Abstract
Tomato yellow leaf curl virus (TYLCV) causes tremendous losses of tomato worldwide. An elicitor Hrip1, which produced by Alternaria tenuissima, can serve as a pathogen-associated molecular patterns (PAMPs) to trigger the immune defense response in Nicotiana benthamiana. Here, we show that Hrip1 can be targeted to the extracellular space and significantly delayed the development of symptoms caused by TYLCV in tomato. In basis of RNA-seq profiling, we find that 1621 differential expression genes (DEGs) with the opposite expression patterns are enriched in plant response to biotic stress between Hrip1 treatment and TYLCV infection of tomato. Thirty-two known differential expression miRNAs with the opposite expression patterns are identified by small RNA sequencing and the target genes of these miRNAs are significantly enriched in phenylpropanoid biosynthesis, plant hormone signal transduction and peroxisome. Based on the Pearson correlation analysis, 13 negative and 21 positive correlations are observed between differential expression miRNAs and DEGs. These miRNAs, which act as a key mediator of tomato resistance to TYLCV induced by Hrip1, regulate the expression of phenylpropanoid biosynthesis and plant hormone signal transduction-related genes. Taken together, our results provide an insight into tomato resistance to TYLCV induced by PAMP at transcriptional and posttranscriptional levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants mount various kinds of defense responses to resist attacks by pathogens, including bacterial, fungal and virus (Jones and Dangl 2006). According to the model of plant immunity defense to bacterial and eukaryotic infection, researchers have proposed that antiviral immune systems can be divided into three categories, ETI (effector-triggered immunity) in antiviral defense, PTI [pathogen-associated molecular pattern (PAMP)-triggered immunity] in antiviral defense and NIK1-mediated antiviral response (Mandadi and Scholthof 2013; Zorzatto et al. 2015). Compared with ETI pathway, the PTI pathway that involves in plant resistance against viruses remains largely unexplored.
Small RNAs play important role in plant antiviral defense, and viral proteins can suppress endogenous defense response by targeting the components of small RNA biogenesis or processing (Sunkar et al. 2012; Alvarado and Scholthof 2009). The miR168 can limit the amount of Argonaute (AGO) 1, which is essential for antiviral RNAi. Monocotyledon-specific miR528 can also be competitively bound by AGO18 to release the target gene ascorbate oxidase (AO), thereby triggering the accumulation of reactive oxygen species (ROS) and initiating downstream antiviral pathways in plant (Wu et al. 2017). Recent study found that miR482 leads to downregulation of dozen of CC-NB-LRRs (CNLs) in response to unrelated viruses in Solanum lycopersicum (Shivaprasad et al. 2012).
Tomato yellow leaf curl disease (TYLCD), which is caused by several species of the Begomovirus genus (Geminiviridae), leads to drastic yield losses in global tomato production (Prasad et al. 2020; Butterbach et al. 2014). Tomato yellow leaf curl virus (TYLCV) is one of the most important causative agents of TYLCD. Hrip1, which is identified in Alternaria tenuissima, causes much higher ROS accumulation and triggers in tobacco systemic acquired resistance to tobacco mosaic virus (TMV) (Kulye et al. 2012). The inducible expression of Hrip1 enhances disease resistance and improves salt and drought tolerance in Arabidopsis thaliana (Peng et al. 2015). Studies have shown that the homologous protein of Hrip1 in A. alternata Strain R2 can be secreted into the cytoplasm of apple leaves as effector. It also improves the disease resistance of apple by interacting with MdNLR16 protein (Meng et al. 2018).
In this study, we found that Hrip1 mainly induces cell death by targeting the extracellular body and delays the development of symptoms during TYLCV infection. Furthermore, the mechanism of tomato resistance to yellow leaf curl virus induced by Hrip1 is elucidated by using RNA-seq and small RNA sequencing profiling at the transcriptional and posttranscriptional regulatory level, respectively. Our results will help to understand the PAMP-mediating plant antiviral mechanism.
Materials and methods
Plant growth conditions
Nicotiana benthamiana and tomato (S. lycopersicum cv. Moneymaker) plants were grown on soil (substrates were obtained from Jiffy company) in a growth chamber condition under a light/dark cycle (16 h of illumination at 120 μmol m−2 s−1 and 8 h dark) at a constant temperature of 27 °C and 60% relative humidity (RH).
Agrobacterium and protein infiltration assays
The transient expression by agroinfiltration was performed according the method described previously (Ma et al. 2015). To test the effect Hrip1-triggered cell death, Agrobacterium tumefaciens strains GV3101 carrying the plasmid pYBA1132-Hrip1, pYBA1132-Hrip118−164 (without signal peptide) and empty vector pYBA1132 were used. Briefly, overnight cultures of different A. tumefaciens containing pYBA1132-Hrip1 pYBA1132-Hrip118−164 and pYBA1132 plasmid were collected by centrifugation at 2000g for 6 min and resuspended in infiltration buffer (10 mM MES, 10 mM MgCl2, 20 μM acetosyringone, pH 5.6) with an optical density (OD600) of 0.6 and kept at room temperature for 3 h. The mixtures were infiltrated into leaves of 6-week-old N. benthamiana. Cell death was observed at three days after infiltration and leaves were isolated for total protein extraction and immunoblot analysis.
