Microorganisms were isolated from the solid fermentative substrate of Liangzhou fumigated vinegar donated by Yunxiao Company in Wuwei (Gansu, China). The solid substrate was ground into powder and stored at 4 °C until use.
The specific isolation process was as follows: Liangzhou fumigated vinegar solid substrate (5 g) was shaken (THZ-82 N desktop Shakers, Yuejin medical instrument co., LTD, China ) for 1 h with 50 mL sterile deionized water in a 250-mL flask to suspend the cells. For microorganism enrichment, 0.2 mL solid substrate cell suspension was inoculated into 20 mL of basal medium (10 g/L glucose, 5 g/L yeast extracts, 5 g/L NaCl at pH 7.0) at 30 °C for 24 h with agitation, and then sub-cultured six times (1:10 dilution) on glucose-yeast extract-CaCO3 (GYC) agar (glucose, 20 g/L; yeast extracts, 10 g/L; CaCO3, 5 g/L; NaCl, 5 g/L; agar, 20 g/L) at 30 °C for 96 h. According to morphologies and whether there was formation of a transparent zone on GYC agar, acid-producing pure cultures were obtained (Wu et al. 2012). The acetic-acid-producing strains were selected by a reddish-brown precipitate reaction of FeCl3 solution (Liu 2000), and then pure cultures were maintained on GYC agar for further analysis.
Screening of ACT-producing strains
Pure cultures were incubated in 250-mL Erlenmeyer flasks containing 50 mL of Voges–Proskauer (V-P) medium (10 g/L glucose - autoclaved separately at 121 °C for 15 min, 10 g/L fish peptone, 2 g/L potassium dihydrogen phosphate at pH 7.0) at 30 °C for 48 h with shaking at 150 rpm. The fermentation broth was used for ACT measurement by the V-P colorimetric assay (Meara 1931) with modification. Briefly, 0.1 mL creatine (3 % in water, w/v) and 1 mL NaOH (40 %, w/v) were added sequentially to 1 mL of the 1:10 diluted fermentation broth with vortex, and the mixture was incubated at 30 °C for 15 min. Finally, the maximum absorbance was measured at a wavelength of 516 nm (A516) in a spectrophotometer (U-3010, Hitachi, Japan). The concentrations of ACT and TTMP were measured by gas chromatography (GC) analysis (see analytical methods). All positive strains were selected for further optimization experiments.
Identification of ACT-producing strains
According to Bergey’s Manual of Determinative Bacteriology (Buchanan and Gibbons 1984; Dong and Cai 2001), we performed different analyses to examine morphology, physiology and biochemistry for the highest ACT-producing strain, including Gram’s stain, spore formation, catalase, oxidase, motility, glucose fermentation, oxidation of ethanol, hydrolysis of starch, tert-butyl hydroperoxide, ketogenesis from glycerol, production of gluconic acid, production of 5-ketogluconate, production of 2,5-diketogluconate, water soluble brown pigment, γ-pyrones from D-glucose.
All bacterial isolates producing ACT were identified according to similarities of the 16S rRNA partial gene sequences. Nearly full length 16S rRNA genes were amplified by PCR using the universal primers 27f and 1492r (Lane 1991) under conditions described by Prat et al. (2009). Sequencing in both directions was performed by the Sangon Biotech co., Ltd (Shanghai, China) and then manually corrected for ambiguities using BioEdit v.7.0 (Hall 1999). BLAST, a sequence alignment program, was used for strain selection according to pairwise similarity with sequences in public databases (http://www.ncbi.nlm.nih.gov/BLAST/) (Altschul 1997).
Optimization for conditions of ACT production
Flask experiments were performed in 250-mL Erlenmeyer flasks containing 50 mL of modified PYG medium (100 g/L glucose - autoclaved separately at 121 °C for 15 min, 30 g/L fish peptone, 10 g/L yeast extract, 30 g/L ammonium sulfate, 100 g/L potassium dihydrogen phosphate). First, the best fermentation time was selected by examining the ACT-producing ability by GC. The experiment conditions were: 7.0 % inoculum in modified PYG medium at pH 7.0, 30 °C, shaking at 150 rpm for 72 h. The selected fermentation time was applied in the study of other conditions.
