Abstract
This study evaluated in vivo anti-diabetic and anti-obesity activity of a polyherbal formulation’s methanolic extract containing an optimized ratio of edible seeds (Salvia hispanica, Chenopodium quinoa, Nelumbo nucifera). Diet-induced obese mice model (C57BL/6) was developed by feeding the mice a high-fat diet for 10 weeks resulting in hyperglycemia and obesity. Different doses (125, 250 and 500 mg/kg of body weight) of formulation were administered orally daily for 6 weeks. Fasting blood glucose and body weight were monitored throughout the study. At the end of the study, serum parameters were analyzed and histological examinations were performed. There was a significant reduction in fasting blood glucose levels and body weight in animal groups receiving polyherbal formulation. Lipid profile was improved as revealed by a reduction in serum triglycerides and total cholesterol. Histological study showed an improvement in liver, kidney and pancreatic sections of treated mice. High-performance thin layer chromatography was performed to identify the phytochemicals responsible for the above-mentioned bioactivities. The results revealed the presence of flavonoid (rutin) in seeds of N.nucifera and in the polyherbal formulation. For the first time, this study demonstrated the anti-diabetic and anti-obesity potential of the optimized formulation. The formulation can be used as a potential therapy for management of diabesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and hyperlipidemia as a result of altered carbohydrate and lipids metabolism occurring due to defects in insulin secretion, insulin action or both (Ojiaco et al. 2016; Ben Salem et al. 2017). In recent years, sedentary lifestyle and consumption of calorie-rich diet has resulted in increased incidence of diabetes and obesity also known as diabesity. Consumption of calorie-rich diet causes abnormal lipid metabolism, increased production of reactive oxygen species and oxidative stress, development of hyperinsulinemia and insulin resistance resulting in secondary diabetic complications (Bansal et al. 2012; Tan et al. 2018; Kopp 2019). Thus, considering these conditions, the treatment of type 2 diabetes mellitus (T2DM) should focus on modulating both glucose and lipid metabolism (Ben Salem et al. 2017).
There are various synthetic drugs available for treatment of T2DM, out of which intermediate-acting insulin, glibenclamide and metformin are accepted in almost all countries (Bazargani et al. 2014). These drugs are helpful in the management of T2DM, but there is no drug available for holistic management of T2DM (Rawat et al. 2013). Additionally, the adverse effect associated with currently available synthetic medicines has created the necessity for finding alternatives, mainly from natural sources, as these are easily available and possess less or even no side effects (Tran et al. 2020).
Herbal formulations developed from versatile combinations of pharmacologically potent herbs are being used in the treatment of several ailments including diabetes and obesity (De et al. 2019). The safety and efficacy of these herbs in producing multiple target action have made them a potential source of treatment for T2DM (Chang et al. 2013). Among various natural sources for T2DM management, some edible seeds (Salvia hispanica, Chenopodium quinoa, Nelumbo nucifera) have gained attention as super foods/functional foods (food exhibiting therapeutic effects apart from providing nutrition). S. hispanica (Chia) seeds are commonly consumed as salad sprouts, beverages, cereals, salad dressing or eaten raw (Kulczy´nski et al. 2019). Polyphenols from S.hispanica have been reported to be potent inhibitors of α-glucosidase and pancreatic lipase with antioxidant potential (Marineli et al. 2014; Martínez-Cruz and Paredes-López, 2014; Rahman et al. 2017; Rubavathi et al. 2020). Furthermore, S.hispanica seeds also improved glucose and insulin tolerance and reduced adiposity in animal models (Rosario et al. 2020; Enes et al. 2020; Oliva et al. 2021). Consumption of the seeds has shown to exhibit potent activities against dyslipidemia, cardiovascular disease, glucose homeostasis and insulin resistance in T2DM patients (Vuksan et al. 2017a, b; Cassia et al. 2022). C. quinoa (Quinoa) is known as “golden grain” and has been exploited in the development of nutrient-enriched novel food products, gluten-free products and can be eaten as rice replacement, soups, yogurt, salads, popcorn, flour and even sprouted (Brend et al. 2012; Angeli et al. 2020; Del Hierro et al. 2020). Recently, C. quinoa has gained attention due to its high nutritional value, phytochemical content and therapeutic activities including anti-hyperglycemic and lipase inhibitory potential (Graf et al. 2014; Tang et al. 2016; Lin et al. 2019; Song et al. 2021a, b; Oluwagunwa et al. 2021; Chen et al. 2022). Additionally, antioxidant potential of the seeds has also been reported (Park et al. 2017; Liu et al. 2020b, a; Sampaio et al. 2020; Enciso Roca et al. 2021; García-Parra et al., 2021). N.nucifera (lotus) seeds exhibit various flavonoids and alkaloids which contribute to its antioxidant and anti-diabetic potential (Rai et al. 2006; Pan et al. 2009; Kim and Shin 2012; You et al. 2014; Zhu et al. 2017). The seeds have been reported to exhibit hepatoprotective and anti-obesity activity (Sohn et al. 2003; Raajeswari and Meenakshi 2017; Wang et al. 2019).
