Abstract
Exosomes are biological extracellular vesicles that are released by both prokaryotic and eukaryotic cells. Their size ranges between 40 and 160 nm, and their role, although not fully clarified yet, seems to be important for intracellular homeostasis and intercellular communication. As exosomes are enriched in a plethora of payloads, such as cytosolic or surface proteins, lipids, and nucleic acids, their interaction with target cells has proved significant in the progression of many diseases. Cardiovascular, neurodegenerative, or immune pathological conditions are only some examples, where the effect of exosomes is studied, either as disease modulators or as powerful diagnostic tools. On the other hand, exosomes, due to their ability to deliver different types of information, have numerous advantages as drug delivery systems. These nanoplatforms are currently under preclinical and clinical evaluation for the therapy of many diseases, including the novel coronavirus disease. However, production, purification, and compliance with the good manufacturing practice (GMP) issues limit the wide clinical use of exosomes as therapeutic delivery systems. For this reason, the area of artificial exosomes is rapidly evolving. Novel nanosystems that mimic the functionality of exosomes but lack their disadvantages have started to be developed. The aim of this chapter is to present the recent work on the development approaches and the possible payloads of the artificial exosomes so that they can be utilized as safe and effective drug delivery nanosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the last few decades, researchers of Pharmaceutical and Medical Sciences have realized that the development of new active pharmaceutical ingredients (APIs) is less than enough to address current medicinal needs. It is not a rare phenomenon that potentially efficacious APIs do not have a favorable pharmacodynamic and pharmacokinetic profile. This is the main reason why the science of drug delivery systems has flourished. In this area, Pharmaceutical Nanotechnology and Nanomedicine are pioneers due to their unique properties that are inextricably connected with the size of the delivery systems (1 nm = 10−9 m). Drug delivery nanosystems function as a Trojan horse that protects the API from biodegradation, drives it to the tissue or organ of desire, and eliminates its toxicity to healthy tissues. The first medicine based on pharmaceutical nanotechnology was approved in 1995 and since then many others have followed [1]. Lipid-based and polymeric nanoparticles are the main platforms that are used, though other platforms such as inorganic nanoplatforms and carbon nanotubes are also studied [2]. All of these nanosystems have undoubtedly optimized drug delivery. But what happens when the API is not just a classic small molecule? The recent example of coronavirus disease 2019 (COVID-19) has proved there is a need for innovative platforms that can encapsulate and deliver bioactive molecules, such as proteins or RNAs. Indeed, the majority of the authorized COVID-19 vaccines utilize nanoplatforms to induce human immunity and achieve their protective role [3].
In this chapter, we will discuss exosomes as API delivery nanovesicles, their limitations in clinical practice, and the development of artificial exosomes. The artificial exosomes are fully- or semisynthetic nanoplatforms that can encapsulate a variety of different biomolecules while being free of the main disadvantages of cell-derived exosomes.
2 Exosomes
Extracellular vehicles (EVs) are cell-derived lipid bilayer structures that were first observed by P. Wolf in human plasma [4]. They are produced by both prokaryotic and eukaryotic cells and are classified into three main categories, based on their biogenesis mechanism, cargo, and size: (i) apoptotic bodies (1000–10,000 nm), (ii) microvesicles (100–1000 nm), and (iii) exosomes (50–150 nm). All types of EVs carry bioactive molecules, such as proteins, lipids, and nucleic acids, that are connected to their parent cell. Thus, depending on the endogenous messages they carry, each of these EV types leads to the activation of different biological signaling pathways [5]. The aim of this unit is the presentation of the biological behavior of exosomes, the smallest group of EVs, and their potential role as well as their main difficulties as effective therapeutic delivery platforms.
2.1 Biogenesis
In contrast with the other types of EVs, which are formed by the outward bending of the plasma membrane, exosomes are released after the fusion of the plasma membrane with the endogenous multivesicular bodies. The term “exosome” comes from the Greek words “έξω” (exo), meaning outside and “σώμα” (soma), meaning body. It was first proposed by Johnstone and her team, due to their formation mechanism: these bodies are formed mainly into the cytoplasm and afterward, they are released in the extracellular plasma [6].
2.1.1 ESCRT-Dependent Pathway
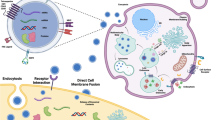
Exosome biogenesis is connected with the endosomal formation mechanism. Extracellular molecules, such as proteins, ions, and lipids, are inserted into the cell through endocytosis. In this way, the early endosomes are formed. Interactions between the early endosomes and intercellular organelles, mitochondria, and trans-Golgi network lead to the insertion of intercellular constituents into the endosomes and their transformation into late endosomes [7]. Some additional modifications and content exchange between these blebs and the cytoplasm give rise to the multivesicular bodies (MVBs). During the formation of the late endosomes and the MVBs, the inward invagination of the bleb membrane results in the development of the intraluminal vesicles (ILVs), which are the future exosomes (Fig. 1). Two pathways are responsible for the production of the ILVs: (a) the endosomal sorting complex required for transport (ESCRT)-dependent and (b) the ESCRT-independent, as analyzed below. MVBs can either undergo a degradation process, direct interaction with lysosomes, or degradation after fusion of MVBs with the autophagosomes first, or anchor into the plasma membrane and release their constituents into the extracellular environment [8].
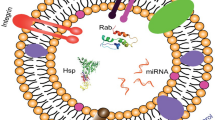
(a) Exosome biogenesis. Extracellular molecules, such as proteins, ions, and lipids, are inserted into the cell through endocytosis and early endosomes are formed. Early endosomes interact with organelles (i.e., mitochondrion) and are converted into late endosomes. Further modifications and interactions with the trans-Golgi network lead to the formation of multivesicular bodies (MVBs) that encapsulate small vesicles that are called intralumilar bodies (ILVs). MVBs can be either degraded or attach to the cell membrane and release their cargo, which contains the modified ILVs known as exosomes, in the extracellular matrix. (b) Structure of exosomes. Exosomes carry a variety of biomolecules such as proteins, lipids, and nucleic acids that are produced in the parent cells. (Figure (b) adapted from [27])
Possible mechanisms of exosomes internalization by the target cells: (a) clathrin-mediated endocytosis, (b) lipid raft-mediated endocytosis, (c) caveolin-mediated endocytosis, (d) phagocytosis, (e) micropinocytosis. (Adapted from [8])
Four protein complexes (ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III), as well as the AAA ATPase Vps4 complex (VPS4, ALIX, TSG101), play a key role in the biogenesis of the exosomes via the ESCRT-dependent pathway. ESCRT-0 complex recognizes and binds to the mono-ubiquitinated proteins of the endosomal surface [9]. ESCRT-0 and phosphatidylinositol 3-phosphate (an endosome-enriched lipid) recruit ESCRT-I and ESCRT-II into this area. Subsequently, ESCRT-I and ESCRT-II induce membrane deformation and the development of small blebs into the surface. These blebs enclose soluble molecules and cytoplasm into their center [10, 11]. ESCRT-III and VPS4 (vacuolar protein sorting-associated protein 4) are necessary for the inward endosomal membrane abscission and the release of the ILVs into the late endosomes or MVBs [12]. Next, de-ubiquitylating enzymes de-ubiquitylate the ubiquitylated proteins so that the vesicles will not be degraded [13]. During all of the mentioned processes, the role of ESCRT-accessory proteins ALIX (apoptosis-linked gene 2-interacting protein X), TSG101 (tumor susceptibility gene 101), and SNARE [soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor] complex proteins is of extreme importance as they control the production, the homogeneity, and the value of the ILVS content [14]. For instance, ALIX binds to the de-ubiquitylated proteins and transfer them in the ILVs, with the help of syndecans and tetraspanin CD63 [15]. SNARE complex proteins are responsible for the fusion of MVBs with the plasma membrane and subsequently in the release of exosomes [11].
