Abstract
Acetic acid bacteria (AAB) are important microorganisms in the vinegar industry. However, AAB have to tolerate the presence of ethanol and high temperatures, especially in submerged fermentation (SF), which inhibits AAB growth and acid yield. In this study, seven AAB that are tolerant to temperatures above 40 °C and ethanol concentrations above 10 % (v/v) were isolated from Chinese vinegar Pei. All the isolated AAB belong to Acetobacter pasteurianus according to 16S rDNA analysis. Among all AAB, AAB4 produced the highest acid yield under high temperature and ethanol test conditions. At 4 % ethanol and 30–40 °C temperatures, AAB4 maintained an alcohol–acid transform ratio of more than 90.5 %. High alcohol–acid transform ratio was still maintained even at higher temperatures, namely, 87.2, 77.1, 14.5 and 2.9 % at 41, 42, 43 and 44 °C, respectively. At 30 °C and different initial ethanol concentrations (4–10 %), the acid yield by AAB4 increased gradually, although the alcohol–acid transform ratio decreased to some extent. However, 46.5, 8.7 and 0.9 % ratios were retained at ethanol concentrations of 11, 12 and 13 %, respectively. When compared with AS1.41 (an AAB widely used in China) using a 10 L fermentor, AAB4 produced 42.0 g/L acetic acid at 37 °C with 10 % ethanol, whereas AS1.41 almost stopped producing acetic acid. In conclusion, these traits suggest that AAB4 is a valuable strain for vinegar production in SF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vinegar is used as food flavoring agent and preservative worldwide. In addition, vinegar has been demonstrated to possess healthful properties (Verzelloni et al. 2007). The production of acetic acid is usually composed of three steps, that is, starch saccharification, alcohol fermentation, and acetic acid fermentation, which is the most important of the three (Liu et al. 2004; Raspor and Goranovic 2008). Because excellent properties of acetic acid bacteria (AAB) play key roles in yielding high acetic acid and producing quality vinegar, the isolation of potential AAB species is necessary for improving vinegar production.
Solid-state fermentation (SSF) is an age-old and traditional process that is widely used to produce vinegar from cereals in China and other Asian countries. The basic process steps of SSF include crushing and steaming of cereals; addition of water and Qu, a specific cereal preparation containing molds, yeasts, and bacteria; saccharification and alcoholic fermentation; and acetic acid oxidation, during which wheat bran and rice (or other cereals) hulls are mixed with old Pei (acetic acid-fermented product from the last batch used as seed vinegar) (Chen et al. 2009). Several species of AAB are often present in Chinese vinegar SSF process. Acetobacter pasteurianus is the predominant species in Zhenjiang vinegar due to the high initial ethanol concentration (Huang and Cai 1999). Acetobacter aceti dominates the Sichuan bran vinegar, and Acetobacter xylinum is the main bacterium in Jiangzhe vinegar and Fujian vinegar (Lei 2000). Some isolates from Shanxi aged vinegar were identified as A. pasteurianus, Acetobacter senegalensis, Acetobacter indonesiensis, Acetobacter malorum, and Gluconobacter (Wu et al. 2012). Given the rich microorganism in Chinese vinegar Pei, excellent AAB can be potentially isolated from it.
To date, submerged fermentation (SF) is widely used for vinegar production. SF has several advantages over SSF, including high yield and process speed. In general, SF requires robust AAB strains that are able to produce high-titer acetic acid under strict conditions (Gullo et al. 2014). During the SF process, high temperature and ethanol are extremely important limiting factors. AAB must endure the high initial ethanol concentration, which inhibits AAB cell growth and limits acid productivity (Yuan et al. 2013). As fermentation continues, temperatures rise more than the optimal value for AAB growth because of the rapid heat accumulation during SF. At high temperatures, essential enzymes are denatured, and cell membrane damage causes cellular constituents to distribute (De Ory et al. 1998). With these limiting factors, the growth of AAB cells is restricted and the acid yield is reduced. Therefore, to overcome these problems, ethanol-tolerant and thermotolerant AAB must be isolated. Numerous Acetobacters with good acetic acid tolerance or thermotolerance have been reported (Trcek et al. 2006; Ndoye et al. 2006; Perumpuli et al. 2014; Nakano and Fukaya 2008), but little is known about the isolation of ethanol-tolerant AAB (Yuan et al. 2013).
