Abstract
The difficulty of releasing nutrients from soils in karst areas limits the yield of local crops and leads to poverty. In this study, two strains of plant growth-promoting rhizobacteria (PGPR) were isolated from the rhizosphere soil of typical plants in karst areas, which were both identified as Bacillus sp. and named GS1 and N1. And two isolates were used to construct a composite PGPR named MC1. These three strains of PGPR were used for soil inoculation in the pot experiment and field trial and their capacity to promote rice development was assessed. The results showed that MC1 inoculation exhibited notable rice growth-promoting ability in pot experiments, and, respectively, had an increment of 16.96, 18.74, and 11.50% in shoot biomass, total biomass, and rice height compared with control. This is largely attributed to PGPR’s capacity to secrete phytohormones and soil enzymes, particularly urease (UE) in GS1, whose secreted UE content was significantly higher by 12.18% compared to the control. When applied to the field, MC1 inoculation not only increased rice yield by 8.52% and the available nutrient content in rice rhizosphere soil, such as available phosphorus (AP) and exchangeable magnesium (EMg); but also improved the abundance of beneficial rhizobacteria and the diversity of microbial communities in rice rhizosphere soil. Results in this study revealed that inoculated PGPR played a major role in promoting rice growth and development, and a new strategy for facilitating the growth of rice crops in agriculture was elucidated.
Article Highlights
-
Two stains of Bacillus sp. isolated from the rhizosphere soil of typical plants in karst areas could promote rice growth and yield.
-
PGPR exhibited the great potential of secreting phytohormones and soil enzymes.
-
PGPR released the available nutrient for rice plants and alleviated the imbalance of nutrients in karst soils.
-
Co-inoculation of PGPR could be a promising approach to improving crop yield and applied to agriculture production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst ecosystems currently cover 15% of the total global land area and are delicate ecosystems governed by soluble rock environments (Yuan 2001). Due to the high concentration of calcium ions and the resulting high and stable amounts of humic acid in karst soil, they are alkaline and calcium-rich. This leads to a slower rate of nutrient supplementation (Wang 1998), and along with the accumulation of calcium and magnesium, it exacerbates the trace element content imbalance, which greatly hinders the growth of crops in this area (Xu et al. 2000). Therefore, to improve crop yields in agricultural production, farmers increase the dosage of chemical fertilizers and pesticides, leading to problems such as soil structure damage and pollution. Over the past few years, this method has been gradually replaced by the use of plant growth-promoting rhizobacteria (PGPR) as a biofertilizer.
The rhizosphere is the microenvironment most strongly influenced by plants and is where soil–plant interactions occur (Venturi and Keel 2016; Jones and Hinsinger 2008). Plant growth-promoting rhizobacteria (PGPR) are present in such particular microcosms, which play a key role in plant growth by altering the rhizosphere soil environment, promoting nutrient uptake by plants, and defending against pathogenic bacteria (Shi et al. 2016). Typically, host plants affect the microbial community structure through root exudates and supply PGPR with nutrients via the root system (Angers and Caron 1998). In return, PGPR protects host plants from pathogens by secreting phytohormones (e.g., indole acetic acid) and siderophores or by improving the environment of rhizosphere soils (Berendsen et al. 2012), which enhances plant disease resistance and abiotic stress tolerance (e.g., high temperature, high salt, drought) (Ouhaddou et al. 2022; Khan 2022; Jiménez-Mejía et al. 2022).
Nutrient supply and phytohormone secretion are direct ways by which PGPR promotes plant growth (Ahmed and Hasnain 2014; Orozco-Mosqueda et al. 2019). They provide available nutrients to plants through the ability of PGPR to solubilize phosphorus and potassium (Naseer et al. 2020; Granada et al. 2018). For example, three previously studied PGPR capable of dissolving phosphorus and secreting phytohormones have been shown to increase rice plant height, root length, and yield in pot experiments (Liu et al. 2022). In another study, a novel strain of native rhizobacteria, Flavobacterium pokkalii, was isolated from pokkali rice planted in a coastal saline field and was found to promote rice growth under salt stress (Menon et al. 2020). Additionally, the microbial community structure in rhizosphere soil is susceptible to being influenced by the physicochemical properties of the soil (Xue et al. 2018; Shi et al. 2016). Conversely, changes in the soil environment caused by PGPR can indirectly promote plant growth, such as an increase in the nutrient content and the number and species of beneficial rhizobacteria in the soil (Nihorimbere et al. 2011). This is because nutrients such as N and P are required for the production of proteins, cell division, and metabolism (Bakhshandeh et al. 2020). Therefore, PGPR is considered an essential tool for bolstering eco-friendly crop productivity enhancement (Trivedi et al. 2020; Haney et al. 2015; Nihorimbere et al. 2011; Yang et al. 2009).
Rice is an important cereal crop, as it produces the third-largest amount of food in the world. (Bakhshandeh et al. 2017). The ecological benefits of plant inter-root microorganisms have gained attention in recent years, with many studies having applied PGPR as natural agricultural biofertilizers to promote the growth of rice with desirable results (Singh et al. 2016; Banik et al. 2019; Bakhshandeh et al. 2020). However, relatively few studies have investigated the growth promotion effect of PGPR isolated from karst areas on local crops. and little attention has been paid to the effect of such biofertilizers on crop yield improvement in karst areas.