Since diabesity is a multi-variant metabolic disorder, a single herb may be insufficient to exhibit the desired therapeutic effect. Just as combinational dosage forms of synthetic drugs are promoted in the treatment of T2DM with a multidimensional approach, polyherbal formulations (PF) have gained more attention over single herb for management of T2DM due to their synergistic therapeutic effects (Parasuraman et al. 2014; De et al. 2019). Various studies have reported the potential of PF in management of T2DM (Kiani et al. 2018; Virk et al. 2020; Pérez Gutiérrez et al. 2021; Perumal et al. 2022). Even though these studies proved the efficacy of herbal formulation treatment on glycemic control to be significant, their effect on lipid profile needs to be proved (Suvarna et al. 2021). Furthermore, identification of phytochemicals present in a newly developed herbal formulation is essential for supporting its therapeutic potential and to increase their acceptability prior to commercialization (Shinde et al. 2016). High-performance thin layer chromatography (HPTLC) is an established method for the analysis of various phytochemicals (Kustrin and Hettiarachchi 2014).
There are different reports to prove the anti-diabetic effect of these edible seeds, but there is no report demonstrating the anti-diabesity effect of methanolic extract of these three seeds combination on high-fat diet (HFD)-induced diabetic mice. For the first time, this research work reports the anti-diabesity effect of a PF, which was developed from methanolic extract of three edible seeds (S.hispanica, C.quinoa, N. nucifera) in a chemometrically optimized ratio with the aid of Design Expert Software 11.0 in a diet-induced obese (DIO) mice model (C57BL/6). The study also made an effort to identify the phytochemicals responsible for therapeutic effect of the PF using HPTLC.
Materials and method
Seeds collection
Seeds of S. hispanica, C. quinoa and N. nucifera were purchased from local shops (Bangalore) and authenticated at the Regional Ayurveda Research Institute for Metabolic Disorders (RARIMD), Bangalore, India. The authentication number for S. hispanica, C. quinoa and N. nucifera was RRCBI- mus189, RRCBI- mus213 and RRCBI-10394, respectively.
Preparation of polyherbal formulation
The individual seeds were powdered and mixed in a ratio of 1:1:1 according to earlier performed optimization studies (Tanisha and Majumdar 2019) and extracted with methanol using the Soxhlet apparatus. Extracts were then filtered through Whatman filter paper No. 1. The filtrates were concentrated by a rotary evaporator and the extracts were stored at 4 °C for further analysis. Methanol was chosen as a solvent for the preparation of PF extract, as methanol is reported to be the best solvent for achieving a maximum yield of phytochemicals due to higher solubility of these compounds (Truong et al. 2019; Kumar et al. 2020). Furthermore, studies have shown methanol as a choice of solvent for anti-diabetic studies (Chandran et al. 2017; Singh et al. 2018).
