Abstract
Production costs of bacterial cellulose (BC) can be reduced using alternative fermentation media, e. g., various agricultural by-products including whey. This study focuses on whey as an alternative growth medium for BC production by Komagataeibacter rhaeticus MSCL 1463. It was shown that the highest BC production on whey was 1.95 ± 0.15 g/L, which is approximately 40–50% lower that BC production on standard HS media with glucose. It was also confirmed that K. rhaeticus MSCL 1463 can utilise both lactose and galactose as the sole C source in the modified HS medium. Different whey pre-treatment methods showed that the highest BC synthesis with K. rhaeticus MSCL 1463 was achieved in undiluted whey after standard pre-treatment procedure. Moreover, BC yield from substrate on whey was significantly higher (34.33 ± 1.21%) compared to the HS medium (16.56 ± 0.64%), which shows that whey can be used as a potential fermentation medium for BC production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC) is an extracellular biopolymer synthesised by certain strains of acetic acid bacteria (AAB) (Raspor and Goranovič 2008). It is assumed that BC is synthesised by AAB as a self-defence mechanism from UV rays and to help bacteria float in an air–liquid interface (Cannon and Anderson 1991). BC is an environmentally friendly biopolymer with high potential for replacing different synthetic materials (Azeredo et al. 2019). Primarily due to its excellent mechanical properties, it can be used in several industries, such as biomedicine, food, packaging materials, water treatment or electronics (Azeredo et al. 2019; Blanco Parte et al. 2020).
Despite the potentially wide spectrum of applications, the commercialization of BC is still difficult due to high production costs (Ul-Islam et al. 2015). It is known that the costs associated with the use of synthetic media can reach approximately 30% of total production costs (Revin et al. 2018). The use of alternative substrates such as whey can significantly reduce total production costs and lead to significant improvement in BC yields (Jozala et al. 2015; Revin et al. 2018; Kolesovs and Semjonovs 2020). Due to the fact that only 50% of its volume is appropriately processed, whey is an environmentally problematic by-product of the dairy industry, which is associated with significant environmental damage. If utilised inappropriately, whey may cause severe pollution in the surrounding environment, resulting in significant reduction of biodiversity (Panghal et al. 2018). The use of whey in the process of obtaining BC can significantly reduce the cost of BC production, as well as reduce the environment degrading effect (Kolesovs and Semjonovs 2020).
At present, there are still only a few studies on producing BC on whey as a fermentation medium and the possibilities of optimizing its composition with the aim of increasing BC productivity and reducing its production costs (Revin et al. 2018; Kolesovs and Semjonovs 2020). A significant number of these studies show that different AAB strains either do not support BC growth on whey or lactose-based substrates, or produce a significantly smaller amount of BC compared with standard medium (Thompson and Hamilton 2000; Nguyen et al. 2008; Jozala et al. 2015). This is mostly associated with the strains' inability to use lactose as the main C source in whey. In addition, many research articles lack the necessary information on whey composition used in the experiment and give an insufficient amount of information regarding whey pre-treatment methods. Moreover, BC production experiments are rarely accompanied by lactose and galactose uptake control.
In previous research, K. rhaeticus MSCL 1463 showed the highest BC productivity (0.2 g/L/d) among studied AAB strains (Semjonovs et al. 2017). However, important parameters such as lactose and galactose uptake and influence of whey pre-treatment procedures have not been analysed in detail. The aim of this study is to further investigate BC production by K. rhaeticus MSCL 1463 on whey as a growth medium.
Materials and methods
Whey composition
Cheese whey was received from local milk producers (Rankas piens, JSC, Latvia). The composition of whey is summarized in Table 1. Analyses of the composition of raw whey were carried out in the laboratory of J.S. HAMILTON. Ltd., Latvia. Lactose, glucose and galactose concentrations were determined using enzymatic assay kits for lactose and its monomers—glucose and galactose K-LACGAR 03/20 (Megazyme, Wicklow, Ireland). After acquiring whey, it was divided into 500 mL portions and stored frozen at – 20 ℃. All experiments were carried out using one batch of whey.