In the current work, the rhizosphere soil of typical plants in the karst area of the Huixian wetland was collected to isolate plant growth-promoting rhizobacteria (PGPR), and a composite rhizobacterium (MC1) was constructed for application in pot and field experiments to explore the effects of PGPR on rice growth performance. The effect of PGPR on rice yield and soil nutrient levels was further conducted in the field, where the composition and structure of soil microbial communities in the rice rhizosphere were analyzed.
Materials and methods
Description of the sampling area and soil collection
The Huixian Wetland is the world's and China's most representative mid- and low-altitude karst wetland ecosystem. The location of this region, which has a subtropical monsoon climate, is located at longitude 110°08′15″-110°18′00″ E, latitude 25°01′30″-25°11′15″ N (Cai 2012).
Three typical plants (Avena fatua, Arundo donax, and Echinochloa crusgalli) were selected from Huixian Wetland (latitude 25°06′10″ N, longitude 110°13′7″ E) to collect their rhizosphere soil (Cai 2012), which was then used for subsequent rhizosphere microbial isolation. Three of the above plants were looked for and dug up at each sampling point (Fig. 1), and their rhizosphere soil was scraped with a sterilized scalpel. Each sample was sampled three times in parallel. Soil samples were collected in sterile centrifuge tubes, brought back to the lab at low temperatures, and mixed in equal amounts for screening and separating of PGPR. The paddy soil used in the pot experiment was collected from a 0–10 cm soil layer in the field.
Isolation and rhizobacterial preparation
Rhizobacteria were isolated from the homogeneous rhizosphere soil of three typical plants. One gram of soil samples was weighed and deposited in a flask containing 100 mL of sterile enrichment medium with glass beads, then placed in a shaker at 35 °C, 120 rpm for 3 days. The enriched suspension was serially diluted up to 10−3–10−8, and 0.2 mL of culture was plated on the solid medium, then placed and incubated for 3 days in a 35℃ incubator. Colonies with different morphologies were selected from the plates and streaked on fresh solid medium until single and pure colonies were obtained. These strains were stored in glycerol at − 80 °C for use (Majumder et al. 2013). Isolates were evaluated by plant growth-promoting tests, and then two best-performing isolates were screened as effective growth-promoting rhizobacteria (PGPR), which were named GS1 and N1, respectively.
A composite PGPR recorded as MC1 was constructed by GS1 and N1 to confirm the potential for promoting rice growth. The process was as follows: the cultures of GS1 and N1 were inoculated with 1% sterilized beef paste peptone liquid medium and incubated in a shaker for 24 h at 35℃, 160 rpm. Then the culture was centrifuged for 10 min at 8000 rpm, and the bacteria were resuspended three times with sterilized saline. The OD600 of the suspension was adjusted to 1, i.e., the total effective live bacteria count was 9 × 108 CFU mL−1 (Chen et al. 2010), stored in glycerol at − 80 °C for use.
Identification of isolates
DNA was extracted with the genomic DNA extraction kit (Sangon Biotech Co., Ltd., China). The 16S rDNA sequencing was performed by Sangon Biotech. The 16S rDNA gene of bacterial identification was amplified using the primers (341F: 5’-CCTACGGGNGGCWGCAG-3’ and 805R: 5’-GACTACHVGGGTATCTAATCC-3’). Then the polymerase chain reaction (PCR) and gel electrophoresis on a 1% agarose gel were performed. Sequence similarity was compared with the reference species of bacteria in GeneBank using NCBI BLAST. MEGA 5.0 and the adjacency algorithm were used for the phylogenetic analysis (Thulasi et al. 2018).
Assessment of plant growth-promoting ability
One gram of fresh soil was collected and homogenized by adding 9 mL of phosphate buffer saline solution (pH = 7.2–7.4, c = 0.01 mol L−1), followed by centrifugation at 5000 rpm for 15 min, and the supernatant was taken for measurement. Then the content of acid phosphatase (ACP) and urease (UE) in the soil was determined using an enzyme-linked immunosorbent assay. Indole acetic acid (IAA), gibberellin (GA), cytokinin (CTK), and ACC deaminase (ACCD) levels were also determined using an enzyme-linked immunosorbent assay. The assay was performed according to the instructions. The absorbance was determined with an enzyme-labeled instrument at 450 nm, and the content of each soil enzyme and phytohormone was calculated by standard curve.
Germination of seeds
Rice seeds were placed outdoors for sun exposure, then placed in sterile flasks containing water. The seed sterilization method was performed according to Marques et al. (2013): first sterilized for 30 s with 75% anhydrous ethanol, then three times with 2.5% sodium hypochlorite for 15 min, and finally three times with sterile water. Seeds were soaked in sterilized petri dishes with 0.5 mM CaCl2 solution, and placed in a sterile incubator at 26 °C. Soak for 48 h at 8 h/16 h dark/light conditions by changing the CaCl2 solution every 6 h, and the temperature of the incubator was raised to 35 °C for 24 h after that. When the seeds had just germinated, adjusted the incubator temperature back to 26 °C for growth. Finally, seeds with consistent growth (1 cm of shoot length, 2 cm of root length) were selected for subsequent experiments. The rice seed variety used for the experiment was South Japonica 9108, which was purchased from Jiangsu Hi-Tech Seed Technology Co.