Expression and purification of recombinant Hrip1 protein
Pichia pastoris KM71H was used as the expression host and was cultured on yeast extract–peptone–dextrose (YEPD) medium. For expression of recombinant Hrip1 protein, the plasmid pPICZαA-Hrip1 was linearized with PmeI and transformed into P. pastoris KM71H. The yeast transformant contained plasmid pPICZαA-Hrip1 was grown and induced on BMGY (buffered glycerol-complex medium) and BMMY (buffered methanol-complex medium), respectively. The recombinant Hrip1 protein expression was performed according to the manufacturer’s instructions. Purification of recombinant Hrip1 protein from the culture supernatant was performed by affinity chromatography using GE HisTrap FF. The purified Hrip1 was kept in protein buffer (20 mM Tris, pH 8.0) and then stored at −80 °C. The purified Hrip1 was measured by SDS-PAGE and Western blotting and the concentration of Hrip1 was detected using Easy II Protein Quantitative Kit (BCA).
Viral infection assays in tomato
A. tumefaciens strains EHA105 carrying TYLCV-[CN:SH2], an infectious clone of tomato yellow leaf curve virus, was cultured on YEP medium. The cultures were collected and resuspended in infiltration buffer (10 mM MES, 10 mM MgCl2, 20 μM acetosyringone, pH 5.6) with an optical density (OD600) of 1.0 and kept at room temperature for 3 h. Two-week-old tomato seedlings were sprayed with the 20 μM Hrip1 protein solution or with a buffer control for two days prior to viral infection. The infectious clone was inoculated into the petioles of the tomato using a 1-mL syringe. After inoculation, the incidence of the disease was detected every day until the number of infected plants did not change. According to the recorded data, the percentage of tomato disease in different days after inoculation was calculated. Each group planted 15 tomato plants, and then combined the results of three parallel experiments to calculate the mean and standard deviation. Finally, the curve was generated for the incidence of tomato under different conditions.
Analysis of RNA sequencing and small RNA sequencing
Two-week-old tomato seedlings were sprayed with the 20 μM Hrip1 protein solution or with a buffer control for 2 h before tomato leaf was collected. The TYLCV infectious clone was inoculated into the petioles of 2-week-old tomato seedlings using a 1-mL syringe. After inoculation, the tomato leaf was collected when leaf appears curl and yellowing symptom. For libraries construction, samples of tomato seedlings were prepared from three independent biological replicates. Total RNA (RNeasy Plant Mini Kit, Qiagen, USA) was extracted from different treatment with three independent samples. A cDNA library was constructed for sequencing on the MGI DNBSEQ system (BGI Inc.). Bowtie2 was used to map clean reads to the reference genome of S. lycopersicum in the Tomato Sol Genomic Network database (http://solgenomics.net/). The gene expression levels were calculated by the RSEM. Differentially expressed genes (DEGs) were detected using the DESeq2 package with the following parameters: 1.5-fold and q < 0.05. Gene ontology (GO) functional enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed between different samples. For small RNA sequencing, sRNA libraries were constructed as described previously with minor modifications (Gao et al. 2015). Bowtie2 is used to map clean reads to the reference genome of S. lycopersicum and the other sRNA databases, such as miRBase, Rfam, siRNA, piRNA and snoRNA. The conserved miRNAs were identified by perfectly mapping all the reads to tomato genome and checked using the mireap (https://sourceforge.net/projects/mireap/). The miRA was used to predict novel miRNA by exploring the characteristic hairpin structure of miRNA precursor. Furthermore, psRobot, TAPIR or TargetFinder was used to predict miRNAs target gene in tomato.
Correlation analysis between miRNA and mRNA
The correlation analysis between miRNA and mRNA was determined using Pearson’s correlation coefficient with threshold more than 0.6. According to the correlation coefficients, correlation of DEGs and miRNAs was further analyzed with the following parameters. The correlation coefficient was negative and the expression of miRNAs and DEGs displayed the opposite trends in the same sample, suggesting that miRNAs were negatively correlated with target genes. Conversely, the correlation coefficient was positive and the expression of miRNAs and DEGs showed consistent trends in the same sample, suggesting that miRNAs were positively correlated with target genes.
Verification of miRNA and mRNA expression by RT-qPCR
About 2 µg of total RNA free of DNA contamination was reverse transcribed with oligo(dT)18 primer by EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen). Quantitative RT-PCR assays were performed using the Applied Biosystems 7500 Real-Time PCR System with PerfectStart® Green qPCR SuperMix (TransGen). The relative expression values were determined by using SlACTIN as a housekeeping gene. For miRNAs expression, miRNAs were extracted by the MiPure Cell/Tissue miRNA Kit (Vazyme) and miRNAs were reversed by miRNA first Strand cDNA Synthesis Kit (by stem-loop) (Vazyme). The relative expression values of miRNAs were determined by the same sample for both interest miRNAs and U6 small nuclear RNA using the miRNA Universal SYBR qPCR Master Mix (Vazyme) according to the instructions. Relative expression was determined by the 2−△△Ct method as described previously (Li et al. 2015). Three biological replicates were performed for all experiments. Moreover, each replicate comprised of three technical repetitions. Statistically significant differences were analyzed by ANOVA and Student’s t tests. All primers used herein are listed in Supplemental Table S1.
Data availability
High throughput sequence raw data for this study were deposited to the National Center for Biotechnology Information website SRA under PRJNA796154 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA796154?reviewer=ciaba2qtjmm0vqrp5j3f26m5l8).
Results
Hrip1 is an apoplastic elicitor of cell death
The hrp1 gene (Gene bank accession number HQ713431.1) in Alternaria tenuissima encodes a protein elicitor with 164 amino acids and a molecular weight of 17.53 kDa (Fig. 1a). Based on the EffectorP analysis (http://effectorp.csiro.au/), we found that Hrip1 might act as effector (95.1% probability). Transient expression of Hrip1 and Hrip118−164 (without signal peptide) in Nicotiana benthamiana showed that both Hrip1 and Hrip118−164 can cause cell death; nevertheless, cell death activity of Hrip1 with signal peptide was significantly stronger than Hrip118−164 (Fig. 1b). Western blot analysis showed that GFP, Hrip118−164-GFP and Hrip1-GFP could all be expressed in tobacco leaves (Fig. 1c), implying that the apoplastic space may be the main target of Hrip1. The transient expression of Hrip1 in tomato leaves also triggered severe cell death, compared with empty vector (Fig. 1d). In conclusion, we propose that Hrip1 mainly plays a role in the extracellular body.