The pH of the modified PYG medium was adjusted to between 5.0 and 9.0 by addition of NaOH (10 mol/L) or HCl (10 mol/L), to select the optimal pH. The effects of oxygen supply on ACT production were tested shaking at 90, 120, 150, 180, and 210 rpm, respectively. Different incubation temperatures (26, 28, 30, 32, or 34 °C) or inoculation volumes (3, 5, 7, 9 or 11 %) were examined individually in the modified PYG medium (pH 7.0) with shaking at 150 rpm. All experiments were performed in triplicate, and the mean and standard deviations were determined.
The levels of the significant factors and the interaction effects between these factors were analyzed and optimized using Central Composite design as described previously (Azaman et al. 2010; Bezerra et al. 2008; Wu et al. 2010; Farliahati et al. 2010). In this study, 30 trials were included and the independent variables were studied at three different levels. All experiments were performed in duplicate and the mean of ACT was taken as the dependent variable or response (Y). Experimental design and analysis was done using Design Expert software (Version 8.0, Stat-Ease Inc.) to calculate the polynomial coefficients. The relationship between variables and responses was calculated by the quadratic polynomial equation:
where Yi is the predicted response, XiXj are input variables that influence the response variable Y; β0 is the offset term; βi is the ith linear coefficient; βii the iith quadratic coefficient and βij is the ijth interaction coefficient. Statistical analysis of the model was performed to evaluate the analysis of variance (ANOVA), which included lack of fit, Fisher’s F test, its associated probability p (F) and correlation coefficient R to measures the goodness of fit of the quadratic model. For the present study, a total of 30 tests were performed to estimate the coefficients. The generated mathematical model was validated by conducting experiments at given optimal conditions.
Analytical methods
Samples collected from the flask were divided into two aliquots. One aliquot was centrifuged (10,000 g, 10 min) and the precipitate was collected, washed twice with distilled water and dried at 105 °C to constant weight, to determine the biomass of the culture (Ji et al. 2009). The supernatant was used to determine the total sugar amount using the 3, 5-dinitrosalicylate method as described by Miller (1959). The other aliquot was used to examine the amount of ACT. A DVB/CAR/PDMS fiber was used in headspace solid-phase micro-extraction (HS-SPME) analysis (Supelco, USA) for the determination of ACT in the fermentation broth. The SPME fibers were conditioned as recommended by the manufacturer prior to their first use. ACT amount was measured by exposing the SPME absorbent to the headspace of the inoculated vials for 30 min at 50 °C in a water bath. The compounds retained on the fiber were thermally desorbed in the injection port of the GC at 250 °C.
Chromatographic analyses were made on a GC6890 instrument coupled with a flame ionization detector (FID) (Agilent Technologies, USA). The injection was made in the split mode (split ratio1:1) at 250 °C. Each sample was analyzed on a DB-Wax (30 m ×0.25 mm i.d., 0.25 μm film thickness, J&W Scientific) fused-silica capillary column. The column carrier gas was high-purity helium at a constant flow of 3 mL/min. The makeup gas to the detector was nitrogen at 27.5 mL/min. The H2 flow was 1.5 mL/min and the air flow was 144.9 mL/min. The temperature of the detector was held at 250 °C. The oven temperature was held at 50 °C for 2 min, and then it was raised at a rate of 2 °C/min to 85 °C and held for 0.1 min. Finally, the oven temperature was increased at a rate of 5 °C/min to 210 °C and held at 210 °C for 2 min.
Results
Screening of ACT-producing strains
We obtained 200 isolates with different morphologies from Liangzhou fumigated vinegar, suggesting that the method of forming transparent zones on GYC agar was effective for isolating acid-producing bacteria (data not shown).
Among these isolates, 28 showed the ability to produce ACT, and these were defined as V-P positive, including 20 acetic acid-producing isolates. Table 1 shows the ACT produced in the broth of the 28 isolates by GC- FID analysis. In modified YPG medium, the isolate C92 produced the highest amount of ACT (12.23 g/L) after 48 h of cultivation.
