Abstract
Exosomes are nanosized (size ~ 30–150 nm) natural vesicular structures released from cells by physiological processes or pathological circumstances. Exosomes are growing in popularity as a result of their many benefits over conventional nanovehicles, including their ability to escape homing in the liver or metabolic destruction and their lack of undesired accumulation before reaching their intended targets. Various therapeutic molecules, including nucleic acids, have been incorporated into exosomes by different techniques, many of which have shown satisfactory performance in various diseases. Surface-modified exosomes are a potentially effective strategy, and it increases the circulation time and produces the specific drug target vehicle. In this comprehensive review, we describe composition exosomes biogenesis and the role of exosomes in intercellular signaling and cell–cell communications, immune responses, cellular homeostasis, autophagy, and infectious diseases. In addition, we discuss the role of exosomes as diagnostic markers, and their therapeutic and clinical implications. Furthermore, we addressed the challenges and outstanding developments in exosome research and discuss future perspectives. In addition to the current status of exosomes as a therapeutic carrier, the lacuna in the clinical development lifecycles along with the possible strategies to fill the lacuna have been addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the years of modern drug delivery, liposomes, micelles, dendrimers, and polymeric nanoparticles, inorganic nanoparticles as drug delivery vehicles have been exploited to improve the efficacy and therapeutic index concerning the PKPD of the drug, with the aim of reducing the off-target side effects and drug-related toxicity (Butreddy et al. 2021; Khalid et al. 2023). However, many hurdles still exist with these systems, including the specific organ targeting, the chemical and physical features related to toxicity, and the undesired immune response which ultimately obligates to discontinue the treatment in some individuals. Here a new natural nanocarrier system, namely exosome, can address the constraints associated with other nanocarrier systems (Shao et al. 2020).
Exosomes are nanosized membrane vesicles of 30–100 nm size range (Kooijmans et al. 2012). It can be released from various types of cells by either physiological processes or under pathological circumstances. The nanoscale dimensions of exosomes are commanding greater attention at present and are the most promising ones for advanced and targeted drug delivery (Bunggulawa et al. 2018). Research carried out has proved the excellent ability of exosomes to protect the stability of their content and has reported the highest stability among the various other extracellular vesicles. Some shreds of evidence have been shown to protect exosomal content from degradation by enzymes of the digestive and other biological fluids, facilitating to reach the target sites in their active form.
In the last few decades, liposomes and polymeric nanoparticles have been evaluated extensively for anticancer drugs, antifungal drugs, analgesics, and other important biomolecules (Stoicea et al. 2017; Mitchell et al. 2021; Tasharrofi et al. 2022). However, formulating liposomes that can evade the immune system with a long-circulating capability without instability and toxicity is tedious (Sainaga et al. 2022). While polymeric nanoparticles might possess better stability, their biocompatibility and complete safety are still a matter of concern (Sandra et al. 2019; Patel et al. 2021). In this instance, exosomes or exosome mimetics are the epitome of success in the sector of ideal drug delivery as these have intrinsic targeting ability and are minimally or not lethal to cells or tissues. Exosomes, which are small carriers, are native to the animals and redesigned by researchers so that they can escape immune recognition and bypass the engulfment by lysosomes as well as can avoid phagocytosis and fuse with the cell membrane. As a natural carrier, exosomes also protect contained cargo (Antimisiaris et al. 2018). Slightly negative zeta potential of the exosome vesicles helps for longer systemic t1/2. They have a high concentration of non-lamellar-forming lipids in their makeup, which may cause their bilayer to curve in a way that is advantageous for drug delivery. Furthermore, the extremely asymmetrical nature of the exosome lipid bilayer may be favorable for their contact with the plasma membrane, and more specifically with their target cells (Tenchov et al. 2022). Exosomes include a wide variety of integral and peripheral membrane proteins, which is an additional unique benefit in their application over other drug delivery systems. By transferring their contents, such as proteins, mRNAs, and miRNAs to target cells with or without direct cell contact, these membrane vesicles participate in intercellular interactions. Thus, they have an impact on physiological and pathological processes, their own intrinsic therapeutic activity. Moreover, the ability of exosomes to cross blood–brain barriers, improve motor and neural functioning in the nervous system, and allow multiple intravenous doses of drug-loaded exosomes without causing any negative side effects (Amiri et al. 2022). Based on these characteristics, exosomes have been explored in distinguistic domains of applications other than drug delivery including vaccine development (Escudier et al. 2005), photodynamic immunotherapy (Jang et al. 2021), genome editing/gene therapy (Kim et al. 2017; McAndrews et al. 2021), biomedical 3D printing (Chen et al. 2019a), regenerative medicines, and many more.
Currently, protein therapies are being delivered via protein nanocages (Wang et al. 2021). Genetic engineering can be used to simultaneously display a number of epitopes and ligands on the surface of protein nanocages since these structures self-assemble into hollow spherical structures with several protein subunits. However, when the therapeutic protein is a membrane protein in nature, it is challenging to express and purify membrane proteins, especially those with transmembrane domains, because they must connect with a cellular membrane. Due to these restrictions, the majority of membrane proteins are created as ectodomain sections that are water-soluble. The elimination of these proteins' membrane-anchored counterparts, however, often results in a decrease in their biological activity. Similar attempts to capture histidine-tagged, truncated proteins using liposomes containing NTA-Ni lipids have failed because of the low dissociation constants brought on by the unstable immobilization of proteins in lipids (Yang et al. 2018). Utilizing formulations based on exosomes that preserve the membrane proteins' natural shape and conformation is one way to solve this issue. This is also experimentally manifested that exosomes with membrane protein can induce about 68.3% of tumor growth inhibition in comparison with ferritin nanoparticles which is only 17%. The cell binding activity of the exosome vehicle was five times greater than ferritin nanoparticles. The enhanced therapeutic activity is also boosted by the antagonistic “Don’t eat me” signal to cancer cells exhibited more strongly by exosomes (Cho et al. 2018). The delivery efficiency of exosomes is not restricted to the size of the therapeutic molecule, and it can effectively deliver recombinant nerve growth factor protein and its mRNA simultaneously in the ischemic cortex crossing the blood–brain barrier which is unfeasible by most drug delivery technology (Yang et al. 2020a). Even the exosome-mimicking liposomes had antiserum aggregation activity and improved storage stability but less than four times as much cytotoxicity (Lu et al. 2018).
Numerous synthetic and natural nanotransfecting vehicles have been designed to efficiently deliver siRNA. But the success rate is low due to some issues. Synthetic vehicles interact with one another in a variety of ways. For example, stronger binding of the RNA molecule improves protection from degradation but also lowers the delivery rate inside the cell. Biodegradability decreases toxicity but also shortens the serum half-life. Including endosomal release mechanisms may increase cytotoxicity. Thus, creating the ideal transfection vehicle requires not only integrating these criteria into a single system but also bringing these qualities into harmonious balance with one another. Exosomes exhibit this balance which makes these a suitable transfer vehicle, ultimately providing a superior siRNA delivery mechanism when compared to synthetic carriers (Duechler 2013). In case of small molecule delivery, research provided compelling evidence that exosomes demonstrate superior potency with an improved therapeutic index combined with their natural tropism. At the same concentration when free doxorubicin (Dox) was compared with liposomal Dox (Myocet), Dox in pegylated liposomes (Doxil), and Exo-Dox, time lapse video microscopy reveals a rapid beginning of mitochondrial expansion and apoptosis by the exosomal doxorubicin therapy. Doxorubicin delivered from exosomes rather than free or liposomal form is more quickly absorbed by recipient cells. In comparison with free or liposomal doxorubicin, exosomal doxorubicin is rapidly released from endocytic compartments after uptake and builds to higher intracellular levels. As a result, within hours after cell treatment, beginning of mitochondrial enlargement happened followed by apoptotic cell death. It has shown that a 20- to 80-fold higher concentration of these formulations is necessary to provide a signal that is comparable to the one produced by Exo-Dox (Schindler et al. 2019). These all explain that exosomes are a viable technology for drug delivery.
Moreover, exosome-based treatment can be patient-specific which is distinct from other nanoparticular drug delivery systems; for example, if a patient is suffering from a disease, diagnosed to be caused by a missing or defective microRNA, the patient's exosomes can be isolated, modified with the appropriate nucleic acid, and then delivered back to the patient for treatment. Exosomes isolated from the patient's own cells are also less immunogenic (Escudier et al. 2005). Clinical trials are being conducted on three animal sources of exosomes: patient-derived tumor cells, mesenchymal stem cells (MSCs), and dendritic cells (DCs). However, five different cell types have been used in GMP for exosome production: bone marrow mesenchymal stem cells (BMSCs), monocyte-derived dendritic cells (DCs), human cardiac progenitor cells, adipose tissue-derived stem cells, and HEK293 cells (Chen et al. 2019b).
At the site of inflammation, a large number of pro-inflammatory M1 are available but lack enough anti-inflammatory M2 macrophages. Therefore, the establishment of a repolarization equilibrium of macrophages can overcome the disease condition faster. At this point, exosomes secreted from M2 macrophages possess inherent anti-inflammatory functions and are found suitable as an anti-inflammatory drug vehicle for target inflammatory sites (Gao et al 2021). Curcumin-loaded cell penetrating R9 peptide-modified exosomes from the M2 macrophages have been shown to improve the inflammatory microenvironment with significant reversal of M1 macrophages to M2 macrophages approximately by 53% macrophage population repolarization. The degree of axon injury and motor function recovery was improved in spinal cord injured mice. Moreover, the same exosomal system had been shown to reduce different inflammatory marker expressions like tumor necrosis factor (TNF-α), Interleukins (IL-1β, and IL-6) in the peripheral blood of mice induced with rheumatoid arthritis (Li et al. 2023). Therefore, exosomes are rapidly gaining momentum as a novel therapeutic strategy due to its intrinsic therapeutic potential. Substantial growth is expected for the market worldwide as they are integrated into the multiple fields of the healthcare system including liquid biopsy (Ma et al. 2021) and regenerative medicine (Maumus et al. 2013). Regardless of the wide opportunities for exosomes in clinical therapy, the heterogeneity of the isolated exosomes is a barrier. So it is important to understand the advantages and limitations of available technologies to isolate pure and homogeneous exosome populations followed by their precise characterization.
Composition of exosomes
The composition of exosomes is reliant on the parent plasma membrane construction from which they were born, i.e., scientifically termed the endosomal origin. These generally comprise various types of proteins and lipids which reflect their possible physiological functions. Major histocompatibility complex-II, cluster of differentiation, surface proteins like tetraspanins, lactadhesion, integrins, and other types of adhesion molecules, heat-shock protein (HSC70, HSP84/90), Ras-related proteins (Rab-2, Rab-7), cytoskeletal proteins (actin, tubulin, cofilin, moesin, fibronectin, vimentin, talin), ESCRT components comprising approximately thirty proteins (for example, Tsg 101, Alix, etc.), lysosomal proteins (Lamp 2b), fusion proteins, etc. are the different distinguishable protein components found in exosomal structures (Jadli et al. 2020). In the light of the discussion on exosomal protein composition, it is obvious to mention a new technology termed “Exosome Display Technology.” This involves the expression of proteins (soluble, membrane-bound, transmembrane, multimeric antigens) on exosomes by recombinant technology that naturally does not exist on these nanovesicles (Delcayre et al. 2005). More precisely, it is a technology to generate functional proteins in the exosomes to endow new physicochemical properties or new therapeutic or diagnostic characteristics. In terms of physicochemical properties, improved solubility, stability, and extended half-lives are the major advantages of this display technique. Such kind of protein manipulation also enables the production of multiple replicas of antigen and can be extended to generate antibodies against therapeutic targets and tumor biomarkers (Delcayre et al. 2005).
Apart from proteins, lipids are another salient class of constituent thought to have a significant function in the formation and release of exosomes. Quantitative analysis of lipid content by different techniques involving mass spectrometry, thin-layer chromatography, gas–liquid chromatography, and LC–MS has revealed that the nature and percentage of lipids vary in the outer and inner leaflets of the exosome membrane (Skotland et al. 2017). Such asymmetric lipid distribution is also variable with the type of parent cells from where they get released. Some lipids from a wide range of lipid species characterized in different exosomes are phosphatidylinositol, phosphatidylcholine, phosphatidylglycerol, phosphatidylserine, phosphatidylethanolamine, phosphatidylethanolamine ether, sphingomyelin, hexosylceramide, lactosylceramide, cholesteryl ester, cholesterol, diacylglycerol, and globotriaosylceramide (Gb3) (Skotland et al. 2017).