Animals
6-week-old male mice (C57BL/6) weighing 22 ± 3 g were obtained from In Vivo Biosciences, Bangalore, India. Mice were housed in polypropylene cages, maintained at 23 ± 1 °C, 60 ± 10% humidity, exposed to 12 h cycles of light and dark and had access to diet and water ad libitum throughout the study. Before starting the experiment, the mice were allowed to acclimatize for 7 days.
Diet-induced obese (DIO) model development
After the acclimatization period was over, mice were fed either low-fat diet with 10% kcal fat (Research Diets Inc. D12450B) or a high-fat diet with 60% kcal fat (Research Diets Inc. D12492) for 10 weeks to develop the DIO mice model (Shang et al. 2017). Diet formulation for the study was based on nutrient to calorie ratio to ensure that all the diet contains the same level of energy. The composition of commercial diet used in the study is presented (Table 1). The hyperglycemic status of mice was confirmed by fasting glucose levels. Thereafter, the diet-induced obese mice were treated with different doses of PF for another 6 weeks (drug treatment period) with a continuation of high-fat diets. All animal experiment protocols were approved by In Vivo Biosciences Institutional Animal Ethical Committee in agreement with CPCSEA (Committee for Purpose of Control and supervision of Experiments on Animals) guidelines (Regd. No. 1165/PO/RcBiBt-S/NRc-L/08/CPCSEA, Proposal number 62/2018).
Acute toxicity test
Acute oral toxicity test was performed by administering the methanolic extract of PF to C57BL/6 male mice having a weight of 25–30 g (n = 6/group). The test was performed as per the Organization for Economic Co-operation and Development (OECD) guideline 423. Different doses of PF extract (125, 250, 500, 1000 and 2000 mg/kg) were suspended in 0.5% carboxyl methylcellulose (CMC) and administered orally to the HFD-fed mice groups as mentioned in earlier studies (Zhang et al. 2016; Sahu et al. 2018). The normal control group was treated with CMC suspension. Mice were observed initially for 4 h and then for14 days. The limit dose for the study was 2000 mg/kg b.wt., which did not show any toxic effects in the treated mice. Thus, 1/4th (500 mg/kg b.wt.), 1/8th (250 mg/kg b.wt.) and 1/16th (125 mg/kg b.wt.) of the limit dose were chosen for further anti-diabetic studies (Zhang et al. 2016; Sahu et al. 2018).
Grouping and dosing of animals
Male mice were chosen for the study including oral starch tolerance test, oral sucrose tolerance test, oral lipid tolerance test and anti-diabetic activity. It has been shown that HFD feeding for 14 weeks resulted in prominent hyperinsulinemia and alterations in islet size without significant changes in β-cell function in C57BL/6 male mice in comparison to female mice (Pettersson et al. 2012). Therefore, C57BL/6 male mice seem to be an appropriate model for studying metabolic syndromes such as diabetes and obesity.
In the oral starch tolerance test, oral sucrose tolerance test and oral lipid tolerance test mice were randomly divided into five groups (n = 6 mice per group). For starch and sucrose tolerance test, Group I was treated with 1% CMC (1 ml/100 g of body weight (b.wt.)). Group II was treated with the standard drug, glibenclamide (5 mg/kg). Groups III, IV and V were treated with PF extract at a dose of 125 mg/kg, 250 mg/kg and 500 mg/kg, respectively.
For lipid tolerance test, Group I was treated with corn oil emulsion (5 ml/kg b.wt.). The composition of oil emulsion was a combination of cholesteryl oleate (2 mg), cholic acid (80 mg), saline (6 ml) and corn oil (6 ml). Group II was administered with orlistat (50 mg/kg). Groups III, IV and V were treated with PF extract at a dose of 125 mg/kg, 250 mg/kg and 500 mg/kg, respectively.
For the anti-diabetic study, mice were randomly divided into six groups (5 groups of diabetic mice and 1 additional group of normal mice, 6 mice per group) as shown below.