Characteristics of strain C92
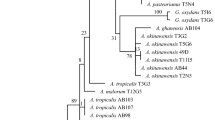
In all cases sequence similarities approached 100 % with publicly available sequences at the GenBank (NCBI, www.ncbi.nlm.nih.gov), indicating a close phylogenetic relationship with previously cultured organism. Acetobacter pasteurianus and Lactobacillus plantarum were the more frequent species and accounted for 32.1 and 17.8 % of the isolates, respectively. The highest ACT-producing strain, C92 (GenBank accession number KR149365), showed a high identity (99.2 %) to the Gram-negative bacterium Acetobacter pasteurianus (GenBank accession number DQ523493) (Table 2), belonging to the family of Acetobacteriaceae.
The physiological and biochemical characteristics of strain C92 are shown in Table 3. Strain C92 is a rod-shaped, non-spore-forming bacterium that also shares several characteristics of A. pasteurianus, such as being Gram-negative, aerobic, catalase reaction-positive, oxidase reaction-positive, and having the following results: oxidation of ethanol – positive, tert-butyl hydroperoxide test – positive, gluconic acid producing test – positive, and water soluble brown pigment – positive.
Effect of fermentation conditions on ACT production
As shown in Fig. 1, while being cultured at 30 °C, the ACT production of strain C92 reached its maximum (14.02 g/L) at 40 h, indicating that the optimum fermentation time was 40 h.
Figure 2a shows the maximum level of ACT in medium at pH 7.0, suggesting that 7.0 is the best pH value. The amount of ACT increased with the increase of shaking speed, reaching a maximum of 15.37 g/L at 180 rpm (Fig. 2b). This result indicates that oxygen supply is positively associated with ACT production. As shown in Fig. 2c, the ACT level increased with an increase of temperature from 26 to 32 °C. The ACT level produced under 32 °C is approximately twofold of that under 26 °C. However, when temperature increased to 34 °C, ACT appeared to decline, which may be caused by abnormal cellular regulation related to metabolism. The amount of ACT increased with an increase in inoculated volume from 3 % (9.05 g/L) to 7 % (14.02 g/L), and then remained at comparable levels (9 and 11 %).
Optimization of ACT production conditions
Based on response surface methodology (Kishore and Kayastha 2012), the best conditions for ACT production in strain C92 are temperature of 31.31 °C, pH of 6.67, shaking speed of 209 rpm, and inoculation volume of 8.27 %. In experiments under such conditions, the ACT yield is 19.04 g/L, which is very close to the theoretical prediction (18.97 g/L), suggesting the reliability of the conditions.
Table 4 shows the relationship between ACT and independent variables estimated using a quadratic polynomial equation. The final equation in terms of actual factors, which governs the response, is as follows:
where A is temperature (°C); B, inoculation volume (%); C, pH and D represents shaking speed (rpm). The equation indicates that ACT production has linear and quadratic relationships with variables. Analysis of variance (ANOVA) was also done to determine the adequacy and significance of the quadratic model. ANOVA for the response surface model is provided in Table 5.
There is only a 0.01 % chance that a “model F Value” of 62.33 could occur due to noise. Regression analysis revealed a high coefficiency (R-squared = 0.9831) and a high adjusted coefficiency (R-squared = 0.9673). The response surface graphs (RSG) are shown in Fig. 3.
The dynamics for ACT production
We measured the amount of ACT at different times to examine the dynamics for ACT production. The weight of dried cells collected 8 h post-culture increased until 24 h post-culture, and then reached stationary phase (Fig. 4). No ACT was detected in the first 8 h and then it accumulated rapidly at logarithmic phase to 2.32 g/L at 24 h. At 40 h, ACT reached the maximum level (19.04 g/L), and then decreased. The pH decreased rapidly until 16 h, then remained constant in the logarithmic phase, and then decreased slowly in late stage.