Exosomes have been shown to contain heterogeneous RNA species or their fragments, for instance Y RNAs, short hairpin RNAs, transfer RNAs, messenger RNA, structural RNAs, small interfering RNAs, and microRNA (Yaghoubi et al. 2019). A precise reassessment of exosome constituents has been done, providing a detailed framework of extracellular vesicle heterogeneity (Ha et al. 2016, Jeppesen et al. 2019) as shown in Fig. 1.
Representative structure and composition of exosomes: Exosome phospholipid bilayer is produced from the plasma membrane that includes elements of the parent cell's cytoplasm. Exosome composition including various types of proteins (Hsp, Rab), lipids (cholesterol, sphingomyelin), and nucleic acids (miRNA) is influenced by the type of source cell, the health of the source cell, and external stimulation. Other vesicular components are antigen-presenting cells (MHCII), adhesion molecules (integrin, tetraspanins), and cluster of differentiation (CD)
Biogenesis of exosomes
Biogenesis of exosomes occurs via several mechanisms, either constitutively or in response to stimuli. The biogenesis from endosomal systems results in vesicles of heterogeneous vesicular architectures. Three broad subclasses of extracellular vesicles (EVs) are (Colao et al. 2018):
-
1.
Microvesicles (50–1000 nm diameter): shed from the cellular membrane involving membrane remodeling and outward blebbing.
-
2.
Apoptotic blebs (100–5000 nm): derived from dying cells during late-stage apoptosis.
-
3.
Exosomes (20–150 nm, variable between research groups): released from multivesicular bodies.
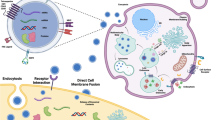
The process begins when early endosomes get matured to late endosomes or multivesicular bodies (MVBs). Then the endosomal membrane of MVBs produces intraluminal vesicles (ILVs) in the lumen of organelles by inward invagination. The next crucial step is to decide the fate of ILVs by lysosomes or generation of exosomes for which different sorting processes are involved. The MVBs when allowed to fuse with the cell membrane. In due course, ILVs are released into the extracellular environment by the mode of exocytosis. These released vesicles are referred to as exosomes (Burkova et al. 2021; Han et al. 2022). They can either be secreted from a wide array of healthy cells or diseased cells under pathological conditions. The endosomal sorting complex (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) are important central machinery required for transport along with VPS4, VTA1, ALIX, and other associated proteins, the key molecules for exosomal genesis. Biogenesis and spatiotemporal traffic of vesicles has been described to be regulated by several other endogenous compounds, including GTPase enzymes (Bunggulawa et al. 2018; Butreddy et al. 2021). Figure 2 shows the complete process of exosome vesicle formation from the endocytosis of the plasma membrane to release by exocytosis.
Biogenesis of exosome vesicle: A, B Early endosomes (EE) created by plasma membrane invasion give rise to exosome production. C EE maturation and late endosome membranes develop an inward bud to create intraluminal vesicles (ILVs), which thereafter change into multivesicular bodies (MVBs), exosomal cargo sorting by endosomal sorting complex (ESCRT). D, E ILVs are released into the extracellular environment to create exosomes once the MVBs have fused with the plasma membrane. F MVB or late endosome (LE) degradation
Isolation or purification methods
Exosomes are novel biotherapeutics, and their production process is like biological production starts with the cell culture, and harvesting the selected cell line from suitable conditioned media. The cell culture media may include MSCs, for example human embryonic stem cell-derived MSCs (Lai et al. 2016), BMSCs (McBride et al. 2017), adipose-derived MSCs (Liu et al. 2019c), cardiac progenitor cells (Andriolo et al. 2018), DCs, mast cells (Valadi et al. 2007), HEK293 cells (Butreddy et al. 2021), T cells (Zakharova et al. 2007), etc.
The first stage of exosome manufacturing is upstream processing involves filtration, the concentration of the cell culture medium, and exosome isolation from that medium. After cell harvest, a variety of downstream processing can be accustomed to purifying exosomes discussed below. The current purification methodologies support the separation of exosome vesicles from cells, culture media, soluble protein aggregates, and undesirable particulates. However, there are no unequivocal protocols for exosomal isolation and purification as each method gives a variable yield of exosomes that could contain humoral protein aggregates or EV subtypes (Sidhom et al. 2020).
Differential centrifugation
One of the most common exosome isolation and purification technique is differential centrifugation. Highly viscous biological samples require a higher speed of centrifugation usually above 100,000×g or longer centrifugation time as the highly viscous biofluids impart higher resistance for sedimentation of exosomes (Cvjetkovic et al. 2014). The purity of the isolated exosomes has a significant correlation with the viscosity of the biofluids because the longer duration of centrifugation may lead to the compromise of exosomal integrity. Exosomes from the pancreatic ductal adenocarcinoma cell line have been reported to be purified by differential centrifugation at the sequence of 800×g, 2000×g, and 100,000×g for 2 h (Capello et al. 2019).
Ultracentrifugation techniques
This is currently used gold standard technique for exosome extraction and separation of high-dose sample components based on the size and density by a series of continuous low–medium- and higher-speed centrifugation. The process optimization fails when there are only minor differences in the sedimentation rates between different fractions of vesicles (Livshits et al. 2015).
Density gradient centrifugation
This involves a combined principle of ultracentrifugation and sucrose density gradient to separate out the particles of different densities, protein–protein aggregates. The optimal centrifugation time is a prime factor to set for the complete separation of contaminating particles from the exosomal fractions of similar densities. The optimal time is dependent upon the rotor’s efficiency used in the centrifuge instrument as well as the buoyant density of the mixture contents other than the pure exosome fractions. Non-exosomal protein impurities levels get elevated in case of 4 h or more time of centrifugation (Konoshenko et al. 2018). At this point, the iodixanol gradient is found to be a perfect process as their sedimentation velocities are significantly different in iodixanol, which enables separation of high-purity exosomes from the HIV-1-infected cells. For example, exosomes are isolated from mouse bone marrow-derived macrophage culture supernatants using 60% iodixanol cushion by modified ultracentrifugation ensures maximal recovery of isolated exosomes. Exosomes from antigen-presenting cells were undergone capturing in a small density cushion [30% sucrose and deuterium oxide in the ultracentrifugation step (Lamparski et al. 2002).
Ultrafiltration
Ultrafiltration is frequently performed during ultracentrifugation or final step of chromatography using a standard membrane with defined size-exclusion limits. Direct flow filtration is a type of ultrafiltration that can separate the floating cells and cell debris using a 0.1 µm filter, preferably from small-volume samples. Tangential or crossflow filtration (TFF), is another type of ultrafiltration process is rated high due to more efficiency and rapidity in isolating exosomes even on a large scale. Sample filtered through a 0.2 µm polyethersulfone membrane is subjected to the TFF, where the sample flows tangentially through a cartridge filter membrane befitting process temperature, definite flow rate, and transmembrane pressure to avoid clogging or cake formation (Cheruvanky et al. 2007).
Size-exclusion chromatography
Exosomes are allowed to pass through a column packed with polymeric beads that build paths of multiple pores and tunnels in size-exclusion chromatography (SEC). Thus, exosomes can be separated precisely from other contaminating molecules depending on their diameter. A potential cause for structural damage that is shear force is absent and hence SEC is more advantageous. However, in the long run, it restrains its application for a handful of biologicals (Yakimchuk 2015).
Flow field-flow fractionation
This is an elution-based separation method combined with a UV analyzer and light-scattering detector. It is performed in a navigable channel allowing the interaction of two streams of parabolic flow (toward the detector) and crossflow (moving across a rectangular channel) to isolate exosomes. When a cell culture-derived exosome sample is added into the AF4 (Asymmetric flow field-flow) channel, the smaller exosome particles move through the down channel with greater velocity than larger exosomes, perpendicular to the crossflow. Therefore, in the fractogram, the first eluted peak is for the smallest exosome subpopulation (Petersen et al. 2014). Zhang H. and his research group have used asymmetric flow field-flow fractionation (AF4) technology to get homogeneous exosome subpopulations. They found two main exosome subpopulations as isolation products like large exosomes (exo-L) and small exosomes (exo-S). A distinct non-membranous nanoparticle of less than 50 nm size was also identified, termed “exomere.” All these subpopulations hold different cellular distribution patterns and distinct biological roles (Zhang et al. 2018). Zhang H and Lyden D have also described the protocol development and optimization of AF4 technology. This fractionation technology offers a high resolution of 1 nm for exosome nanoparticle separation within 1 h. Oh S. and his team also applied the same technology to fractionate exosome vesicles isolated from bone marrow for immunotherapeutic applications (Oh et al. 2007). However, the large-scale utilization of AF4 technology is limited because only a microgram amount of samples can be accommodated at a time (Zhang and Lyden 2019). But it is expected that the scale-up of this instrument will open a new avenue of exosome applications and its subpopulations in diagnostics, prognostics, and therapeutics.
Polymer precipitation
Polymer precipitation was originally used to isolate viruses (Oh et al. 1988); however, this method has been carried out sometimes to isolate and purify exosomes as these share some similar characteristics with viruses. Polyethylene glycol is used as a medium, and the exosomes are harvested under centrifugation (Zhang et al. 2020). ExoQuick-TC is a patented technology available as a commercial kit that uses a proprietary polymer that can precipitate exosomes from low-volume samples (Taylor et al. 2011; Alvarez 2014; Lee et al. 2019).
Immunoaffinity chromatography (IAC)
The authenticity of this purification technology lies in the specific binding of antibodies and ligands, which enables the unambiguous separation of exosomes from a small volume of heterogeneous mixtures (Fitzgerald et al. 2017). This way the target protein that is a unique component on the exosome surface helps IAC to recognize exosomes of different sources with strong specificity. This ensures a high yield without compromising purity (Li et al. 2017). ExoTEST™ (by HasaBioMed Life Sciences) is a commercial platform comprising antibody pre-coated ELISA plates, allowing in vitro quantitative measurement of exosomes (having a lower detection limit of less than 0.35 μg) from different biofluids and cell culture media. The exo-FACS kit of the same company is available for a qualitative Fluorescence-activated cell sorting (FACS) analysis of exosomes (exosomal marker detection) from human plasma and saliva. Limitations arose due to the non-specific interference adsorption of the matrix that produces interfering proteins and the selection of proper storage conditions, which ultimately restricts the scale-up (Zhang et al. 2020).
Microfluidics-based techniques
Microfluidics-based tools are ideal for fast, continuous separation of exosomes from other nanosized particles since they support precise isolation in a cost-effective manner (Salafi et al. 2016). A patented total exosome isolation chip based on this technique (ExoTIC device) is gradually becoming popular due to its exclusive ability to handle a wide range of sample volumes (from 10 μL to 50 mL) while maintaining high purity, yield, and efficiency. The device has been found to be more controllable for extracting exosomes from plasma, lung lavage, and other body fluids in comparison with ultracentrifugation, and PEG precipitation including the ExoQuick™ kit-based method (Lin et al. 2020). Despite its numerous advantages like purity, controllability, tractability, and isolation specificity, the foreseeable challenges are the requirement for complicated devices and the need for high immunoaffinity (Yang et al. 2017a).
Magnetic nanowire-based isolation
From the viewpoint of the requirement of less labor-intensive, less time-consuming, and cost effectivity, scientists have developed a new affordable, ultrasensitive technique using antibody cocktail-conjugated magnetic nanowires. This method can isolate exosomes directly from small-volume biological samples. However, exosomes isolated by this technique are ideal candidates for drug delivery (Lim et al. 2019).
Characterization techniques
The isolated vesicular samples should be characterized conscientiously to validate the isolation process. Accurate assessment of purity and quantification by conventional methods is the utmost challenges in exosome biology.