Pot experiments
The nursery pots used in this experiment were 15 cm in diameter and 20 cm in height, with no light penetration around or at the bottom. One germinated seed was planted in each nursery container, which contained 1000 g of air-dried and previously ground paddy soil. The pot experiment was conducted with four treatments: (1) Non-inoculation: Sterile water was added to the nursery pots regularly to keep the soil moisture consistent with other groups, recorded as the CK group; (2) GS1-inoculum: a single GS1 strain was inoculated into pots, recorded as the GS1 group; (3) N1-inoculum: a single N1 strain was inoculated into pots, recorded as the N1 group: (4) MC1-inoculum: the composite strain was inoculated into pots, recorded as the MC1 group. The amount of rhizobacterial culture added remained consistent at 100 mL. Fifty rice plants were cultivated in each group, and all pots were placed in a greenhouse maintained at 25–28 °C with a photoperiod of 12 h and humidity of 70–80%. All treatments were replicated three times.
Ten rice plants were harvested from each group on days 25, 40, 55, and 70. The height of the rice was measured with a tape measure (vertical distance from the rice rootstock to the top of the main stem), followed by drying the samples in an oven at 80 °C for 48 h, and then parameters such as shoot, root, and total biomass, and height of plants were measured to evaluate the growth of the affected rice.
Field experiments
The field trial site was located in Fengjia village, Guilin, China. Randomly selected two sample plots, marked SC and DC, respectively, whose soil was collected to determine the original physicochemical properties. After planting rice, plot SC and DC were respectively marked as plot SY (inoculated with MC1) and DY (watered as a control). Then the soil was tilled before sowing, mixed, spread evenly, and irrigated to provide sufficient moisture in each plot. After that, the inoculum plot-SY and the control plot-DY were respectively divided into S1, S2, S3 and D1, D2, D3. The area of each plot was about 225 m2 and separated by a certain distance. Three inoculum plots (S1, S2, and S3) were inoculated with composite MC1, and three control plots (D1, D2, and D3) were watered only as controls.
Seeds with the same root and shoot lengths were selected for sowing, as described above. Each plot kept the same sowing density. After the rice was grown for 15 days, inoculum plots (S1, S2, and S3) were evenly inoculated with bacterial culture at a ratio of inoculum to the soil of 1:50 (v/v). The entire field trial began in April and ended in August 2020. Rice was harvested at maturity to measure the yield, and the collected rice plants were washed, placed in an oven at 80 ℃ for 48 h, and then milled to a fine powder for analysis of nutrients in plants. The rice rhizosphere soil was collected to determine its physicochemical properties.
Determination of physicochemical properties
The physicochemical properties of rhizosphere soil were analyzed after rice plants were collected, air-dried, ground, and sieved. Five plants were randomly selected from each group. The potentiometry method was used to determine the pH of the soil. Total nitrogen (TN) was measured by the Kjeldahl method and the alkaline decomposition-diffusion method according to GB 7173-1987. Total phosphorus (TP) and potassium (TK) were determined by the alkali fusion-molybdenum antimony anti-spectrophotometric method according to GB/T13140-2015 and GB9836-88, respectively. The available nitrogen (AN) and phosphorus (AP) were determined according to HJ 704-2014. Total calcium (TCa) and magnesium (TMg) were measured by following NY/T 1121.13-2006, and exchangeable calcium (ECa) and magnesium (EMg) were determined by the same method.
DNA extraction, PCR amplification, and sequencing
The rice rhizosphere soils of SY, DY, SC, DC, and background soil-BC in the field were collected for macrogenome sequencing. SY and DY soil samples were collected before rice planting, and the BC soil sample was the soil that had not been planted with rice. Follow the manufacturer's instructions to extract DNA from soil samples using a DNeasy PowerSoil Pro Kit (Qiagen, Germany). Then, the following steps were completed by Sangon Biotech Co., Ltd. (Shang, China): the concentration of the DNA was evaluated by Fluorometer Nucleic Acid Quantifier (Thermo Fisher Scientific, USA), and the integrity and purity of the DNA were examined using 1% agarose gel electrophoresis (200 V, 30 min). The V3-V4 region of the 16S rRNA gene was amplified using the primers (341F: 5’-CCTACGGGNGGCWGCAG-3’ and 805R: 5’-GACTACHVGGGTATCTAATCC-3’). The sequencing library was created by the NEBNext Ultra DNA Library Prep Kit for Illumina (NEB, USA) according to the manufacturer's instructions and quantified by the Agilent 2100 Bioanalyzer system. The quantified library was sequenced on the Novaseq 6000 sequencing platform (Illumina, USA).