Target site of Hrip1 triggering cell death. a Schematic presentation of the Hrip1 and Hrip118−164. b Representative N. benthamiana leaves 3 days after agroinfiltration containing pYBA1132-Hrip1 pYBA1132-Hrip118−164 and pYBA1132 plasmid. c Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing the indicated proteins from pYBA1132. d The cell death activity triggered by transient expression of Hrip1 in tomato. Representative tomato leaves 3 days after agroinfiltration containing pYBA1132 plasmid and pYBA1132-Hrip1
Hrip1 confers resistance to TYLCV in tomato
To confirm that Hrip1 could trigger cell death and then enhance the resistance to TYLCV in tomato, Hrip1 was expressed in the yeast Pichia pastoris using the pPICZaA vector (pPICZaA: Hrip1). The recombinant protein Hrip1 was purified using Ni–NTA resin, and detected by SDS-PAGE and Western blotting (Fig. S1a). Cell death activity of Hrip1 was assessed by infiltrating 10 μM protein solution into the mesophyll of tomato leaves. Compared with the Buffer and 10 μM BSA, Hrip1 induced distinctly cell death (Fig. S1b, c). Here, we sought to determine whether Hrip1 improves the resistance to TYLCY in tomato. The two-week tomato seedlings were treated foliar spraying with the 20 μM protein solution or with a buffer control and inoculated with the TYLCV-[CN:SH2] infectious clone after two days. The results showed that Hrip1 significantly delayed the development of symptoms until 15 days after inoculation with TYLCV, compared with the control group at 10 days (Fig. 2a, b). Furthermore, the quantity of TYLCV genomic DNA was measured by qPCR. The results demonstrated that the TYLCV replication was temporarily inhibited by Hrip1 treatment (Fig. 2c). In our previous study, a comparative proteomic analysis was performed by infiltration of recombinant protein Hrip1 or protein buffer into tomato leaves. A set of defense-related and stress-responsive proteins were up-regulated in Hrip1 treatment (unpublished results). To examine whether foliar spraying of Hrip1 triggers the plant immune response, the expression of defense-related genes was measured by RT-qPCR (Table 1). The results showed that several genes were significantly up-regulated in Hrip1 treatment, such as pathogenesis-related protein PR-1 (Solyc09g007010), pathogenesis-related protein 4B (Solyc01g097240), thaumatin-like protein (Solyc12g056390) and ascorbate peroxidase (Solyc06g005150) (Fig. 2d). These results suggest that plant immune defense response induced by Hrip1 may involve in tomato resistance to TYLCV.
The resistance effect of Hrip1 on TYLCV. a Phenotype of TYLCV-infected tomato. b Two-week-old tomato seedlings were treated foliar spraying with the 20 μM protein solution or with a buffer control for 2 days prior to viral infection. The infectious clone was inoculated into the petioles of the tomato using a 1-mL syringe. The incidence of the disease on control group and Hrip1 treatment groups was calculated every day. c The quantity of TYLCV was monitored from the total genomic DNA by qPCR. Error bars represented SE of three biological replicates and asterisks indicate significant differences by Student’s t test for p < 0.05. d The relative expression levels of defense response genes were detected by RT-qPCR in tomato treated foliar spraying with the 20 μM Hrip1 solution or with a buffer. Error bars represent standard deviation of three independent replicates. Error bars represented SE of three biological replicates and ** indicate significant differences by Student’s t test for p < 0.05
Hrip1 alters many defense-related genes expression pattern
To elucidate the mechanism of Hrip1-induced tomato resistance to TYLCV at the transcriptional level, the global expression patterns of tomato leaves were investigated by RNA-seq, which were treated with Hrip1, and BSA protein with the same concentration. The distribution of differentially expressed genes is shown in volcano plot (Fig. 3a). The results suggested that the expression of 5701 genes was significantly altered, including 2926 up-regulated and 2775 down-regulated (Fig. 3b). We found that among the top 50 DEGs altered by Hrip1 treatment (based on p values), 11 chlorophyll a/b-binding proteins were identified (Fig. 3c). Chlorophyll a/b-binding protein of light-harvesting complex II type 1 like (LHC II-1L) plays a vital role in maintaining the stability of the electron transport chain (Xu et al. 2022). Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation was performed for pathway analysis, and 125 KEGG pathways were obtained. Eight pathways including 338 genes were detected as closely associated with plant resistance to pathogens including photosynthesis-antenna protein, phenylpropanoid biosynthesis, flavonoid biosynthesis, indole alkaloid biosynthesis, plant hormone signal transduction, mitogen-activated protein kinase (MAPK) signaling pathway, plant–pathogen interaction and brassinosteroid biosynthesis (Fig. 3d). Therefore, these results suggest that differentially expressed genes induced by Hrip1 are involved in the plant disease process.