Nanoparticle tracking analysis (NTA)
This study permits determining both particle size and concentration. Laser wavelengths are used, and a matched long-pass filter allows visualization of the fluorescently labeled particles which get excited by the laser. The particles which are under Brownian motion scatter the laser light, which is collected by a microscope. The camera fitted to the microscope records the particle motion. An NTA software is there to estimate the particle size and concentration from the captured video. The challenge with NTA is that it requires a high concentration level (McNicholas and Michael 2016; Liu et al. 2018).
Dynamic light scattering (DLS)
Particle size and concentration can be determined based on dynamic light scattering. The advantage of DLS is the requirement of minimal sample volume (70 µL) and user-friendliness. While DLS has its benefits over NTA, the major bottleneck is in the analysis of heterogeneous samples. In consequence, it may produce skewness in the data toward larger particles when the particles present in the suspension are full of diverse sizes (Doyle and Wang 2019).
Tunable resistive pulse sensing (TRPS)
TRPS has emerged as a new platform for single-particle characterization in an in situ setting. It can precisely measure the size distribution and concentration specifically when characterizing particles ranging from approximately 50 nm. However, particles that are too small give rise to system sensitivity issues. System stability issues may also arise during TRPS measurement if pores become blocked by particles (Gurunathan et al. 2019). It is a very useful technique to provide surrogate information about the exosomal fate and nano-biointeractions (Vogel et al. 2017).
Atomic force microscopy (AFM)
AFM is a unique nanoscale tool based on optical and electron diffraction techniques. It records the interactions between the sample surface and a probing surface for studying the morphological characteristics, biomolecular makeup of exosomes, molecular dynamics and biomechanics at the single-vesicle and subvesicular levels such as DNA and membrane proteins (Gurunathan et al. 2019).
Transmission electron microscopy (TEM)
TEM can characterize the structure, morphology, and size of various vesicular, fibrous, and other nanomaterials and biological components. To avoid the electron beam generated damage, cryo-EM (cryogenic electron microscopy) has been introduced for exosome analysis as it bypasses the effects of dehydration artifacts and fixation. The key feature of Cryo-TEM is its ability to capture images of exosomes, with lumen and membrane structures, protein structure imaging, and associated macromolecular complexes (Mio and Sato 2018). However, it becomes difficult to distinguish exosomes in some cases because of exaggerated fluorescence signals. As a substitute immunogold EM has emerged where the exosomes can be clearly visualized by specific antibody binding (Gurunathan et al. 2019).
Flow cytometry
Flow cytometry allows for visual observation and size analysis of exosomes along with the detection of surface proteins of exosomes in a quantitative way (Theodoraki et al. 2020). Still, the limitation exists that size estimation is not possible for single exosome due to having a limit of detection of 270–600 nm. To avoid such complications, exosomes can be covalently conjugated to microbeads made up of aldehyde or sulfate latex. However, this strategy may lead to false results as free proteins in the sample can get adsorbed on the beads which also interferes with protein analysis. To overcome this problem pH-triggered exo-cluster strategy can be adopted to increase the overall size of exosomes above the detectable limits of the conventional flow cytometer (Liu et al. 2021). The immobilization of exosomes by the immunocapture method is another possible solution. When the sample passes through the flow cytometer’s laser, it will emit a fluorescence signal which will be detected (Doyle and Wang 2019). Accurate analysis of exosomes is reliably complicated by direct cell sorting for their very small size. Thus, the prepared sample is characterized by FACS, a distict kind of flow cytometry, is able to overcome the difficulty mentioned earlier (Thierry et al. 2006). ImageStream analysis (combined with the method of flow cytometry and fluorescence imaging technology) can be used to distinguish exosomes from other similar-sized contaminants (Mastoridis et al. 2018).
Enzyme-linked immunosorbent assay (ELISA)
ELISA is a qualitative as well as quantitative plate-based assay technique. The ELISA technique is useful to measure exosome counts with accuracy and can also provide good details about exosomal protein content, but it needs a large sample volume (Butreddy et al. 2021). A new single exosome counting immunoassay has been developed by scientists named "droplet digital ExoELISA," which offers absolute quantification for targeting exosomes having specific protein biomarkers (Liu et al. 2018). The method involves the conjugation of magnetic beads with antibodies that can selectively bind to an exosomal membrane protein. When the exosomal suspension is mixed with conjugated magnetic beads, magnetic separation has happened (resulting in the immobilization of the target exosome onto magnetic beads). Tagging of antibodies along with enzymatic reporter allows the detection of antigen of the immobilized exosome by the formation of a single enzyme-linked immunocomplex on the bead. Further co-encapsulation of immunocomplex beads and the enzymatic substrate is performed into microdroplets using microfluidic cheap (the majority of the droplets should not contain more than one bead). The droplets containing exosome–immunocomplex beads emit fluorescence within the droplets due to enzyme catalyzation. Detection of target exosome concentration can be done from the fluorescent readout. This is advantageous being highly sensitive and able to detect as few as nearly 5 exosomes/ µL (Liu et al. 2018).
Western blotting
The western blotting methodology, also called immunoblotting, is a matured method to detect exosomal surface proteins and internal proteins. For example, antiflotillin-1 antibody and mouse antihuman TSG101 have been used in western blotting to identify exosomal markers for flotillin-1, a lipid raft-associated protein, and TSG101, the component of ESCRT protein group in exosomes isolated from cerebrospinal fluid (Street et al. 2012). But intact vesicles cannot be observed; rather, the vesicles are undergone lysis during sample preparation. Densitometric analysis of the western blot can be performed for quantification (Van Niel et al. 2001).
Exosomes drug loading techniques
Currently, the clinical application of exosomes as drug carriers still faces several challenges including methods to import drugs into exosomes efficiently. Exogenous drugs, nucleic acids, and protein molecules have been loaded into exosomes, and their physical mimetics using various methods to date can be categorized into preloading and post-loading methods (Xu et al. 2020c). In preloading, exosomes isolated/secreted from cells are already preloaded with the desired drugs and incorporated in the parent cells by employing transfection, activation, etc. Several methods for drug loading have been explored as discussed below in detail. Currently, several methods like transfection, incubation, saponin-assisted loading, sonication, extrusion, freeze–thaw cycles, electroporation, etc. have been employed to insert drugs into exosomes (Xi et al. 2021).
Transfection
Transfection is the process where different molecules like drugs, therapeutic proteins, or oligonucleotides can be loaded into exosomes by using chemical or biological reagents. Exo-fect, a commercialized exosome transfection kit, is very effective at transferring small molecules and nucleic acids straight into exosomes. Biological agents can also mediate transfection, for example the transfer of pre-miRNA (LV-miR) precursor molecules by lentivirus (Kim et al. 2018). Therapeutic molecules can be packaged into the exosomal lumen using transfection efficiency, and the molecular stability of this method is higher than with other loading methods, but the efficiency is not fixed. Sometimes the issue of inadequate packaging has been reported. Transfection agents may alter gene expression in exosomes produced from donor cells. These may result in altered biological activities of loaded nucleic acid agents. So, this is a big concern for the safe application of transfection methods which are also seriously taken due to some reported toxicity of transfection agents (Antimisiaris et al. 2018; Xi et al. 2021).
Incubation
Two types of incubation methods have been developed so far. The first is the direct incubation of drugs and exosomes, allowing the drug to interact with the lipid layer of the exosome membrane. The second type involves incubation of drugs with donor cells of exosomes and then the isolation of drug-carrying exosomes. The latter is mainly intended for small molecule chemicals or drugs having low cytotoxicity. However, none of the methods was reported to have good loading efficiency (Xi et al. 2021). A co-incubation method is also effective for loading therapeutic RNA into exosomes along with hydrophobically modified small interfering RNAs (Didiot et al. 2016).
Saponin-assisted loading
There are some chemicals that can produce holes/pores to increase cell membrane permeability by different mechanisms. Among these saponin, a type of natural glycoside is an efficient membrane-penetrating agent, able to make pores in exosome membrane-forming complexes with membrane cholesterol (Orczyk and Wojciechowski 2015). Saponin treatment does not alter the size distribution or zeta potential, which is common in extrusion, hypotonic dialysis, and some other loading techniques (Fuhrmann et al. 2015). However, there are limitations to the in vivo application of saponins due to the hemolysis effect. Thus, exosomes must undergo a purification procedure after incubation with saponins (Lin and Wang 2010).
Sonication
Donor cells or target cell-derived exosomes are mixed with drugs of interest and sonicated by a homogenizer probe. Exosomal membrane deformation is mediated by sonication-induced mechanical shear. This allows the deformed exosomes to flow across the relatively tight lipid layer and release the drug at the target site. After ultrasound treatment, the exosomes were incubated to restore the integrity of the membrane. There is a chance of exosomal aggregation (Xi et al. 2021). Efficient drug loading into the macrophage-released exosomes by sonication has been shown to favor the sustained release of the loaded drug (Kim et al. 2016). Loading efficiency is larger, using ultrasound than incubation (Salarpour et al. 2019).
Extrusion
This method has the merit of high drug loading efficiency and production of a uniformly sized mixture of exosomes where the drug is loaded into a lipid extruder with a porous membrane (aperture: 100–400 nm). When porphyrins of different hydrophobicities were loaded into exosomes with the help of a syringe-based mini-extruder equipped with polycarbonate membranes (400 nm pore), a notable result was found that the extruded exosomes were cytotoxic. In contrast, no significant toxicity has been reported or published when the same porphyrin is loaded by other techniques (Fuhrmann et al. 2015).
Freeze–Thaw cycles
This strategy involves the incubation of exosomes with a targeted drug at room temperature or at 37 °C for the optimum time experimentally checked to aid higher encapsulation of drugs. After that rapid freezing at a very low temperature (− 80 °C) or in liquid nitrogen, subsequently, the mixture is thawed at RT again (Sato et al. 2016; Kumar et al. 2022). Thus freeze–thaw cycles are repeated for better drug encapsulation. The encapsulation efficiency of loaded cargo depends upon the number of repetitive cycles, which are regulated by key components of the exosomes. Few studies have reported exosome aggregation promoted by freeze–thaw cycles, leading to a high polydispersity index of the drug-loaded exosomes (Haney et al. 2015; Sato et al. 2016).
Electroporation
Through electroporation, small pores for the passage of therapeutic cargoes in the membrane of exosomal vesicles are created by disturbing the phospholipid bilayer under the action of an electric field. This method is the preferred one to load large nucleotides, such as siRNAs or miRNAs into exosomes, but care should be taken during the loading as it is prone to reducing drug loading efficiency, being vulnerable to RNA precipitation or exosome aggregation (Xi et al. 2021). Exosome structural distortion/aggregation may happen if an improper electroporation buffer system is used. The retention of the original exosome size and prevention of aggregation can be obtained by using a trehalose pulse medium (TPM) when aggregation is induced by cytomix electroporation buffer (Johnsen et al. 2016). TPM has allowed heavy cargo loading into exosomes like SPION5 (superparamagnetic iron oxide nanoparticles) which provides advanced opportunities in targeted drug delivery and theranostics investigations (Hood et al. 2014).
Surface modification of exosomes
Exosome biodistribution and cell-targeting capabilities are integrated with several proteins expressed on the exosome surface. Exosome components can be engineered either at the cellular level (involving parenteral cell-based engineering) or subjected to modification after their isolation (post-isolation engineering). At the cellular level modification, one approach is to develop a modified exosome membrane protein by cellular transgene expression by the coding sequence insertion of the desired ligand into the sequence frame of the signal peptide and the peptide –NH2 terminus of specific membrane protein (Mentkowski et al. 2018). The ultimate goal to search for an efficient technique or suitable ligand for surface protein modification is to attain targeting specificity and efficiency to the tissues and cell types of interest.
Aptamer conjugation, peptide modification and genetic engineering of exosomes
When drug-loaded exosomes with or without surface modifications had been compared, about 3 times increased tumor inhibition was there with aptamer–drug–exosome than with drug-exosome because of high affinity for the overexpressed receptors on tumor. C26 tumor ectopic model has shown a higher therapeutic index of Aptamer–doxorubicin@exosome compared to doxorubicin@exosome (Bagheri et al. 2020). Similar kinds of selective and potent toxic effects have been found in leukemic CEM cells by diacyllipid–aptamer sgc8-decorated doxorubicin-loaded exosomes (immature dendritic cell-derived) (Zou et al. 2019). Targeting the disease environment is critical to obtain the maximum therapeutic benefits of drug candidates. It has been observed that intravenous injection of aptamer functionalized exosomes was efficiently internalized in the bone marrow of mice suffering from postmenopausal osteoporosis. Further, there was an increase in bone mass via osteogenesis. Similar way it accelerated callus tissue formation in a femur fracture mouse model. So, it found to be a promisable tool for targeting different bone diseases (Luo et al. 2019). A synergistic improvement of disease can be attained by attaching an aptamer to an exosome. 5′ modified LJM-3064 aptamer conjugation to bone marrow mesenchymal stem cell-derived exosomes has shown to exert synergistic immunomodulatory action and remyelination effect. This suggests that exosomes can be a good candidate in the advanced therapy of neurodegenerative diseases (Shamili et al. 2019).