Statistical analysis
Data are expressed as means ± standard deviation. Excel 2010 and SPSS (version 25.0) statistical software were used for statistical analysis [analysis of variance (ANOVA)]. One-Way ANOVA was used to determine the experimental error and the effect of different treatments. The Duncan test was used for measuring the significant difference between different groups at the 5% level of significance.
Results
Isolated strains
Two strains of plant growth-promoting rhizobacteria (PGPR) were isolated from the rhizosphere soil of three typical plants (Avena fatua, Arundo donax, and Echinochloa crusgalli) in karst areas, which were recorded as GS1 and N1, respectively. Through 16S rDNA gene sequencing of rhizobacterial isolates GS1 and N1, the two strains both exhibited 100.00% sequence similarity with Bacillus sp. strain 6063 (GeneBank Accession No. MT393628.1) and Bacillus safensis strain 25 (GeneBank Accession No. KY020051.1), respectively. Therefore, the isolates were respectively identified as Bacillus sp. strain 6063 (GS1) and Bacillus safensis strain 25 (N1).
As seen in Table 1, all the PGPR had four plant growth-promoting traits, and the ability to secrete phytohormones was sorted as follows: MC1 > N1 > GS1. The yield of ACCD increased the most, followed by IAA, the yield of the two in MC1 increased by 18.81–20.40% and 12.73–14.91% compared to that in GS1 and N1, respectively. The GA and CTK also had a slight increase in production, with their production in MC1 increasing by 10.54–11.98% and 9.47–10.21% compared to GS1 and N1, respectively. It is noteworthy that the combination of two rhizobacteria strengthened phytohormones’ synthesis potential, suggesting that MC1 has the potential to be a PGPR for promoting plant growth. Therefore, based on their good performance on the secretion of phytohormone, two isolates (GS1 and N1) were used to construct a composite PGPR and recorded as MC1 to apply to subsequent experiments.
Effects of PGPR on rice growth-promoting in pots
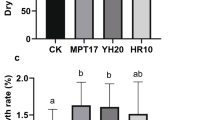
As illustrated in Fig. 2, compared with the control group, three PGPR groups exhibited different promoting effects on the rice shoot, root, and total biomass, and height. And the most significant growth effect was found in the treatment of MC1. On day 70, the shoot biomass of GS1, N1, and MC1 (Fig. 2a) reached a maximum of 1.22 ± 0.045, 1.17 ± 0.055, and 1.31 ± 0.070 g, respectively, compared with the CK group (1.12 ± 0.050 g), with an increment of 8.93, 4.46, and 16.96%. The growth of total biomass in each group showed the same trend as shoot biomass, on day 70, GS1, N1, and MC1 respectively increased by 8.35, 7.05, and 18.74% compared to the CK group (Fig. 2c). The root biomass in GS1, N1, and MC1 (Fig. 2b) was respectively up to 1.95 ± 0.045, 1.92 ± 0.10, and 2.13 ± 0.065 g after 70 days, which respectively increased by 4.85, 9.22, and 19.90% compared with the CK group (1.80 ± 0.010 g). The results revealed that inoculation with MC1 had the greatest impact on rice biomass.
The effects of different treatments on rice growth on a shoot biomass; b root biomass; c total biomass; d rice height. CK is the non-inoculation group; GS1 is the group inoculated with a single strain of GS1; N1 is the group inoculated with a single strain of N1; MC1 is the group inoculated with a composite strain of MC1. Values are mean ± standard deviation. Different letters indicate the treatments have significantly different effects (P < 0.05)
In the growth trend of plant height (Fig. 2d), on day 25, there was little difference among the four groups, and then the growth rate of each group increased over time. On day 70, the average plant height in the MC1 group was the highest, reaching 73.53 ± 0.31 cm, which was notably taller than GS1, N1, and CK with 71.53 ± 0.25, 68.50 ± 0.30, and 65.93 ± 0.61 cm, respectively, with an increment of 11.50% compared to the CK group. And the total growth rate of each group (CK, GS1, N1, and MC1 group) was 0.94, 1.02, 0.97, and 1.05 cm d−1, respectively, demonstrating that MC1 notably promoted rice growth not only in biomass but also in plant height.
Effects of PGPR on the rice rhizosphere soil
In the pot experiment, rice was cultivated for 70 days under different treatments, and the groups that were inoculated with PGPR exhibited better acid phosphatase (ACP) and urease (UE) secretion abilities. The results in Table 2 showed that the content of ACP and UE in rice rhizosphere soil varied with the different inoculated strains. The ACP level in the rice rhizosphere soils of GS1, N1, and MC1 reached 7.56 ± 0.17, 7.21 ± 0.20, and 7.94 ± 0.21 IU g−1, respectively. The MC1 group exhibited the most significant increase in secretory capacity, which was 18.83% compared with the CK group. The UE content in the rhizosphere soils of GS1, N1, and MC1 was increased by 12.18, 8.86, and 8.05%, respectively.