Group I: Normal mice treated with 1% CMC (1 ml/ 100 g b.wt.).
Group II: DIO mice: HFD without any treatment.
Group III: DIO mice: HFD + glibenclamide (25 mg/kg/ b.wt.).
Group IV: DIO mice: HFD + PF extract 125 mg/kg b.wt.
Group V: DIO mice: HFD + PF extract 250 mg/kg/day b.wt.
Group VI: DIO mice: HFD + PF extract 500 mg/kg/day b.wt.
The DIO mice were treated with the above-mentioned dose of PF extract and standard drug (glibenclamide) for 6 weeks. Glibenclamide was selected as a standard drug based on reports of different studies (Tamiru et al. 2012; Belayneh and Birru 2018). The three doses of the PF extract were determined based on the results of the acute oral toxicity study and according to earlier reported studies (Shin and Yoon 2012; Sharma et al. 2014; Araújo et al., 2016; Sarega et al. 2016). The oral route of administration was used in the study because plants are taken orally (Belayneh and Birru 2018). All the doses were given using an oral gavage after dissolving the PF extract in CMC at a volume of 1 ml/100 g body weight of the mice.
Oral carbohydrate challenge tests
The oral carbohydrate tolerance tests were conducted according to standard protocol, which has been discussed below. The tests were carried out using starch and sucrose in normal and diabetic mice, respectively.
Oral starch tolerance tests
The oral starch tolerance test was conducted according to standard protocol (Ye et al. 2002). Mice were kept on fasting for 16 h before the experiment, but had access to water ad libitum. Mice were divided into five groups (n = 6) and treated as described above. 30 min after dosing, the mice were loaded with corn starch (3 g/kg b.wt.) with an oral gavage. Blood was taken from tip of the tail vein at 0, 0.5, 1 and 2 h. Blood glucose concentration was determined by Accu-Chek glucometer (Roche Diagnostics) according to earlier reported studies (Mudgal et al. 2016; Tang et al. 2018).
Oral sucrose tolerance test
The oral sucrose tolerance test was carried out with a similar protocol mentioned in the oral starch tolerance test (Ye et al. 2002). However, instead of starch, the mice were loaded with sucrose at a dose of 4 g/kg. Blood glucose concentration was determined by Accu-Chek glucometer (Roche Diagnostics).
Oral lipid tolerance test
Oral lipid tolerance test (OLTT) was conducted as per the standard protocol (Zhang et al. 2008; Ghelani et al. 2017). The animals were kept fasting for 16 h before the experiment, but had access to water ad libitum. Mice were divided into five groups (n = 6) and treated as described above. 30 min after dosing, the mice were loaded with corn oil emulsion (5 ml/kg b.wt.) with an oral gavage. Blood was taken from the tip of the tail vein at 0, 1.5, 3 and 4 h. The level of plasma triglyceride (TG) was measured using the Accutrend® Plus system (Roche).
Assessment of anti-diabetic activity of the extract in DIO mice
For anti-diabetic evaluation of the PF extract, mice were fasted overnight (16 h). Mice were divided into six different groups (n = 6). Then the animals were treated according to their respective groups as mentioned above. The animals were supplemented with PF (via oral gavage) every day at the same time (9AM–10 AM) till the end of the study (6 weeks). For blood glucose measurement, the blood was obtained from the tip of the tail and measured using the Accu-Chek glucometer (Roche Diagnostics) till the end of the study. The blood glucose level of the treated animals was measured once a week throughout the study, i.e., for 6 weeks. The body weight of the animals was measured on a weekly basis throughout the study. On completion of the study, mice were fasted overnight and blood was obtained from the tip of the tail by pricking the tail and the TG level was measured using Accutrend Plus (Roche) triglyceride meter. Blood was drawn by cardiac puncture. Blood was collected in the microfuge tube, allowed to stand for 30 min at room temperature and centrifuged at 10,000 rpm for 10 min. The supernatant was collected in a fresh tube and stored at −80 °C until further use.