Intravenous injection of exosome mimetics from U937 cells (engineered with WQPDTAHHWAT antispecific membrane antigen peptide) has shown greater tumor accumulation in prostate cancer (Severic et al. 2021). TRAIL-engineered exosomes (by novel non-virus vectorPEI25Pyr50%) demonstrated a significant decrease in tumor size via intravenous administration and higher delaying of tumor growth upon multiple-dosing than single-dose administration (Shamili et al. 2018). Besides chemotherapy, a new possibility has arisen in cancer immunotherapy where covalent anchoring of CP05 peptide to tumor-derived exosomes shown to heighten the immunogenicity of DCs when pulsed to dendritic cells. Persistent antitumor immunity was contributed by increased protective T cell memory response as well as increased homing to lymphoid tissues (Yuan et al. 2017; Zuo et al. 2020).
Modification of exosomes with biotinylated CpG DNA by genetic engineering exhibited strong antigen-specific immune response and antitumor activity when given as intradermal vaccination (Morishita et al. 2016). Preclinical study results are significantly indicating the modified exosome system as a new star in cancer therapeutics. Further assessment of safety and pharmacokinetic evaluation can confirm its clinical translation. Efficient internalization of MSC-derived exosomes in the hypoxia injured H9C2 cells has been obtained after lentivirus transduction directed genetic modification of exosome membrane protein with CSTSMLKAC peptide fusion (Wang et al. 2018). Based on this evidence, exosomes as novel drug carrier can be explored for different cardiac issues related to ischemia, and myocardial infarction.
Surface coating and complexation of exosomes
Surface coating of the exosomes with a suitable modifier, for example polydopamine, makes the exosome tailorable for reactive group functionalization via secondary reactions which opened new avenues for novel applications extending toward multidrug therapy or theranostic approach (Wang and Douglas 2021). Electrostatic complexation of fusogenic peptide (GALA) and cationic lipid on exosomes exerted increased cytosolic delivery of dextran, a large molecule with enhanced bioactivity of a ribosome-inactivating protein. This can be extended to develop an intracellular visualization tool (Nakase and Futaki 2015). Anchoring of SPMN (superparamagnetic nanoparticle cluster) to exosomes has been shown effective sustained delivery of active molecules in the specific region even with low blood velocity over a prolonged period. So, possible expansion in biomedical applications has been further strengthened by magnetic nanoparticle technology (Qi et al. 2016).
Surface labeling of exosomes
Surface modification of exosomes by different labeling moieties is especially significant to studying their in vivo fate (Salunkhe et al.2020), the internalization mechanism of exosomes in cancer cells vs. normal healthy cells (Greco et al. 2016) and evaluating the effect of routes of administration on the biodistribution (Kim et al. 2007). In light of the above-mentioned biodistribution, it is important to note that surface charges and lipophilicity of exosomes are indeed critical for the overall kinetic profile, controlling the translocation rate, hepato-billiary and renal excretion, and specific uptake by distinct immune cells. Labeling of exosomes from human lung adenocarcinoma cells with dye via in situ biorthogonal click chemistry enabled the preservation of intrinsic tumor-homing functions as well as allowed effective in vivo tracking of exosomes, confirmed by fluorescence imaging and quantification (Song et al. 2020). Even azide functionalized AlexaFluor®488 fluorophore labeling has been shown to favor a higher accumulation of exosomes in pancreas tumor mass (Xu et al.2020a). Thus, labeling of exosomes is a remarkable strategy for theranostics development due to the absolute specificity of the system.
Exosome-based drug delivery systems
Exosomes are in the emerging research trend in diagnosis and therapy, being a winner to overcome many of the obstacles observed with the other nanovesicular delivery systems as discussed in earlier introduction section. Some reported studies to unlock the power of exosomes to deliver therapeutic molecules in different diseases are listed in Table 1.
Exosomes as delivery vehicles for therapeutic small molecules
Natural and engineered exosomes have been proved for having access to penetration through several barriers including blood–brain, blood–labyrinth, blood–air, blood–lymph, blood–retinal, placental, and stromal barriers. This provides state of the art of delivery vehicle design. Among this extensive research has been carried out to deliver drugs like small molecules and nucleic acids to the brain and to the tumor region, explaining their transport mechanisms using a different animal model (Elliott and He 2021).
Exosomes as delivery vehicles for therapeutic large molecules
Exosomes are also reliably good delivery vehicles for large molecules like proteins and peptides in addition to small molecules. A novel nanocarrier has been developed to deliver enkephalin (a pentapeptide) across the BBB for reducing cerebral apoptosis and neuronal recovery. Enkephalin-loaded exosomes containing transferrin were increased to 223.5/mm2 and 492.5/mm2 after 4 h and 12 h, respectively, in the ischemic zone of the brain and available at a sufficient amount than exosome–enkephalin without transferrin to exert protective effect (Liu et al. 2019b). An antibody–drug conjugate [Trastuzumab emtansine (T-DM1)] delivery vehicle has been prepared from HER2-positive cancer cells, shown to have significant growth inhibitory action and caspases three and/or seven activations (Barok et al. 2018). This research can be further extended to design noninvasive stable insulin delivery devices using exosomes.
Exosomes as the delivery vehicle for natural products
Experimental campaigns are going on to evaluate the suitability of exosomes as natural carriers for natural products. The incorporation of curcumin in the exosome helped to overcome some barriers related to the clinical use of curcumin due to poor systemic bioavailability, solubility, and stability. Intraperitoneal injection of exosome curcumin has shown a higher level of curcumin in plasma even after 12 h of post-injection. However, there was no detectable curcumin in the blood when it was injected without exosome encapsulation which suggests that exosome increases the systemic stability of curcumin. The in vivo assessment indicates that mice given exosome-loaded curcumin have lower mortality than the liposome-loaded curcumin of equivalent concentration treatment. The data revealed that such efficacy of protecting mice from LPS-induced septic death is due to enhanced cellular uptake of curcumin (Sun et al. 2010). Several anticancer natural molecules and herbal bioactive constituents have been shown to exert increased apoptotic effect when loaded in exosomes, for example triptolide-loaded exosomes in ovarian cancer (Liu et al. 2019a) and celastrol-loaded exosomes in lung cancer (Aqil et al. 2016).
Exosomes as protein delivery vehicles
Therapeutic proteins loading in a carrier in adequate quantity for optimal therapeutic efficacy is challenging to a formulation scientist while keeping the protein intact and stable. Brain delivery of protein is one of these challenges due to physiological or anatomical barriers, and the large size and high molecular weight of protein to load in a carrier in adequate quantity for optimal therapeutic efficacy. A human cerebral microvascular endothelial cell model study has shown that the macrophage exosomes are able to penetrate the brain and deliver brain-derived neurotrophic factors in inflamed conditions after intravenous administration (Yuan et al. 2017). A recent study showed that exosomes loaded with the antioxidant protein catalase (exoCAT) resulted in an improved state in Parkinson’s disease by being able to deliver loaded elements across the BBB (Haney et al. 2015).
An optogenetically engineered exosome system (EXPLORs) from HEK293T cells has been developed by a group of scientists, where protein, namely mCherry-CRY2 protein (84 kDa), has been loaded through the endogenous biogenesis process (via optically reversible protein–protein interactions in presence of continuous blue light illumination) allowing controllable detachment of proteins inside target cells. Most interestingly, EXPLORs can be upgraded to personalized protein-based therapy (Yim et al. 2016). A novel genetically encoded pseudotyping approach has also been adopted to facilitate efficient loading of therapeutic protein, using a set of virus glycoproteins fusion reporters, resulting in a dramatic increase in exosome uptake by a variety of mammalian cells (Meyer et al. 2017).
Exosomes as nucleic acid delivery vehicles
It has been discussed previously that exosomes naturally carry nucleic acids and have a natural ability to deliver these to cells. These features are in the investigation to be used in different sets of treatments involving genetic therapy. It is providing hope to fill the shortage of gene delivery vehicles that are non-immunogenic to host, efficient, and target-specific (Koppers-Lalic et al. 2013). Some of the studies to evaluate the usefulness of exosomes to deliver exogenous genetic material successfully and their functionality are mentioned in Table 1.
Safety and pharmacokinetic aspects of exosomes
The cellular origin of exosomes is one of the most important variables influencing their biodistribution and toxicity. Tumor-derived exosomes have been demonstrated to be effective at delivering anticancer therapies to their parental tumor (Yang et al. 2015). While still tumor-derived exosomes provide tumor-targeting advantages, these can pose safety concerns when injected systemically: Tumor-derived exosomes may supply tumorigenic stimuli to normal tissues while also promoting tumor spread by commencing pre-metastatic niche development in normal tissue (Guo et al. 2019).
Mirzaaghasi et al. have discovered that a large percentage of exosomes were transported to the lung in sepsis-induced mice following intravenous infusion, with more than 30% transferred to the lung after 1 h after injection, but nearly none were observed in the lung of healthy mice. Exosomes were also shown to be retained in the bloodstream for an extended period of time owing to liver failure (Mirzaaghasi et al. 2021). Clearance of fluorophore-labeled exosomes (intravenous) from blood in normal mice was 0.0054–0.0154 mL/min (Hwang et al. 2019), whereas Gaussia luciferase (gLuc)-lactadherin (LA) labeled exosomes (intravenous) in macrophage-depleted mice (Imai et al. 2015) and 125I labeled exosomes (intravenous) in Parkinson’s disease mouse model (Hwang et al. 2019) were around 0.651 mL/h and 0.016 mL/min, respectively. The distribution of the payload after systemic administration has been shown to involve different major organs; although the extent of distribution is largely variable based on the exosome membrane chemistry, its origin represents the molecular signature necessary for cellular interaction as well as the pathophysiological condition of the subject. Biodistribution studies have been made aware that the injection dose of the exosomes exceeding 400 μg leads to unwanted aggregation and accumulation in the lungs followed by asphyxiation of the test animal (Fu et al. 2020). To distribute therapeutic exosomes to specific cells or tissues, either active or passive therapeutic exosome targeted techniques can be used. Passive targeting of exosomes makes use of exosomes' natural cellular tropism, whereas active targeting accomplishes targeted delivery of exosomes via exosomal surface modification using numerous technological strategies. However, in both strategies, the route of administration plays a major role in desired effects as a few examples are enlisted in Table 2.
Bioluminescence and fluorescence imaging are now the most widely used techniques for evaluating the in vivo behavior of given exosomes. Considering recent technical breakthroughs in deep tissue penetration imaging, alternative clinical imaging modalities such as magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography are now being used for exosome biodistribution and PK research (Sokolova et al. 2011).
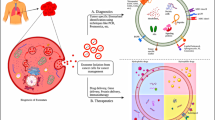
For the purpose of visualization, exosomes are labeled directly or indirectly by different dyes like fluorescent proteins—RFP (red fluorescent proteins), GFP (green fluorescent proteins) lipophilic fluorescent dyes—cyanine 7 (Cy7), PKH26, DiOC18(7)(DiR), DiIC18(5)(DiD), by luminogens, radiolabeling agent—99mTc-HMPAO, 111-indium/125-iodine, bioluminescent imaging labeling agent—Gaussia luciferase, immunofluorescent molecule—antibody + dye, magnetic contrast agents—superparamagnetic iron oxide nanoparticles (SPIONs), gold–iron nanoparticles (GIONs). Even gold nanoparticles are used as a labeling agent for Computed tomography (Sokolova et al. 2011). The available in vivo noninvasive imaging methods of the present to track the exosome biodistribution and metabolic transfer are shown in Fig. 3.
