Abstract
The present study aimed to identify the differentially expressed genes (DEGs) and enriched pathways in docetaxel (DTX) resistant breast cancer cell lines by bioinformatics analysis. The microarray dataset GSE28784 was obtained from gene expression omnibus (GEO) database. The differentially expressed genes (DEGs), gene ontology (GO), and Kyoto Encyclopedia of gene and genome (KEGG) pathway analyses were performed with the help of GEO2R and DAVID tools. Furthermore, the protein–protein interaction (PPI) and hub-gene network of DEGs were constructed using STRING and Cytohubba tools. The prognostic values of hub genes were calculated with the help of the Kaplan–Meier plotter database. From the GEO2R analysis, 222 DEGs were identified of which 120 are upregulated and 102 are downregulated genes. In the PPIs network, five up-regulated genes including CCL2, SPARC, CYR61, F3, and MFGE8 were identified as hub genes. It was observed that low expression of six hub genes CXCL8, CYR61, F3, ICAM1, PLAT, and THBD were significantly correlated with poor overall survival of BC patients in survival analysis. miRNA analysis identified that hsa-mir-16-5p, hsa-mir-335-5p, hsa-mir-124-3p, hsa-mir-20a-5p, and hsa-mir-155-5p are the top 5 interactive miRNAs that are commonly interacting with more hub genes with degree score of greater than five. Additionally, drug-gene interaction analysis was performed to identify drugs which are could potentially elevate/lower the expression levels of hub genes. In summary, the gene-miRNAs-TFs network and subsequent correlation of candidate drugs with hub genes may improve individualized diagnosis and help select appropriate combination therapy for DTX-resistant BC in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the most frequently diagnosed malignancies, and the second leading cause of cancer-related death among females worldwide (14%), with an estimated 2.1 million cases and 627,000 deaths in 2018 (Ferlay et al. 2013; Organization 2016). Current BC treatment strategies include surgery, cytotoxic chemotherapy, radiotherapy, and hormonal therapy. The backbone of current chemotherapeutic regimens includes taxanes (docetaxel, and paclitaxel), anthracyclins (doxorubicin and epirubicin), anti-estrogens (tamoxifen, fulvestrant), and aromatase inhibitors (anastrozole, letrozole). Despite achievements made in treating BC during the past decades, resistance to classical anticancer agents is a major problem in cancer therapies and is responsible for cancer relapses and deaths (Wang et al. 2020; Geng et al. 2020). Docetaxel (DXT), a taxane class of anticancer drug used in BC chemotherapy, acts as a microtubule destabilizer and ultimately inhibits cell proliferation. However, half of the patients not showing benefits from the treatment after a prolonged period, as a result of acquired drug resistance (Zhang et al. 2020). Thus drug resistance constitutes a major clinical problem in the successful treatment of BC. Therefore, there is a need to identify novel therapeutic targets, including mRNA, microRNAs (miRNAs), and Transcription factors (TFs) that can improve the therapeutic efficacy of resistant drugs. Some progress has been made in determining the mechanism of mRNA-mediated DXT resistance in BC. For example, Huang et al. identified that up-regulated expression of ABCB1 exhibited resistance to DXT (Huang et al. 2018). Similarly, a study conducted by Miyoshi et al. revealed that overexpression of CYP3A4 mRNA in BC is responsible for increased inactivation of DXT, which ultimately results in acquired drug resistance (Miyoshi et al. 2002). In another study, Brown et al. identified a novel mechanism for DXT resistance, in which the reduced expression of p27 was associated with acquired drug resistance (Brown et al. 2004). Likewise, Egawa et al. and colleagues identified that reduced expression of BRCA2 was positively correlated with improved DXT response in BC (Egawa et al. 2001). All these studies supported the possible role of mRNA expression in drug-resistant cancers. Recently, trends have been shifted towards miRNAs which are small non-coding RNAs and can control 30% of overall gene expression through transcriptional repression or mRNA cleavage (Lewis et al. 2005). The miRNAs have recently emerged as important players in different biological processes such as cancer progression, metastasis, and drug resistance. Various studies identified the possible role of miRNAs in DXT-resistant BC including miRNA-34a, miRNA-3646, miRNA-462, and miRNA-129-3p (Kastl et al. 2012; Zhang et al. 2016, 2015; Hu et al. 2014). Nevertheless, these studies are not enough to understand the mechanism of DXT resistance in BC. Therefore, the identification of reliable biomarkers and an understanding of the molecular mechanism responsible for acquired DTX resistance are urgently needed for effective diagnosis and treatment. In this study, the microarray dataset of GES28784 was collected and analyzed using gene expression omnibus (GEO) database. Differentially expressed genes (DEGs) were screened and gene ontology (GO), Kyoto Encyclopedia of genes and genome (KEGG) pathway studies were performed. We also performed protein–protein interaction network studies (PPI) and identified the hub genes involved in the development of DTX chemoresistance in BC patients. Afterward, the prognostic effect of identified hub genes on the overall survival of BC patients was also analyzed. Further, gene-miRNAs-TFs interaction was constructed. Finally, gene-drug interactions between hub genes and available chemotherapeutic drugs were identified. This study could provide a better understanding of the molecular mechanism underlying DXT-resistant BC development and recurrence. The identified genes, miRNAs, and TFs and networking among them may explore to address DTX in BC.