Analysis of differentially expressed (DE) gene between control and Hrip1 treatment. a Volcano plot showing the expression level change of genes after foliar spraying with the 20 μM Hrip1 solution, compared with a buffer control for two days. b Statistical analysis of differentially expressed genes (DEGs). FC fold change. FPKM fragments per kilobase of exon model per million mapped fragments. Fold Change ≥ 1.5 and q value ≤ 0.05 threshold were considered statistically significant. c Heat map of RNA sequencing showing the expression pattern of Top 50 DEGs in control and Hrip1 treatment. d KEGG analysis of differential expression genes in Hrip1 treatment. The x axis is the rich ratio and the y axis denotes the pathway terms. The rich factor represents the ratio of DEGs numbers annotated in our data to all gene numbers annotated in this pathway term. q value is the corrected p value and the lower q value indicates the greater level of enrichment of DEGs
Hrip1 treatment and TYLCV infection change many DEGs with opposite expression patterns
In order to further explore whether the DEGs induced by Hrip1 are involved in tomato resistance to TYLCV, we detected transcriptional changes induced by TYLCV infection infected by virus. Applying q < 0.05 and 1.5-fold as a cutoff, the transcriptomic data showed that TYLCV infection significantly altered gene expression, including 9938 up-regulated and 1960 down-regulated (Fig. S2). As mentioned above, Hrip1 can distinctly decrease viral infection in tomato. Therefore, we speculated that there were some genes with opposite expression patterns, which are closely associated with Hrip1-mediated antiviral activity. To test this hypothesis, we analyzed the common DEGs between Hrip1 treatment and TYLCV infection, and found that 3458 genes were common (Fig. 4a). These common genes were further divided into two group according to the opposite expression patterns. A total of 358 DEGs were identified in up-regulated by Hrip1 treatment and down-regulated by TYLCV infection (Fig. 4b). A total of 1263 DEGs were identified in down-regulated by Hrip1 treatment and up-regulated by TYLCV infection (Fig. 4c). A total of 1621 DEGs with the opposite expression patterns were selected for KEGG pathway analysis. The results demonstrated that these genes were significantly enriched in metabolic pathway, biosynthesis of secondary metabolites, biosynthesis of antibiotics, phenylpropanoid biosynthesis pathway and plant hormone signal transduction (Fig. 4d). Some DEGs, such as 8-HYDROXYGeraniol dehydrogenase-like (Solyc11g011330), anthocyanidin 3-O-glucosyltransferase 5-like (Solyc02g063000), peroxidase 15-like (Solyc11g072920), peroxidase 51 (Solyc02g094180), shikimate O-hydroxycinnamoyl transferase (Solyc03g117600) and vinorine synthase-like (Solyc07g006670) involved in p-hydroxyphenyl lignin, coniferin, and 5-hydroxy-guaiacyl lignin (syringin), syringin and syringyl lignin, were enriched in the phenylpropanoid biosynthesis pathway (Fig. S3). Furthermore, we also found that some DEGs of plant hormone signaling pathways were significantly enriched, including D-type Cyclin-2 (Solyc01g107730), IAA13(Solyc09g090910), LAX5 protein (Solyc10g055260), abscisic acid receptor PYL4 (Solyc10g085310), auxin-induced protein 10A5 (Solyc01g091030), histidine kinase 4 (Solyc04g008110), histidine-containing phosphor-transfer protein 4-like (Solyc08g066350), small auxin-up protein 58 (Solyc06g053260) and two-component response regulator ARR15-like protein (Solyc03g113720) (Fig. S4). In summary, our results suggest that Hrip1 mediates tomato resistance to TYLCV through the regulation of relevant genes in the phenylpropanoid biosynthesis and plant hormone signaling pathways.
Analysis of differential expression genes with opposite expression patterns. a Venn diagram of total DEGs between virus infection and Hrip1 treatment. The number in the circles show DEGs count. b Venn diagram of down-regulated DEGs by viral infection and up-regulated DEGs by Hrip1 treatment. c Venn diagram of up-regulated DEGs by viral infection and down-regulated DEGs by Hrip1 treatment. d KEGG analysis of DEGs with opposite expression patterns between viral infection and Hrip1 treatment. The x axis is the rich ratio and the y axis is the pathway terms. The rich factor represents the ratio of DEGs numbers annotated in our data to all gene numbers annotated in this pathway term. q value is the corrected p value and the lower q value indicates the greater level of enrichment of DEGs
Posttranscriptional regulation mediated by Hrip1 contributes tomato resistance to TYLCV
To further elucidate the mechanism of Hrip1-induced tomato resistance to TYLCV at the posttranscriptional level (PTGS), miRNAs were identified by small RNA sequencing. A total of 30 families including 145 known miRNAs were identified during the Hrip1 treatment and viral infection (Table S2). A total of 346 novel miRNAs were identified using the miRDeep2 and miRA for novel miRNA prediction. The differentially expressed analysis of miRNA was performed using DEGseq package. Here, we identified differentially expression miRNAs with q value ≤ 0.05. A total of 145 differentially expressed miRNAs were identified in Hrip1 treatment of tomato, including 90 and 55 up-regulated and down-regulated, respectively (Fig. 5a). A total of 157 differentially expressed miRNAs were found in TYLCV infection, including 116 up-regulated and 41 down-regulated (Fig. 5b), and 91 miRNAs were common between Hrip1 treatment and TYLCV infection (Fig. 5c). Among them, 32 miRNAs showed the opposite expression patterns. Nine miRNAs showed up-regulated by Hrip1 treatment and down-regulated by TYLCV infection, and 23 displayed the opposite trends (Fig. 5d, e). Some miRNAs, including sly-miR156e-5p, sly-miR166c-5p, sly-miR167b-3p, sly-miR171c, sly-miR171f, sly-miR390a-3p, sly-miR390a-5p, sly-miR477-5p, sly-miR6027-5p, sly-miR164a-5p and sly-miR164b-5p, were significantly changed with upregulation by TYLCV infection and downregulation by Hrip1 treatment (Fig. 5f), whereas sly-miR162, sly-miR171e, sly-miR396a-5p, sly-miR403-5p, sly-miR482e-5p, sly-miR9471a-3p and sly-miR9478-3 showed an opposite trend (Fig. 5g). These results implied that Hrip1 altered gene expression pattern to improve the tomato resistance to TYLCV by these miRNAs. We further predicted targets of miRNAs using the psRobot, TAPIR and TargetFinder (Fahlgren and Carrington 2010; Wu et al. 2012; Bonnet et al. 2010). KEGG pathway enrich showed that phenylpropanoid biosynthesis, starch and sucrose metabolism, plant hormone signal transduction, peroxisome, cyano-amino acid metabolism occur significantly enriched (Fig. 5h). These results were coincident with the change of tomato transcriptional level treated by Hrip1, implying that Hrip1 enhanced the tomato resistance to TYLCV by influencing phenylpropanoid biosynthesis and plant hormone signal transduction-related gene expression.