Time course of ACT fermentation under the modified YPG medium and optimized conditions by A. pasteurianus C92. Opened triangle: ACT, filled square: pH, opened square: residue sugar, filled triangle: biomass, filled circle: TTMP. Data are the means of triplicate experiments. Standard errors are less than 5.0 % of the means
Discussion
ACT, an important metabolic product secreted by various microorganisms, could function as the precursor of TTMP (Kim et al. 1994; Besson et al. 1997; Larroche et al. 1999; Huang et al. 1999). ACT has been found in 35 Chinese black vinegars for flavor enhancement (Chen et al. 2009).
In our study, we isolated Strain C92 with good ACT-production ability from the solid fermentative substrate of Liangzhou fumigated vinegar. We found that C92 exhibited increased production of ACT with increase in oxygen supply, which is consistent with two previous reports. Moes (1985) reported a beneficial effect on ACT production by the dissolved oxygen in Bacillus subtilis culture, which might be mediated through the reversible transformation between ACT and 2, 3-butanediol. Similarly, Nakashimada et al. (1998) found an association between ACT production and oxygen supply in Paenibacillus polymyxa ATCC 12321.
Acetobacter pasteurianus C92 isolated in our study has been shown to be an important strain for the acetification, which may affect the acidity value required for the final product. In a previous study, Acetobacter pasteurianus was selected for colonization of musts and acetification for traditional balsamic vinegar acetification, an Italian aged condiment produced by “seed vinegar” (Gullo et al. 2006; Gullo et al. 2009). Taken together with the ACT-production ability in vinegar, A. pasteurianus C92 would be a promising strain not only in acetification, but also in flavor improvement in the industrial production of vinegar.
We isolated 20 acetic acid-producing strains belonging to Acetobacter and Staphylococcus, including A. pasteurianus and A. pomorum, important strains in industrial vinegar production (Sengun and Karabiyikli 2011). Although these acetic acid-producing strains could exhibit different functions, they may collectively result in a final improved taste of Liangzhou fumigated vinegar.
Research by Larroche et al. (1999) revealed that ACT was one of intermediary metabolites of Bacillus subtilis in the conversion of sugar to TTMP, and that the ACT synthesis rate could be affected by fermentation time. We observed that ACT was detected at the 16th h; afterward, it showed an increase with a decrease in sugar. In addition, the pH value decreased rapidly from 0 to 16 h, but remained stable from 16 to 40 h, and decreased slowly late in the culture period. Therefore, our conclusion is that ACT is the primary metabolite from sugar in C92. ACT level increased with the increase of biomass until 40 h. Afterward, the ACT level decreased while biomass remained stable. TTMP was not detected until 40 h, and then began to increase. Therefore, ACT production was associated with biomass and it probably decreased due to the the combined effect of being converted to TTMP or some other substances as well as product evaporation. Taken together, ACT produced in C92 is a typical growth-associated product, which serves as a biosynthesis precursor of TTMP. Our conclusion is supported by a previous study showing the dynamic biosynthesis model of ACT and TTMP in Bacillus sp. from Chinese high temperature Daqu (Zhu and Xu 2010b).
We also observed that there was an increased reduction in precursor ACT levels after 56 h, which might be caused by the energy-storing function of ACT derived from pyruvate (Johansen et al. 1975; Grundy et.al 1994). In Bacillus subtilis, ACT is mainly catalyzed by the acetoin dehydrogenase enzyme system (AoDH ES) (Huang et al. 1999), and the aco operon encoding AoDH ES is subject to direct and indirect CcpA-dependent glucose transcriptional repression (Xiao and Xu 2007). In early stage of culture with abundant glucose, ACT is synthesized as a primary metabolite, while other catabolic pathways are transcriptionally repressed. However, in late stage when glucose is depleted, ACT was consumed as a successive energy and carbon source to keep the cells alive.