As shown in Fig. 3a, the AP content of rhizosphere soil in the GS1 group was relatively constant during rice cultivation. After 70 days, the AP content in GS1 was still significantly higher than that of CK, and the content was respectively 8.16 ± 0.68 and 7.58 ± 0.63 mg kg−1. During cultivation, rice rhizosphere soil in GS1 exhibited the highest level of AN. On day 70, the AN content in the CK, GS1, N1, and MC1 groups reached 91.28 ± 5.29, 189.93 ± 6.95, 94.28 ± 5.60, and 98.69 ± 7.73 mg kg−1 (Fig. 3b). The AN content in the GS1 group increased by about two times compared to the CK group, showing that PGPR inoculation greatly enhanced the level of AN in the rice rhizosphere soil, which was largely attributed to the ability of PGPR to secrete soil enzymes (e.g., UE and ACP).
Effects of different PGPR on nutrient contents in rice rhizosphere soil in pot experiments. a Available phosphorus; b Available nitrogen. CK is the non-inoculation group; GS1 is the group inoculated with a single strain of GS1; N1 is the group inoculated with a single strain of N1; MC1 is the group inoculated with a composite strain of MC1. Values are mean ± standard deviation. Different letters indicate the treatments have significantly different effects (P < 0.05)
Field experiments
The inoculum plot-SY was divided into S1, S2, and S3, and the control plot-DY was divided into D1, D2, and D3 in the field. MC1 inoculation was performed at plots S1, S2, and S3. Plots SC and DC were respectively represented as the soil plot before planting rice in SY and DY, and the soil of plots SC and DC was sampled for monitoring the original soil parameters before the trial. As illustrated in Table 3, the rice yield of plots S1, S2, and S3 was 208, 213, and 216 kg, respectively, which were all higher than the control plots (195, 199, and 193 kg), with an average increase of 8.52%. This indicated that the application of PGPR to the field could also promote rice growth and increase rice yield.
In Fig. 4a, it is worth noting that the level of total phosphorus (TP) in the inoculum plot-SY was notably higher than that in the control plot-DY, and the contents of the plots were 2.33 ± 0.04 and 2.04 ± 0.03 g kg−1, respectively. As illustrated in Fig. 4b, the AP concentration in plot-SY was approximately 1.15 times that of plot-DY, and the contents were, respectively, 29.62 ± 1.38 and 13.78 ± 1.05 mg kg−1. Compared with the original plot-SC, the AP content in the soil of plot SY was increased by two times, while the AP level in plot DY was only 0.75 times that of plot DC (Table S1), which further proved that PGPR has a certain phosphorus-soluble ability when applied to the field. Moreover, MC1 inoculation also promoted the uptake of Mg, whose content increased by 17.07% (Fig. 4a). In plot SY, the level of total calcium (TCa) was lower than the original plot SC, while plot DY exhibited the opposite trend, and the exchangeable calcium (ECa) content in the plot SY increased by 11.76% compared with the original soil SC. This indicated that PGPR could alleviate the severity of calcium imbalances in karst soil.
a Nutrient content in rice crops; b Physicochemical properties of rice rhizosphere soil in field experiments. SY is the MC1-inoculum plot; DY is the uninoculated plot; SC stands for the original plot before SY planted rice; DC stands for the original plot before DY planted rice. Values are mean ± standard deviation. Different letters indicate the treatments have significantly different effects (P < 0.05)
The microbial community of rice rhizosphere soil
The results in Fig. 5 showed that the Shannon index in the inoculum plot-SY (12.71) was higher than not only the control plot-DY (12.64) but also the original plot-SC (12.50), suggesting that the introduction of PGPR would enhance the diversity of soil microbial communities in the rice rhizosphere. On the other hand, the average Simpson index of plot SY (4.33e-06) was lower than that of plot DY (5.00e-06) and plot SC (6.0e-06), which evidenced that the soil inoculated with PGPR had an even larger microbial community (Fig. 5). In Fig. 6a, the principle coordinate analysis (PCoA) of the rhizosphere microbial community structure data showed that there was a slight difference between the microbial communities in plot SY (S1-S3) and DY (D1-D3). However, the difference was much smaller than that in the original plot (SC and DC) and the background plot (BC).
The boxplots of alpha diversity index of rhizosphere soil microbial communities in each plot. a the Shannon index; b the Simpson index. SY is the MC1-inoculum plot; DY is the uninoculated plot; SC is the original plot before SY planted rice; DC is the original plot before DY planted rice; BC is the plot that has not been planted with rice
a Principal coordinate analysis of microbial community structure data in the rhizosphere soil. b Relative abundance of rhizosphere soil microbial communities of different samples at the phylum level. c Heatmap of relative abundance at the genus level. S1-S3 are the MC1-inoculum plots; D1-D3 are the uninoculated plots; SC is the original plot before SY planted rice; DC is the original plot before DY planted rice; BC is the plot that has not been planted with rice. Values of *P < 0.05 was considered to represent statistically significant differences
The Proteobacteria, Acidobacterium, and Gemmatimonadetes phyla were the three dominant phyla in the rice rhizosphere soil, as illustrated in Fig. 6b. The average abundance of the Proteobacteria and Firmicutes phylum in plot SY (51.93%, 3.66%) significantly increased compared to plot DY (48.42%, 2.19%) after inoculating PGPR, and these two are beneficial microorganisms in the plant rhizosphere. The top 50 genera in all soil samples were selected to further study the effect of PGPR on the rhizosphere microbial community of rice at the genus level. The heat map (Fig. 6c) showed plot SY had a much greater abundance of Bacillus sp. than plot DY, and since Bacillus are important for pathogen control and plant growth. Therefore, PGPR inoculation contributes to the increase in species abundance, thereby boosting rice growth and production.