Isolation was conducted from Chinese vinegar Pei at high temperature (40 °C) and subsequently at high ethanol concentration (10 %, v/v) to select AAB that is resistant to high temperature and ethanol for SF. Seven ethanol-tolerant and thermotolerant AAB belonging to A. pasteurianus were selected for characterization of their potential. AAB4 was characterized as resistant to 43 °C and 12 % ethanol, with the highest acetic acid yield reaching 61.2 g/L at 30 °C and 10 % ethanol, during the shake flask fermentation process. In a 10 L fermentor with 10 % ethanol at 37 °C, AAB4 produced 42.0 g/L acetic acid, whereas a widely used AAB in China called AS1.41 almost stopped producing acetic acid. Therefore, AAB4 could be considered as a valuable strain for production of vinegar through SF.
Materials and methods
Vinegar Pei and AAB enrichment culture
Vinegar Pei is prepared as follows. At the end of the alcoholic fermentation, the fermented starch material was mixed with wheat bran, millet chaff, and AAB. A solid mixture, Pei, containing a large amount of AAB and some acetic acid was then formed through static fermentation. Vinegar Pei was collected from the workshop of Fengyang Vinegar Factory (Fengyang, China) and Danyang Vinegar Factory (Danyang, China), where vinegar is produced through traditional SSF. The samples were collected at a depth of approximately 20 cm from the surface at the fifth day of acetic acid fermentation and stored at 4 °C immediately. From the two factories, 1 g of each vinegar Pei was mixed and inoculated into 100 mL enrichment medium broth (1 % absolute ethanol, 1 % glucose and 1 % yeast extract) for AAB enrichment at 180 rpm for 24 h at 30 °C.
Screening AAB strains under high temperature and high ethanol
GYEC medium (1 % glucose, 1 % yeast extract, 2 % calcium carbonate and 2 % agar, w/v) was used for plate selection of AAB. GY medium (1 % glucose and 1 % yeast extract) was used as the fermentation medium. AS1.41, an A. pasteurianus strain, was used as a reference strain.
The enrichment culture was diluted, and 100 μL each of 10−5 and 10−6 dilutions was spread onto the GYEC agar plates containing 4 % ethanol and incubated at 40 °C for 72 h. Acid-producing microorganisms resistant to 40 °C showed clear zones around the colonies on GYEC agar plates. Colonies from the plate were scattered into a 200 μL GY medium. Then 100 μL of 10−5 dilution was spread onto GYEC agar plates with ethanol (10 %). The plate was incubated at 30 °C for 72 h. The isolates with clear zones were considered as strains tolerant to high temperature (40 °C) and ethanol (10 %).
adhA gene amplification
DNA extraction was performed according to the method described by Ruiz et al. (2000). For rapid molecular detection of AAB, the target adhA gene-specific primers (NuniADHfw: 5′-TGG(T/C)(A/T) CGG(C/T)AT(T/C)CC(G/C)GG-3′ and NuniADHrev: 5′-GT(G/C/A)GCGTC(A/G)TA(A/G)GC(A/G) TGGAA-3′) designed by Trcek (2005) were used. 50 μL PCR system included 5 μL of 10 × buffer, 2 μL of 2.5 mmol/L dNTP, 0.5 μL of template, 1.0 μL of NuniADHfw (10 mmol/L) and NuniADHrev (10 mmol/L), 0.5 μL of 5 U/μL taq DNA polymerase, and 40 μL of double distilled water. PCR amplification was conducted as follows: degeneration for 5 min at 94 °C, 30 circulations for degeneration at 94 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 30 s. Lastly, a final extension was performed at 72 °C for 10 min, followed by cooling to 4 °C. Amplified DNA was separated by electrophoresis with 0.8 % (w/v) agarose and 1 × TAE running buffer. After ethidium bromide dyeing, the product was examined using fluorescence-visible gel imaging analysis system.
According to adhA gene amplification results, a typical band of approximately 370 bp was most likely to be AAB, whereas non-AAB isolates were discriminated and eliminated (Trcek 2005).
Shake flask culture and determination of growth and acid yield
Cells from slant cultures were transferred to 200 mL shake flasks containing 30 mL of a pre-culture medium for seed preparation. Incubation was performed at 30 °C and 180 rpm in a shaker. Cells for all experiments were used when the optical density at 600 nm was approximately 1.2. The 500 mL shake flask (containing 100 mL GY medium with 4 % ethanol and 8 % inoculum) was used to measure AAB properties at different temperatures. The shake flask culture was incubated at 30, 33, 35, 37, 40, 41, 42, 43, or 44 °C. The properties of AAB at different ethanol concentrations were detected in 500 mL shake flask (containing 100 mL GY medium at 30 °C) with 4, 6, 8, 10, 11, 12, 13, or 14 % ethanol. The shake flask culture was performed at 180 r/min for eight days.