There are mostly available ADME case studies are based on the systemic administration of the exosomes. Intravenously injected unmodified exosomes from PC3 and MCF-7 cell origin have shown to be cleared from the systemic pool in a rapid manner in the case of both healthy and tumor-bearing mice, which is attributed due to the slower uptake of the exosomes by the reticuloendothelial system and may also be contributed by complement opsonization along with innate immunity system (Wang et al. 2009; Fu et al.2020). However, upon intratumoral injection, these are shown to be accumulated inside the tumor. This suggests that these kinds of exosomes are not suitable to be developed for the intravenous drug delivery system. More examples presented in Table 2 revealed the need to establish the pharmacokinetic behavior of exosome formulation along with an investigation of the effect of different routes. Fast tear turnover on ophthalmic application, salivation on buccal application, and body sweating limit exosomes’ topical or mucosal administration due to shorter half-lives and swift ejection. To overcome these barriers and achieve the supremacy of these routes, designing, and fabrication of exosome incorporated hydrogel matrix has been under investigation (Riau et al. 2019).
Challenges in production of exosomes
The availability of exosomes in global clinical platforms is only possible when there will be optimized, standardized protocols without deviating from the regulatory guidelines for large-scale manufacturing of exosomes. It is obvious that all the modern technology transfer-related issues will exist for the development of scale-up, including all the steps of process optimization, validation, performance qualification activities, and implementation of a fileable quality control testing program. The first challenge in the product development cycle is the limitation of scalable cell culture methods concerning controlling environmental parameters and reactor design or construction (Colao et al. 2018).
Challenges in upstream production
Large-scale upstream production demands large numbers of manufacturing devices like dozens of large volume flasks and multiple stacked array multilayer culture flasks, which increase cost expenses prior to the production phase. Moreover, these conventional platforms suffer from the limitation of high-density culture effects during scale-up where the cell division is limited by reduced availability or elimination of added growth factors or surface-active agents in the culture medium. The use of porous 3D scaffofiber, fiber-based packed bioreactor, and fixed bed bioreactor can ameliorate this issue, but a large volume bioreactor is required. Continuous cell culture for which hollow fiber perfusion bioreactors can also be employed to allow incessant addition of nutrients and removal of waste products but retain exosomes and oversized products (Colao et al. 2018; Whitford and Guterstam 2019).
Concerning stem cell culture progression (cellular differentiation, apoptosis ability), exosome production efficiency is dependent on the maximum surface area for maximum efficiency. The large surface area will allow the parent cells to be in maximal contact with the culture media for efficient nutritional transfer. With regard to the adaptation of perfusion-based production, technology can increase the gross production of exosomes by minimizing manual interference as an alternative one to the current T-flask culture. Higher surface area can be provided by using hollow fiber bioreactors or stirred bioreactors which allow sufficient cell-to-microcarrier binding for next-level transformation. However greater control over the endo-environment of the bioreactor during the whole process is very stringent to prevent the phenotype changes in cells due to generated shears or stresses by impeller mixer or oxygen sparging. A probable way to prevent this risk is the use of membrane-based flasks/bioreactors. Although the newly designed bioreactors permit continuous biomanufacturing over a longer period in a closed controlled environment, there is a need to verify culture conditions at time intervals whether are reproducible or not to ensure consistency in the exosome population (Colao et al. 2018; Whitford and Guterstam 2019).
Challenges in downstream processing for efficient purification
There are no available techniques that can completely discriminate exosomes beyond size or density which encourages co-isolation of other vesicles or impurities sharing overlapping attributes, which may lead to more trouble when there is a higher cell death rate in large-scale production. Need to develop purification methods based on biochemical and biophysical characteristics of exosomes in parallel to the current physical methods like the use of immunoaffinity method using antibody-conjugated magnetic beads (but the problem may arise to remove beads from large-scale purification systems and demands for special equipment). Another way is to shift toward advanced purification platforms like tangential flow filtration, hollow fiber ultrafiltration coupled to microfiltration, etc. However, there should not be any associated yield loss or disturbance of exosome vesicle integrity. A robust virus filtration mode as a final step should be designed before undergoing the drug loading process (Colao et al. 2018; Whitford and Guterstam 2019).
For large-scale production, one of the possible solutions is continuous cell culture for which hollow fiber perfusion bioreactors can be employed which allows continuous addition of nutrients and removal of waste products but retain the exosomes, oversized secreted products (Whitford and Guterstam 2019).
Challenges in the preservation and stability of exosomes
Presently, the exosomes are preserved and protected by three methods, namely freezing or cryopreservation, freeze-drying, and spray-drying. In cryopreservation, exosomes are stored below the temperature (usually 4 °C, − 80 °C, and − 196 °C) at which there will be no possible chemical reaction/degradation or loss of functionality. The integrity of exosomes isolated from biological fluids (e.g., plasma) can be retained maximally for up to 5 days in a glass container, whereas; a safe storage period is longer (20 months) for exosomes from human saliva without enzymes, and 28 days in the presence of salivary enzymes at the same temperature of 4 °C (Kumeda et al. 2017). This indicates the complexity of the storage issues. Therefore, to expand the clinical use of exosomes, there is a need for more in-depth knowledge on stability for long-term preservation and more research on preservation techniques which must be relatively cost-efficient. Lyophilization preservation technology stores exosomes in liquid nitrogen in the presence of trehalose (cryoprotective agent) has been shown to prohibit aggregation and increase colloidal stability (Zhang et al. 2020). Other protectants, such as DMSO (1%) and glycerin (5%), cannot protect the exosomes fully from degradation and even sometimes lead to lysis. Structural and physicochemical properties of the exosomes could be affected by pressure, nature of the solvent used in the different preservation processes, repetition of the freeze–thaw cycle, and storage duration (Charoenviriyakul et al. 2018; Akuma et al. 2019).
Current scenario and future prospect of exosomes
At present, the industry is witnessing a surging number of scientific publications investigating exosomes and their potential applications along with rising numbers of clinical trials (investigating exosomes as a therapeutic and diagnostic tool). If the studies result in positive outcomes exosomes will serve as a blueprint for new drug delivery vehicles. A list of clinical trials completed and currently registered ongoing trials involving exosomes as therapeutic agents or drug delivery tools is summarized in Table 3.
Recently, the Aruna Biocompany has decided to commercialize pharmaceutical exosomes for CNS-specific drug delivery and is also seeking a solution for efficient large-scale production of exosomes to meet the future market demand. A new neural-derived exosome, namely AB126, has been introduced by ArunaBio which has been found to unlock the BBB. It is having the dual advantage of innate therapeutic properties (like neuroprotection, antineuroinflammation, and neuro-regeneration) and innate functional properties (neural cell targeting). This can deliver monoclonal antibodies, proteins, siRNAs, and allele-specific oligonucleotides by breaching the BBB. Exosome-based delivery platforms of this company, for example AB127 (siRNA), AB128 (protein), and AB129 (mRNA), are in the development pipeline (Tenchov et al. 2022). An antigen-presenting cell-targeting agent exoSTING has been developed by Codiak, where interferon gene stimulators were loaded inside the exosome lumen. It has also been shown to preserve memory responses against tumor cells. Tumor macrophage-targeting exosome product—exoASO™-STAT6—has been also engineered by Codiak (Lee and Kim 2021). Exosomal topical suspension for diabetic ulcers chronic wounds, and exosomal eye serum for age-related macular degeneration, and glaucoma are in progress for clinical availability by Vittilabs. Many research articles have reported the beneficial role of exosomes as ocular pharmacotherapeutics for age-related macular degeneration, autoimmune uveoretinitis, corneal injury, Sjögren’s syndrome, diabetic retinopathy, retinal ischemia, etc. (Li et al. 2019; Zhang et al. 2021). The scientific teams of evox therapeutics are engaged in developing protein engineering technology especially to load proteins in the exosome and nucleic acids as well which will allow the display of loaded cargo to the specific organ. Evox has also established a portfolio for intellectual property (IP) and also made safeguards to protect its IP by trademarks, copyrights, and trade secrets for its different exosome DDS assets (intended to deliver mRNA, siRNA, antibodies, receptors, antisense oligonucleotides, enzyme replacement therapies, CRISPR technologies by tailored targeting technology) and for proprietary cellular engineering technology, upstream process optimization, downstream processing. Few other leading companies like TEVAC Pharmaceuticals, OmniSpirant, ILIAS, XOSTEM, etc. are also engaged in solving the engineering and manufacturing challenges enabling the production of transformative exosome medicine with reproducibility. Most highly representative diseases for which companies are expanding their therapeutic research on exosomes are bone diseases, fibrotic diseases, alopecia, cardiac diseases, skin problems, gastrointestinal disorders, kidney diseases, rare genetic disorders, autism spectrum disorder, neurodegenerative diseases, cancer, diabetes, wounds as well as in transplant rejection (Tenchov et al. 2022).
In a recent non-randomized open-label cohort research, EXOFlo, an exosomal-based product generated from bone marrow, was evaluated for the treatment of severe COVID-19. No adverse reactions were noticed within 72 h of treatment in the twenty-four patients who received these exosomes. Exosome therapy was seen to significantly improve these patients' conditions by restoring oxygen storage capacity, reducing cytokine storm, and boosting immunity. This work, however, is the first of its type to assess the COVID-19 therapeutic potential of exosomes produced from MSCs (Sengupta et al. 2020). In a recently completed study, authors evaluated the effects of systemically delivered MSC exosomes in a mouse model of acute lung damage caused by E. coli endotoxin. These exosomes were observed to decrease pulmonary edema and lung protein permeability while reducing extravascular lung water by 43%. Later, the team showed that these exosomes might improve alveolar fluid clearance (AFC) (Zhu et al. 2014). These exosomes found as a novel approach to lung disease treatment and prevention because of the regenerative immunomodulatory cargo they carry and the antiviral medications they combine with. In this direction, Fortrexo CoV is an antiviral therapy that has been designed by ExoPharm [Products (exopharm.com)] where naive cell-derived EVs have been engineered by attaching CoV Spike on EV Surface that targets ACE2 + cells. Using the LOAD technology, RNAi has been loaded into the Fortrexo CoV that disrupts the replication of SARS-CoV-2 within a cell, enabling a reduction of duration and severity of COVID infection. In comparison with liposomes, recombinant SARS-CoV-2 receptor-binding domain (RBD) coupled to lung-derived exosomes can improve the RBD's retention in the mucus-lined respiratory airway and in the lung parenchyma (Wang et al. 2022). This design can also be mimicked to target other RNA viruses responsible for rabies, dengue, hepatitis C, Ebola, etc. This novel approach shows the ability to obtain vaccine templates to transfer antigens, drugs, and/or stimulating factors from immune cell-derived exosomes and even from patient-derived exosomes by integrating them into a customer-grade 3D printer (Zhao et al. 2019).
The potential application of exosomes as drug delivery agents is not restricted to targeting specific diseases or a specific delivery site in the body. Exosome technology is now engaged to improve the solubility issues of hydrophobic drugs and modify the dissolution rate. Aspirin, a well-known over-the-counter drug has been transformed to a promising anticancer moiety by converting it into a hydrophilic–hydrophobic nanostructure using an amphiphilic polymer matrix. The nanotransformed amorphous aspirin–polymer pre-exosomes when incubated with exosomes, it can embed in both hydrophilic and lipophilic sections of the exosome vesicles which eventually increases the encapsulation efficiency of the drug. Exosomes have been shown to exhibit higher toxicity to their respective parent cancer cells (breast cancer and colorectal cancer) than the pre-exosomes of aspirin along with the increased magnitude of solubility by approximately 40-fold. However, nanoamorphous exosomes is very less toxic to normal intestinal cells (Tran et al. 2019a). Thus, carefully balancing the drug’s hydrophilic and hydrophobic properties, selecting the right pre-exosome components, and creating a specified nanostructure for that drug will appear to build the groundwork for a good outcome.