2.1.2 ESCRT-Independent Pathway
The ESCRT-independent pathway is mainly driven by some areas in the surface of lipidic bilayer membranes that are called lipid rafts or microdomains. Lipid rafts have a very sticked composition and conformation. They are enriched in cholesterol, sphingolipids, glycosylphosphatidylinositol (GPI), ceramides, and saturated phospholipids. The architecture of lipid rafts is stabilized by the high quantity of tetraspanins or other GPI-anchored proteins [16]. These areas seem to be rich in information content, encrypted into the so-called thermodynamic epitopes [17]. The information is translated into an enthalpic content that is capable of interacting with the neighbor molecules [18]. Indeed, lipid rafts are responsible for the encapsulation of certain RNA molecules into exosomes due to enhanced affinity for specific RNA motifs. This process seems to be lipid- and not protein-mediated, while entropic phenomena of the lipidic surface are translated into a “bind-here” message for the RNA motifs [19]. Although this type of information content may be unusual in biological sciences, it is mathematically explained by the Shannon equation of information [20]:
where H represents the entropy for xi possible outcomes. p(xi) represents the probability of the possible outcomes and b is the base of the logarithm used. The corresponding entropy units are bits when b = 2.
The above equation can explain the thermodynamic behavior of biological epitopes taking into account the quality and quantity of information (bits) they carry. Thus, the information content of the lipid rafts activates an early-exosome production mechanism that is thermodynamically favored.
Although the complete mechanism of exosome production and release is currently not fully understood, both ESCRT-dependent and ESCRT-independent pathways have an important role on exosomes’ biogenesis. Recent studies support that these two processes act complementary to each other, providing a complex biogenesis algorithm [21,22,23,24].
2.2 Biological Role
In the early ages of their discovery, exosomes were thought to be cellular waste carriers so that the unwanted cell cargo is released outside of the cell compartment. However, recent research has proved that their role in cell-to-cell communication is much more complex [25]. Figure X presents possible mechanisms of exosomes’ internalization into the receiver cells. As they are carriers of several molecules (i.e., proteins, lipids, or nucleic acids) of the parent cells and have the ability to transfer these contents intercellularly, they affect both physiological or pathological biochemical routes [26]. For instance, their role in the progress of different types of cancer or metastasis [13, 27], in the activation of immune responses [28], or even in progress of the neurodegenerative diseases has been detected [14].
2.2.1 The Role of Exosomes in Carcinogenesis
The function of tumor-derived exosomes (TDEs), also known as oncosomes, is one of the most investigated topics concerning their pathogenesis-associated function. Different cancer types have been studied for this purpose. Breast, gastric, and melanoma cancers are some examples [29,30,31].
Tumor-derived exosomes that are produced and released by the malignant cells affect the tumor microenvironment and thus the progression of the pathogenicity. Specifically, stress tumor conditions such as hypoxia and oxidative stress increase the rate of exosomal biogenesis and the release by the tumor cells. These exosomes interact with the neighboring area, which subsequently results in the remodeling of the microenvironment [13]. The main cells of the tumor microenvironment (fibroblasts, immune and stromal cells) receive the tumorigenic content of the TDE.
Furthermore, TDE seem to have a crucial role in the metastasis process. TDE can transit their content in more or less distant areas (in transit or satellite metastasis) or even organs [32]. As the development of the pre-metastatic niche is equally important with the cells’ mutations in the metastasis formation, the TDE should be taken into account when the secondary tumor tissues are studied [33]. Indeed, according to Emmanouilidi et al., pancreatic TDE were found to carry more than 300 tumorigenic proteins [34], while Hoshino et al. claim that the unique integrins of the TDE drive the organotropic selection of the metastasis site [35]. Moreover, exosomal miR-25-3p induces the modeling of the pre-metastatic niche in foreign sites [36].
Finally, TDE are of importance due to their active role in drug resistance of tumors. On the one hand, TDE can actively remove the chemotherapeutic drugs outside the malignant cells. Notably, HER-2 overexpressing exosomes bind with trastuzumab and inhibit its action [37]. Nevertheless, the basic role of exosomes in drug resistance is attributed to biochemical pathways. For instance, TDE transmit the information for upregulation of certain active molecules such as P-glycoprotein [38, 39] and GSTP1 [40]. Most of the times, nucleic acids are responsible for this upregulation, mainly miRNAs [13, 41, 42].
2.2.2 The Role of Exosomes in the Immune System
As exosomes regulate intercellular communication, they may trigger or suppress the immune response. Interestingly, exosomes that are produced by infected, malignant, or immune cells exert influence on both innate and adaptive immunity.
The innate system, which is the nonselective way of the human organism to fight exogenous, and harmful, factors is composed of macrophages, dendritic and natural killer cells, granulocytes, as well as complement and chemoattractants. Exosomes derived from infected macrophages are considered to enhance pro-inflammatory conditions and activation of the innate system [43, 44]. Zhou et al. suggest that exosomes derived from pancreatic cancer cells are uploaded with miR-203 that downregulate the expression of the membrane toll-like receptor 4 (TLR4) of dendritic cells [35] and thus decrease the capability of dendritic cells to identify bacterial components or host heat shock proteins [45]. Recent studies show that activation of natural killer cells, which are the responsible cells to recognize and cytolyze infected, cancer, or allogenic cells, by TDE might have a beneficial role in the elimination of tumors [46, 47].