Preparation of the medium
Standard Hestrin-Schramm (HS) medium with different C sources was used for the preparation of inoculum and as control for comparison with whey medium. Composition of HS medium was as follows: glucose, 20.0 g/L, yeast extract, 5.0 g/L, bacteriological peptone, 5.0 g/L, disodium phosphate, 2.7 g/L, citric acid, 1.15 g/L (Hestrin and Schramm 1954). To investigate lactose and galactose metabolism, the modified HS medium with 20.0 g/L lactose or galactose were used to substitute glucose in the medium. Prior to sterilisation of media, pH was regulated to 7.0 ± 0.1 with 1 M sodium hydroxide and 1 M hydrogen chloride. All experimental media were sterilised at 121 ℃ for 15 min at 1.2 atm.
Standard whey medium was prepared by heating whey for 10 min at 90 ℃ to achieve protein precipitation. After cooling, the whey was centrifuged at 8000 rpm for 10 min to separate the precipitated proteins. It provides a twofold reduction of total protein concentration from approximately 8 to 4 g/L, which is more suitable for bacterial growth (data not shown). After centrifugation, the whey supernatant was sterilized at 121 ℃ at 1.2 atm for 15 min.
Different thermal whey pre-treatment methods were used during the experiments. For the first method, whey was heated for 30 min at 60 ℃, which corresponds to the low-temperature pasteurization procedure (Lau et al. 1991). This whey medium was not autoclaved further. For the second method—whey was heated for 1 min at 80 ℃, which corresponds to the high temperature pasteurization procedure (Barrett et al. 1999), with no further autoclaving. In this case too, the whey medium was not autoclaved. For the third method—whey was autoclaved at 121 ℃ for 15 min.
Different alternative media were also tested to compare BC productivity on whey with some of the alternative media. Mannitol medium composition was as follows: mannitol, 15.0 g/L; beef bacteriological peptone, 5.0 g/L; yeast extract, 20.0 g/L (Oikawa et al. 1995). Corn steep liquor (CSL) was obtained from C.C. Moore & Co Limited, (Ltd, Great Britain). Sugar concentrations were determined by Sucrose, D-fructose and D-glucose assay procedure K-SUFRG 04/18 (Megazyme, Ireland). Glucose, 55.2 g/L, sucrose, 4.3 g/L, fructose, 3.3 g/L, total proteins were specified by the manufacturer and correspond to 37 g/L. CSL was dissolved in distilled water at 5 g/L concentration.
For apple juice medium preparation, off-market apples (Malus domestica) sourced from a local household (Bauska District, Latvia) were used. Apple juice medium prepared in accordance with the methodology described by Kolesovs et al. (2022). The concentrations of sugars in apple juice with 1:2 (apple juice: distilled water) were as follows: glucose 6.73 ± 0.14, fructose 30.25 ± 1.02, sucrose 14.21 ± 0.23 g/L.
Bacterial strain
BC synthesis on whey was carried out with Komagataeibacter rhaeticus MSCL 1463 isolated by the Laboratory of Industrial Microbiology and Food Biotechnology, Institute of Biology, of the University of Latvia because of its ability to synthesise BC on whey medium (Semjonovs et al. 2017; Semjonovs et al. 2018). The strain was deposited in the Microbial Strain Collection of Latvia (MSCL) and previously reported as K. rhaeticus P 1463.
BC synthesis experiments
Inoculum was prepared in 0.5 L Erlenmeyer flasks with 100 mL standard HS medium, under shaking conditions at 150 rpm and 30 ℃ for 24 h. Consequently, 5% of the inoculum was added to the main fermentation media (50 mL of medium in 250 mL wide-neck Erlenmeyer flasks). Experiments were carried out with air surface/media volume ratio at ~ 30 cm2/50 mL, which corresponds to the optimal ratio for static BC synthesis (Ruka et al. 2012). The experiment was carried out statically in thermostats for 10 days at 30 ℃.