The acid yield and cell growth were measured at indicated time points. Acid yield was determined by titration with 0.1 M NaOH using phenolphthalein reagent as indicator. Cell growth was determined with a spectrophotometer (i8 Double Beam Ultraviolet Visible Spectrophotometer; Jinan Hanon Instruments Co., Ltd., China) at 600 nm.
Comparison of the characteristics of AAB4 to AS1.41 in a 10 L fermentor
AAB4 was compared with AS1.41 in a 10 L fermentor to determine the characteristics of AAB4 in the SF process. Agitation rate was 120 rpm, and dissolved oxygen concentration was maintained above 22 %. The GY culture (containing 8 % inoculum) was incubated at 30 or 37 °C for eight days with 4, 6, 8 and 10 % ethanol. Acid was measured for every 24 h.
DNA extraction and 16S rDNA PCR enlargement
DNA extraction was performed according to the method described by Ruiz et al. (2000). PCR amplification of 16S rDNA was performed using primers 27f (5′-AGAGTTTGATCCTGGCAG-3′) and 1492r (5′-GGCTACCTTGTTACGACTT-3′) (Hiraishi 1992). Fifty μL PCR system included 5 μL of 10 × buffer, 2 μL of 2.5 mmol/L dNTP, 0.5 μL of template, 1.0 μL of each primer (27f and 1492r), 0.5 μL of 5 U/μL taq DNA polymerase, and 40 μL of double distilled water. PCR amplification was conducted. The reaction was initiated at 94 °C for 5 min, and 30 circulations were performed at 94 °C for 30 s to denature the template DNA, annealing at 54 °C for 30 s, and extension at 72 °C for 90 s. A final extension was performed at 72 °C for 10 min, followed by cooling to 4 °C. The PCR product underwent electrophoresis with 0.8 % agarose.
Phylogenetic analysis
The 16S rDNA amplification was sequenced in Sangon Biological Engineering Co., Ltd. (Shanghai, China). For phylogenetic analysis of the isolated strains, the 16S rDNA gene sequences of ten A. pasteurianus, eight Gluconobacter strains, and other Acetobacter species were obtained from the NCBI website (http://www.ncbi.nlm.nih.gov). Multiple-sequence alignments and phylogenetic analysis were carried out using MEGA version 5.0 software (Tamura et al. 2011). The phylogenetic tree based on 16S rDNA was constructed according to the neighbor-joining (NJ) approach (González and Mas 2011). The homology of all isolates to other AAB species was analyzed according to their evolutionary divergence.
Nucleotide sequence accession number
The nucleotide sequence of 16S rDNA reported in this paper have been deposited in National Center for Biotechnology Information nucleotide sequence databases under Accession Numbers KT724707 (AAB1), KT724708 (AAB2), KT724709 (AAB3), KT724710 (AAB4), KT724711 (AAB5), KT724712 (AAB6) and KT724713 (AAB7).
Results
Preliminary screening of AAB strains with high temperature and high ethanol resistance
AAB are microorganisms that are fastidiously isolated and cultivated despite the great number of growth media available (De Vero et al. 2006), but GYEC solid medium is recommended as an ideal medium because it enables AAB recovery from traditional vinegar. The halo formation caused by acid hydrolysis of CaCO3 contained in GYEC is one of the most basic and dominant characteristics that associate an unknown colony with acetic acid bacterial group (Sengun and Karabiyikli 2011). To isolate potential thermotolerant and ethanol-tolerant AAB strains, we initially incubated GYEC agar plates at 40 °C with 4 % ethanol as selective stress. When 100 μL of 10−5 dilution of enriched culture was spread onto the GYEC agar plates with 4 % ethanol, many separate colonies showing distinct clear zones appeared at 40 °C (Fig. 1a) and were considered as thermotolerant strains. From these candidate thermotolerant strains, seven isolate strains presenting distinct clear zones were further selected using GYEC agar plates with 10 % ethanol and were considered as ethanol-tolerant strains (Fig. 1b). Thus, the seven isolate strains could tolerate 40 °C and 10 % ethanol (Table 1).