Histological studies
Vital organs (kidney, liver and pancreas) were excised and stored in cold 10% formalin containing phosphate-buffered saline for at least 24 h. Tissues were embedded in paraffin wax and 4 μm sections of tissues were stained with hematoxylin and eosin (H&E). Tissue sections were observed using an inverted microscope (Nikon Eclipse TE2000-5, Japan) at a magnification of 100× to find the morphological changes in treated and untreated groups.
HPTLC
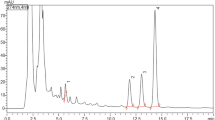
HPTLC was performed using standard protocol (Varkey and Kasthuri, 2016). The standard rutin was dissolved in methanol (1 mg/ml). 10 mg of S.hispanica, C.quinoa and N.nucifera seed extracts and PF extract were dissolved in 1 ml of methanol. HPTLC was performed on silica gel 60F254, 200X100 mm HPTLC plates (Merck, Darmstadt, Germany) with ethyl acetate:formic acid:acetic acid:water (14.4:1.4:1.4:2.8 (v/v) as a mobile phase. Standard solution rutin (10 μl) and test solution (10 μl) were loaded as 5 mm band length on HPTLC plate with the help of a CAMAG LIWOMAT 5 sample applicator at a distance of 10 mm from the edge of the plates. The sample-loaded plate was kept in a twin trough glass chamber (saturated with mobile phase for 30 min). The plate was developed in mobile phase up to 90 mm. The developed plate was dried in hot air to evaporate the remaining solvents. The plate was kept in a photo-documentation chamber (CAMAG REPROSTAR 3) to capture the images in white light, UV 254 nm and UV 366 nm. For derivatization, the plates were sprayed with a specific natural product reagent (1 g of 2-aminoethyl diphenylborinate in 200 ml of ethyl acetate). The plate was heated at 110º C for 3 min, then after cooling the plates were kept in photo-documentation chamber (CAMAG REPROSTAR 3) and the images were captured at 254 nm and 366 nm and white light. The peak table, peak densitogram and peak display were recorded. The retention factors (Rf) and % area were calculated by the WIN CATS software (version 4X).
Statistical analysis
Experimental values were expressed as mean ± SD (n = 6). Statistical analysis was performed using the one-way analysis of variance (ANOVA), followed by Dunnett’s test in GraphPad Prism 6.0. Results were considered statistically significant at p < 0.001 (Gao et al. 2016).
Results and discussion
Toxicological studies
During the observation period, no abnormality and mortality in the behavior of animals was seen at a dose of 2000 mg/kg.
DIO model development
HFD supplementation resulted in increased body weight (~ two fold) of DIO mice compared to lean control. Serum TG was significantly high compared to the lean control. 10 weeks of HFD supplementation resulted in impaired glucose tolerance in mice. It has been reported that the DIO model mimics the progression of impaired carbohydrate metabolism, increased hepatic glucose production, insulin resistance, compromised β-cell function, obesity and diabetes when fed with HFD as seen in humans (Kakimoto and Kowaltowski 2016).
Oral starch and sucrose tolerance test
Herbal extracts have been shown to control the elevation of starch and sucrose-associated postprandial blood glucose levels by inhibiting α-amylase and α-glucosidase enzymes. Thus, these extracts influence the absorption and metabolism of starch and sucrose (Subramanian et al. 2008). Similar to the above-mentioned literature, the present study showed that PF administration lowered elevated blood glucose levels in starch- and sucrose-fed DIO mice. PF extract significantly (p < 0.001) lowered elevated blood glucose levels in a dose-dependent manner when compared to diabetic control which received only starch and sucrose solution, respectively, for starch and sucrose tolerance test (Fig. 1 and Fig. 2). Also, at 2 h the blood glucose-lowering effect of the middle and higher dose (250 and 500 mg/kg b.wt.) of PF was comparable to glibenclamide. It can be said that PF delays the digestion of starch and sucrose, thus, lengthening the time needed for carbohydrate absorption. The tendency of the PF to suppress the increased blood glucose levels in starch-loaded HFD mice suggests the involvement of α-glucosidase inhibition as mentioned in earlier studies (Subramanian et al. 2008). Different studies have performed oral starch and sucrose tolerance tests for single herbs (De La Garza et al. 2014; Dakam et al. 2021; Solares-Pascasio et al. 2021), but rarely any reports available for oral starch and sucrose tolerance tests of a PF. To our knowledge, the present study for the first time demonstrates α-amylase and α-glucosidase inhibitory potential of the optimized PF methanolic extract using oral starch and sucrose tolerance test.