On the other hand, the function of adaptive immunity is affected by exosomal messages too. Exosomes produced by activated dendritic cells were found to be more efficient in the immunomodulation than the ones from immature dendritic cells [48]. These vesicles carry in their surface MHC-I and MHC-II complexes that are bound with pathogenic molecules [49]. Notably, humoral immunity (CD4+ T-lymphocytes and B-lymphocytes) induced by the vaccination with dendritic cell-derived exosomes that were infected with Eimeria tenella proved to be more efficient than the antigenic-subunit vaccine when intramuscularly administrated in chicken [50]. Moreover, cellular immunity, mainly caused via the Th1 route and the production of CD8+ T-lymphocytes, is also synergistically activated by exosomes from immune cells [51, 52].
Except for the physiological role of exosomes in the immune system, they seem to trigger or control autoimmune diseases under certain conditions. For instance, miRNAs that are released under rheumatoid arthritis can either up- or downregulate the progress of the disease [53,54,55]. Miao and colleagues review their role in a variety of autoimmune diseases, such as systemic lupus and sclerosis [56].
2.2.3 The Role of Exosomes in the Central Nervous System and Neurodegeneration
The mechanism of action, communication, and interaction between the central nervous system (CNS) cells is highly complicated and not yet fully understood. However, exosomes have proved to be important information transporters for these mechanisms. Indeed, great studies show that CNS-derived exosomes that were isolated from human fluids such as plasma carry miRNAs and protein aggregates that could help in the very early diagnosis of CNS neurodegenerative diseases like Alzheimer’s or Parkinson’s disease [57,58,59,60].
Microglia, neurons, astrocytes, and oligodendrocytes all produce exosomes that are important factors for neuronal health and normal neural functionality [61,62,63,64,65,66]. Interestingly, Venturini et al. have recently shown that astrocyte-derived exosomes can be selectively up-taken by neuron cells although the mechanism of action for the above activity is not yet clear. These exosomes transferred neuroglobin, a molecule that is assumed to have a neuroprotective role [67]. Likewise, exosomes from cortical neurons were found to interact only with neurons and not glial cells [68]. This selectivity empowers the perspective that exosomes are not just “junk carriers” but they do support neural homeostasis by selective cell-to-cell communication.
A controversy exists of whether exosomes provide a protective or aggressive profile in the case of neurodegeneration. Recent studies prove that brain-derived exosomes of AD patients have increased rates of pathological proteins and protein complexes such as Aβ oligomers and hyperphosphorylated tau (p-tau) [69, 70]. Some research groups support that these exosomes that are produced by pathological AD parent cells promote the spread of the above toxic proteins and consequently, the evolution of neurodegeneration [69, 71, 72]. On the contrary, some other groups found that exosomes might also have a neuroprotective functionality under certain circumstances: recently, Wei and colleagues proved that in in vitro experiments mesenchymal stem cell-derived exosomes evoke neuronal cell apoptosis in AD models [73]. Moreover, neuronal exosomes were found to aid in the Aβ clearance in nonhuman primates with AD pathologies [74]. Contrariwise, glia-derived exosomes, enriched in α-synuclein, a main pathological protein found in patients with Parkinson’s disease, are accused of causing α-synuclein aggregation and low protein clearance of the neurons [75, 76].
2.3 Applications in Preclinical and Clinical Stages
The most common way to develop exosomes as innovative medicinal platforms is through genetic modification of the parent cell. In this way, exosomes are modified to carry increased or decreased quantities of certain cargoes such as proteins or nucleic acids. Notably, Duarte-Sanmiguel and colleagues developed a protocol for the isolation of exosomes derived from modified dendritic cells, mentioning the importance of these exosomes as up- or downregulators of protein biogenesis in the receiver cells [77]. A pioneering study by Alvarez-Erviti et al. achieved more than 60% knockdown of the enzyme beta-secretase 1 (BACE-1) in mice by administration of exosomes derived from genetically modified dendritic cells [78]. Mesenchymal stem cell-derived exosomes that are overexpressing Anti-miRNA-221 or miR-34a proved to be beneficial in the treatment of different cancer types [79,80,81].
Another way to develop effective therapeutic exosomes is by loading them with drug molecules. The loading procedure takes place after the exosome production via passive or active cargo loading [82]. For instance, a passive process is the incubation of the parent cell with the drug molecule [83], while active ones are sonication [84] or electroporation [78, 85].
Last but not least, certain categories of exosomes, such as mesenchymal stem cell-derived exosomes, show a therapeutic effect due to their inherent regenerative properties without the need of any biochemical or drug loading modifications. For instance, bone marrow mesenchymal stem cell-derived exosomes have recently proved through in vitro and ex vivo models to promote tendon regeneration [86]. Similar regenerative properties have been found in preclinical and clinical trials against musculoskeletal pathologies such as osteoarthritis [87, 88]. Furthermore, mesenchymal stem cell-derived exosomes could have a therapeutic effect in wound healing [89]. Interestingly, Yang et al. showed promising results in the treatment of diabetic wounds after the administration of polymeric hydrogel loaded with umbilical cord-derived mesenchymal stem cell-derived exosomes [90].
Till today, no medicine based on exosomes has been approved by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Nevertheless, as of February 2022, by using the keyword “exosome” in the US National Institutes of Health (NIH) clinicaltrials.gov, 251 trials appear. Most of these trials concern the diagnosis of diseases, while a few targets in the evaluation of exosomal therapeutic products. Table 1 presents the clinical trials that evaluate the exosomes as advanced therapeutic medicines. Only two trials appear to be in phase III, from whom only one is active (NCT05216562). The active one evaluates the safety and efficacy of mesenchymal stem cell-derived exosomes to reduce hyper-inflammation in COVID-19 patients. The effect of the intravenous administration of the exosomes will be evaluated additionally with the administration of standard COVID-19 treatments. As it is shown, the majority of the clinical trials presented in Table 1 involve mesenchymal stem cell-derived exosomes. Mesenchymal stem cell-derived exosomes are ideal delivery platforms as they are easily modified, nontoxic, and biodegradable [91]. However, other parent cells have also been utilized such as dendritic cells (NCT01159288) or plant cells (NCT01294072, NCT01668849).
2.4 Difficulties and Disadvantages
Although exosomes show tremendous advantages as innovative medicinal platforms, some serious limitations also exist. Firstly, because the production of exosomes requires a parent cell culture, specific quality and safety procedures should be followed. Different cell banks, e.g., mesenchymal stem cells, dendritic cells, or plant cells, require different culture conditions and have unique proliferation rates. Unique microenvironment conditions such as pH, oxygen, or specialized growth factors shall be applied in each case [92]. As it is necessary for every type of medicinal biological factor and in appliance with the guidelines of the worldwide health administrations [93], the culture of cell lines should be done under good manufacturing practice (GMP) conditions, which are stricter for biological products than conventional drug formulations. Indeed, according to the EMA, exosomes belong to the category of advanced therapy medicinal products (ATMPs), and their evaluation follows EMA/CAT/852602/2018 guidelines [94]. As this category of therapeutics is new, there are not yet certain advice or recommendations from the medicine agencies [95].