Conclusion
In summary, we isolated a highly acetoin-producing strain of Acetobacter pasteurianus strain C92 from Liangzhou fumigated vinegar substrate. Its fermentation characteristics show that it can be regarded as a strain with promising ACT-production ability in vinegar production. We examined ACT production of strain C92 under different conditions, and obtained the best conditions, including temperature, pH value, shaking speed and inoculation volume. By analysis of the relationship between ACT and time, we suggest that ACT is a primary metabolite from sugar. Studies of molecular mechanisms underlying the metabolic capabilities of strain C92 are needed in the future before its application in the industrial production of vinegar.
References
Altschul SF, Madden TF, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Azaman SN, Ramanan RN, Tan JS, Rahim RA, Abdulla MP, Ariff AB (2010) Optimization of an induction strategy for improving interferon-alpha 2b production in the periplasm of Escherichia coli using response surface methodology. Biotechnol Appl Biochem 56:141–150
Besson I, Creuly C, Gros JB, Larroche C (1997) Pyrazine production by Bacillus subtilis in solid-state fermentation on soybeans. Appl Microbiol Biotechnol 47:489–495
Bezerra MA, Santelli RE, Oliveira EP, Villar SL, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Buchanan RE, Gibbons NE (1984) Bergey’s Manual of Determinative Bacteriology. Williams and Wilkins Co., Baltimore, pp 276–278
Chen QA, Zhai DY (2001) The production technology of Liangzhou fumigated vinegar. Chin Condiment 9:25–26
Chen F, Li L, Qu J, Chen C (2009) Cereal vinegars made by solid-state fermentation in China. In: Solieri L, Giudici P (eds) Vinegars of the World. Springer Publishing Inc, Berlin, pp 243–259
Chen JC, Chen QH, Guo Q, Ruan S, Ruan H, He GQ, Gu Q (2010) Simultaneous determination of acetoin and tetramethylpyrazine in traditional vinegars by HPLC method. Food Chem 122:1247–1252
Dong XZ, Cai MY (2001) Manual of Systematic Determination for the Common Bacteria. Science Press, Beijing, pp 133–137
Durán E, Palma M, Natera R, Castro R, Barroso CG (2010) New FT-IR method to control the evolution of the volatile constituents of vinegar during the acetic fermentation process. Food Chem 121:575–579
Fan WL, Xu Y, Zhang YH (2007) Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J Agric Food Chem 55:9956–9962
Farliahati MR, Ramanan NR, Mohamad R, Puspaningsih NNT, Ariff AB (2010) Enhanced production of xylanase by recombinant Escherichia coli DH5 through optimization of medium composition using response surface methodology. Ann Microbiol 60:279–285
Faveri DD, Torre P, Molinari F, Perego P, Converti A (2001) Carbon material balances and bioenergetics of 2,3-butanediol bio-oxidation by Acetobacter hansenii. Enzym Microb Technol 33(5):708–719
Grundy FJ, Turinsky AJ, Henkin TM (1994) Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J Bacteriol 176:4527–4533
Gullo M, Caggia C, De Vero L, Giudici P (2006) Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int J Food Microbiol 106:209–212
Gullo M, De Vero L, Giudici P (2009) Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl Environ Microbiol 75:2585–2589
Hall TA (1999) Bio Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Han QH, Yun JM, Song YQ, Wen Y (2012) Changes of volatile composition during fumigating process of Liangzhou fumigated vinegar. Sci Technol Food Ind 22:146–151
Huang M, Oppermann-Sanio FB, Steinbuchel A (1999) Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway. J Bacteriol 181:3837–3841
Ji XJ, Huang H, Du J, Zhu JG, Ren LJ, Hu N (2009) Enhanced 2,3-butanediol production by Klebsiella oxytoca using a two-stage agitation speed control strategy. Bioresour Technol 100:3410–3414
Johansen L, Bryn K, Stormer FC (1975) Physiological and biochemical role of the butanediol pathway in Aerobacter (Entero bacter) aerogenes. J Bacteriol 123:1124–1130