Molecular detection of real AAB by adhA gene amplification
Trcek (2005) reported that traditional AAB identification based on an array of phenotypic tests was not completely reliable and are time-consuming methods. The adhA gene-encoding subunit I of pyrroloquinoline-quinone-dependent alcohol dehydrogenase (ADH) is used for constructing DNA probes for quick and accurate identification of AAB. According to adhA gene amplification, the strain with a typical band of approximately 370 bp was most likely an AAB, and non-AAB isolates could be discriminated (Trcek 2005). Referring to this method, 370 bp band was amplified from seven isolate strains and reference strain AS1.41 (Fig. 2). Thus, the tentative identification indicated that the seven strains could be classified under the AAB group as AAB1, AAB2, AAB3, AAB4, AAB5, AAB6 and AAB7.
Determination of AAB traits at high temperature and high ethanol
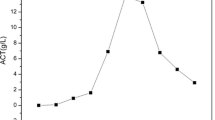
AAB were cultured in shake flask with 4 % ethanol at 30–40 °C for eight days to determine the traits of AAB at different temperatures. Cell growth (Fig. 3a) and acid yield (Fig. 3b) were measured daily. As temperature increased from 30–40 °C, the growth and acid yield of four AAB (AAB3, AAB4, AAB6 and AAB7) suffered less change, but the growth and acid yield of the other four (AAB1, AAB2, AAB5 and AS1.41) declined significantly. The acid yield of AAB4 were almost maintained at the same high level (not <38.4 g/L, alcohol–acid transform ratio was 90.5 %) from 30 to 40 °C. AAB4 maintained high alcohol–acid transform ratio at 41 °C (87.2 %) and 42 °C (77.1 %). Moreover, AAB4 still maintained 14.5 % efficiency at 43 °C, and 2.9 % efficiency at 44 °C. However, the growth and acid yield of AAB3, AAB6 and AAB7 almost disappeared at 42 °C, and AS1.41 completely stopped growing at 41 °C.
To identify the properties of AAB at different ethanol concentrations, we incubated AAB at 30 °C with 4–13 % ethanol for 8 days. The growth (Fig. 4a) and acid yield (Fig. 4b) were measured. As the ethanol concentration increased, the growth of all AAB was inhibited by 2–8 %. AAB1, AAB2, AAB5, AAB6, AAB7 and AS1.41 decreased sharply at 11 % ethanol, whereas AAB3 and AAB4 grew well at 11 % ethanol and showed tolerance to 12 % ethanol. AAB3 and AAB4 maintained almost the same acid yield larger than the other AAB at 8–11 % ethanol test concentrations. Although the alcohol–acid transform ratio of both AAB3 and AAB4 declined from 4 % (88.7 and 98.1 % ratio) to 10 % ethanol (56.8 and 59.7 % ratio), the amount of the acid increased. The maximum acid yields of AAB3 and AAB4 were 58.6 and 61.2 g/L, respectively, at 10 % ethanol. AAB3 and AAB4 could even maintain good alcohol–acid transform ratio (44.4 % AAB3 and 46.5 % AAB4) at 11 % ethanol and a certain amount of efficiency (4.5 % AAB3 and 8.7 % AAB4) at 12 % ethanol.
The fermentation process of AAB4 was further analyzed by comparisons with AS1.41. AAB4 maintained relatively high growth and acid yield throughout the fermentation at 4–11 % ethanol (Fig. 5a, b) and 30–40 °C (Fig. 5c, d). However, both growth and acid yield of AS1.41 decreased sharply at 10 % ethanol (Fig. 5a, b) and 40 °C (Fig. 5c, d). At 8 % ethanol concentration, the maximum acid yield of AS1.41 was 47.2 g/L, whereas the acid yield of AAB4 was 59.1 g/L, which is 1.25 times that of AS1.41. When the ethanol concentration was 8–11 %, AS1.41 showed a long lag phase, whereas AAB4 was almost unaffected.
Comparison of characteristics of AAB4 and AS1.41 in SF
When fermentation was performed in a 10 L fermentor, AAB4 showed greater alcohol–acid transform ratio than AS1.41 at different combinations of temperature and ethanol concentration (Fig. 6). At 30 °C with 10 % ethanol, the acid yield of AAB4 (64.2 g/L) was 2.89 times than that of AS1.41 (22.2 g/L). At 37 °C with 10 % ethanol, the acid yield by AAB4 decreased but still was retained at 42.0 g/L, whereas AS1.41 almost stopped producing acetic acid (Fig. 6d). Therefore, AAB4 could be simultaneously resistant to high temperature and high ethanol. These results suggest that AAB4 has a good application prospect for SF.