The analysis of differential expression (DE) miRNAs involved in Hrip1-induced tomato resistance to TYLCV. a Volcano plot represents the profile of differentially expressed miRNAs during foliar spraying with Hrip1 (HT) and buffer (control). b Volcano plot represents the profile of differentially expressed miRNAs during infected and uninfected tomato. c Venn diagram of total DE miRNAs between virus infection (Virus) and Hrip1 treatment (Hrip1). The number in the circles show DE miRNAs count. d Venn diagram of down-regulated DE miRNAs by viral infection and up-regulated DE miRNAs by Hrip1 treatment. e Venn diagram of up-regulated DE miRNAs by viral infection and down-regulated DE miRNAs by Hrip1 treatment. f The heat map analysis of miRNAs with upregulation in viral infection and downregulation in Hrip1 treatment. g The heat map analysis of miRNAs with downregulation in viral infection and upregulation in Hrip1 treatment. h KEGG analysis of target genes of DE miRNAs during foliar spraying with Hrip1 (HT) and buffer (control). The x axis is the rich ratio and the y axis is the pathway terms. The rich factor represents the ratio of DEGs numbers annotated in our data to all gene numbers annotated in this pathway term. q value is the corrected p value and lower q value indicates the greater level of enrichment of DEGs
Differentially co-expressed miRNAs associate with tomato resistance to TYLCV
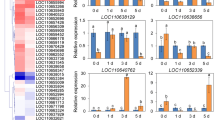
To further elucidate the mechanism of Hrip1-induced tomato resistance to TYLCV at both transcriptional and posttranscriptional levels, the Pearson correlation analysis was performed. The results indicated that 30 genes were predicted to be the candidate targets of 11 differentially expressed miRNAs. There were 13 negative correlations, including 6 miRNAs and 12 target genes. The number of positive correlations was 21, including 9 miRNAs and 19 target genes (Fig. 6a). The expression level of sly-miR166c-5p was significantly down-regulated with Hrip1 treatment and led to the upregulation of Solyc01g066957 and Solyc10g076510, which encodes Flowering-promoting factor 1-like protein 3 and pyruvate decarboxylase/indolepyruvate decarboxylase, respectively. sly-miR172a and sly-miR172b coordinately regulated the expression of Solyc10g006710, which encodes a G-type lectin S-receptor-like serine/threonine-protein kinase (Fig. 6b, Table 2). A total of 12 target genes of sly-miR1917 were identified. Among them, 9 genes were positive correlation, including Solyc09g090970, Solyc09g074270, Solyc11g007370, Solyc09g011490, Solyc08g021870, Solyc12g088170, Solyc11g044840, Solyc09g091550 and Solyc02g062390 (Fig. 6b, Table 2). Three genes were negative correlation, including Solyc05g055970, Solyc12g008980 and Solyc02g080400 (Fig. 6c, Table 2). DEGs encoding phenylpropanoid biosynthesis related proteins included Solyc12g088170 and Solyc11g007370. DEGs involving in plant hormone signal transduction-related proteins included Solyc09g090970, Solyc05g055970, Solyc08g021870, Solyc09g091550, Solyc10g006710 and Solyc11g044840. Solyc12g008980, Solyc09g074270 and Solyc10g076510 participated in phenylpropanoid biosynthesis and plant hormone signal transduction pathway. To verify the accuracy of RNA-seq and small RNA sequencing data, sly-miR1917, sly-miR172d and sly-miR166c-5p and their corresponding target genes Solyc05g055970, Solyc10g076940 and Solyc01g066957 were verified using RT-qPCR. The expression trend of RT-qPCR was consistent with that of sequencing, proving that the data obtained by high-throughput sequencing were reliable (Fig. 6d, e). In summary, our results suggest that these miRNA-regulated metabolic pathways play an important role in Hrip1-induced tomato resistance to TYLCV.
Correlation analysis regulatory network of miRNA and transcriptome. a Correlation analysis of DEGs in transcriptome data and target genes of DE miRNAs in small RNA sequencing data during foliar spraying with Hrip1 and buffer. b Positive correlation shows DE miRNAs and DEGs with the same expression patterns. c Negative correlation shows DE miRNAs and DEGs with the opposite expression patterns. The pentagram represents the DE miRNA and the oval represents the DEGs. Red color indicates up-regulated expression, and green color indicates down-regulated expression. d RT-qPCR validation of the expression level of miRNA. e RT-qPCR validation of the expression level of DEGs. Error bars represented SE of three biological replicates and ** indicate significant differences by Student’s t test for p < 0.05
Discussion
Plants perceive various danger signals by pattern recognition receptors (PRRs) and integrate this information to generate an appropriate immune response in order to block the invasion of pathogens (Gust et al. 2017; Couto and Zipfel 2016). Danger signals are derived from the infectious pathogens or from the plant itself. Previous studies have confirmed that Hrip1, a novel protein elicitor secreted by Alternaria tenuissima, can be recognized by plants (Kulye et al. 2012). In this study, we transiently expressed the Hrip1 and Hrip118−164 (without signal peptide) in Nicotiana benthamiana, and found that Hrip1 could trigger more obvious necrosis response. The homologous protein of Hrip1 derived from A. alternata interacted with MdNLR16 in cytoplasm and involved in apple disease resistance (Meng et al. 2018). Interestingly, Hrip118−164 can also cause a slight hypersensitivity reaction, implying that Hrip1 may also exist intracellular target. We also found that Hrip1 can interacted with the SlCSN5B (Solyc06g073150), which encoded a subunit of constitutive photomorphogenesis 9 (COP9) signalosome complex in tomato (our unpublished data). Previous study had demonstrated that TYLCSV C2 influences the activity of SKP1-CULLIN1-F-box (SCF) by interacting with COP9 signalosome 5 (CSN5) (including Arabidopsis CSN5A; S. lycopersicum cultivar Moneymaker SlCSN5) (Lozano-Duran et al. 2011). In addition, high-throughput sequencing data showed that many common genes existed in Hrip1 treatment and viral infection, especially with opposite expression patterns. These results demonstrated that the immune response mediated by Hrip1 plays an important role in tomato resistance to TYLCV.