Materials and methods
Data collection and identification of DEGs
To identify DEGs in DTX-resistant BC cells, the public gene expression dataset GSE28784 was downloaded from the NCBI-GEO database (https://www.ncbi.nlm.nih.gov/geo/) using specific keywords of “Breast cancer”, “Docetaxel resistance” and “Taxane resistance”. Gene expression profiles in this dataset were recorded by considering three sensitive and three DTX-resistant MDA-MB-231 BC cell lines, using GPL 96 platform (Affymetrix GeneChip HU133A arrays). The series matrix file of the aforementioned GSE28784 dataset was downloaded from the NCBI-GEO database. Genes with multiple probes were removed from the dataset. GEO2R is a freely available web tool that can compare and analyze two different groups of samples under the same experimental conditions. The statistical package GEO2R was applied to screen the DEGs between sensitive and DTX-resistant cell lines. Subsequently, results were downloaded and genes that met the cutoff criteria of the adjusted P value < 0.02 and LogFC > 1 based on the Benjamini-Hochberg (BH) procedure were considered DEGs. A volcano plot was constructed for graphically distinguishing up-regulated and down-regulated genes using R studio. Additionally, an online tool, ClustVis (https://biit.cs.ut.ee/clustvis/) was used to draw the heat map of the top 200 DEGs for analyzing their expression profiles.
GO and KEGG pathway analysis of DEGs
Gene Ontology (GO) provides a computational model of biological systems from the molecular level in three different categories including biological process (BP), molecular function (MF), and cellular component (CC). Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database for high-level functions of biological systems. The online biological tool DAVID (https://david.ncifcrf.gov/; version 6.8) was used to carry out GO and KEGG pathway analysis of DEGs. Up-regulated and down-regulated genes were submitted to the DAVID online program. The top 10 items of biological process (BP), cellular component (CC), and molecular functions (MF) categories and KEGG pathways were sorted and illustrated in the form of bubble maps. The bubble plots were drawn using the ggplot2 package based on P-value through the statistical software R (Version 6.6.2). GO terms and KEGG pathways were considered significant when P < 0.05.
PPI network construction and hub-gene identification
The PPI network of identified DEGs was constructed by an online tool Search Tool for the Retrieval of Interacting Genes, STRING (https://string-db.org/) with an interaction score > 0.4. Subsequently, the Molecular Complex Detection (MCODE) plugin in Cytoscape software (Version 3.7.2) (https://cytoscape.org/) was applied to identify significant PPI modules in the network using default parameters including degree cutoff = 2, node score cutoff = 0.2, k-score = 2, and max. Depth = 100. In this study, the criteria of the top 2 modules were set with MCODE score > 5.5 and nodes > 5. Finally, the top 20 hub genes were identified, based on the highest degree of connectivity by applying the Cytoscape plugin CytoHubba. STRING software was again utilized to construct the PPI network of the hub genes using a confidence score > 0.4 as a criterion of selection.
Survival analysis and validation of the hub genes
Kaplan–Meier plotter is an online database capable of assessing the prognostic survival of 54 K genes on different types of cancers including breast (n = 6234), ovarian (n = 2190), lung (n = 3452), and gastric (n = 1440) cancer (https://kmplot.com/analysis/). In the current study, the Kaplan–Meier Plotter online database was used to perform a survival analysis of the hub genes. Patient with BC was categorized into two groups namely, low expression and high expression group based on the expression of a particular gene. The general inclusion criteria of survival analysis are as follows: (1) an user-defined probeset was used; (2) the median value of gene expression was considered for splitting patients into two different groups. Then, the overall survival was analyzed for the above two groups for each hub gene. The analysis was shown in the form of prognostic curves, according to the hazard ratio, 95% confidence interval, and log-rank P-value ≤ 0.05).
Hub gene-miRNA-TFs network construction
Freely available online tool miRNet (https://www.mirnet.ca/) was used to identify the potential mRNA-miRNA interactions in the PPI network. miRNet utilizes eleven established miRNA-target prediction databases including miRTarBase, TarBase, miRecords, SM2miR, Pharmaco-miR, miR2Disease, PhenomiR, StarBase, EpimiR, miRDB, and miRanda. In this study, Predicted miRNAs were considered the targeted miRNAs of hub genes. Simultaneously, hub gene-regulated TFs were also predicted, and an interaction network of mRNA-miRNAs-TFs was constructed using miRNet.
Drug-hub gene interaction network
The Comparative Toxicogenomics Database (CTD) database was used to construct hub-gene and drug interaction networks (Mattingly et al. 2003), the identified chemotherapeutic drugs that could potentially serve as promising agents for hub genes. The identified drugs may reduce or elevate the mRNA or protein expression levels of hub genes.