Moreover, the isolation of exosomes necessitates the development of elegant techniques that are able to distinguish them from other extracellular vesicles, like microvesicles or apoptotic bodies. Fortunately, in 2015, the International Society for Extracellular Vesicles (ISEV) published a position paper regarding the isolation methods that should be applied for EVs that are studied in clinical trials [96], and this paper brought a new era in the exosome clinical trials. However, till today, the distinguished line between the different types of EVs is not yet fully understood and more research is necessary. Recognizing the need for more certain guidelines, ISEV recently published another position paper to promote the need for a legislative framework concerning EV medicinal products. In this paper, ISEV report that according to current EMA guidelines, EV products are categorized in ATMPs, though it supports that certain details differentiate them from the other ATMPs and is correct to be taken into account [97].
Last but not least, exosomes are biological vesicles that contain sensitive cargo. Thus, the storage conditions might be a limitation too. For instance, especially when exosomes are used as RNA carriers that can be affected and inactivated very easily, special storage is required. Indeed, Yamashita and colleagues support that the optimized storage temperature is −80 °C [98]. Such a temperature would dramatically increase the price of these therapeutical products that could eliminate their use in low-income countries.
3 Artificial Exosomes
All of the above create obvious difficulties in the manufacturing production of exosomal medicines. As a result, scientists are currently trying to go a step further and develop new platforms that could acquire the advantages of exosomes and eliminate their disadvantages. The category of artificial exosomes as innovative therapeutic medicinal products is a new area in nano-biotherapeutics with promising results in the development of safe and effective platforms that could fight currently incurable diseases. In this section, we will discuss the developmental processes of artificial exosomes and their loading efficiency.
3.1 Development Approaches
Three main approaches for producing artificial exosomes can be found in the literature: (a) the bottom-up, (b) the top-down, and (c) the hybrid method (Fig. 3). Each procedure has unique characteristics and limitations that shall be taken into account in each case.
Development approaches of artificial exosomes. (a) Top-down method: cells pass through polycarbonate filters or microfluidics devices and cell membranes are isolated and form pseudospherical vesicles. (b) Bottom-up method: block molecules, e.g., phospholipids, cholesterol, proteins, or peptides, are mixed in the appropriate ratios to self-assemble into exosome-like particles. (c) Hybrid method: exosome-like particles or liposomes and biologically derived exosomes are mixed through fusion techniques and hybrid liposomal-exosomal particles are formed. (Adapted from [102])
3.1.1 Bottom-Up Method
The bottom-up approach is a method to develop fully artificial exosomes. Building block molecules, e.g., phospholipids, cholesterol, proteins, or peptides, are mixed in the appropriate ratios to self-assemble into biomimetic platforms. The main advantage of the present methodology is that it utilizes the same manufacturing processes with the development of liposomes and other lipid nanoparticle formulations that are already authorized [99,100,101]. For example, thin-film hydration, microfluidics, or extrusion can be applied for the production of artificial exosomes. This approach, though, requires deep knowledge of the physicochemical properties of exosomes. As it is not possible to utilize all the components of biologically derived exosomes, the choice of certain key molecules leads to a “clean” result when we have targeted certain exosomal properties of interest [102]. Proof-of-concept experiments on the role of each molecule in the surface of exosomes or extracellular vesicles as well as lipidomics analysis are necessary tools for the design of experiment (DoE) methodology to develop such innovative biomimetic nanoplatforms [103,104,105,106]. The work of Peña et al. in 2009 is one of the first in the area of artificial exosome development. Although the lipids and the ratios that were utilized do not approach the ones of exosomes, the researchers achieved the complexation of their liposomes with major histocompatibility complex-1 (MHC-1) peptide complexes, enhancing the interaction with T-lymphocytes in a similar way with exosomes [107]. In another study, artificial exosomes carrying DEC205 antibody were developed by a microemulsion process. The conjugation of the antibody, which is negatively charged, is favored due to electrochemical interactions with the positively charged lipids. Interestingly, these platforms do not only have high encapsulation efficiency of the API, but they also show decreased cytotoxicity because of the charge neutralization [108]. Similarly, Staufer et al. developed fully synthetic extracellular vesicles (fsEVs) decorated with tetraspanins, which are naturally present in EVs’ membranes and loaded with miRNA molecules. The wound-healing abilities of fsEVs were evaluated by in vitro and ex vivo experiments showing promising results as therapeutic platforms. Although this study is the most accurate concerning the membrane composition, the physicochemical characteristics of the fsEVs need further optimization as the polydispersity index (PDI) of the empty platforms is approximately 0.8 [109]. Finally, it is worth mentioning that there is a gray area in the literature of the definition of artificial exosomes which should be based on their functionality and their morphological characteristics. For instance, Aday et al. mention the development of artificial exosomes loaded with RNA molecules [110]. However, the developed platforms are basically lipid nanoparticles (LNPs) that carry an miRNA that is found in small EVs. In our point of view, the functionality of such nanomorphologies based on their surface characteristics, i.e., the lipid rafts, could be the road map for characterizing such nanoplatforms as artificial exosomal membranes and not as artificial exosomes. However, the platform cargo, such as a single nucleic acid, is not sufficient to characterize the nanovesicles as artificial exosomes without proving their functionality.
3.1.2 Top-Down Method
The top-down method to produce exosome-like particles, also known as nanovesicles, is based on cell membrane fractions that assemble into pseudospherical blebs. Two main processes, extrusion over polycarbonate filters and microfluidics, are utilized for the production. Both methodologies require a cell culture. These cells will then be driven in membrane pressure that conclude in the formation of small nanovesicles. In this way, cell membranes as it is, including the transmembrane proteins and other signal molecules, are part of the final platforms. Thus, the produced artificial exosomes have a very similar proteomic and lipidomic consistency as biologically derived exosomes. Goh et al. presented those data and compared cell-derived nanovesicles (CDNs) with exosomes resulting that CDNs might play an important role as ATMPs [111]. Although a similarity between them is well-established, someone should take into account that differences do exist. For example, in this study, an important differentiation in the percentage of sphingomyelin is observed. Such an observation might seem insignificant, but we believe that it is possibly connected with the formation of lipid rafts, areas of high importance for exosomes, as mentioned above. Various producer cells, such as U937 cells [112], NIH3T3 fibroblasts [113], hMSF10A [113], or ASC cells [114], are cultured to produce NVs. Depending on the desired pharmacological effect, the origin of the NVs is decided as the final vesicles appear with high affinity to their parent cells. Such an approach has tremendous advantages in artificial exosome development. However, many of the limitations that appear in the classic exosome development are also present here.