Purification of BC pellicles
At the end of the BC cultivation cycle, pellicles were removed from the fermentation media, rinsed in distilled water (100 mL), then washed in distilled water for 20 min in an orbital spinner to remove excess media and bacterial cells. The procedure was then repeated three times. Afterwards BC pellicles were placed in 100 mL of 0.1 M sodium hydroxide solution and heated to 90 ℃ for 20 min. After heating procedure, BC pellicles were purified from sodium hydroxide by rinsing in distilled water until pH 7.0 was reached. Purified BC was placed in weighting bottles and dried overnight at 110 ℃ before estimating the dry weight of pellicles gravimetrically. The BC concentration was then calculated as the mass of BC per litre (g/L) of medium.
Determination of sugars and organic acids concentrations
Concentrations of C sources (glucose, lactose, and galactose) and organic acids (acetic and gluconic) in the fermentation media were determined using enzymatic assay kits (Megazyme, Wicklow, Ireland) as described by Kolesovs et al. (2022).
Protein concentration
Protein concentration in whey was determined using the Bradford reagent colour reaction (Kruger 2009), monitoring the intensity of the colour reaction with a spectrophotometer (UV5Bio, Mettler Toledo, Greifensee, Switzerland).
Determination of BC production biotechnological parameters
At the beginning and at the end of cultivation samples of 500 μL were collected from the fermentation media. Sugar consumption (ΔS) during fermentation was calculated as the difference between the initial sugar concentration (S0) in the medium and at the end of cultivation (S1). Using data from the changes in the amount of BC or organic acids (P0—product amount at the beginning; P1—amount at the end) and the ΔS for the respective time points, the yield of BC production (YBC/ΔS,%) was calculated according to the following formula:
The productivity (QP, g/L/d) of BC was calculated in a similar way, where t corresponds to cultivation time (10 days):
Data statistical analysis
Results were analysed using SPSS (BM SPSS Statistics for Windows, Version 21.0) at significance level p = 0.05. BC production (difference in dry weight) was analysed with a one-way ANOVA test. Changes in sugar and organic acid concentrations in the fermentation media were analysed with a paired-sample T-test.
Results
BC synthesis experiments
The ability of K. rhaeticus MSCL 1463 ability to produce BC on lactose and galactose as a sole C source in modified HS medium had not been previously determined. Therefore, BC synthesis was examined on HS with glucose, HS with lactose, HS with galactose, HS without C media. As shown in Fig. 1, HS medium with glucose showed the highest BC synthesis (3.05 ± 0.06 g/L BC dry weight). It is worth noting that BC production on whey medium was significantly lower (1.65 ± 0.03 g/L) compared with the standard HS medium (p < 0.01). Moreover, it is possible to conclude that BC synthesis takes place in the media, where the only source of C was lactose or galactose, although BC production was found to be low (Fig. 3). In a modified HS medium with lactose and in HS medium with galactose, BC dry weight was 0.26 ± 0.01 and 0.25 ± 0.03 g/L respectively, with no significant statistical difference between two groups (p = 1). On the other hand, the HS medium without sugar showed none or minimal BC synthesis (0.08 ± 0.01 g/L) due to glucose residues from the inoculum (Table 2).
To understand the lactose and galactose uptake by K. rhaeticus MSCL 1463, enzymatical analysis for sugars was performed and the data summarised in Table 2. The data confirms that K. rhaeticus MSCL 1463 utilises both lactose and galactose as the sole C source in a modified HS medium. However, the assimilation of both lactose and galactose was minimal compared with glucose, which resulted in a significantly lower BC production. In whey, all three sugars (glucose, lactose, and galactose) were assimilated and both glucose and galactose residues were fully depleted.