Discussion
Over the past several years, the link between plants and rhizobacteria has received attention (Philippot et al. 2013; Cordovez et al. 2019; Fields and Friman 2022). But to our knowledge, few studies have applied PGPR to karst areas to promote crop growth, though many researchers have demonstrated that inoculation with PGPR significantly facilitates rice growth both in greenhouses and outdoors (Liu et al. 2022; Bakhshandeh et al. 2020). The findings in this study demonstrated that PGPR inoculation boosted rice growth in simulation pots and fields by enhancing crop biomass, height, and yield. These positive impacts were due to the phytohormones (IAA, GA, CTK, and ACCD) and effective soil enzymes (ACP and UE) secreted by PGPR on the one hand, which are indispensable and one of the pathways through which PGPR directly promotes the growth of crops (Bakhshandeh et al. 2020). On the other hand, the PGPR inoculation improved the microbial community structure in the plant rhizosphere environment.
The isolated strains in this study were both identified as Bacillus sp., which is a beneficial genus that releases helpful substances, such as siderophores and phytohormones, to promote crop growth through more than 20 mechanisms (Kim et al. 2018; Lyu et al. 2022). For example, Liu et al. (2022) isolated three strains of Bacillus sp. from the soil of a farm, which displayed the great capability of secreting IAA (55.66–75.89 mg L−1), GA (16.33–23.58 mg L−1), and siderophores (38.77–52.88%) to promote the shoot and root of rice seedlings in the pot experiment. In this research, the results in pot experiments demonstrated that PGPR inoculation greatly boosts rice growth compared to the control, especially when inoculated with MC1, as evidenced by the increase in shoot and total biomass and rice plant height (Fig. 2). This was not only attributed to the potential of isolated strains (GS1 and N1) to synthesize phytohormones but also due to the enhanced secretory ability of the two isolates when they were co-inoculated, as the results in Table 1 showed. This result is consistent with that achieved by Bakhshandeh et al. (2020), who demonstrated that PGPR can increase the total biomass, the uptake and utilization efficiency of P and K in rice, especially under P. ananatis and P. indica co-inoculation.
Additionally, AP and AN were essential nutrients during plant growth, thereby the ability of PGPR to secrete soil acid phosphatase (ACP) and urease (UE) was a factor to be reckoned with. The increase in ACP and UE content in the rhizosphere soil after inoculation with PGPR led to an enhancement in AP and AN content, which was also one of the factors promoting rice growth (Table 2, Fig. 3). As the results of Yu et al. (2019) showed, an isolated strain of Bacillus megaterium YLYP1 could effectively solubilize tricalcium phosphate and produce 716 mg L−1 AP in 6 days, showing strong potential for application in agriculture to limit the demand for chemical fertilizers. In this study, it was found that PGPR inoculation not only enhanced the uptake of P and Mg by rice crops but also alleviated the calcium-rich nature of karst soils to some extent in field experiments.
Given the complex mechanisms by which PGPR promotes rice growth and the way rhizobacteria alter their specializations as the soil environment changes (Lyu et al. 2022; Abbasi et al. 2011), it is reliable that Bacillus isolated specifically from the rhizosphere soil of typical plants in karst would have better stability and adaptability when applied as PGPR to karst soils. Moreover, previous investigations showed that Bacillus sp. is highly resistant to stress (Naseer et al. 2020; Liu et al. 2022). This explained why PGPR could be stably applied in the field and increase rice yield by 8.52% (Table 3). In addition, the application of PGPR in the field could also stably promote the release of AP and AN and improve the utilization of nutrients by rice plants (Fig. 4). It is noteworthy that the diminution in total calcium (TCa) and the increment in exchangeable calcium (ECa) in the rice rhizosphere soil of plot SY were more significant than those in plot DY, indicating that PGPR inoculation alleviated the calcium-rich nature of karst areas and enhanced the tolerance of rice to abiotic stresses to a certain extent.
Different environments have different impacts on rice growth. Karst soil environments are more extreme and complex than normal ones. Plant growth, nutrient uptake, and the composition of the rhizosphere soil microbial community are closely related to soil physicochemical properties (Angers and Caron 1998; Xue et al. 2018). Therefore, it is necessary to observe the change in the rhizosphere soil microbial community in the local field. Our results showed that PGPR inoculation affected the physicochemical properties of rice rhizosphere soil, further altering the abundance of Proteobacteria, Firmicutes, and Bacteroidetes phyla (Fig. 6b). These three species are beneficial rhizobacteria, which usually promote crop growth by producing phytohormones, inducing crop resistance to stress, and inhibiting pathogenic bacteria (Berendsen et al. 2012; Orozco-Mosqueda et al. 2019; Bakhshandeh et al. 2020). Moreover, according to the Shannon and Simpson index of rhizosphere soil, inoculation with PGPR improved the diversity and evenness of rhizosphere soil. We also found a notable increment in the abundance of Bacillus sp. after PGPR was inoculated (Fig. 6c), suggesting that the inoculated PGPR may be a contributor to the increase in rice yield. Therefore, the results obtained from large field experiments in karst areas are informative.