Classification of AAB4 by 16SrDNA sequencing and phylogenetic analysis
Fragments of 16S rDNA were sequenced and subjected to phylogenetic analysis to further identify the AAB. Nucleotide-nucleotide BLAST results showed that all AAB have more than 99 % sequence similarity with the first hit of A. pasteurianus. A phylogenetic NJ tree was constructed to understand the taxonomic position of all AAB used. Through the phylogenetic tree, AAB1 to AAB7 were shown to be closest with A. pasteurianus (Fig. 7). All AAB have greater than 99 % sequence similarity with the reference strain A. pasteurianus AS1.41 (AAB1, AAB2, AAB3, AAB4, AAB5, AAB6 and AAB7 have 99.98, 99.18, 99.33, 99.32, 99.23, 99.99 and 99.34 % similarity to A. pasteurianus AS1.41, respectively). Thus, this observation suggests that AAB1 to AAB7 belonged to A. pasteurianus.
Discussion
Screening potential AAB which could be tolerant to different drastic conditions is important to improve industrial vinegar fermentation. In the study, seven strains tolerant to temperatures above 40 °C and ethanol above 10 % were isolated from Chinese vinegar Pei using GYEC agar plate. Although AAB strains that are usually isolated with GYEC plates show distinct clear zones, the methods based on the presence of a clear zone was not completely believable because other strains, such as some lactic acid bacteria, could also form distinct clear zones. The fast molecular detection was proven to be efficient and accurate (Trcek 2005). According to this method, the seven strains were classified to AAB groups. These isolates were further proven to be A. pasteurianus by 16S rDNA sequencing and phylogenetic tree analysis. Thus, the method using GYEC agar plate directional isolation and molecular identification strategy adopted was efficient and accurate, and it also proved that Chinese vinegar Pei is a rich source of AAB.
During the fermentation process, ethanol and temperature can become extreme limiting factors. Although ethanol is the substrate of acetic acid fermentation, the concentration must be strictly controlled because high concentrations of ethanol can affect AAB growth. In SF, high-titer acetic acid requires high ethanol-tolerant traits for AAB (Gullo et al. 2014). In addition, high ethanol fermentation can improve the content of organic acid and the quality of vinegar (Wei et al. 2012). In this study, AAB4 showed a higher ethanol tolerance and produced more acetic acid than other AAB isolated from industrial fermented vinegar (Yuan et al. 2013). The maximum acid yield of AAB4 was 61.2 g/L at 10 % ethanol, whereas the reported ethanol-tolerant AAB (Yuan et al. 2013) lost the ability to grow at 10 % ethanol. Even with 12 % ethanol, AAB4 still produced 10.5 g/L acetic acid. Therefore, AAB4 is potential for the industrial application in high ethanol fermentation.
The optimum temperature for acid production for most AAB is usually at 30 °C. Heat accumulated in fermentation could rapidly increase the temperature to above 37 °C, which could restrict industrial vinegar yield. Cooling equipment must be used to overcome high temperatures, which would require additional costs. This is a serious problem in high-temperature regions. Many researches focused on the screening of thermotolerant AAB, such as those isolated from tropical products of Sub-Saharan Africa (Ndoye et al. 2006), coconut water vinegar in Sri Lanka (Perumpuli et al. 2014), and fruits and flowers in tropical Thailand (Kanchanarach et al. 2010). As can be seen, the maximum acid yield of these thermotolerant AAB was approximately 30 g/L at 40 °C, whereas AAB4 was able to produce 38 g/L acetic acid at the same conditions. Even at 42 °C, AAB4 produced more than 30 g/L acetic acid. Thus, AAB4 was a more excellent thermotolerant strain.
Although the selection of excellent AAB is in progress, many studies focused on one extreme feature, such as temperature, ethanol, or acetic acid tolerance (Trcek et al. 2006; Perumpuli et al. 2014; Yuan et al. 2013). When faced with high temperature and high ethanol at the same time, whether they could still perform well become a problem. In this regard, AAB4 was compared with AS1.41 fermentation in a 10 L fermentor. The results showed that when faced with high temperature (37 °C) and high ethanol concentration (10 %) at the same time, AAB4 still produced a large amount of acetic acid (42 g/L), whereas AS1.41 almost stopped acid production. In the SF process, the raw alcohol used for acetic acid fermentation may be more than 8 %, and the temperature inevitably exceeds 37 °C especially in the summer. The use of thermotolerant and ethanol-tolerant AAB would reduce the cooling cost and enhances the quality of acetic acid. Therefore, AAB4 should be considered as a valuable strain to be introduced in SF for vinegar production.