Results
Identification of DEGs
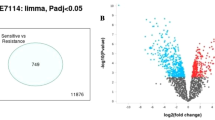
The gene expression dataset GSE28784 was downloaded from the NCBI GEO database with three sensitive (GSM712682, GSM712684, GSM712684) and DTX resistant (GSM712685, GSM712686, GSM712687) BC cell lines. Analysis of microarray dataset GSE28784 was performed by GEOR2 tool and data were visualized in volcano plot as described in Fig. 1A. Blue and red dots represent the significantly up-regulated (logFC > 1, P < 0.02) or down-regulated (logFC < − 1, P < 0.02) genes, respectively. After the DEG analysis of microarray data, a total of 222 genes were identified to be DEGs, among which 120 genes were up-regulated and 102 genes were down-regulated. Heat map as displayed in Fig. 1B was generated for top20 DEGs using the ClustVis tool based on the expression level of DEGs in the GSE28784 dataset. In the heat map, each column represents a biological sample, i.e. three DTX-sensitive and three resistant cell lines, and each row represents a DEG. This plot describes the expression level of DEG in sensitive and resistant BC cells using color coding as red indicates high expression and blue indicates low expression level with some intermediate members ranging from light red to light blue via white color. Different color patterns were observed in different columns, i.e. different cell lines.
A Volcano plot of differentially expressed genes (DEGs). Blue dots represent up-regulated genes, red dots represent down-regulated genes, and green dots represent non-DEGS. B Heat map of the top 20 DEGs. Red color indicates a high expression level of DEG; Blue color indicates a low expression level of DEG
GO and KEGG pathway analysis of DEGs
For a more functional of understanding the 222 DEGs, GO and KEGG pathway analyses were performed using DAVID software. As shown in Fig. 2, the DEGs were classified into four functional groups including BP, CC, MF, and KEGG. The top 5 significant terms from the GO enrichment analysis showed that in the biological process (BP) category, the up-regulated DEGs were involved in male gonad development, high-density lipoprotein particle Whereas, the down-regulated DEGs were significantly enriched in angiogenesis, positive regulation of neutrophil chemotaxis, cellular response to lipopolysaccharide, and extracellular matrix organization Supplementary file1 (S1); Fig. S1A. For the CC category, the up-regulated DEGs were involved in the cell surface, plasma membrane, extracellular exosome, proteinaceous extracellular matrix, and phosphorylase kinase complex (Fig. 2B). On the other hand down-regulated genes were associated with extracellular space, extracellular exosome, extracellular matrix, extracellular region, and cell surface S1; Fig. S1B. For the MF category up-regulated genes were enriched in extracellular matrix binding, protein dimerization activity, phosphatidylcholine-translocating ATPase activity, xenobiotic-transporting ATPase activity, protein binding, protease binding, peptide antigen binding, MHC class-II receptor activity (Fig. 2C). Whereas the down-regulated genes were correlated with protease binding, integrin binding, serine-type endopeptidase inhibitor activity, thyroid hormone transmembrane transporter activity, and chemokine activity S1; Fig. S1C. For KEGG pathway analysis up-regulated genes were significantly involved in ABC transporters, Influenza A, and insulin signaling pathway (Fig. 2D). Whereas the top 5 significant KEGG pathways of down-regulated genes include Complement and coagulation cascades, Cell adhesion molecules (CAMs), Rheumatoid arthritis, Malaria, and Staphylococcus aureus infection S1; Fig. S1D.
Bubble map for GO and KEGG pathway analyses of upregulated DEGs. The top 10 items of the GO and KEGG pathway enrichment analyses are illustrated in the form of a bubble plot using the ggplot2 package for R software. A P-value < 0.05 was considered statistically significant. A biological process, B cellular components, C molecular function, and D KEGG pathways. GO gene ontology, KEGG Kyoto encyclopedia of genes and genomes
PPI network construction and hub-gene identification
The PPI network was further constructed using the STRING database to analyze the correlation among DEGs in the DTX-resistant BC cell line. All 222 DEGs were submitted to the STRING database as displayed in Fig. 3A, only 216 genes were mapped with 435 edges corresponding to PPI enrichment P-value lower than 1.0e–16. The top 2 functional clusters were identified using the Cytoscape plugin MCODE based on the MCODE score. Module 1, as displayed in Fig. 3B, is consisted of 18 nodes and 86 edges with an average node degree of 18.5 corresponding to an MCODE score of 10 whereas module 2 as displayed in Fig. 3C consists of 6 nodes and 11 edges with an average node degree of 3.6. Subsequently, KEGG pathway analysis was performed for each module by DAVID software. In KEGG pathway analysis, module 1 indicated the involvement of nodes in Complement and coagulation cascades and Malaria S1; Fig. S2A, whereas, nodes of module 2 are significantly enriched in the TNF signaling pathway, PI3K-Akt signaling pathway, and Pathways in cancer S1; Fig. S2B. Twenty genes including IL6, FN1, CCL2, CXCL8, PECAM1, SERPINE1, SERPINA1, ICAM1, SPARC, CYR61, MMP14, TIMP3, F3, IGFBP3, MFGE8, SERPIND1, THBD, PLAT, LAMB1, and CXCL1 having the highest degree score (> 13) were identified as the hub genes in DTX resistance BC by applying Cytohubba plugin Fig. 3D. In these 20 hub genes, five were up-regulated including CCL2, SPARC, CYR61, F3, and MFGE8, and the remaining fifteen hub genes were down-regulated such as IL6, FN1, CXCL8, PECAM1, SERPINE1, SERPINA1, ICAM1, MMP14, TIMP3, IGFBP3, SERPIND1, THBD, PLAT, LAMB1, and CXCL1.