3.1.3 Hybrid Method
Some recent studies focus on a more complex design by mixing synthetic liposomal formulations with biologically derived exosomal membranes. That semisynthetic approach is a result of the technological combination of both top-down and bottom-up methods and targets the design and development of ATMPs that will take advantage of both synthetic and biological platforms while eliminating their problems in clinical use [110]. A pioneering study that took place in 2016 by Sato et al. presents the successful development of hybrid exosomes by freeze-thaw and their in vitro evaluation. Notably, the researchers pointed out that the lipids used for the liposomal platforms affected the final behavior of the hybrid systems. Thus, the variety of synthetic lipids could lead to unique biological behavior of the final platform [115]. For instance, the incorporation of PEGylated lipids could eliminate the recognition and neutralization by the mononuclear phagocyte system (MPS), correspondingly to liposome’s behavior [116]. At the same time, another research group studied the endogenous production of hybrid EVs after infection of the producer cells with membrane fusogenic liposomes [117]. Results concerning the loading of both hydrophobic and hydrophilic molecules into the produced EVs showed that the efficiency was higher when membrane fusogenic liposomes are utilized in comparison with non-fusogenic highly cationic liposomes.
Two years after, two different research groups applied simple incubation methods to mix synthetic liposomes with mesenchymal-derived EVs [118] or HEK293FT exosomes [119]. According to Piffoux et al., the hybrid systems maintain the intrinsic properties of the EVs while adopting the high loading efficiency and prolonged circulation time from the synthetic cells [118]. Lin et al. benefit from this technology to deliver the CRISPR/CAS9 system into cells. The isolated exosomes were incubated with liposomes that carried the above gene modification tool and created hybrid exosomes that could transfer CRISPR/CAS9 intracellular without the need of a viral vector, as it is often presented [119].
Another method to produce hybrid exosomes is by extrusion through polycarbonate filters. Ryamajhi et al. developed hybrid anticancer exosomes from macrophage-derived EVs and doxorubicin-loaded liposomes. Their results showed enhanced in vitro internalization of the hybrid exosomes into the tumor cells in comparison to the normal cell lines. That observation attributed to the ability of macrophage EVs to actively interact with the cancer cells over the physiological cultures [120]. Moreover, Jhan et al. succeeded to encapsulate siRNA molecules into hybrid EVs. They follow a protocol that the hybrid EVs were first developed by simultaneous extrusion of lung carcinoma (A549) or mouse fibroblast (3T3) cell lines with the liposomal formulation. Afterward, the hybrid systems were loaded with the siRNA via an electroporation process. The scientists achieved an eightfold increase of the hybrid EVs’ quantity over the natural production, but believe that further system optimization could lead to less toxic platforms (a parameter that is correlated with the electroporation method) and more effective and “clean” delivery systems [121]. On the other hand, Evers et al. achieved a siRNA encapsulation efficiency of 50–60% into hybrid EV-liposomal systems by adding the siRNA during the liposomes’ thin film hydration stage [122].
3.2 Cargo Loading
As it is mentioned above, artificial exosomes have been used for a plethora of different drug molecules or biomolecules. First of all, artificial exosomes have proved beneficial for the loading of small molecules. For instance, Wu et al. developed advanced platforms that carry doxorubicin. In vitro and in vivo models showed that these novel platforms might potentially aid in the treatment of glioblastoma [123]. Moreover, another study by Go et al. achieved the production of EV-mimetic platforms loaded with anti-inflammatory drug molecules (dexamethasone). The researchers support that their ghost nanovesicles preserve the properties of the EVs, produced by U937 cell cultures. At the same time, the quantity of vesicles is significantly increased and the loading capacity is enhanced [124].
Furthermore, surface molecules of various molecular weights are able to attach in the surface of artificial exosomes. Notably, Yenerni et al. designed a click chemistry methodology that enables the binding of biomolecules in the exosomal surface. A cholesterol-DNA tether is responsible for the interaction with the bioactive molecules. In that study, the researchers attached an immunomodulatory protein, FasL. Nevertheless, due to the nature of click chemistry methodology, the experimental protein is just an example and many other molecules could replace it [125].
Lastly, different nucleic acids have also been delivered in in vivo preclinical experiments by artificial biomimetic vesicles. For example, EV-mimetic nanovesicles loaded with long noncoding RNA H19 that are produced through a top-down extrusion procedure provide promising results in the treatment of diabetic wounds [126]. Similarly, in vivo and in vitro uptake studies showed that artificial exosomes can effectively deliver siRNA [127].
4 Future Perspectives and Conclusion
The development of artificial exosomes provides new possibilities and opens new roads in the area of ATMPs and the technology of delivery systems. Due to their smart design, they combine the benefits of both biological systems and liposomal vesicles. First of all, their small size allows the easy drug or biomolecule delivery when they are in vivo administrated. A lower degree of interaction with the MPS has also been observed, compared to classical liposomal formulations. Moreover, membrane EV proteins that are incorporated in the surface of artificial exosomes might lead to enhanced active targeting to the desired tissue. Finally, the well-established liposome technology allows the production of platforms that can carry a variety of different types of molecules. Small drugs, proteins, nucleic acids, or even complexes, as in the case of the CRISPR/Cas9 complex, could be effectively loaded into artificial exosomes. Although the encapsulating efficiency of the drugs or biomolecules might be lower than in the case of classic liposomes (especially in the case of hydrophilic molecules), the artificial platforms eliminate the toxicity and adverse effects as they provide higher targeting efficiency. Thus, these bio-mimicking platforms might be morphologically complicated, due to the variety of their components, but they present a very clear, noncomplex functionality that we believe is strongly connected with the biophysical routes of biosystems.
However, a lot of points are yet to be cleared or optimized. For instance, although the top-down approaches are the most common ones, as mentioned above, they often need advanced manufacturing equipment and supplies that increase the cost of the final product. In addition, stricter instructions concerning GMPs are necessary for comparison with fully synthetic nanoparticles. Thus, the acceptance process is more demanding and currently under discussion. On the other hand, bottom-up approaches are still on an early stage, and thus, highly functional nanoplatforms are yet to be developed.
The hybrid systems present interesting behavior as advanced nano-therapeutic medicinal products. They combine the benefits of both synthetic and biological nanoplatforms resulting in the development of innovative procedures in the area of therapeutics. However, thorough design and targeted experiments are necessary to achieve the development of platforms that present the advantages and lack the limitations of these two technologies.
Nevertheless, exosomes and especially artificial exosomes breathe new life into the area of therapeutics, as they bring together the classic synthetic techniques of Pharmaceutical Sciences and the biological processes that are used in bio-medicinal products.
References
Barenholz Y. (Chezy). Doxil® — The first FDA-approved nano-drug: lessons learned. J Control Release [Internet]. 2012;160(2):117–34. Available from: https://www.sciencedirect.com/science/article/pii/S0168365912002301
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143.
Tsakiri M, Naziris N, Demetzos C. Innovative vaccine platforms against infectious diseases: under the scope of the COVID-19 pandemic [Internet]. Int J Pharm. 2021;610:121212. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517321010188
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–88.