Further BC synthesis was evaluated in dependence of medium sterilization procedure (thermal pre-treatment). The results of BC synthesis are summarized in Fig. 2. The highest BC synthesis by K. rhaeticus MSCL 1463 was observed in the medium that underwent the standard whey thermal pre-treatment and achieved 1.78 ± 0.05 g/L BC (dry weight). However, the statistical analysis of the data showed that BC in other three groups—60 ℃ for 30 min, 80 ℃ for 1 min and 121 ℃ for 15 min—showed no significant difference (p > 0.05). These results can be attributed to the lower protein concentration in the group with standard pre-treatment—heating of whey for 10 min at 90 ℃, then centrifugation at 8000 rpm for 10 min, and sterilization of supernatant at 121 ℃ at 1.2 atm for 15 min. It is noteworthy that the protein assay by Bredford method did not show any significant changes in protein concentration during the experiment. For further experiments the standard pre-treatment procedure was applied for all experimental whey media.
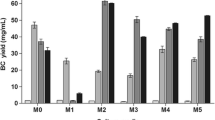
It is known that sugar concentration can significantly affect BC synthesis (Embuscado et al. 1994). It has been established that whey contains a high concentration of lactose (47–49 g/L), which may significantly inhibit BC synthesis. Therefore, different whey concentrations were evaluated in this study. During the experiment, it was found that whey dilution with distilled water significantly reduced BC yield from 1.68 ± 0.05 g/L in undiluted whey to 0.57 ± 0.05 g/L (1:1 dilution) and 0.46 ± 0.05 g/L (1:2 dilution) (Fig. 3).
A comparison of whey (standard—pre-treated) with alternative media showed moderate BC synthesis compared with other media. Mannitol medium showed that the highest BC synthesis among evaluated alternative media (2.39 ± 0.05 g/L BC). CSL was also found to be more suitable substrate for BC synthesis due to main C source—sucrose. However, apple juice provided significantly lower BC synthesis compared with the whey medium, probably due to the insufficient presence of N in the fermentation medium (Kurosumi et al. 2009).
Determination of biotechnological parameters of BC grown on whey
For estimation of biotechnological parameters, separate trials were carried out, where BC was grown on the HS medium with glucose and whey medium. Changes in sugars and organic acids were evaluated and summarised in Tables 3 and 4.
The data shown in Table 3 confirmed the results of previous experiments, when lactose was only partly assimilated. Both monosaccharides (glucose and galactose) were fully utilised in HS and whey media. Organic acid analysis showed that a significantly higher concentration of both gluconic and acetic acids was achieved in the HS medium on glucose. Whey did not support high organic acid synthesis which also resulted in final pH being significantly higher (Fig. 4).
Table 5 summarises the BC production biotechnological parameters for K. rhaeticus MSCL 1463 on HS and whey media. It was demonstrated that BC dry weight on whey was significantly lower (~ 40%). However, BC yield from substrate in the whey medium was found to be significantly higher (34.33 ± 1.21%) compared to HS (16.56 ± 0.64%). It is also worth noting that that the organic acid yield was significantly higher in the standard HS medium.
Additionally, formation of BC pellicle with characteristic 3D fibres produced by K. rhaeticus MSCL 1463 on whey medium was observed at × 5,000 magnification using scanning electron microscopy (Fig. 5).
Discussion
In the course of the experiments, whey was studied as a cheap and promising alternative to replace more expensive BC synthesis media e.g. HS. Based on the obtained results, it can be concluded that whey can serve as an alternative medium for BC synthesis, although K. rhaeticus MSCL 1463 strain showed the lower BC production (1.95 ± 0.15 g/L) compared with HS.
Analysis of literature shows that whey does not provide higher BC synthesis compared to other alternative media, e. g. containing such C sources as crude glycerol, molasses, apple juice (Vazquez et al. 2013; Semjonovs et al. 2017; Zikmanis et al. 2021). As can be seen, the highest BC synthesis was achieved in media with easily assimilated sugars such as glucose, fructose, and sucrose.