Survival analysis of hub genes in BC
The prognostic information of these 20 hub genes in BC patients and normal patients was analyzed using the Kaplan–Meier plotter database. Among the twenty hub genes, nine were significantly associated with the overall survival of patients (Fig. 4A–I). Low expression of PECAM1 [HR = 0.80 (0.65–0.99), P = 0.044] Fig. 4E, SERPINA1 [HR = 0.66 (0.53–0.82), P = 0.00012] Fig. 4G, TIMP3 [HR = 0.76 (0.62–0.94), P = 0.012] Fig. 4I, were significantly associated with better overall survival of BC patients. Whereas, low expression of CXCL8 [HR = 1.33 (1.08–1.65), P = 0.0082] Fig. 4A, CYR61 [HR = 0.81 (0.65–1), P = 0.05] Fig. 4B, F3 [HR = 0.78 (0.63–0.97), P = 0.022] Fig. 4C, ICAM1 [HR = 0.78 (0.63–0.97), P = 0.023] Fig. 4D, PLAT [HR = 1.67 (0.54–0.83), P = 0.00027], and THBD [HR = 0.75 (0.6–0.93), P = 0.0078] Fig. 4H, were associated with poor overall survival of BC patients.
Overall survival analysis of hub genes. A–I Survival curves were constructed by the Kaplan–Meier plotter online database based on the high and low expression of hub genes in BC patients. The black line represents low expression and the red line represents the high expression of hub genes in BC. Log rank < 0.05 was considered statistically significant
Analysis of gene-mRNAs-TFs interactions
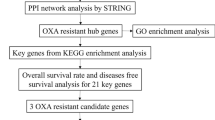
Interactions among gene-mRNAs-TFs are required to understand the mechanism of acquired DTX resistance in BC patients. The miRNet tool was applied to screen the targeted miRNAs and TFs of hub genes. Then, the miRNA-hub gene-TFs interaction network was constructed using the miRNet visualization program. As illustrated in Fig. 5 interaction network consists of 9 hub genes, 311 miRNAs, and 58 TFs. The contribution level of miRNAs and TFs to their surrounding hub genes is represented as several edges. In this network analysis, nodes were ranked based on the degree of connectivity as described in Table 1 From this table, it is clear that genes CXCL8 (121), ICAM1 (96), F3 (75), TIMP3 (69), and SERPINA1 (49) are highly interactive hub genes followed by PLAT (46), THBD (44), CYR61 (37) and PECAM1 (15). Furthermore, miRNA including has-mir-16-5p, hsa-mir-335-5p, hsa-mir-124-3p, hsa-mir-20a-5p and hsa-mir-155-5p were the top 5 interactive ones with connectivity degree score ≥ 5, that would target most of the target hub genes Table 2. Similarly, SP1, NFKB1, RELA, JUN, and STAT3, were the top 5 TFs that simultaneously regulate more than two hub genes in the network Table 3. Finally, by considering the significance of survival prognosis, and degree of hub gene-miRNA-TFs interactions, one up-regulated (F3) and five down-regulated hub gene genes (CXCL8, ICAM1, TIMP3, SERPINA1, and PLAT) were selected for exploration of Drug-gene interaction profiles.
Drug-gene interactions
To explore interactions between hub genes and available chemotherapeutic drugs, the hub gene-drug interaction was constructed using CTD, and visualization of drug-gene interaction was done by Cytoscape. As shown in Fig. 6, a variety of chemotherapeutic drugs could affect the expression of 6 hub genes, including CXCL8, ICAM1, TIMP3, SERPINA1, PLAT, and F3. For example, doxorubicin and dacarbazine could elevate the CXCL8 expression level, while daunorubicin and dasatinib could reduce the CXCL8 expression level.