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol [Internet]. 2020;21(10):585–606. Available from: https://doi.org/10.1038/s41580-020-0251-y.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem [Internet]. 1987;262(19):9412–20. Available from: https://doi.org/10.1016/S0021-9258(18)48095-7.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci [Internet]. 2018;75(2):193–208. Available from: https://doi.org/10.1007/s00018-017-2595-9.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal [Internet]. 2021;19(1):47. Available from: https://doi.org/10.1186/s12964-021-00730-1.
Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J [Internet]. 2010;29(6):1045–54. Available from: https://doi.org/10.1038/emboj.2010.6.
Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature [Internet]. 2009;458(7237):445–52. Available from: https://doi.org/10.1038/nature07961.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol [Internet]. 2018;19(4):213–28. Available from: https://doi.org/10.1038/nrm.2017.125.
Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol [Internet]. 2013;5(9). Available from: http://cshperspectives.cshlp.org/content/5/9/a016766.abstract
Mashouri L, Yousefi H, Aref AR, Mohammad AA, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer [Internet]. 2019;18(1):75. Available from: https://doi.org/10.1186/s12943-019-0991-5.
Raghu K, S LV. The biology, function, and biomedical applications of exosomes. Science (80- ) [Internet]. 2020;367(6478):eaau6977. Available from: https://doi.org/10.1126/science.aau6977.
Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res [Internet]. 2015;25(4):412–28. Available from: https://doi.org/10.1038/cr.2015.29.
Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif [Internet]. 2020;53(7):e12857. Available from: https://pubmed.ncbi.nlm.nih.gov/32578911
Demetzos C. Nanoplatforms as information carriers and thermodynamic epitopes in neurodegenerative and immune diseases BT. In: Vlamos P, Kotsireas IS, Tarnanas I, editors. Handbook of computational neurodegeneration. Cham: Springer International Publishing; 2020. p. 1–13. Available from: https://doi.org/10.1007/978-3-319-75479-6_59-2.
Naziris N, Chountoulesi M, Stavrinides S, Hanias M, Demetzos C. Chaotic dynamics and stability of liposomal Nanosystems [Internet]. Curr Nanosci. 2021;17:1–16. Available from: http://eurekaselect.com/article/117502
Janas T, Janas MM, Sapoń K, Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett [Internet]. 2015;589(13):1391–8. Available from: https://www.sciencedirect.com/science/article/pii/S001457931500294X
Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27(3):379–423.
Liao P-C, Garcia EJ, Tan G, Tsang CA, Pon LA. Roles for L o microdomains and ESCRT in ER stress-induced lipid droplet microautophagy in budding yeast. Mol Biol Cell [Internet]. 2021;32(22):br12. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85121947917&doi=10.1091%2Fmbc.E21-04-0179&partnerID=40&md5=f3e35ce09b52b9d3dd570a93ace984af
Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep [Internet]. 2018;8(1) Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85047824214&doi=10.1038%2Fs41598-018-26411-7&partnerID=40&md5=74e76040d7bf59be1de35a1444172ccb
Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol [Internet]. 2011;23(4):452–7. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-79960743373&doi=10.1016%2Fj.ceb.2011.04.008&partnerID=40&md5=75cf19e1ebd6bfdbac5d452a2f9f67b0
Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic [Internet]. 2009;10(7):925–37. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-67249110996&doi=10.1111%2Fj.1600-0854.2009.00920.x&partnerID=40&md5=ee716e1f2bdf5df58ed217b3bfb2e7b0
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol [Internet]. 2019;21(1):9–17. Available from: https://doi.org/10.1038/s41556-018-0250-9.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917.
Rios-Colon L, Arthur E, Niture S, Qi Q, Moore JT, Kumar D. The role of exosomes in the crosstalk between adipocytes and liver cancer cells. Cell. 2020;9(9):1988.
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cell. 2019;8(12):1605.
Famta P, Shah S, Khatri DK, Guru SK, Singh SB, Srivastava S. Enigmatic role of exosomes in breast cancer progression and therapy. Life Sci [Internet]. 2022;289. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85120755597&doi=10.1016%2Fj.lfs.2021.120210&partnerID=40&md5=9da49648ba37ed683347ed7d711d7990
Li Q, Wang D, Ding D, Feng Y, Hou R, Liu D, et al. The role and application of exosomes in gastric and colorectal cancer. Front Pharmacol [Internet]. 2022;12. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123799436&doi=10.3389%2Ffphar.2021.825475&partnerID=40&md5=4adcbb654b73077c7dada2ebbee6e488
Vignard V, Labbe M, Marec N, Andre-Gregoire G, Jouand N, Fonteneau J-F, et al. MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunol Res [Internet]. 2020;8(2):255–67. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85079018393&doi=10.1158%2F2326-6066.CIR-19-0522&partnerID=40&md5=c7c1d1f6fccc1da130343d462bcba9bd
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell [Internet]. 2019;49(3):347–60. Available from: https://doi.org/10.1016/j.devcel.2019.04.011.
Qian JJ, Akçay E. Competition and niche construction in a model of cancer metastasis. PLoS One [Internet]. 2018;13(5):e0198163. Available from: https://doi.org/10.1371/journal.pone.0198163.
Emmanouilidi A, Paladin D, Greening DW, Falasca M. Oncogenic and non-malignant pancreatic exosome cargo reveal distinct expression of oncogenic and prognostic factors involved in tumor invasion and metastasis. Proteomics. 2019;19(8):1800158.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun [Internet]. 2018;9(1):5395. Available from: https://pubmed.ncbi.nlm.nih.gov/30568162
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–67.
Muralidharan-Chari V, Kohan HG, Asimakopoulos AG, Sudha T, Sell S, Kannan K, et al. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget. 2016;7(31):50365.
Ning K, Wang T, Sun X, Zhang P, Chen Y, Jin J, et al. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J Surg Oncol. 2017;115(8):932–40.
Checa-Rojas A, Delgadillo-Silva LF, del Castillo V-HM, Andrade-Domínguez A, Gil J, Santillán O, et al. GSTM3 and GSTP1: novel players driving tumor progression in cervical cancer. Oncotarget. 2018;9(31):21696.
Zhang C, Ji Q, Yang Y, Li Q, Wang Z. Exosome: function and role in cancer metastasis and drug resistance. Technol Cancer Res Treat [Internet]. 2018;17:1533033818763450. Available from: https://pubmed.ncbi.nlm.nih.gov/29681222
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther [Internet]. 2020;5(1):145. Available from: https://pubmed.ncbi.nlm.nih.gov/32759948
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood J Am Soc Hematol. 2007;110(9):3234–44.
Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282(35):25779–89.