Kim KS, Lee HJ, Shon DH, Chung DK (1994) Optimum conditions for the production of tetramethylpyrazine flavor compound by aerobic fed-batch culture of Lactococcus lactis subsp. lactis biovar. diacetylactis FC1. J Microbiol Biotechnol 4:327–332
Kishore D, Kayastha AM (2012) Optimisation of immobilisation conditions for chick pea β-galactosidase (CpGAL) to alkylamine glass using response surface methodology and its applications in lactose hydrolysis. Food Chem 134:1650–1657
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley, New York, pp 115–175
Larroche C, Besson I, Gros JB (1999) High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soy-beans. Process Biochem 34:667–674
Liu SJ (2000) The experiment technology of food microbiology. Chinese Agricultural Science and Technology Press, Beijing, pp 12–14
Meara RAQO (1931) A simple delicate and rapid method of detecting the formation of acetylmethylcarbinol by bacteria fermenting carbohydrate. J Pathol Bacteriol 34:401–406
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moens F, Lefeber T, De Vuyst L (2014) Oxidation of metabolites highlights the microbial interactions and role of Acetobacter pasteurianus during cocoa bean fermentation. Appl Environ Microbiol 80:1848–1857
Moes J, Griot M, Keller J, Heinzle E, Dunn IJ, Bourne JR (1985) A microbial culture with oxygen-sensitive product distribution as a potential tool for characterizing bioreactor oxygen-transport. Biotechnol Bioeng 27:482–489
Nakashimada Y, Kanai K, Nishio N (1998) Optimization of dilution rate, pH and oxygen supply on optical purity of 2, 3-butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol Lett 20:1133–1138
Prat C, Ruiz-Rueda O, Trias R, Anticó E, Capone D, Sefton M (2009) Molecular fingerprinting by PCR-denaturing gradient gel electrophoresis reveals differences in the levels of microbial diversity for musty-earthy tainted corks. Appl Environ Microbiol 75(7):1922–1931
Romano A, Gandolfi R, Nitt P, Rollin M, Molinari F (2002) Acetic acid bacteria as enantioselective biocatalysts. J Mol Catal B Enzym 17:235–240
Schrader J (2007) Microbial flavor production. In: Berger RG (ed) Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer, Berlin, pp 507–574
Sengun IY, Karabiyikli S (2011) Importance of acetic acid bacteria in food industry. Food Control 22:647–656
Wu JA, Wang JL, Li MH, Lin JP, Wei DZ (2010) Optimisation of immobilisation for selective oxidation of benzyl alcohol by Gluconobacter oxydans using response surface methodology. Bioresour Technol 101:8936–8941
Wu JJ, Ma YK, Zhang FF, Chen FS (2012) Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of Shanxi aged vinegar, a traditional Chinese vinegar. Food Microbiol 30:289–297
Xiao ZJ, Xu P (2007) Acetoin metabolism in bacteria. Crit Rev Microbiol 33:127–140
Xu W, Huang ZY, Zhang XJ, Li Q, Lu ZM, Shi JS, Xu ZH, Ma YH (2011) Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol 28:1175–1181
Zhao LQ, Li L (2005) The history, present status, development trend of the production technology of Chinese vinegar. Chin Condiment 2:15–17
Zhu BF, Xu Y (2010a) A feeding strategy for tetramethylpyrazine production by Bacillus subtilis based on the stimulating effect of ammonium phosphate. Bioprocess Biosyst Eng 33:953–959
Zhu BF, Xu Y (2010b) Production of tetramethylpyrazine by batch culture of Bacillus subtilis with optimal pH control strategy. J Ind Microbiol Biotechnol 37:815–821
Zhu BF, Xu Y, Fan WL (2010) High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J Ind Microbiol Biotechnol 37:179–186
Acknowledgments
Financial support from the Ministry of Science and Technology, P. R. China under the Nature Science Foundation of China (NSFC) (NO. 31360405), and the Gansu Province Key Laboratory of Food Science and Technology, Gansu Agriculture University, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Yun, J. Isolation, identification and fermentation conditions of highly acetoin-producing acetic acid bacterium from Liangzhou fumigated vinegar in China. Ann Microbiol 66, 279–288 (2016). https://doi.org/10.1007/s13213-015-1106-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-015-1106-1