Gene-drug interaction network constructed with six hub genes and chemotherapeutic drugs. Panels A–F show available chemotherapeutic drugs decrease or increase the expression levels of hub genes in mRNA or protein. A CXCL8, B ICAM1, C TIMP3, D SERPINA1, E PLAT, and F F3. Green color arrows: chemotherapeutic drugs increase the expression of hub genes: Purple color arrows: chemotherapeutic drugs decrease the expression of hub genes
Discussion
Taxane class of drugs (DTX and Paclitaxel) are first-line drugs used to treat BC patients. Acquired drug resistance limits the therapeutic efficacy of taxanes, which ultimately decreases the patient's survival. Therefore, the identification of reliable biomarkers and molecular mechanisms in acquired drug resistance is urgently needed for improving the treatment and survival of BC patients. In the present study, bioinformatics analysis was performed to investigate gene-miRNAs-TFs mediated mechanism of breast cancer chemoresistance and to identify molecular targets which can address the problem of DTX resistance in BC patients. In the present study, the GEO dataset of GSE28784 was selected to screen out DEGs. By applying the GEO2R tool, 222 DEGs (120 up-regulated and 102 down-regulated) were identified. To understand the effect of DEGs on molecular biology, GO and KEGG pathway analysis was performed using DAVID. In this analysis, KEGG pathway results showed that the up-regulated genes were enriched in influenza A, ABC transporters, and insulin signaling pathway whereas, down-regulated genes associated with a compliment and coagulation cascades, CAMs, rheumatoid arthritis, malaria, and staphylococcus aureus infection. These results may provide significant clues to understanding the molecular interactions in the progression of BC and drug resistance as these show some kind of correlation with reported studies. Many studies have reported that ABC transporters, insulin signaling, complement and coagulation cascades, and staphylococcus aureus infection, are highly associated with the progression of BC and drug resistance. It has been reported that the expression of ABC transporters in BC contributes to resistance of neoadjuvant anticancer drugs like DTX via ATP- dependent drug efflux pumps (Leonard et al. 2003). Although insulin and insulin-like growth factor signaling pathways are associated with acquired resistance to anti-estrogen therapy and trastuzumab therapy, a correlation with DTX is still needed to be established (Yang and Yee 2012). The latest reports suggested that complement and coagulation cascade pathways are correlated with drug resistance in soft tissue sarcoma. Furthermore, a PPI network with 216 nodes and 435 edges was constructed based on the DEGs, and 20 hub genes with the highest degree of connectivity in the PPI network were identified as potential genes in the DTX resistance of BC. Survival analysis of these hub genes subsequently demonstrated that low expression of TIMP3, SERPINA1, and PECAM1 is correlated with positive progression of overall survival of BC patients whereas low expression of CXCL8, ICAM1, PLAT, THBD, and PECAM1are significantly associated with poor overall survival. The functional enrichment and survival analysis showed that these target genes may participate in many important cancer-related biological processes, drug resistance, and signaling pathways. In terms of the increasingly prominent role of miRNAs and TFs in drug resistance, a gene-miRNAs-TFs network was constructed. In this study, it was observed that expression of all hub genes was dysregulated in drug resistance cell lines as compared to sensitive cell lines, therefore, it can be anticipated that these genes may be crucial for tumourigenesis, and acquired drug resistance in BC. Since many genes, miRNAs, and TFs identified in our study have functions in the development of drug resistance in BC, these can be explored to select appropriate potential drug targets. In our study, six hub genes, five miRNAs, and five TFs are found to be well connected in the established network therefore these were considered to be crucial. The hub-genes were found to be regulated by different families of miRNAs, top 5 miRNAs are hsa-mir-16-5p, hsa-mir-335-5p, hsa-mir-124-3p and hsa-mir-20a-5, and hsa-mir-155-5p. Since regulation of corresponding hub genes by miRNAs may reverse the DTX resistance in BC and the sensitivity of tumor cells to DTX may be restored, these miRNAs may consider potential targets for chemoresistance BC. Among the top six identified hub genes including CXCL8, ICAM1, TIMP3, SERPINA1, PLAT, and F3, the CXCL8 chemokine (C-X-C motif) ligand 8 or IL8 gene produces IL8 chemokine by macrophages and endothelial cells that have been reported to be associated with multidrug-resistant to BC (Hedges et al. 2000; Wolff et al. 1998). It was reported that up-regulation of CXCL8 is associated with the chemoresistance of doxorubicin (Shi et al. 2012). In this study, the down-regulated expression of CXCL8 was identified in the DTX resistance cell line, and low expression of CXCL8 was significantly correlated with poorer overall survival of BC patients. We also identified 9 potential miRNAs and 9 TFs that might regulate the expression of CXCL8 Tables 2, 3. Apart from chemoresistance, CXCL8 is also reported to be involved in different cancers, such as breast cancer, prostate cancer, lung cancer, and melanoma (Liu et al. 2016). Similarly, intracellular adhesion molecule 1 (ICAM1) was also found to be down-regulated in DTX-resistant cell lines. ICAM1 is mainly involved in pathways of BC, myeloma, squamous cell carcinoma, and ovarian cancer (Schröder et al. 2011; Zheng et al. 2013; Tsai et al. 2015; de Groote et al. 2014). High expression of ICAM1 in BC can activate several intracellular signaling pathways, ultimately triggering the tumor progression in BC (Schröder et al. 2011). From the literature, it was evident that high expression of ICAM1 was associated with doxorubicin resistance in colon cell carcinoma (Rivoltini et al. 1991; Zheng et al. 2013). In this study, downregulation of ICAM1 was identified, and low expression of ICAM1 was significantly associated with poor overall survival. The role of ICAM1 in DTX resistance breast cancer remains to be established. Studies suggested that ICAM1 signaling molecules may be involved in the different biological processes of tumor development, and could play an important role in the resistance of BC. We also identified that 9 potential miRNAs and 8 TFs might regulate the expression of ICAM1 Tables 2, 3.