Comparing the results obtained in this study with the literature, it can be noted that in several studies BC synthesis on whey was relatively low or not observed at all (Thompson and Hamilton 2000; Battad-Bernardo et al. 2004; Nguyen et al. 2008). This was attributed to lactose being a problematic source of C, which is consumed significantly slower by BC producers than glucose or sucrose (Jozala et al. 2015; Kolesovs and Semjonovs 2020). This was attributed to the lack of the lacZ gene, which is responsible for the production of the β-galactosidase enzyme and the breakdown of lactose to glucose and galactose (Battad-Bernardo et al. 2004). Given that lactose uptake was observed in this study (Tables 2 and 3), the presence of the lacZ gene in the genome of K. rhaeticus MSCL 1463 can be assumed. However, lactose consumption during the experiment was significantly lower than for G. sucrofermentans B-11267, which was able to reduce the lactose concentration in the medium from 42.1 g/L to 22.4 g/L on the first day of cultivation (Revin et al. 2018). Genome sequencing of K. rhaeticus strain MSCL 1463 would provide a complete picture of the genes involved in lactose metabolism. In addition, there have been few attempts to create genetically modified BC producer strains to date. A potential solution for improving the efficiency of the use of lactose-containing substrates for BC production could be the use of genetically engineered strains. By inserting genes responsible for lactose hydrolysis and protease activity into the producer's genome, higher BC yields can be achieved on whey (Battad-Bernardo et al. 2004). Moreover, in further studies whey enzymatic and acidic hydrolysis should be evaluated.
The analysis of literature sources and the results of this work show that it is important to continue studying BC-synthesizing strains with the aim of selecting more efficient BC producers. The study showed that BC synthesis on whey is lower compared to the standard HS medium and other alternative carbon sources such as mannitol or CSL. BC dry weight on whey still remains 40–50% lower than the yield on standard HS medium. Despite the fact that whey is a cheap substrate, its use still remains problematic due to low BC productivity without prior hydrolytic treatment procedures.
As it follows from the changes in metabolites in the media, K. rhaeticus MSCL 1463 consumed approximately 6–8% of the lactose in the whey media during 10 days of cultivation. By increasing the duration of cultivation, it could be possible to increase the consumption of lactose. An important observation is that galactose was consumed during bacterial cultivation in all experiments (Tables 2 and 3). Without excluding the possibility of other explanations, which should be tested in further studies, this could mean that the bacterium has characteristic lactose transport in the intracellular space with the further hydrolysis of lactose into glucose and galactose. This could explain why no accumulation of galactose was observed in the culture medium, as should be the case when the bacteria's extracellular enzymes break down lactose, primarily consuming glucose, while galactose remains in the culture medium until a significant portion of the free glucose is consumed. To verify the latter, it is necessary to confirm it experimentally in future studies. Most likely, all the free monosaccharides in the whey medium are consumed during the first days of the experiment and only then does the bacteria begin to assimilate lactose. At the same time, it has been clearly shown (Tables 2 and 3) that K. rhaeticus MSCL 1463 uses galactose and lactose as the only C sources in the artificial HS medium. However, in another study it was shown that G. xylinus ATCC 53524 did not assimilate galactose, and no BC production was observed on galactose medium beyond 0.09 g/L, presumably due to the presence of residual inoculum in the analysed culture fluid (Mikkelsen et al. 2009), which indicates the specificity of a particular BC producer strain. To obtain unambiguously interpretable data on carbohydrate metabolism pathways, studies on the metabolism of K. rhaeticus MSCL 1463 are being continued, including the study of the genome structure of this strain during the subsequent stages of research.
Comparing the synthesis of organic acids (acetic acid and gluconic acid) on HS and whey media, it can be established that the accumulation of acetic acid and gluconic acid occurred significantly slower on the whey media (Table 4). This may be associated with the fact that BC synthesis occurs in parallel with cell biomass synthesis (Hornung et al. 2007). As can be seen in Table 5, in the case of both BC and organic acids, synthesis is also lower compared with the HS medium. As it follows from the pH changes, it can be stated that in the whey medium, the pH dropped more slowly than in the HS medium (Table 4). This is due to lower synthesis of organic acids in the whey medium. When calculating the yield of products from substrate (YP/S), whey provides significantly higher BC yields (34.33 ± 1.21%) compared with HS medium (16.56 ± 0.64%) and lower organic acids yields—3.87 ± 0.42% compared with 14.02 ± 0.57%. This could indicate that, despite the fact that the synthesis of BC is lower in the whey medium, the substrate is consumed more efficiently directly for the synthesis of BC than for the synthesis of organic acids.