Frazão JB, Errante PR, Condino-Neto A. Toll-Like Receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch Immunol Ther Exp (Warsz) [Internet]. 2013;61(6):427–43. Available from: https://doi.org/10.1007/s00005-013-0243-0.
Borrelli C, Ricci B, Vulpis E, Fionda C, Ricciardi MR, Petrucci MT, et al. Drug-induced senescent multiple myeloma cells elicit NK cell proliferation by direct or exosome-mediated IL15 trans-presentation. Cancer Immunol Res [Internet]. 2018;6(7):860–9. Available from: https://doi.org/10.1158/2326-6066.CIR-17-0604.
Li Q, Huang Q, Huyan T, Wang Y, Huang Q, Shi J. Bifacial effects of engineering tumour cell-derived exosomes on human natural killer cells. Exp Cell Res [Internet]. 2018;363(2):141–50. Available from: https://www.sciencedirect.com/science/article/pii/S001448271730647X
Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–68.
Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol [Internet]. 2018 Apr 26;36(1):435–59. Available from: https://doi.org/10.1146/annurev-immunol-041015-055700.
del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, et al. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine [Internet]. 2011;29(21):3818–25. Available from: https://www.sciencedirect.com/science/article/pii/S0264410X11003744
Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun [Internet]. 2004;72(7):4127–37. Available from: https://pubmed.ncbi.nlm.nih.gov/15213158
Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect [Internet]. 2007;9(14):1614–22. Available from: https://www.sciencedirect.com/science/article/pii/S1286457907002602
Meng Q, Qiu B. Exosomal microRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression. Front Physiol. 2020;11:441.
Maeda Y, Farina NH, Matzelle MM, Fanning PJ, Lian JB, Gravallese EM. Synovium-derived microRNAs regulate bone pathways in rheumatoid arthritis. J Bone Miner Res. 2017;32(3):461–72.
Tavasolian F, Moghaddam AS, Rohani F, Abdollahi E, Janzamin E, Momtazi-Borojeni AA, et al. Exosomes: effectual players in rheumatoid arthritis. Autoimmun Rev [Internet]. 2020;19(6):102511. Available from: https://www.sciencedirect.com/science/article/pii/S156899722030063X
Miao C, Wang X, Zhou W, Huang J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res [Internet]. 2021;169:105680. Available from: https://www.sciencedirect.com/science/article/pii/S1043661821002644
Niu M, Li Y, Li G, Zhou L, Luo N, Yao M, et al. A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur J Neurol. 2020;27(6):967–74.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol [Internet]. 2019;25(5):702–9. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85059569151&doi=10.1007%2Fs13365-018-0695-4&partnerID=40&md5=97debd381bd593581199f7ebd8879d09
Nogueras-Ortiz CJ, Mahairaki V, Delgado-Peraza F, Das D, Avgerinos K, Eren E, et al. Astrocyte- and neuron-derived extracellular vesicles from Alzheimer’s disease patients effect complement-mediated neurotoxicity. Cells [Internet]. 2020;9(7). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85087721239&doi=10.3390%2Fcells9071618&partnerID=40&md5=2c5aa5d41ca90860402d4d31f068df3a.
Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29(2):589–96.
Reynolds JL, Mahajan SD. Transmigration of Tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J Neuroimmune Pharmacol. 2020;15(3):554–63.
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512–28.
Wang JKT, Langfelder P, Horvath S, Palazzolo MJ. Exosomes and homeostatic synaptic plasticity are linked to each other and to Huntington’s, Parkinson’s, and other neurodegenerative diseases by database-enabled analyses of comprehensively curated datasets [Internet]. Front Neurosci. 2017;11. Available from: https://www.frontiersin.org/article/10.3389/fnins.2017.00149
Kalani A, Tyagi N. Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res [Internet]. 2015;10(10):1565–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26692841
Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U, et al. Astrocytes at the hub of the stress response: potential modulation of neurogenesis by miRNAs in astrocyte-derived exosomes. Stem Cells Int. 2017;2017:1–13.
Frühbeis C, Fröhlich D, Krämer-Albers E-M. Emerging roles of exosomes in neuron-glia communication. Front Physiol [Internet]. 2012;30(3):119. Available from: https://pubmed.ncbi.nlm.nih.gov/22557979
Venturini A, Passalacqua M, Pelassa S, Pastorino F, Tedesco M, Cortese K, et al. Exosomes from astrocyte processes: signaling to neurons [Internet]. Front Pharmacol. 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fphar.2019.01452
Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell vesicles. 2014;3(1):24722.
Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136(1):41–56.
Chiarini A, Armato U, Gardenal E, Gui L, Dal Prà I. Amyloid β-exposed human astrocytes overproduce phospho-tau and overrelease it within exosomes, effects suppressed by calcilytic NPS 2143-further implications for Alzheimer’s therapy. Front Neurosci. 2017;11:217.
Baker S, Polanco JC, Götz J. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. J Alzheimers Dis. 2016;54(3):1207–17.
Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement Diagnosis, Assess Dis Monit [Internet]. 2016;3:63–72. Available from: https://www.sciencedirect.com/science/article/pii/S2352872916300252
Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11(4):1–11.
Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589(1):84–8.
Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis [Internet]. 2019;10(3):174. Available from: https://doi.org/10.1038/s41419-019-1404-9.
Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain [Internet]. 2020;143(5):1476–97. Available from: https://doi.org/10.1093/brain/awaa090.
Duarte-Sanmiguel S, Higuita-Castro N, Gallego-Perez D. Nanoelectroporation and collection of genetically modified exosomes in primary cultures of dendritic cells. Methods Mol Biol. 2020;2050:79–84.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol [Internet]. 2011;29(4):341–5. Available from: https://doi.org/10.1038/nbt.1807.
Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281–94.
Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871.
Han S, Li G, Jia M, Zhao Y, He C, Huang M, et al. Delivery of anti-miRNA-221 for colorectal carcinoma therapy using modified cord blood mesenchymal stem cells-derived exosomes. Front Mol Biosci. 2021;8:743013.
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin [Internet]. 2017;38(6):754–63. Available from: https://pubmed.ncbi.nlm.nih.gov/28392567
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release [Internet]. 2014;192:262–70. Available from: https://www.sciencedirect.com/science/article/pii/S0168365914005239
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12(3):655–64.
Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res [Internet]. 2012/05/22. 2012;40(17):e130. Available from: https://pubmed.ncbi.nlm.nih.gov/22618874
Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater [Internet]. 2020;106:328–41. Available from: https://www.sciencedirect.com/science/article/pii/S1742706120300696
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis?. Stem Cell Res Ther [Internet]. 2019;10(1):340. Available from: https://doi.org/10.1186/s13287-019-1445-0.
Kim YG, Choi J, Kim K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol J. 2020;15(12):2000082.
Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell-derived exosomes: a key player in wound healing. Tissue Eng Regen Med [Internet]. 2021;18(4):537–48. Available from: https://pubmed.ncbi.nlm.nih.gov/33547566
Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined Pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed [Internet]. 2020;15:5911–26. Available from: https://pubmed.ncbi.nlm.nih.gov/32848396
Lee B-C, Kang I, Yu K-R. Therapeutic features and updated clinical trials of Mesenchymal Stem Cell (MSC)-derived exosomes. J Clin Med [Internet]. 2021;10(4):711. Available from: https://pubmed.ncbi.nlm.nih.gov/33670202
Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36(8):2051–9.
EMA. Biological guidelines. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/biological-guidelines
EMA. Guideline on quality, non-clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials. 2019.
Song Y, Kim Y, Ha S, Sheller-Miller S, Yoo J, Choi C, et al. The emerging role of exosomes as novel therapeutics: biology, technologies, clinical applications, and the next. Am J Reprod Immunol [Internet]. 2020;85(2):e13329. Available from: https://pubmed.ncbi.nlm.nih.gov/32846024
Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4(1)
Silva AKA, Morille M, Piffoux M, Arumugam S, Mauduit P, Larghero J, et al. Development of extracellular vesicle-based medicinal products: a position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs – EVOLVE France.” Adv Drug Deliv Rev [Internet]. 2021;179:114001. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X2100394X.
Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–42.
Roces CB, Lou G, Jain N, Abraham S, Thomas A, Halbert GW, et al. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. 2020;12(11):1–19.
Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27
Šturm L, Poklar UN. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int J Mol Sci. 2021;22(12): 6547.
Li Y-J, Wu J-Y, Liu J, Xu W, Qiu X, Huang S, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology [Internet]. 2021;19(1):242. Available from: https://doi.org/10.1186/s12951-021-00986-2.
Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles [Internet]. 2016;5(1):32570. Available from: https://doi.org/10.3402/jev.v5.32570.
Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic [Internet]. 2021;22(7):204–20. Available from: https://pubmed.ncbi.nlm.nih.gov/34053166
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res [Internet]. 2017;66:30–41. Available from: https://www.sciencedirect.com/science/article/pii/S0163782716300492
Katarina T, Chieh H, Salvatore C, Lawrence R, Dirk W, Felix W, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (80- ) [Internet]. 2008;319(5867):1244–7. Available from: https://doi.org/10.1126/science.1153124.
De La Peña H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GWV, Selman A, et al. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods [Internet]. 2009;344(2):121–32. Available from: https://www.sciencedirect.com/science/article/pii/S0022175909000775
Li K, Chang S, Wang Z, Zhao X, Chen D. A novel micro-emulsion and micelle assembling method to prepare DEC205 monoclonal antibody coupled cationic nanoliposomes for simulating exosomes to target dendritic cells. Int J Pharm [Internet]. 2015;491(1):105–12. Available from: https://www.sciencedirect.com/science/article/pii/S0378517315004974
Oskar S, Franziska D, Rahul R, Martin S, Sebastian F, Heike B, et al. Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci Adv [Internet]. 2022;7(36):eabg6666. Available from: https://doi.org/10.1126/sciadv.abg6666.
Aday S, Hazan-Halevy I, Chamorro-Jorganes A, Anwar M, Goldsmith M, Beazley-Long N, et al. Bioinspired artificial exosomes based on lipid nanoparticles carrying let-7b-5p promote angiogenesis in vitro and in vivo. Mol Ther [Internet]. 2021;29(7):2239–52. Available from: https://www.sciencedirect.com/science/article/pii/S1525001621001441
Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep [Internet]. 2017;7(1):14322. Available from: https://doi.org/10.1038/s41598-017-14725-x.
Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano [Internet]. 2013;7(9):7698–710. Available from: https://doi.org/10.1021/nn402232g.
Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lässer C, et al. RNAi delivery by exosome-mimetic nanovesicles–implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–8.
Kim Y-S, Kim J-Y, Cho R, Shin D-M, Lee SW, Oh Y-M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med. 2017;49(1):e284.
Sato YT, Umezaki K, Sawada S, Mukai S, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep [Internet]. 2016;6(1):21933. Available from: https://doi.org/10.1038/srep21933.
Foteini P, Pippa N, Naziris N, Demetzos C. Physicochemical study of the protein–liposome interactions: influence of liposome composition and concentration on protein binding. J Liposome Res. 2019;29(4):313–21.
Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, et al. Cellular engineering with membrane Fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mater Interfaces [Internet]. 2016;8(11):6790–5. Available from: https://doi.org/10.1021/acsami.6b01315.
Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano [Internet]. 2018;12(7):6830–42. Available from: https://doi.org/10.1021/acsnano.8b02053.
Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5(4):1700611.
Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater [Internet]. 2019;94:482–94. Available from: https://www.sciencedirect.com/science/article/pii/S1742706119303812
Jhan Y-Y, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharm [Internet]. 2020;573:118802. Available from: https://www.sciencedirect.com/science/article/pii/S0378517319308476
Evers MJW, van de Wakker SI, de Groot EM, de Jong OG, Gitz-François JJJ, Seinen CS, et al. Functional siRNA delivery by extracellular vesicle–liposome hybrid nanoparticles. Adv Healthc Mater. 2021;11(5):e2101202.
Wu J-Y, Li Y-J, Hu X-B, Huang S, Luo S, Tang T, et al. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J Control Release [Internet]. 2021;336:510–21. Available from: https://www.sciencedirect.com/science/article/pii/S0168365921003473
Go G, Lee J, Choi D-S, Kim SS, Gho YS. Extracellular vesicle–Mimetic Ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle–induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8(4): e1801082.
Yerneni SS, Lathwal S, Shrestha P, Shirwan H, Matyjaszewski K, Weiss L, et al. Rapid on-demand extracellular vesicle augmentation with versatile Oligonucleotide tethers. ACS Nano [Internet]. 2019;13(9):10555–65. Available from: https://doi.org/10.1021/acsnano.9b04651.
Tao S-C, Rui B-Y, Wang Q-Y, Zhou D, Zhang Y, Guo S-C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv [Internet]. 2018;25(1):241–55. Available from: https://doi.org/10.1080/10717544.2018.1425774.
Yang Z, Xie J, Zhu J, Kang C, Chiang C, Wang X, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release [Internet]. 2016;243:160–71. Available from: https://www.sciencedirect.com/science/article/pii/S0168365916306526
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tsakiri, M., Naziris, N., Mahairaki, V., Demetzos, C. (2022). Artificial Exosomes as Targeted Drug Delivery Systems. In: Barabadi, H., Mostafavi, E., Saravanan, M. (eds) Pharmaceutical Nanobiotechnology for Targeted Therapy. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-12658-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-12658-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12657-4
Online ISBN: 978-3-031-12658-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)