TIMP3-tissue inhibitor of metalloproteinase 3, a secreted glycoprotein, plays a significant role in the protection of the extracellular matrix from degradation. Downregulation of TIMP3 was identified in different malignancies including melanoma, renal cell carcinoma, BC, and choriocarcinoma (Das et al. 2016; Masson et al. 2010; Song et al. 2010; Feng et al. 2004). In this study, downregulation of TIMP3 was identified, and low expression of TIMP3 was highly correlated with poor overall survival. A study conducted by Xiu et al. identified that decreased TIMP3 expression was associated with cisplatin resistance in osteosarcoma whereas increased expression of TIMP3 reverses the cisplatin resistance in osteosarcoma (Han et al. 2018). Till now there are no studies reported regarding the association of TIMP3 with DTX resistance BC, experimental investigation of TIMP3 low expression in DTX resistance BC needed to be established. The overexpression of serpin peptidase inhibitor clade A member 1 (SERPINA1) has been reported in BC and gastric cancer (Chan et al. 2015; Kwon et al. 2014). The high expression of SERPINA is associated with positive progression survival of BC patients, and poor progression survival of gastric cancer patients. In this study, the down-regulated expression of SERPINA1 was identified, and survival analysis results indicated that low expression of SERPINA1 was significantly associated with poor OS of BC patients. A study reported by Wen Juan et al. identified that high expression of SERPINA1 was associated with cisplatin resistance in epithelial ovarian cancer (Wu et al. 2016). However, there are no relevant studies on the involvement of SERPINA1 in DTX resistance BC. High intratumoral levels of tissue plasminogen activator (PLAT) were associated with poorer OS of colon cancer patients whereas decreased expression of PLAT was associated with aggressiveness and poorer OS of BC (Corte et al. 2005). In our study, down-regulated expression of PLAT was significantly associated with poorer progression of BC. Further experimental studies on the role of PLAT on DTX resistance are needed to be investigated. The overexpression of tissue factor (F3) is reported in different tumors such as BC and ovarian cancer (Vrana et al. 1996; Cocco et al. 2011). The F3-mediated coagulation cascade pathways play a significant role in BC cancer progression and metastasis (Ueno et al. 2000). In our study, up-regulated expression of F3 was identified and survival analysis studies indicating that low expression of F3 was correlated with poor progression. However, the association between F3 and DTX resistance is not reported. To further explore the possibility of these hub genes as potential therapeutic targets, we analyzed the hub gene and chemotherapeutic drug interactions. Gene-drug interaction network may suggest the suitable chemotherapeutic agents required for induction and suppression of those hub gene which are found to be down-regulated and up-regulated, respectively, and responsible for DTX resistance BC. All these targets still need further experimental investigation. Despite this, these studies could provide accurate results based on integrated bioinformatics analysis.
Conclusion
In the present study, we analyzed a dataset of microarray profiles from DTX resistance BC cell lines and chemosensitive samples using integrated bioinformatics tools. In summary, a total of 222 DEGs, including 120 up-regulated DEGs and 102 down-regulated DEGs in DTX-resistant BC, were screened out through the GEO2R tool, and 9 hub-genes namely, CXCL8, ICAM1, F3, TIMP3, SERPINA1, PLAT, THBD, CYR61, and PECAM1 may play a crucial role in BC progression, metastasis, and DTX resistance. Our data suggest that these 9 genes could be useful to predict the progression survival of BC. Furthermore it was observed from hub-gene-miRNA-TFs interactions network that top 5 miRNAs (hsa-mir-16-5p, hsa-mir-335-5p, hsa-mir-124-3p, hsa-mir-20a-5, and hsa-mir-155-5p) and TFs (SP1, NFKB1, RELA, JUN, and STAT3) simultaneously regulating multiple hub-genes. Targeting these miRNAs and TFs may reverse the problem of DTX resistance in BC. From the gene-drug interaction network, it was identified that CXCL8, ICAM1, TIMP3, SERPINA1, PLAT, and F3 may serve as a potential therapeutic target for DTX-resistant BC, which remains to be validated by further experimental studies.
Data availability
Data could be provided as per request.