Plant hormones, such as ethylene (ET), salicylic acid (SA), jasmonic acid (JA), involve in plant–virus interactions (Zhao and Li 2021). sly-miR1917 was involved in ethylene response regulation by negatively regulating the target gene SlCTR4svs (Wang et al. 2018). After perception of PAMPs, plants can rapidly synthesize ethylene. Previous study has demonstrated that the APETALA2 (AP2) and ethylene response factor (ERF) respond to the TYLCV infection (Huang et al. 2016). Our results demonstrated that Hrip1 significantly altered the expression level of sly-miR1917 and its target genes in tomato, implying that ethylene may be involved in the process of Hrip1-mediated viral resistance. Solyc09g090970 encoded Major allergen Mal d 1 protein, which belongs to pathogenesis-related proteins PR-10 (Ahammer et al. 2017). Previous study found that the Cytokinin-Specific Binding Proteins (CSBP) subfamily of PR-10 proteins might link with general phytohormone-binding properties and termed phytohormone-binding proteins (PhBP) (Ruszkowski et al. 2014). Solyc05g055970 encoded accelerated cell death 6 (ACD6) protein, and ACD6-1 mutant exhibited spontaneous cell death and increased disease resistance in A. thaliana. Acd6-1 also enhanced the response to salicylic acid (SA) (Lu et al. 2003). Solyc08g021870 was a homologous to BDA1 in A. thaliana. SNC2 is the receptor or coreceptor of bacterial PAMP signaling, and BDA1 plays a role downstream of SNC2 to activate the defense response triggered by PAMP. It might activate downstream defense pathways dependent on SA and NPR1 (Yang et al. 2012). Solyc09g091550 encoded a salicylic acid carboxyl methyltransferase and catalyzed salicylic acid to methyl salicylate (Koo et al. 2007). Solyc11g044840 encoded an LL-diaminopimelate aminotransferase, which catalyzed L-lysine to generate cyclic dehydropiperic acid and subsequently reduced to piperic acid (Pip) by SARD4 in A. thaliana. N-hydroxypiperidinic acid (NHP) derived from l-piperidinic acid can induce the expression of plant immune defense genes and act synergistically with the salicylic acid to promote cell death (Hartmann and Zeier 2018; Abeysekara et al. 2016; Navarova et al. 2012). Solyc10g006710, which was the target of sly-miR172a and sly-miR172b, encoded a G-type lectin S-receptor-like serine/threonine-protein kinase, which plays a crucial role in plant response to salt stress (Sun et al. 2013). Salicylic acid (SA) biosynthesis and signaling are required for plant systemic acquired resistance (SAR) that confers long-lasting protection against a broad spectrum of microorganisms (Hu et al. 2017). RNA-seq data showed that the expression of SlNRP1 (Solyc07g040690.3) was significantly increased 1.6-fold in Hrip1 treatment. In addition, Solyc02g032850.3 encoded Phytoalexin deficient 4 (PAD4) was also significantly increased 1.4-fold in Hrip1 treatment. Therefore, these results demonstrated that Hrip1 may activate the SA pathway to enhanced tomato resistance to TYLCV.
Plant defense compounds are divided into three main groups, including signaling molecules, phytoargins and phytoalexins (VanEtten et al. 1994). Phenylpropanoid biosynthesis is one of the most important pathways in plant and generates a series of secondary metabolites, such as lignin, anthocyanin and tannin, which play an important role in plant growth and development and response to biotic and abiotic stress (Li et al. 2021; Geng et al. 2020). In this study, we found that the target genes Solyc11g007370 (UDP-glucosyltransferase) and Solyc12g088170 (hydroxycinnamoyl CoA quinate transferase, HQT) of sly-miR1917 are involved in phenylpropanoid biosynthesis. Previous study demonstrated that UDP-glucosyltransferase (UGT84A2) is a novel component of Arabidopsis mesophyll nonhost resistance (NHR) to Phakopsora pachyrhizi. The phenylpropanoid metabolism is involved in resistance to pathogen infection through UDP-glucosyltransferase (Langenbach et al. 2013). Solyc12g088170 is the key enzyme catalyzing chlorogenic acid (CGA) biosynthesis and involves in phenylpropanoid biosynthesis. CGA has been implicated in resistance to both microbes and insects in tomato (Cle et al. 2008; Dixon et al. 2002).