The comprehensive review on exosomes directs that future research must focus more on the research gaps specially in vivo studies to shed light on the differential exosome responses of communication between normal and malignant cells, to decipher exosomal transport, and physiological controls exercised by target cells. Furthermore, more systematic in vivo studies should be carried out to understand the toxicology elaboratively and to direct the therapeutic potency, which could help to transform these novel nanoplatforms to a footstep closer to clinical application. Although exosome-based clinical trials are in an infant stage, unable to meet the advanced understanding of clinical transition issues. Even at the stage of preclinical evaluation, it is critical to select and develop a proper animal model that will also meet economic sustainability. But in this instance, organ-on-a-chip can be a powerful, robust tool to look further into the mechanism of action of the exosome, its ability for drug delivery, and assess its potency (Wu et al. 2022). Exosomes are not bounded to animals but plants and plant parts, fruit juices have been found to be a potential origin (Yang et al. 2020b; Bruno et al. 2021). Exosomes from grapes can be used to treat oral mucositis brought on by chemotherapy or radiation. In a preclinical investigation, it has shown that grape-derived exosomes could regenerate intestinal tissue and take part in tissue remolding after pathological damage (Ju et al. 2013). Another clinical trial study was enrolled to investigate the effects of exosomes from ginger or aloe for the treatment of polycystic ovarian syndrome patients in the hope that it would reduce insulin resistance and chronic inflammation (trial number NCT03493984). Additionally, Donar Millar et al. employed plant-derived exosomes as a hydrophobic drug delivery carrier to encapsulate curcumin for the treatment of intestinal disorders due to the hydrophobic nature of molecules (NCT01294072) (Chen YS et al. 2019). More efforts should be given for cell free sources or to produce from animal free sources to make the exosome isolation process more easier.
It has been observed that most of the exosomes that eventually get the ticket for human clinical studies are derived from mesenchymal stem cells or other types of stem cells. So, there are an increased number of biobanks for the storage of stem cells worldwide to satisfy the exponentially growing demands of more and more advanced research. With the need, the science of biobanking is not only concerned about the exosomal origin but also focused on the exosome lifecycle, including collection, storage, and distribution. Indeed, the information about the exosomal life cycle will fill the gap of lack of information on some processes, transport variables, isolation yields, preclinical parameters, etc. that are unavailable in the literature. Thus, biobank operation plays a central role in between bench research and clinical application transition by helping to overcome the current exosome research hurdles (Mora et al. 2015). However, there is a need for more regulated standardized protocols with precise data archiving for biobanking to assure the quality of exosomes. Just as importantly the adverse reaction should be monitored after the exosomes are getting entry into the market (Nisa et al. 2020).
Conclusion
Exosomes provide an enormous promise in the area for delivery of a diverse range of synthetic, natural as well as biological molecules. Exosomes are in vogue due to some benefits over conventional nanovehicles like avoidance of homing in the liver or metabolic degradation and no undesired accumulation before reaching target sites. The exosomes with a well-established long-term safety profile and with a proved inherent ability to carry intercellular therapeutic molecules and nucleic acids across membranes barriers like BBB direct its practical relevance. But before these drug delivery systems are revealed as clinical reality, thorough characterization and in-depth clarification of immunological reactions are needed.
The point to be highlighted is the regulatory aspects that have not been tackled thoroughly to date for exosome therapeutics. The linking the gap between nature, synthetic biology, and biophysics we can uphold the viability of exosomes into safe, personalized drug delivery systems which will also vindicate the therapeutic reconfiguration and clinical evolution. Although considerable technological hurdles need to be addressed to generate clinical-grade exosomes, the activities of the leading firms creating exosome medicines will pave the road for mastering the technical and scientific problems. Finally, we may propose exosome as an appealing next-generation therapeutic platform for treating a variety of human disorders with unmet medical needs. However, the scale-up manufacturing lifecycle is still complicated. Scientists are looking for suitable modifications of conventional platforms for isolation of consistent exosome population, efficient purification, quantification and quality checking, and simultaneous evaluation of exosomes’ long-term stability. The journey of exosomes drug delivery systems from laboratory to market, which is already started with baby steps, needs more control with respect to regulatory aspects and clinical trials.
Data availability
As the manuscript is a review article, data are collecetd from published work from different sources.
Abbreviations
- AF4:
-
Asymmetric flow field-flow fractionation
- ADME:
-
Absorption, distribution, metabolism and excretion
- BBB:
-
Blood–brain barrier
- BMSCs:
-
Bone marrow mesenchymal stem cells
- CNS:
-
Central nervous system
- DCs:
-
Dendritic cells
- DDS:
-
Drug delivery systems
- DMSO:
-
Dimethylsulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- EVs:
-
Extracellular vesicles
- ESCRT:
-
Endosomal sorting complex
- FACS:
-
Fluorescence-activated cell sorting
- ILVs:
-
Intraluminal vesicles
- LC–MS:
-
Liquid chromatography–mass spectroscopy
- LPS:
-
Lipopolysaccharides
- miRNAs:
-
MicroRNA
- MSCs:
-
Mesenchymal stem cells
- MVBs:
-
Multivesicular bodies
- NTA-Ni:
-
Nitriloacetic acid-Nickel
- PKPD:
-
Pharmacokinetic pharmacodynamic
- RT:
-
Room temperature
- SEC:
-
Size-exclusion chromatography
- siRNAs:
-
Small interfering RNAs
References
Akuma P, Okagu OD, Udenigwe CC (2019) Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2019.00023
Alvarez ML (2014) Isolation of urinary exosomes for RNA biomarker discovery using a simple, fast, and highly scalable method. Methods Mol Biol 1182:145–170. https://doi.org/10.1007/978-1-4939-1062-5_13
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Amiri A, Bagherifar R, Ansari Dezfouli E, Hossein Kiaie S, Jafari R, Ramezani R (2022) Exosomes as bio-inspired nanocarriers for RNA delivery: preparation and applications. J Transl Med 20:125. https://doi.org/10.1186/s12967-022-03325-7
Andriolo G, Provasi E, Lo Cicero V et al (2018) Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Front Physiol 9:1169. https://doi.org/10.3389/fphys.2018.01169
Antimisiaris SG, Mourtas S, Marazioti A (2018) Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 10:218. https://doi.org/10.3390/pharmaceutics10040218
Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga A-H, Munagala R, Gupta R (2016) Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol 101:12–21. https://doi.org/10.1016/j.yexmp.2016.05.013
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R (2017) Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J 19:1691–1702. https://doi.org/10.1208/s12248-017-0154-9
Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M (2020) Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci 261:118369. https://doi.org/10.1016/j.lfs.2020.118369
Barok M, Puhka M, Vereb G, Szollosi J, Isola J, Joensuu H (2018) Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer 18:1–12. https://doi.org/10.1186/s12885-018-4418-2
Bruno SP, Paolini A, D’Oria V, Sarra A, Sennato S, Bordi F, Masotti A (2021) Extracellular vesicles derived from Citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Front Nutr 8:778998. https://doi.org/10.3389/fnut.2021.778998
Bunggulawa EJ, Wang W, Yin T et al (2018) Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol 16:1–13. https://doi.org/10.1186/s12951-018-0403-9
Burkova EE, Sedykh SE, Nevinsky GA (2021) Human placenta exosomes: biogenesis, isolation, composition, and prospects for use in diagnostics. Int J Mol Sci 22:2158. https://doi.org/10.3390/ijms22042158
Butreddy A, Kommineni N, Dudhipala NJN (2021) Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials 11:1481. https://doi.org/10.3390/nano11061481
Capello M, Vykoukal JV, Katayama H et al (2019) Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat Commun 10:1–13. https://doi.org/10.1038/s41467-018-08109-6
Charoenviriyakul C, Takahashi Y, Nishikawa M, Takakura Y (2018) Preservation of exosomes at room temperature using lyophilization. Int J Pharm 553(1–2):1–7. https://doi.org/10.1016/j.ijpharm.2018.10.032
Chen P, Zheng L, Wang Y et al (2019a) Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9:2439. https://doi.org/10.7150/thno.31017
Chen YS, Lin EY, Chiou TW, Harn HJ (2019b) Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi 32(2):113–120. https://doi.org/10.4103/tcmj.tcmj_182_19
Cheruvanky A, Zhou H, Pisitkun T et al (2007) Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol 292:F1657–F1661. https://doi.org/10.1152/ajprenal.00434.2006
Cho E, Nam G-H, Hong Y, Kim YK, Kim D-H, Yang Y, Kim I-S (2018) Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Control Release 279:326–335. https://doi.org/10.1016/j.jconrel.2018.04.037
Colao IL, Corteling R, Bracewell D, Wall I (2018) Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med 24:242–256. https://doi.org/10.1016/j.molmed.2018.01.006
Cvjetkovic A, Lötvall J, Lässer C (2014) The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles 3:10. https://doi.org/10.3402/jev.v3.23111
Delcayre A, Estelles A, Sperinde J, Roulon T et al (2005) Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis 35:158–168. https://doi.org/10.1016/j.bcmd.2005.07.003
Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A (2016) Exosome-mediated delivery of hydrophobically modified siRNA for Huntington mRNA silencing. Mol Ther 24:1836–1847. https://doi.org/10.1038/mt.2016.126
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8:727. https://doi.org/10.3390/cells8070727
Duechler M (2013) Vehicles for small interfering RNA transfection: exosomes versus synthetic nanocarriers DNA and RNA. Nanotechnology 5:5. https://doi.org/10.2478/rnan-2013-0002
Elliott RO, He M (2021) Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 13(1):122. https://doi.org/10.3390/pharmaceutics13010122
Escudier B, Dorval T, Chaput N, André F, Caby M-P et al (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 3:1–13. https://doi.org/10.1186/2F1479-5876-3-10
Fitzgerald J, Leonard P, Darcy E, Sharma S, O’Kennedy R (2017) Immunoaffinity chromatography: concepts and applications. Methods Mol Biol. https://doi.org/10.1007/978-1-4939-6412-3_3
Fu S, Wang Y, Xia X, Zheng JC (2020) Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 20:100261. https://doi.org/10.1016/j.impact.2020.100261
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029
Gao Z-S, Zhang C-J, Xia N, Tian H, Li D-Y, Lin J-Q, Mei X-F, Wu C (2021) Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater 126:211–223. https://doi.org/10.1016/j.actbio.2021.03.018
Gomari H, Moghadam MF, Soleimani M, Ghavami M, Khodashenas S (2019) Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomed 14:5679–5690. https://doi.org/10.2147/ijn.s210731
Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN (2016) PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology 91:241. https://doi.org/10.1016/j.urology.2016.01.028
Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z, Sun Z (2019) Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer 18:1–11. https://doi.org/10.1186/s12943-019-0995-1
Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H (2019) Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8:307. https://doi.org/10.3390/cells8040307
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6(4):287–296. https://doi.org/10.1016/j.apsb.2016.02.001
Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang JH, Zhang SJ (2022) Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer 21:207. https://doi.org/10.1186/s12943-022-01671-0
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
He R, Jiang Y, Shi Y, Liang J, Zhao L (2020) Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl 117:111314. https://doi.org/10.1016/j.msec.2020.111314
Hood JL, Scott MJ, Wickline SA (2014) Maximizing exosome colloidal stability following electroporation. Anal Biochem 448:41–49. https://doi.org/10.1016/j.ab.2013.12.001