References
Brown I, Shalli K, McDonald SL, Moir SE, Hutcheon AW, Heys SD, Schofield AC (2004) Reduced expression of p27 is a novel mechanism of docetaxel resistance in breast cancer cells. Breast Cancer Res 6(5):R601. https://doi.org/10.1186/bcr918
Chan HJ, Li H, Liu Z, Yuan Y-C, Mortimer J, Chen S (2015) SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget 6(28):25815. https://doi.org/10.18632/oncotarget.4441
Cocco E, Varughese J, Buza N, Bellone S, Lin K-Y, Bellone M, Todeschini P, Silasi D-A, Azodi M, Schwartz PE (2011) Tissue factor expression in ovarian cancer: implications for immunotherapy with hI-con1, a factor VII-IgGF c chimeric protein targeting tissue factor. Clin Exp Metastasis 28(7):689–700. https://doi.org/10.1007/s10585-011-9401-0
Corte MD, Vérez P, Rodríguez JC, Roibás A, Domínguez ML, Lamelas ML, Vázquez J, Muñiz JLG, Allende MT, González LO (2005) Tissue-type plasminogen activator (tPA) in breast cancer: relationship with clinicopathological parameters and prognostic significance. Breast Cancer Res Treat 90(1):33–40. https://doi.org/10.1007/s10549-004-2624-x
Das AM, Bolkestein M, van der Klok T, Ophuis CMO, Vermeulen CE, Rens JA, Dinjens WN, Atmodimedjo PN, Verhoef C, Koljenović S (2016) Tissue inhibitor of metalloproteinase-3 (TIMP3) expression decreases during melanoma progression and inhibits melanoma cell migration. Eur J Cancer 66:34–46. https://doi.org/10.1016/j.ejca.2016.06.020
de Groote ML, Kazemier HG, Huisman C, van der Gun BT, Faas MM, Rots MG (2014) Upregulation of endogenous ICAM-1 reduces ovarian cancer cell growth in the absence of immune cells. Int J Cancer 134(2):280–290. https://doi.org/10.1002/ijc.28375
Egawa C, Miyoshi Y, Takamura Y, Taguchi T, Tamaki Y, Noguchi S (2001) Decreased expression of BRCA2 mRNA predicts favorable response to docetaxel in breast cancer. Int J Cancer 95(4):255–259. https://doi.org/10.1002/1097-0215(20010720)95:4%3c255::aid-ijc1043%3e3.0.co;2-o
Feng H, Cheung AN, Xue W-C, Wang Y, Wang X, Fu S, Wang Q, Ngan HY, Tsao S-W (2004) Down-regulation and promoter methylation of tissue inhibitor of metalloproteinase 3 in choriocarcinoma. Gynecol Oncol 94(2):375–382. https://doi.org/10.1016/j.ygyno.2004.04.019
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh J-WW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403. https://doi.org/10.1016/j.ejca.2012.12.027
Geng W, Song H, Zhao Q, Dong K, Pu Q, Gao H, Lv Y (2020) miR-520h stimulates drug resistance to paclitaxel by targeting the OTUD3-PTEN axis in breast cancer. Biomed Res Int. https://doi.org/10.1155/2020/9512793
Han X-g, Mo H-m, Liu X-q, Li Y, Du L, Qiao H, Fan Q-m, Zhao J, Zhang S-h, Tang T-t (2018) TIMP3 overexpression improves the sensitivity of osteosarcoma to cisplatin by reducing IL-6 production. Front Genet 9:135. https://doi.org/10.3389/fgene.2018.00135
Hedges JC, Singer CA, Gerthoffer WT (2000) Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol 23(1):86–94. https://doi.org/10.1165/ajrcmb.23.1.4014
Hu Q, Chen W-x, Zhong S-l, Zhang J-y, Ma T-f, Ji H, Lv M-m, Tang J-h, Zhao J-h (2014) MicroRNA-452 contributes to the docetaxel resistance of breast cancer cells. Tumor Biol 35(7):6327–6334. https://doi.org/10.1007/s13277-014-1834-z
Huang P, Li F, Li L, You Y, Luo S, Dong Z, Gao Q, Wu S, Brünner N, Stenvang J (2018) lncRNA profile study reveals the mRNAs and lncRNAs associated with docetaxel resistance in breast cancer cells. Sci Rep 8(1):1–15. https://doi.org/10.1038/s41598-018-36231-4
Kastl L, Brown I, Schofield AC (2012) miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res Treat 131(2):445–454. https://doi.org/10.1007/s10549-011-1424-3
Kwon C, Park H, Lee J, Kim H, Jeon T, Jo H, Kim D, Kim G, Park D (2014) Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. Br J Cancer 111(10):1993–2002. https://doi.org/10.1038/bjc.2014.490
Leonard GD, Fojo T, Bates SE (2003) The role of ABC transporters in clinical practice. Oncologist 8(5):411–424. https://doi.org/10.1634/theoncologist.8-5-411
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20. https://doi.org/10.1016/j.cell.2004.12.035
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K (2016) The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Fact Rev 31:61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002
Masson D, Rioux-Leclercq N, Fergelot P, Jouan F, Mottier S, Théoleyre S, Bach-Ngohou K, Patard J-J, Denis MG (2010) Loss of expression of TIMP3 in clear cell renal cell carcinoma. Eur J Cancer 46(8):1430–1437. https://doi.org/10.1016/j.ejca.2010.01.009
Mattingly CJ, Colby GT, Forrest JN, Boyer JL (2003) The comparative toxicogenomics database (CTD). Environ Health Perspect 111(6):793–795