Furthermore, there are three genes involved in both phenylpropanoid biosynthesis and plant hormone signal transduction pathway. Solyc12g008980 encoded a lycopene epsilon cyclase, which a key branch point enzyme in the carotenoid biosynthetic pathway (Yin et al. 2020). Downregulation of lycopene epsilon cyclase can enhance carotenoid synthesis and abscisic acid (ABA) via the β-branch-specific pathway (Kim et al. 2013). Solyc09g074270 probably involved in regulating perception of gibberellic acid (Hollender et al. 2016). Solyc10g076510 encoded a pyruvate decarboxylase/indolepyruvate decarboxylase, which a key enzyme in the production of indole-3-acetic acid (IAA) (Vande Broek et al. 2005). The treatment of IAA and GA enhanced growth parameters and accumulation of flavonoids and other phenolic compounds in buckwheat sprouts (Park et al. 2017).
Conclusions
Taken together, our results demonstrated that tomato perceived Hrip1 to improve the resistance to TYLCV by altering the gene expression of phenylpropanoid biosynthesis and plant hormone signal transduction pathway, which were modulated by some crucial miRNAs such as sly-miR1917, sly-miR172a, sly-miR172b and sly-miR166c-5p (Fig. 7). Therefore, our results provide an insight into Hrip1-induced tomato resistance to TYLCV.
A schematic model representing modes of action of Hrip1 in tomato. In this model, Hrip1 can be recognized by unknown receptor in cytoplasm membrane and the signal was transmitted into the cell. The expression of phenylpropanoid biosynthesis and plant hormone signal transduction pathway-related genes was regulated by some essential miRNAs to improve the resistance to TYLCV. The genes with same color as miRNA represent target of respective miRNA
References
Abeysekara NS, Swaminathan S, Desai N, Guo L, Bhattacharyya MK (2016) The plant immunity inducer pipecolic acid accumulates in the xylem sap and leaves of soybean seedlings following Fusarium virguliforme infection. Plant Sci 243:105–114
Ahammer L, Grutsch S, Kamenik AS, Liedl KR, Tollinger M (2017) Structure of the major apple allergen Mal d 1. J Agric Food Chem 65(8):1606–1612
Alvarado V, Scholthof HB (2009) Plant responses against invasive nucleic acids: RNA silencing and its suppression by plant viral pathogens. Semin Cell Dev Biol 20(9):1032–1040
Bonnet E, He Y, Billiau K, Van de Peer Y (2010) TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26(12):1566–1568
Butterbach P, Verlaan MG, Dullemans A, Lohuis D, Visser RG, Bai Y, Kormelink R (2014) Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. Proc Natl Acad Sci USA 111(35):12942–12947
Cle C, Hill LM, Niggeweg R, Martin CR, Guisez Y, Prinsen E, Jansen MA (2008) Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 69(11):2149–2156
Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16(9):537–552
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3(5):371–390
Fahlgren N, Carrington JC (2010) miRNA target prediction in plants. Methods Mol Biol 592:51–57
Gao C, Ju Z, Cao D, Zhai B, Qin G, Zhu H, Fu D, Luo Y, Zhu B (2015) MicroRNA profiling analysis throughout tomato fruit development and ripening reveals potential regulatory role of RIN on microRNAs accumulation. Plant Biotechnol J 13(3):370–382
Geng D, Shen X, Xie Y, Yang Y, Bian R, Gao Y, Li P, Sun L, Feng H, Ma F, Guan Q (2020) Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic Res 7(1):1–11
Gust AA, Pruitt R, Nurnberger T (2017) Sensing danger: key to activating plant immunity. Trends Plant Sci 22(9):779–791
Hartmann M, Zeier J (2018) L-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants. Plant J 96(1):5–21
Hollender CA, Hadiarto T, Srinivasan C, Scorza R, Dardick C (2016) A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol 210(1):227–239
Hu J, Yang H, Mu J, Lu T, Peng J, Deng X, Kong Z, Bao S, Cao X, Zuo J (2017) Nitric oxide regulates protein methylation during stress responses in plants. Mol Cell 67(4):702-710.e704
Huang Y, Zhang BL, Sun S, Xing GM, Wang F, Li MY, Tian YS, Xiong AS (2016) AP2/ERF transcription factors involved in response to tomato yellow leaf curly virus in tomato. Plant Genome 9 (2):plantgenome2015.09.0082.
Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329
Kim SH, Kim YH, Ahn YO, Ahn MJ, Jeong JC, Lee HS, Kwak SS (2013) Downregulation of the lycopene epsilon-cyclase gene increases carotenoid synthesis via the beta-branch-specific pathway and enhances salt-stress tolerance in sweetpotato transgenic calli. Physiol Plant 147(4):432–442
Koo YJ, Kim MA, Kim EH, Song JT, Jung C, Moon JK, Kim JH, Seo HS, Song SI, Kim JK, Lee JS, Cheong JJ, Choi YD (2007) Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant Mol Biol 64(1–2):1–15
Kulye M, Liu H, Zhang Y, Zeng H, Yang X, Qiu D (2012) Hrip1, a novel protein elicitor from necrotrophic Fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Environ 35(12):2104–2120
Langenbach C, Campe R, Schaffrath U, Goellner K, Conrath U (2013) UDP-glucosyltransferase UGT84A2/BRT1 is required for Arabidopsis nonhost resistance to the Asian soybean rust pathogen Phakopsora pachyrhizi. New Phytol 198(2):536–545
Li X, Huang L, Hong Y, Zhang Y, Liu S, Li D, Zhang H, Song F (2015) Co-silencing of tomato S-adenosylhomocysteine hydrolase genes confers increased immunity against Pseudomonas syringae pv. tomato DC3000 and enhanced tolerance to drought stress. Front Plant Sci 6:eCollection 2015
Li C, Kaituo W, Lei C, Cao S, Huang Y, Ji N, Xu F, Zheng Y (2021) Alterations in sucrose and phenylpropanoid metabolism affected by BABA-primed defense in postharvest grapes and the associated transcriptional mechanism. Mol Plant Microbe Interact 34(11):1250–1266
Lozano-Duran R, Rosas-Diaz T, Gusmaroli G, Luna AP, Taconnat L, Deng XW, Bejarano ER (2011) Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 23(3):1014–1032
Lu H, Rate DN, Song JT, Greenberg JT (2003) ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell 15(10):2408–2420
Ma Z, Song T, Zhu L, Ye W, Wang Y, Shao Y, Dong S, Zhang Z, Dou D, Zheng X, Tyler BM, Wang Y (2015) A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27(7):2057–2072
Mandadi KK, Scholthof KB (2013) Plant immune responses against viruses: how does a virus cause disease? Plant Cell 25(5):1489–1505
Meng D, Li C, Park HJ, Gonzalez J, Wang J, Dandekar AM, Turgeon BG, Cheng L (2018) Sorbitol modulates resistance to Alternaria alternata by regulating the expression of an NLR resistance gene in apple. Plant Cell 30(7):1562–1581
Navarova H, Bernsdorff F, Doring AC, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24(12):5123–5141
Park CH, Yeo HJ, Park YJ, Morgan AM, Valan Arasu M, Al-Dhabi NA, Park SU (2017) Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 22(3):374
Peng XC, Qiu DW, Zeng HM, Guo LH, Yang XF, Liu Z (2015) Inducible and constitutive expression of an elicitor gene Hrip1 from Alternaria tenuissima enhances stress tolerance in Arabidopsis. Transgenic Res 24(1):135–145
Prasad A, Sharma N, Hari-Gowthem G, Muthamilarasan M, Prasad M (2020) Tomato yellow leaf curl virus: Impact, Challenges, and Management. Trends Plant Sci 25(9):897–911
Ruszkowski M, Sliwiak J, Ciesielska A, Barciszewski J, Sikorski M, Jaskolski M (2014) Specific binding of gibberellic acid by cytokinin-specific binding proteins: a new aspect of plant hormone-binding proteins with the PR-10 fold. Acta Crystallogr D Biol Crystallogr 70(7):2032–2041
Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24(3):859–874
Sun XL, Yu QY, Tang LL, Ji W, Bai X, Cai H, Liu XF, Ding XD, Zhu YM (2013) GsSRK, a G-type lectin S-receptor-like serine/threonine protein kinase, is a positive regulator of plant tolerance to salt stress. J Plant Physiol 170(5):505–515
Sunkar R, Li YF, Jagadeeswaran G (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17(4):196–203
Vande Broek A, Gysegom P, Ona O, Hendrickx N, Prinsen E, Van Impe J, Vanderleyden J (2005) Transcriptional analysis of the Azospirillum brasilense indole-3-pyruvate decarboxylase gene and identification of a cis-acting sequence involved in auxin responsive expression. Mol Plant Microbe Interact 18(4):311–323
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus “Phytoanticipins.” Plant Cell 6(9):1191–1192
Wang Y, Zou W, Xiao Y, Cheng L, Liu Y, Gao S, Shi Z, Jiang Y, Qi M, Xu T, Li T (2018) MicroRNA1917 targets CTR4 splice variants to regulate ethylene responses in tomato. J Exp Bot 69(5):1011–1025
Wu HJ, Ma YK, Chen T, Wang M, Wang XJ (2012) PsRobot: a web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res 40(W1):W22–W28
Wu J, Yang R, Yang Z, Yao S, Zhao S, Wang Y, Li P, Song X, Jin L, Zhou T, Lan Y, Xie L, Zhou X, Chu C, Qi Y, Cao X, Li Y (2017) ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants 3(1):1–7
Xu S, Zhang X, Xu K, Wang Z, Zhou X, Jiang L, Jiang T (2022) Strawberry vein banding virus movement protein P1 interacts with light-harvesting complex II Type 1 like of fragaria vesca to promote viral infection. Front Microbiol 13:884044
Yang Y, Zhang Y, Ding P, Johnson K, Li X, Zhang Y (2012) The ankyrin-repeat transmembrane protein BDA1 functions downstream of the receptor-like protein SNC2 to regulate plant immunity. Plant Physiol 159(4):1857–1865
Yin L, Liu JX, Tao JP, Xing GM, Tan GF, Li S, Duan AQ, Ding X, Xu ZS, Xiong AS (2020) The gene encoding lycopene epsilon cyclase of celery enhanced lutein and β-carotene contents and confers increased salt tolerance in Arabidopsis. Plant Physiol Biochem 157:339–347
Zhao S, Li Y (2021) Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog 17(2):e1009242
Zorzatto C, Machado JP, Lopes KV, Nascimento KJ, Pereira WA, Brustolini OJ, Reis PA, Calil IP, Deguchi M, Sachetto-Martins G, Gouveia BC, Loriato VA, Silva MA, Silva FF, Santos AA, Chory J, Fontes EP (2015) NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 520(7549):679–682
Acknowledgements
We are grateful to Professor Xueping Zhou and Xiuling Yang (Institute of Plant Protection, Chinese Academy of Agricultural Sciences) for the generous gift of TYLCV-[CN:SH2] plasmid.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31701782) and the GDAS’ Project of Science and Technology Development (2021GDASYL-20210103016).
Author information
Authors and Affiliations
Contributions
YD and DQ designed the research. YD performed experiments and data analyses. YD and HZ wrote and revised the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, Y., Zhu, H. & Qiu, D. Hrip1 enhances tomato resistance to yellow leaf curl virus by manipulating the phenylpropanoid biosynthesis and plant hormone pathway. 3 Biotech 13, 11 (2023). https://doi.org/10.1007/s13205-022-03426-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03426-6