Hwang DW, Jo MJ, Lee JH, Kang H, Bao K, Hu S, Baek Y, Moon HG, Lee DS, Kashiwagi S, Henary M, Choi HS (2019) Chemical modulation of bioengineered exosomes for tissue-specific biodistribution. Adv Ther (weinh) 2:1900111. https://doi.org/10.1002/adtp.201900111
Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y (2015) Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles 4:26238. https://doi.org/10.3402/jev.v4.26238
Jadli AS, Ballasy N, Edalat P, Patel VB (2020) Inside (sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem 467:77–94. https://doi.org/10.1007/s11010-020-03703-z
Jang Y, Kim H, Yoon S et al (2021) Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. JCR 330:293–304. https://doi.org/10.1016/j.jconrel.2020.12.039
Jeong K, Jeong S, Kim JA, Rhee WJ (2019) Exosome-based antisense locked nucleic acid delivery for inhibition of type II collagen degradation in chondrocyte. J Ind Eng Chem 74:126–135. https://doi.org/10.1016/j.jiec.2019.02.017
Jeppesen DK, Fenix AM, Franklin JL et al (2019) Reassessment of exosome composition. Cell 177:428–445. https://doi.org/10.1016/j.cell.2019.02.029
Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M (2016) Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 68:2125–2138. https://doi.org/10.1007/s10616-016-9952-7
Ju S, Mu J, Dokland T et al (2013) Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther 21(7):1345–1357. https://doi.org/10.1038/mt.2013.64
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2016) Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 79:360–369. https://doi.org/10.1016/j.biocel.2016.09.002
Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, Gopal A, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC (2018) A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol 9:1116. https://doi.org/10.3389/fphar.2018.01116
Khalid AD, Ur-Rehman N, Tariq GH et al (2023) Functional bioinspired nanocomposites for anticancer activity with generation of reactive oxygen species. Chemosphere 310:136885. https://doi.org/10.1016/j.chemosphere.2022.136885
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12:655–664. https://doi.org/10.1016/j.nano.2015.10.012
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M (2017) Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release 266:8–16. https://doi.org/10.1016/j.jconrel.2017.09.013
Kim R, Lee S, Lee J, Kim M, Kim WJ, Lee HW, Lee MY, Kim J, Chang W (2018) Exosomes derived from microRNA-584 transfected mesenchymal stem cells: novel alternative therapeutic vehicles for cancer therapy. BMB Rep 51:406–411. https://doi.org/10.5483/bmbrep.2018.51.8.105
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res Int. https://doi.org/10.1155/2018/8545347
Kooijmans SAA, Vader P, Dommelen SMV, Solinge WWV, Schiffelers RM (2012) Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed 7:1525–1541. https://doi.org/10.2147/ijn.s29661
Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM (2013) Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv Drug Deliv Rev 65:348–356. https://doi.org/10.1016/j.addr.2012.07.006
Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, Gupta RC, Agrawal AK (2022) Exosomes as emerging drug delivery and diagnostic modality for breast cancer: recent advances in isolation and application. Cancers 14(6):1435. https://doi.org/10.3390/cancers14061435
Kumeda N, Ogawa Y, Akimoto Y, Kawakami H, Tsujimoto M, Yanoshita R (2017) Characterization of membrane integrity and morphological stability of human salivary exosomes. Biol Pharm Bull 40:1183–1191. https://doi.org/10.1248/bpb.b16-00891
Lai RC, Yeo RWY, Padmanabhan J, Choo A, De Kleijn DP, Lim SK (2016) Isolation and characterization of exosome from human embryonic stem cell-derived C-Myc- immortalized mesenchymal stem cells. Methods Mol Biol 1416:477–494. https://doi.org/10.1007/978-1-4939-3584-0_29
Lamparski HG, Metha-Damani A, Yao J-Y et al (2002) Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 270:211–226. https://doi.org/10.1016/s0022-1759(02)00330-7
Lee JY, Kim HS (2021) Extracellular vesicles in regenerative medicine: potentials and challenges. Tissue Eng Regen Med 18(4):479–484. https://doi.org/10.1007/s13770-021-00365-w
Lee YR, Kim G, Tak WY et al (2019) Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer 144:1444–1452. https://doi.org/10.1002/ijc.31931
Li P, Kaslan M, Lee SH, Yao J, Gao Z (2017) Progress in exosome isolation techniques. Theranostics 7:789–804. https://doi.org/10.7150/thno.18133
Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, Hu C, Zhang L, Guo H, Gao S (2018) A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine 14:1973–1985. https://doi.org/10.1016/j.nano.2018.05.020
Li N, Zhao L, Wei Y, Ea VL, Nian H, Wei R (2019) Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res Ther 10(1):278. https://doi.org/10.1186/s13287-019-1372-0
Li J, Li N, Wang J (2020a) M1 macrophage-derived exosome-encapsulated cisplatin can enhance its anti-lung cancer effect. Minerva Med. https://doi.org/10.23736/s0026-4806.20.06564-7
Li Y-J, Wu J-Y, Wang J-M, Hu X-B, Cai J-X, Xiang D-X (2020b) Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater 101:519–530. https://doi.org/10.1016/j.actbio.2019.10.022
Li Z, Yuan Y, Zhang Z, Zhang X, Yang H, Li H, Han B, Deng Z, Zhou Z, Fan X (2023) Cell penetrating peptide modified M2 macrophage derived exosomes treat spinal cord injury and rheumatoid arthritis by loading curcumin. Mater Des 225:111455. https://doi.org/10.1016/j.matdes.2022.111455
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, Wang D, Xia J (2020) Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces 12:36938–36947. https://doi.org/10.1021/acsami.0c10458
Lim J, Choi M, Lee H, Kim Y-H et al (2019) Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnology 17:1–12. https://doi.org/10.1186/s12951-018-0433-3
Lin F, Wang RX (2010) Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. J Mol Model 16:107–118. https://doi.org/10.1007/s00894-009-0523-0
Lin S, Yu Z, Chen D et al (2020) Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16:1903916. https://doi.org/10.1002/smll.201903916
Liu C, Xu X, Li B, Situ B, Pan W et al (2018) Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett 18:4226–4232. https://doi.org/10.1021/acs.nanolett.8b01184
Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding C, Li H (2019a) The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 Cells. BioMed Res Int. https://doi.org/10.1155/2019/2595801
Liu Y, Fu N, Su J, Wang X (2019ab) Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/caspase-3. Biomed Res Int. https://doi.org/10.1155/2019/4273290
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J (2019c) Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov 5:1–7. https://doi.org/10.1038/s41420-019-0159-5
Liu X, Zong Z, Xing M, Liu X, Li J, Liu D (2021) pH-Mediated Clustering of Exosomes: Breaking Through the Size Limit of Exosome Analysis in Conventional Flow Cytometry. Nano Lett 21(20):8817–8823. https://doi.org/10.1021/acs.nanolett.1c03211
Livshits MA, Khomyakova E, Evtushenko EG et al (2015) Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 5:1–14. https://doi.org/10.1038/srep17319
Lu M, Zhao X, Xing H, Xun Z, Zhu S, Lang L, Yang T, Cai C, Wang D, Ding P (2018) Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm 550(1–2):100–113. https://doi.org/10.1016/j.ijpharm.2018.08.040
Luo Z-W, Li FX, Liu Y-W, Rao S-S, Yin H, Huang J, Chen CY, Hu Y, Zhang Y, Tan YJ, Yuan LQ, Chen TH, Liu HM, Cao J, Liu ZZ, Wang ZX, Xie H (2019) Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 11:20884–20892. https://doi.org/10.1039/c9nr02791b
Ma Y-S, Yang X-L, Xin R, Liu J-B, Fu D (2021) Power and promise of exosomes as clinical biomarkers and therapeutic vectors for liquid biopsy and cancer control. Biochim Biophys Acta Rev Cancer 1875:188497. https://doi.org/10.1016/j.bbcan.2020.188497
Mastoridis S, Bertolino GM, Whitehouse G, Dazzi F, Sanchez-Fueyo A, Martinez-Llordella M (2018) Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front Immunol 9:1583. https://doi.org/10.3389/fimmu.2018.01583
Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie 95:2229–2234. https://doi.org/10.1016/j.biochi.2013.04.017
McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, Kalluri R (2021) Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci Alliance 4:e202000875. https://doi.org/10.26508/lsa.202000875
McBride JD, Rodriguez-Menocal L, Guzman W, Candanedo A, Garcia-Contreras M, Badiavas EV (2017) Bone marrow mesenchymal stem cell-derived CD63+ exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis in vitro. Stem Cells Dev 26:1384–1398. https://doi.org/10.1089/scd.2017.0087
McNicholas K, Michael MZ (2016) Immuno-characterization of exosomes using nanoparticle tracking analysis exosomes and microvesicles. Methods Mol Biol 5:5. https://doi.org/10.1007/978-1-4939-6728-5_3
Melzer C, Rehn V, Yang Y, Bähre H, Jvd O, Hass R (2019) Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (basel) 11:798. https://doi.org/10.3390/cancers11060798
Mentkowski KI, Snitzer JD, Rusnak S, Lang JK (2018) Therapeutic potential of engineered extracellular vesicles. AAPS J 20:1–17. https://doi.org/10.1208/s12248-018-0211-z
Meyer C, Losacco J, Stickney Z, Li L, Marriott G, Lu B (2017) Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int J Nanomedicine 12:3153–3170. https://doi.org/10.2147/ijn.s133430
Mio K, Sato C (2018) Lipid environment of membrane proteins in cryo-EM based structural analysis. Biophys Rev 10:307–316. https://doi.org/10.1007/s12551-017-0371-6
Mirzaaghasi A, Han Y, Ahn S-H, Choi C, Park J-H (2021) Biodistribution and pharmacokinectics of liposomes and exosomes in a mouse model of sepsis. Pharmaceutics 13:427. https://doi.org/10.3390/pharmaceutics13030427
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Mora EM, Álvarez-Cubela S, Oltra E (2015) Biobanking of exosomes in the era of precision medicine: are we there yet? Int J Mol Sci 17(1):13. https://doi.org/10.3390/Fijms17010013
Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y (2016) Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 111:55–65. https://doi.org/10.1016/j.biomaterials.2016.09.031
Munagala R, Aqil F, Jeyabalan J, Kandimalla R, Wallen M, Tyagi N, Wilcher S, Yan J, Schultz DJ, Spencer W, Gupta RC (2021) Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett 505:58–72. https://doi.org/10.1016/j.canlet.2021.02.011
Nakase I, Futaki S (2015) Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep 5:1–13. https://doi.org/10.1038/srep10112
Nisa ZU, Zafar A, Zafar F, Pezaro S, Sher F (2020) Adverse drug reaction monitoring and reporting among physicians and pharmacists in Pakistan: a cross-sectional study. Curr Drug Saf 15(2):137–146. https://doi.org/10.2174/1574886315666200427120322
Nooshabadi VT, Khanmohammadi M, Shafei S, Banafshe HR, Malekshahi ZV, Ebrahimi-Barough S, Ai J (2020) Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochem Biophys Rep 23:100792. https://doi.org/10.1016/j.bbrep.2020.100792
Nordmeier S, Ke W, Afonin KA, Portnoy V (2020) Exosome mediated delivery of functional nucleic acid nanoparticles (NANPs). Nanomedicine 30:102285. https://doi.org/10.1016/j.nano.2020.102285
Oh DK, Hyun CK, Kim JH, Park YH (1988) Production of penicillin in a fluidized- bed 873 bioreactor: control of cell growth and penicillin production by phosphate limitation. Biotechnol Bioeng 32:569–573. https://doi.org/10.1002/bit.260320421
Oh S, Kang D, Ahn S-M, Simpson RJ, Lee B-H, Moon MH (2007) Miniaturized asymmetrical flow field-flow fractionation: application to biological vesicles. J Sep Sci 30(7):1082–1087. https://doi.org/10.1002/jssc.200600394
Orczyk M, Wojciechowski K (2015) Comparison of the effect of two Quillaja bark saponin extracts on DPPC and DPPC/cholesterol Langmuir monolayers. Colloids Surf B Biointerfaces 136:291–299. https://doi.org/10.1016/j.colsurfb.2015.09.018
Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, Wang L, Zhao W, Ma L, Zhang Q, Cui D (2021) Urinary exosomes-based engineered nanovectors for homologously targeted chemo-hemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials 275:120946. https://doi.org/10.1016/j.biomaterials.2021.120946
Patel P, Vyas N, Raval M (2021) Safety and toxicity issues of polymeric nanoparticles. Nanotechnol Med. https://doi.org/10.1002/9781119769897.ch7
Pei X, Zhang X, Zhang L, Yuan M, Sun L, Yu F, Wang B, Zhao J, He H, Yang VC (2021) Targeted exosomes for co-delivery of siFGL1 and siTGF-β1 trigger combined cancer immunotherapy by remodeling immunosuppressive tumor microenvironment. Chem Eng J 421:129774. https://doi.org/10.1016/j.cej.2021.129774
Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK (2014) A review of exosome separation techniques and characterization of B16–F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem 406(30):7855–7866. https://doi.org/10.1007/s00216-014-8040-0
Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, Qian X, Jia H, Zhao J, Sun J, Hou X, Yuan X, Kang C (2016) Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 10:3323–3333. https://doi.org/10.1021/acsnano.5b06939