Miyoshi Y, Ando A, Takamura Y, Taguchi T, Tamaki Y, Noguchi S (2002) Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int J Cancer 97(1):129–132. https://doi.org/10.1002/ijc.1568
Organization WH (2016) World health statistics 2016: monitoring health for the SDGs sustainable development goals. World Health Organization
Rivoltini L, Cattoretti G, Arienti F, Mastroianni A, Melani C, Colombo MP, Parmiani G (1991) The high lysability by lak cells of colon-carcinoma cells resistant to doxorubicin is associated with a high expression of ICAM-1, LFA-3, NCA and a less-differentiated phenotype. Int J Cancer 47(5):746–754. https://doi.org/10.1002/ijc.2910470521
Schröder C, Witzel I, Müller V, Krenkel S, Wirtz RM, Jänicke F, Schumacher U, Milde-Langosch K (2011) Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin 137(8):1193–1201. https://doi.org/10.3389/fimmu.2023.1176647
Shi Z, Yang W-M, Chen L-P, Yang D-H, Zhou Q, Zhu J, Chen J-J, Huang R-C, Chen Z-S, Huang R-P (2012) Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135(3):737–747. https://doi.org/10.1007/s10549-012-2196-0
Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X (2010) MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res 29(1):1–8. https://doi.org/10.1186/1756-9966-29-29
Tsai S-T, Wang P-J, Liou N-J, Lin P-S, Chen C-H, Chang W-C (2015) ICAM1 is a potential cancer stem cell marker of esophageal squamous cell carcinoma. PLoS One 10(11):e0142834. https://doi.org/10.1371/journal.pone.0142834
Ueno T, Toi M, Koike M, Nakamura S, Tominaga T (2000) Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer 83(2):164–170. https://doi.org/10.1054/bjoc.2000.1272
Vrana JA, Stang MT, Grande JP, Getz MJ (1996) Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cellderived members of the transforming growth factor β family. Cancer Res 56(21):5063–5070
Wang X, Pei X, Guo G, Qian X, Dou D, Zhang Z, Xu X, Duan X (2020) Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J Cell Physiol. https://doi.org/10.1002/jcp.29585
Wolff B, Burns AR, Middleton J, Rot A (1998) Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med 188(9):1757–1762. https://doi.org/10.1084/jem.188.9.1757
Wu W, Wang Q, Yin F, Yang Z, Zhang W, Gabra H, Li L (2016) Identification of proteomic and metabolic signatures associated with chemoresistance of human epithelial ovarian cancer. Int J Oncol 49(4):1651–1665. https://doi.org/10.3892/ijo.2016.3652
Yang Y, Yee D (2012) Targeting insulin and insulin-like growth factor signaling in breast cancer. J Mammary Gland Biol Neoplasia 17:251–261. https://doi.org/10.1007/s10911-012-9268-y
Zhang Y, Wang Y, Wei Y, Li M, Yu S, Ye M, Zhang H, Chen S, Liu W, Zhang J (2015) MiR-129-3p promotes docetaxel resistance of breast cancer cells via CP110 inhibition. Sci Rep 5:15424. https://doi.org/10.1038/srep15424
Zhang X, Zhong S, Xu Y, Yu D, Ma T, Chen L, Zhao Y, Chen X, Yang S, Wu Y (2016) MicroRNA-3646 contributes to docetaxel resistance in human breast cancer cells by GSK-3β/β-catenin signaling pathway. PLoS One 11(4):e0153194. https://doi.org/10.1371/journal.pone.0153194
Zhang C, Wang J, Zhang J, Qu H, Tang X (2020) LINC00461 overexpression can induce docetaxel resistance in breast cancer by interacting with miR-411-5p. Onco Targets Ther 13:5551–5562. https://doi.org/10.2147/OTT.S247776
Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, Wang Z, Liu Z, Li H, He J (2013) PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 27(3):702–710. https://doi.org/10.1038/leu.2012.272
Acknowledgements
Baddipadige Raju and Gera Narendra would like to acknowledge the Indian Council of Medical Research (ICMR), New Delhi for providing a Senior Research Fellowship (SRF).
Funding
This work was supported by the Indian Council of Medical Research (ICMR), New Delhi; Sanction No. ISRM/12(10)/2019.
Author information
Authors and Affiliations
Contributions
Baddipadige Raju: Concept, Designing of Work, Original Manuscript Writing, Editing, Software usage, Analysis, and Interpretation of Data. Gera Narendra: Designing of work, Software usage, and Data interpretation. Himanshu Verma: Data interpretation, Manuscript Editing. Om Silakari: Designing of work, Editing, and Proofreading.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared no conflict of interest.
Ethical statement
This article does not contain any studies with human participants performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raju, B., Narendra, G., Verma, H. et al. Identification of chemoresistance associated key genes-miRNAs-TFs in docetaxel resistant breast cancer by bioinformatics analysis. 3 Biotech 14, 128 (2024). https://doi.org/10.1007/s13205-024-03971-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-03971-2