At present, there is a lack of research on the pre-treatment of whey with the aim of improving BC synthesis (Kolesovs and Semjonovs 2020). Oftentimes, there is also a shortage of data on concentrations of substances present in whey, such as lactose, galactose and protein concentrations. It is important to note that the composition of whey is quite variable, which can significantly affect the results of the experiment. Data on the initial composition of whey are not always represented even in the few publications that are available on this topic (Jozala et al. 2015). Sometimes, information on whey pre-treatment procedures is also incomplete, which can also affect the comparative evaluation of BC productivity.
In general, it can be considered that unless it is subjected to pre-treatment and/or its composition is optimized, whey is a relatively problematic source of BC synthesis. The production of BC on whey is significantly lower than on glucose or mannitol-containing media (HS). Nevertheless, the yield in YP/S whey media is higher than in the standard HS medium.
Conclusions
K. rhaeticus MSCL1463 uses galactose and lactose as the only C substrates in the semisynthetic l HS medium, as well as in whey for BC synthesis. As an alternative medium, whey is suitable for obtaining BC, although it results in approximately 40–50% lower BC production than in the standard HS medium with glucose. It was shown that K. rhaeticus MSCL1463 can utilise both galactose and lactose in whey and modified HS media, although galactose and lactose consumption is relatively low. BC yield YP/S from an amount of consumed substrate in whey medium without additives and no hydrolytic procedures was 40–50% higher than on the standard HS medium. BC synthesis can be significantly improved by thermal pre-treatment of whey.
Data availability
All data generated or analysed during this study are included in this published article.
References
Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM (2019) Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2019.00007
Barrett NE, Grandison AS, Lewis MJ (1999) Contribution of the lactoperoxidase system to the keeping quality of pasteurized milk. J Dairy Res 66:73–80. https://doi.org/10.1017/S0022029998003252
Battad-Bernardo E, McCrindle SL, Couperwhite I, Neilan BA (2004) Insertion of an E. coli lacZ gene in Acetobacter xylinus for the production of cellulose in whey. FEMS Microbiol Lett 231:253–260. https://doi.org/10.1016/S0378-1097(04)00007-2
Blanco Parte FG, Santoso SP, Chou CC, Verma V, Wang HT, Ismadji S, Cheng KC (2020) Current progress on the production, modification, and applications of bacterial cellulose. Crit Rev Biotechnol 2:1–18. https://doi.org/10.1080/07388551.2020.1713721
Cannon RE, Anderson SM (1991) Biogenesis of bacterial cellulose. Crit Rev Microbiol 17:435–447. https://doi.org/10.3109/10408419109115207
Embuscado ME, Marks JS, BeMiller JN (1994) Bacterial cellulose. I. Factors affecting the production of cellulose by Acetobacter xylinum. Top Catal 8:407–418. https://doi.org/10.1016/S0268-005X(09)80084-2
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352. https://doi.org/10.1042/bj0580345
Hornung M, Ludwig M, Schmauder HP (2007) Optimizing the production of bacterial cellulose in surface culture: a novel aerosol bioreactor working on a fed batch principle (Part 3). Eng Life Sci 7:35–41. https://doi.org/10.1002/elsc.200620164
Jozala AF, Pértile RAN, dos Santos CA, de Carvalho S-E, Seckler MM, Gama FM, Pessoa A (2015) Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl Microbiol Biotechnol 99:1181–1190. https://doi.org/10.1007/s00253-014-6232-3
Kolesovs S, Semjonovs P (2020) Production of bacterial cellulose from whey—current state and prospects. Appl Microbiol Biotechnol 104:7723–7730. https://doi.org/10.1007/s00253-020-10803-9