Conclusion
Our study revealed that two strains of rhizobacteria isolated from the rhizosphere soil of typical plants in karst areas can synthesize phytohormones and raise the content of ACP and UE in rice rhizosphere soil. All PGPR (including composite MC1) increased the biomass and yield of rice crops. The plant-available nutrient content in the rice rhizosphere soil, particularly the AN and AP, increased in both the pot and field experiments This finding suggests that PGPR inoculation encourages the release of nutrients in the rice rhizosphere soil and improves the soil nutrient balance in karst ecosystem areas. Furthermore, PGPR in the field also altered the distribution of microbial communication in the long term, which was verified by the heatmap. The PGPR identified in this study will therefore contribute to an increase in agricultural yield and ecological restoration in karst areas. In our future work, we will concentrate on the impacts of plant rhizosphere secretions on soil microorganisms and plant growth so as to explore the mechanism of plant-soil-microbe interactions in greater depth.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abbasi MK, Sharif S, Kazmi M, Sultan T, Aslam M (2011) Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst 145(1):159–168. https://doi.org/10.1080/11263504.2010.542318
Ahmed A, Hasnain S (2014) Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol J Microbiol 63(3):261–266. https://doi.org/10.33073/pjm-2014-035
Angers DA, Caron J (1998) Plant-induced changes in soil structure: processes and feedbacks. Biogeochemistry 42(1):55–72. https://doi.org/10.1023/A:1005944025343
Bakhshandeh E, Pirdashti H, Lendeh KS (2017) Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol Eng 103:164–169. https://doi.org/10.1016/j.ecoleng.2017.03.008
Bakhshandeh E, Pirdashti H, Shahsavarpour Lendeh K, Gilani Z, Khanghahi MY, Crecchio C (2020) Effects of plant growth promoting microorganisms inoculums on mineral nutrition, growth and productivity of rice (Oryza sativa L.). J Plant Nutr 43(11):1643–1660. https://doi.org/10.1080/01904167.2020.1739297
Banik A, Dash GK, Swain P, Kumar U, Mukhopadhyay SK, Dangar TK (2019) Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition. Microbiol Res 219:56–65. https://doi.org/10.1016/j.micres.2018.11.004
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17(8):478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Cai DS (2012) Research on Huixian karst wetland ecosystem. Geology Press, Beijing
Chen L, Luo SL, Xiao X, Guo HJ, Chen JL, Wan Y, Li B, Xu TY, Xi Q, Rao C (2010) Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol 46(3):383–389. https://doi.org/10.1016/j.apsoil.2010.10.003
Cordovez V, Dini-Andreote F, Carrión VJ, Raaijmakers JM (2019) Ecology and evolution of plant microbiomes. Annu Rev Microbiol 73(1):69–88. https://doi.org/10.1146/annurev-micro-090817-062524
Fields B, Friman VP (2022) Microbial eco-evolutionary dynamics in the plant rhizosphere. Curr Opin Microbiol 68:102153. https://doi.org/10.1016/j.mib.2022.102153
Granada CE, Passaglia LMP, De Souza EM, Sperotto RA (2018) Is phosphate solubilization the forgotten child of plant growth-promoting rhizobacteria? Front Microbiol 9:2054. https://doi.org/10.3389/fmicb.2018.02054
Haney CH, Samuel BS, Bush J, Ausubel FM (2015) Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nature Plants 1(6):1–9. https://doi.org/10.1038/nplants.2015.51
Jiménez-Mejía R, Medina-Estrada RI, Carballar-Hernández S, Orozco-Mosqueda MDC, Santoyo G, Loeza-Lara PD (2022) Teamwork to survive in hostile soils: use of plant growth-promoting bacteria to ameliorate soil salinity stress in crops. Microorganisms 10(1):150. https://doi.org/10.3390/microorganisms10010150
Jones DL, Hinsinger P (2008) The rhizosphere: complex by design. Plant Soil 312(1):1–6. https://doi.org/10.1007/s11104-008-9774-2
Khan N (2022) Molecular communication between plants and plant-growth-promoting microorganisms for stress tolerance. Microorganisms 10(6):1088. https://doi.org/10.3390/microorganisms10061088
Kim JS, Lee JE, Nie H, Lee YJ, Kim ST, Kim SH (2018) Physiological and proteomic analysis of plant growth enhancement by the rhizobacteria Bacillus sp. JS Genes Genom 40(2):129–136. https://doi.org/10.1007/s13258-017-0615-7
Liu ZP, Zhang XL, Li LB, Xu N, Hu Y, Wang C, Shi Y, Li DS (2022) Isolation and characterization of three plant growth-promoting rhizobacteria for growth enhancement of rice seedling. J Plant Growth Regul 41(3):1382–1393. https://doi.org/10.1007/s00344-021-10393-4