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X (2018) Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release 287:156–166. https://doi.org/10.1016/j.jconrel.2018.08.035
Riau AK, Ong HS, Yam GHF, Mehta JS (2019) Sustained delivery system for stem cell-derived exosomes. Front Pharmacol 10:1368. https://doi.org/10.3389/fphar.2019.01368
Sainaga Jyothi VGS, Bulusu R, Venkata Krishna Rao B, Pranothi M, Banda S, Kumar Bolla P, Kommineni N (2022) Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int J Pharm 624:122022. https://doi.org/10.1016/j.ijpharm.2022.122022
Salafi T, Zeming KK, Zhang Y (2016) Advancements in microfuidics for nanoparticle separation. Lab Chip 17:11–33. https://doi.org/10.1039/C6LC01045H
Salarpour S, Forootanfar H, Pournamdari M, Ahmadi-Zeidabadi M, Esmaeeli M, Pardakhty A (2019) Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru J Pharm Sci 27:533–539. https://doi.org/10.1007/s40199-019-00280-5
Salunkhe S, Dheeraj BM, Chitkara D, Mittal A (2020) Surface functionalization of exosomes for target-specific delivery and in vivo imaging and tracking: Strategies and significance. J Control Release 326:599–614. https://doi.org/10.1016/j.jconrel.2020.07.042
Sandra J, Mélanie S, Claudia S, Gerrit B, Peter W, Olga B (2019) Hazard assessment of polymeric nanobiomaterials for drug delivery: what can we learn from literature so far. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00261
Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N, Shiku H, Akiyoshi K (2016) Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep 6:21933. https://doi.org/10.1038/srep21933
Schindler C, Collinson A, Matthews C, Pointon A, Jenkinson L, Minter RR, Vaughan TJ, Tigue NJ (2019) Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 14(3):e0214545. https://doi.org/10.1371/journal.pone.0214545
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 29(12):747–754. https://doi.org/10.1089/scd.2020.0080
Severic M, Ma G, Pereira SG, Ruiz A, Cheung CC, Al-Jamal WT (2021) Genetically-engineered anti-PSMA exosome mimetics targeting advanced prostate cancer in vitro and in vivo. J Control Release 330:101–110. https://doi.org/10.1016/j.jconrel.2020.12.017
Shamili FH, Bayegi HR, Salmasi Z, Sadri K, Mahmoudi M, Kalantari M, Ramezani M, Abnous K (2018) Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int J Pharm 549:218–229. https://doi.org/10.1016/j.ijpharm.2018.07.067
Shamili FH, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, Taghdisi SM, Ramezani M (2019) Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release 299:149–164. https://doi.org/10.1016/j.jconrel.2019.02.032
Shao J, Zaro J, Shen Y (2020) Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomed 15:9355. https://doi.org/10.2147/IJN.S281890
Shi Y, Guo S, Liang Y, Liu L, Wang A, Sun K, Li Y (2022) Construction and evaluation of liraglutide delivery system based on milk exosomes: a new idea for oral peptide delivery. Curr Pharm Biotechnol 23(8):1072–1079. https://doi.org/10.2174/1389201022666210820114236
Sidhom K, Obi PO, Saleem A (2020) A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci 21(18):6466. https://doi.org/10.3390/ijms21186466
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Cells 66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001
Sokolova V, Ludwig A-K, Hornung S, Rotan O, Horn PA, Epple M, Giebel B (2011) Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces 87:146–150. https://doi.org/10.1016/j.colsurfb.2011.05.013
Song S, Shim MK, Lim S, Moon Y, Yang S, Kim J, Hong Y, Yoon HY, Kim I-S, Hwang KY, Kim K (2020) In situ one-step fluorescence labeling strategy of exosomes via bioorthogonal click chemistry for real-time exosome tracking in vitro and in vivo. Bioconjug Chem 31:1562–1574. https://doi.org/10.1021/acs.bioconjchem.0c00216
Stoicea N, Fiorda-Diaz J, Joseph N, Shabsigh M, Arias-Morales C, Gonzalez-Zacarias A, Mavarez-Martinez A, Marjoribanks S, Bergese SD (2017) Advanced analgesic drug delivery and nanobiotechnology. Drugs 77:1069–1076. https://doi.org/10.1007/s40265-017-0744-y
Street JM, Barran PE, Mackay CL et al (2012) Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10:1–7. https://doi.org/10.1186/1479-5876-10-5
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614. https://doi.org/10.1038/mt.2010.105
Tasharrofi N, Nourozi M, Marzban A (2022) How liposomes pave the way for ocular drug delivery after topical administration. J Drug Deliv Sci Technol 67:1035. https://doi.org/10.1016/j.jddst.2021.103045
Taylor DD, Zacharias W, Gercel-Taylor C (2011) Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol 728:235–246. https://doi.org/10.1007/978-1-61779-068-3_15
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA (2022) Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano 16(11):17802–17846. https://doi.org/10.1021/acsnano.2c08774
Theodoraki M, Hong C, Donnenberg VS, Donnenberg AD, Whiteside TL (2020) Evaluation of exosome proteins by on-bead flow cytometry. Cytom A 99(4):372–381. https://doi.org/10.1002/cyto.a.24193
Thierry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3:1–29. https://doi.org/10.1002/0471143030.cb0322s30
Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S, Gamini A, Canzonieri V, Rizzolio F (2015) Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 10:2963–2971. https://doi.org/10.2217/nnm.15.118
Tran PHL, Wang T, Yin W, Tran TTD, Barua HT, Zhang Y, Midge SB, Nguyen TNG, Lee B-J, Duan W (2019a) Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm 566:697–707. https://doi.org/10.1016/j.ijpharm.2019.06.028
Tran PH, Wang T, Yin W, Tran TTD (2019b) Aspirin-loaded nanoexosomes as cancer therapeutics. Int J Pharm 572:1186. https://doi.org/10.1016/j.ijpharm.2019.118786
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. https://doi.org/10.1038/ncb1596
Van Niel G, Raposo G, Candalh C et al (2001) Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121:337–349. https://doi.org/10.1053/gast.2001.26263
Vogel R, Pal AK, Jambhrunkar S et al (2017) High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Sci Rep 7:17479. https://doi.org/10.1038/s41598-017-14981-x
Wang Y, Douglas T (2021) Protein nanocage architectures for the delivery of therapeutic proteins. Curr Opin Colloid Interface Sci 51:101395. https://doi.org/10.1016/j.cocis.2020.101395
Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH (2009) Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 4:4160. https://doi.org/10.1371/journal.pone.0004160
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z (2018) Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc 7:e008737. https://doi.org/10.1161/jaha.118.008737
Wang C, Kimura K, Li J, Richardson J, Naito M, Miyata K, Ichiki T, Ejima H (2021) Polydopamine-mediated surface functionalization of exosomes. Chemnanomat 7:592–595. https://doi.org/10.1002/cnma.202100078
Wang Z, Popowski KD, Zhu D et al (2022) Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng 6:791–805. https://doi.org/10.1038/s41551-022-00902-5
Whitford W, Guterstam P (2019) Exosome manufacturing status. Future Med Chem 11:1225–1236. https://doi.org/10.4155/fmc-2018-0417
Wu G, Wu J, Li Z et al (2022) Development of digital organ-on-a-chip to assess hepatotoxicity and extracellular vesicle-based anti-liver cancer immunotherapy. Bio-Des Manuf 5:437–450. https://doi.org/10.1007/s42242-022-00188-1
Xi X-M, Xia S-J, Lu R (2021) Drug loading techniques for exosome-based drug delivery systems. Pharmazie 76:61–67. https://doi.org/10.1691/ph.2021.0128
Xu L, Faruqu FN, Liam-Or R, Abu Abed O, Li D, Venner K, Errington RJ, Summers H, Wang JT, Al-Jamal KT (2020a) Design of experiment (DoE)-driven in vitro and in vivo uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. J Extracell Vesicles 9:1779458. https://doi.org/10.1080/20013078.2020.1779458
Xu M, Chen Q, Li J, Peng L, Ding L (2020b) Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. J BUON 25:1413–1422
Xu M, Yang Q, Sun X, Wang Y (2020c) Recent advancements in the loading and modification of therapeutic exosomes. Front Bioeng Biotechnol 8:586130. https://doi.org/10.3389/fbioe.2020.586130
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M (2019) Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci 233:116733. https://doi.org/10.1016/j.lfs.2019.116733
Yakimchuk K (2015) Exosomes: isolation and characterization methods and specific markers. Mater Methods 5:1450–1453. https://doi.org/10.13070/mm.en.5.1450
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32:2003–2014. https://doi.org/10.1007/s11095-014-1593-y
Yang F, Liao X, Tian Y, Li G (2017a) Exosome separation using microfuidic systems: size-based, immunoafnity-based and dynamic methodologies. Biotechnol J 12:4. https://doi.org/10.1002/biot.201600699
Yang J, Zhang X, Chen X, Wang L, Yang G (2017b) Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7:278–287. https://doi.org/10.1016/j.omtn.2017.04.010
Yang Y, Hong Y, Cho E, Kim GB, Kim I-S (2018) Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J Extracell Vesicles 7(1):1440131. https://doi.org/10.1080/20013078.2018.1440131
Yang J, Wu S, Hou L, Zhu D, Yin S, Yang G, Wang Y (2020a) Therapeutic effects of simultaneous delivery of nerve growth factor mRNA and protein via exosomes on cerebral ischemia. Mol Ther Nucleic Acids 21:512–522. https://doi.org/10.1016/j.omtn.2020.06.013
Yang M, Liu X, Luo Q, Xu L, Chen F (2020b) An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol 18(1):100. https://doi.org/10.1186/s12951-020-00656-9
Yang J, Luo S, Zhang J, Yu T, Fu Z, Zheng Y, Xu X, Liu C, Fan M, Zhang Z (2021) Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol Dis 148:105218. https://doi.org/10.1016/j.nbd.2020.105218
Ye Y, Zhang X, Xie F, Xu B, Xie P, Yang T, Shi Q, Zhang CY, Zhang Y, Chen J, Jiang X, Li J (2020) An engineered exosome for delivering sgRNA: Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater Sci 8:2966–2976. https://doi.org/10.1039/d0bm00427h
Yim N, Ryu SW, Choi K (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun 7:12277. https://doi.org/10.1038/ncomms12277
Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X (2019) Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun 10(10):3838. https://doi.org/10.1038/s41467-019-11718-4
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV (2017) Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142:1–12. https://doi.org/10.1016/j.biomaterials.2017.07.011
Zakharova L, Svetlova M, Fomina AFJ (2007) T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol 212:174–181. https://doi.org/10.1002/jcp.21013
Zhang H, Lyden D (2019) Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc 14(4):1027–1053. https://doi.org/10.1038/s41596-019-0126-x
Zhang H, Freitas D, Kim HS et al (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20:332–343. https://doi.org/10.1038/s41556-018-0040-4
Zhang W, Wang Y, Kong Y (2019) Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol vis Sci 60:294–303. https://doi.org/10.1167/iovs.18-25617
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 15:6917–6934. https://doi.org/10.2147/ijn.s264498
Zhang Z, Mugisha A, Fransisca S, Liu Q, Xie P, Hu Z (2021) Emerging role of exosomes in retinal diseases. Front Cell Dev Biol 9:643680. https://doi.org/10.3389/fcell.2021.643680
Zhao Z, McGill J, Gamero-Kubota P, He M (2019) Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip 19:1877–1886. https://doi.org/10.1039/c8lc01279b
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H (2020) Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release 318:1–15. https://doi.org/10.1016/j.jconrel.2019.12.005
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW (2014) Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 32(1):116–125. https://doi.org/10.1002/stem.1504
Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L, Tan W (2019) Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem 91:2425–2430. https://doi.org/10.1021/acs.analchem.8b05204
Zuo B, Qi H, Lu Z, Chen L, Sun B, Yang R, Zhang Y, Liu Z, Gao X, You A, Wu L, Jing R, Zhou Q, Yin HF (2020) Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat Commun 11:1–16. https://doi.org/10.1038/s41467-020-15569-2
Acknowledgements
The authors are thankful to National Institute of Pharmaceutical Education and Research, Guwahati, Assam, India, and National Institute of Pharmaceuticewdsal Education and Research, Hajipur, Bihar, India.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
SS and JX wrote the original draft, NK took part in reviewing and editing, MZA contributed to review and artwork, and OPR was responsible for conceptualization, review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Human or animal subjects
As this is a review article, it does not involve any animal or human study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sen, S., Xavier, J., Kumar, N. et al. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech 13, 101 (2023). https://doi.org/10.1007/s13205-023-03521-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03521-2