Kolesovs S, Neiberts K, Beluns S, Gaidukovs S, Semjonovs P (2022) Bacterial cellulose production by Novacetimonas hansenii MSCL 1646 on apple juice. Appl Microbiol and Biotechnol. https://doi.org/10.1007/s00253-022-12213-5
Kurosumi A, Sasaki C, Yamashita Y, Nakamura Y (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr Polym 76:333–335. https://doi.org/10.1016/j.carbpol.2008.11.009
Kruger NJ (2009) The Bradford method for protein quantification. Basic Protein Pept Pro 17–24
Lau KY, Barbano DM, Rasmussen RR (1991) Influence of pasteurization of milk on protein breakdown in cheddar cheese during aging. J Dairy Sci 74:727–740. https://doi.org/10.3168/jds.S0022-0302(91)78218-0
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107:576–583. https://doi.org/10.1111/j.1365-2672.2009.04226.x
Nguyen VT, Flanagan B, Gidley MJ, Dykes GA (2008) Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Curr Microbiol 57:449–453. https://doi.org/10.1007/s00284-008-9228-3
Oikawa T, Ohtori T, Ameyama M (1995) Production of cellulose from D-mannitol by Acetobacter xylinum KU-1. Biosci Biotechnol Biochem 59:331–332. https://doi.org/10.1271/bbb.59.331
Panghal A, Patidar R, Jaglan S, Chhikara N, Khatkar SK, Gat Y, Sindhu N (2018) Whey valorization: current options and future scenario—a critical review. Nutr Food Sci 48:520–535. https://doi.org/10.1108/NFS-01-2018-0017
Raspor P, Goranovič D (2008) Biotechnological applications of acetic acid bacteria. Crit Rev Biotechnol 28:101–124. https://doi.org/10.1080/07388550802046749
Revin V, Liyaskina E, Nazarkina M, Bogatyreva A, Shchankin M (2018) Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz J Microbiol 49:151–159. https://doi.org/10.1016/j.bjm.2017.12.012
Ruka DR, Simon GP, Dean KM (2012) Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr Polym 89:613–622. https://doi.org/10.1016/j.carbpol.2012.03.059
Semjonovs P, Ruklisha M, Paegle L, Saka M, Treimane R, Skute M, Rozenberga L, Vikele L, Sabovics M, Cleenwerck I (2017) Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl Microbiol Biotechnol 101:1003–1012. https://doi.org/10.1007/s00253-016-7761-8
Semjonovs P, Ruklisha M, Logina P, Saka M, Treimane R, Patetko A, Linde R (2018) Komagataeibacter rhaeticus P 1463 producer of bacterial cellulose. Eur Patent Spec 3:121–265
Thompson DN, Hamilton MA (2000) Production of bacterial cellulose from alternate feedstocks. 22nd Symposium on Biotechnology for Fuels and Chemicals P. O Box 208:526
Ul-Islam M, Khan S, Ullah MW, Park JK (2015) Bacterial cellulose composites: synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol J 10:1847–1861. https://doi.org/10.1002/biot.201500106
Vazquez A, Foresti ML, Cerrutti P, Galvagno M (2013) Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J Polym Environ 21:545–554. https://doi.org/10.1007/s10924-012-0541-3
Zikmanis P, Kolesovs S, Ruklisha M, Semjonovs P (2021) Production of bacterial cellulose from glycerol: the current state and perspectives. Bioresour Bioprocess 8:1–14. https://doi.org/10.1186/s40643-021-00468-1
Funding
This study was funded by Ministry of Agriculture and the Rural Support Service of the Republic of Latvia (Grant No. 19-00-A01612-000004). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
Writing of the manuscript, literature research, experimental design, microorganism cultivation, metabolites measurements and data statistical analysis, editing—SK. Research conceptualisation, critical revision, and supervision—PS and MR. All authors contributed to the study conception and design, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable, since the work does not involve any study with human participants or animals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolesovs, S., Ruklisha, M. & Semjonovs, P. Synthesis of bacterial cellulose by Komagataeibacter rhaeticus MSCL 1463 on whey. 3 Biotech 13, 105 (2023). https://doi.org/10.1007/s13205-023-03528-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03528-9