References
Chen FS, Li L, Qu J, Chen CX (2009) Cereal vinegars made by solid-state fermentation in China. In: Giudici P, Solieri L (eds) Vinegar of the world. Springer, Milan, pp 243–259
De Ory I, Romero LE, Cantero D (1998) Modelling the kinetics of growth of Acetobacter aceti in discontinuous culture: influence of the temperature of operation. Appl Microbiol Biotechnol 49:189–193
De Vero L, Gala E, Gullo M, Solieri L, Landi S, Giudici P (2006) Application of denaturing gradient gel electrophoresis (DGGE) analysis to evaluate acetic acid bacteria in traditional balsamic vinegar. Food Microbiol 23:809–813
González A, Mas A (2011) Differentiation of acetic acid bacteria based on sequence analysis of 16S–23S rRNA gene internal transcribed spacer sequences. Int J Food Microbiol 147:217–222
Gullo M, Verzelloni E, Canonico M (2014) Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem 49:1571–1579
Hiraishi A (1992) Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett Appl Microbiol 15:210–213
Huang Z, Cai M (1999) Vinegar. In: Shanghai Institute of Brewing (ed) The production technology of fermented condiments. Light Industry Publishing House of China, Beijing, pp 289–419
Kanchanarach W, Theeragool G, Yakushi T, Toyama H, Adachi O, Matsushita K (2010) Characterization of thermotolerant Acetobacter pasteurianus strains and their quinoprotein alcohol dehydrogenases. Appl Microbiol Biotechnol 85:741–751
Lei M (2000) Function of microorganisms and enzymes in vinegar production by solid state fermentation. Chin Condiment 9:20–22
Liu D, Zhu Y, Beeftink R, Ooijkaas L, Rinzema A, Chen J, Tramper J (2004) Chinese vinegar and its solid-state fermentation process. Food Rev Int 20:407–424
Nakano S, Fukaya M (2008) Analysis of proteins responsive to acetic acid in Acetobacter: molecular mechanisms conferring acetic acid resistance in acetic acid bacteria. Int J Food Microbiol 125:54–59
Ndoye B, Lebecque S, Dubois-Dauphin R, Tounkara L, Guiro AT, Kere C, Diawara B, Thonart P (2006) Thermoresistant properties of acetic acids bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enzyme Microb Technol 39:916–923
Perumpuli PABN, Watanabe T, Toyama H (2014) Identification and characterization of thermotolerant acetic acid bacteria strains isolated from coconut water vinegar in Sri Lanka. Biosci Biotech Biochem 78:533–541
Raspor P, Goranovič D (2008) Biotechnological applications of acetic acid bacteria. Crit Rev Biotechnol 28:101–124
Ruiz A, Poblet M, Mas A, Guillamon JM (2000) Identification of acetic acid bacteria by RFLP of PCR-amplified 16S rDNA and 16S–23S rDNA intergenic spacer. Int J Syst Evol Microbiol 50:1981–1987
Sengun IY, Karabiyikli S (2011) Importance of acetic acid bacteria in food industry. Food Control 22:647–656
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Trcek J (2005) Quick identification of acetic acid bacteria based on nucleotide sequences of the 16S–23S rDNA internal transcribed spacer region and of the PQQ-dependent alcohol dehydrogenase gene. Syst Appl Microbiol 28:735–745
Trcek J, Toyama H, Czuba J, Misiewicz A, Matsushita K (2006) Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl Microbiol Biotechnol 70:366–373
Verzelloni E, Tagliazucchi D, Conte A (2007) Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem 105:564–571
Wei K, Cao X, Li X, Wang C, Hou L (2012) Genome shuffling to improve fermentation properties of acetic acid bacterium by the improvement of ethanol tolerance. Int J Food Sci Technol 47:2184–2189
Wu JJ, Ma YK, Zhang FF, Chen FS (2012) Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol 30:289–297
Yuan Y, Feng F, Chen L, Yao Q, Chen K (2013) Directional isolation of ethanol-tolerant acetic acid bacteria from industrial fermented vinegar. Eur Food Res Technol 236:573–578
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Bai, Y., Li, D. et al. Screening and characterization of ethanol-tolerant and thermotolerant acetic acid bacteria from Chinese vinegar Pei . World J Microbiol Biotechnol 32, 14 (2016). https://doi.org/10.1007/s11274-015-1961-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-1961-8