Lyu D, Backer R, Smith DL (2022) Three plant growth-promoting rhizobacteria alter morphological development, physiology, and flower yield of Cannabis sativa L. Ind Crops Prod 178:114583. https://doi.org/10.1016/j.indcrop.2022.114583
Majumder A, Bhattacharyya K, Bhattacharyya S, Kole S (2013) Arsenic-tolerant, arsenite-oxidising bacterial strains in the contaminated soils of West Bengal, India. Sci Total Environ 463:1006–1014. https://doi.org/10.1016/j.scitotenv.2013.06.068
Marques AP, Moreira H, Franco AR, Rangel AO, Castro PM (2013) Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria–Effects on phytoremediation strategies. Chemosphere 92(1):74–83. https://doi.org/10.1016/j.chemosphere.2013.02.055
Menon R, Kumari S, Viver T, Rameshkumar N (2020) Flavobacterium pokkalii sp. Nov., a novel plant growth promoting native rhizobacteria isolated from pokkali rice grown in coastal saline affected agricultural regions of southern India Kerala. Microbiol Res 240:126533. https://doi.org/10.1016/j.micres.2020.126533
Naseer I, Ahmad M, Hussain A, Jamil M (2020) Potential of zinc solubilizing Bacillus strains to improve rice growth under axenic conditions. Pak J Agric Sci 57(4):1057–1071. https://doi.org/10.21162/PAKJAS/20.9988
Nihorimbere V, Ongena M, Smargiassi M, Thonart P (2011) Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol Agron Soc Environ 15(2):327–337
Orozco-Mosqueda MDC, Duan J, DiBernardo M, Zetter E, Campos-García J, Glick BR, Santoyo G (2019) The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front Microbiol 10:1392. https://doi.org/10.3389/fmicb.2019.01392
Ouhaddou R, Ben-Laouane R, Lahlali R, Anli M, Ikan C, Boutasknit A, Slimani A, Oufdou K, Baslam M, Ait Barka E, Meddich A (2022) Application of indigenous rhizospheric microorganisms and local compost as enhancers of lettuce growth, development, and salt stress tolerance. Microorganisms 10(8):1625. https://doi.org/10.3390/microorganisms10081625
Philippot L, Raaijmakers JM, Lemanceau P, Putten WHVD (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. https://doi.org/10.1038/nrmicro3109
Shi SJ, Nuccio EE, Shi ZJ, He ZL, Zhou JZ, Firestone MK (2016) The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett 19(8):926–936. https://doi.org/10.1111/ele.12630
Singh N, Srivastava S, Rathaur S, Singh N (2016) Assessing the bioremediation potential of arsenic tolerant bacterial strains in rice rhizosphere interface. J Environ Sci 48:112–119. https://doi.org/10.1016/j.jes.2015.12.034
Thulasi K, Jayakumar A, Balakrishna Pillai A, Gopalakrishnapillai Sankaramangalam VK, Kumarapillai H (2018) Efficient methanol-degrading aerobic bacteria isolated from a wetland ecosystem. Arch Microbiol 200(5):829–833. https://doi.org/10.1007/s00203-018-1509-z
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18(11):607–621. https://doi.org/10.1038/s41579-020-0412-1
Venturi V, Keel C (2016) Signaling in the rhizosphere. Trends Plant Sci 21(3):187–198. https://doi.org/10.1016/j.tplants.2016.01.005
Wang WF (1998) Soil of China. Beijing Agricultural Publishing House of China, Beijing
Xu SP, Tao S, Xu FL, Cao J (2000) Spatial distribution pattern of trace elements contents in the soil in Inner Mongolia. Acta Geogr Sin 55(3):337–345
Xue PP, Carrillo Y, Pino V, Minasny B, Mcbratney AB (2018) Soil properties drive microbial community structure in a large scale transect in South Eastern Australia. Sci Rep 8(1):11725. https://doi.org/10.1038/s41598-018-30005-8
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4. https://doi.org/10.1016/j.tplants.2008.10.004
Yu LY, Huang HB, Wang XH, Li S, Feng NX, Zhao HM, Huang XP, Li YW, Li H, Cai QY (2019) Novel phosphate-solubilising bacteria isolated from sewage sludge and the mechanism of phosphate solubilisation. Sci Total Environ 658:474–484. https://doi.org/10.1016/j.scitotenv.2018.12.166
Yuan DX (2001) World correlation of karst ecosystem: objectives and implementation play. Adv Earth Sci 16(4):461–466
Acknowledgements
This work was financially supported by the Guangxi Science and Technology Program (Grant No. AD19110043).
Author information
Authors and Affiliations
Contributions
Design of the study: TMS. Performed the experiments: CBW, JLN. Statistical analysis: JYP, CBW. Drafted the manuscript: JYP. Contributed reagents/materials/analysis tools: QLX and TMS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article did not contain any studies with human participants or animals.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, JY., Wang, CB., Nong, JL. et al. Plant growth-promoting rhizobacteria are important contributors to rice yield in karst soils. 3 Biotech 13, 158 (2023). https://doi.org/10.1007/s13205-023-03593-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03593-0