Oral lipid tolerance test
OLTT was performed as this assay evaluates lipid metabolism as well as whole-body lipid metabolism. The data from OLTT can be used to access information about the lipase inhibitory activity of pancreas and intestinal lipid absorption when investigating the suppressive effects of a herbal formulation’s extract on postprandial hypertriglyceridemia. Thus, it can be said that OLTT is a useful method to evaluate lipid metabolism (Ochiai 2020). Increased level of lipids is known as one of the major risk factors for obesity, hypercholesterolemia, atherosclerosis and myocardial infarction (Kim et al. 2018). In the present study, elevated plasma TG levels were observed in mice receiving corn oil emulsion alone at 4 h. Standard drug orlistat (5 mg/ kg) was used for this assay. The drug is commonly used to reduce the weight of overweight adults (Drew et al. 2007). Treatment with orlistat showed a reduction in TG when compared with HFD-fed group of animals. The PF extract (250 and 500 mg/kg b.wt.) significantly (p < 0.001) suppressed incremental plasma TG at 3 and 4 h (Fig. 3). The TG suppressing potential of the PF was comparable to standard drug (orlistat) used in the study. The results of the study indicated that administration of PF extract suppressed the intestinal TG absorption in OLTT, which can ameliorate hyperlipidemia as well as obesity-related parameters in HFD-fed obese mice. This finding of the study is important, as there is rarely any PF studied for OLTT. The study may also be helpful in selecting appropriate animal model and standard protocols for OLTT.
Glycemic status
The increase in fasting blood glucose level is an important characteristic of T2DM (Kifle and Belayneh 2020). Anti-hyperglycemic activity of PF in comparison to glibenclamide was estimated by measuring blood glucose levels on weekly basis. C57BL/6 on HFD feeding tended to have a slower glucose clearance compared to other groups. The PF extract at a dose of 250 and 500 mg/kg was found to be effective in lowering blood glucose levels. Furthermore, at a lower dose (125 mg/kg), PF extract did not show any significant effect on the blood glucose level. The glycemic control was nearly similar in animal groups treated with glibenclamide (5 mg/kg) and PF extract (250 and 500 mg/kg b.wt.). The decrease in fasting blood glucose level of PF treated DIO mice was observed from the 4th week and continued till the end of the study, i.e., for 6 weeks. The data obtained were comparable to that of the standard drug, glibenclamide (Fig. 4). The improvement in blood glucose levels observed in the present study correlated with the results obtained from the oral starch and sucrose tolerance and the same was reported in an earlier study (De La Garza et al. 2014). The anti-diabetic effect of herbal formulations (containing 3 herbs) has been reported earlier and the results of this study were similar to those reported in an earlier study, which has been discussed. A herbal formulation Gyeongshingangjeehwan 18 (composed of 3 herbs Ephedra sinica, Laminaria japonica and Rheum palmatum) showed anti-diabetic effect in HFD-fed mice when treated for 13 weeks. The formulation was given at a dose of 125, 250 and 500 mg/kg b.wt., out of which the higher dose (500 mg/kg b.w) inhibited hyperglycemia and hyperinsulinemia and improved glucose and insulin tolerance (Jang et al. 2018). A herbal formulation (C-DM4) containing a combination of Coptidis rhizome, Salviae miltiorrhizae radix and cinnamomi cortex was evaluated for its anti-diabetic and anti-obesity potential in HFD-fed mice. C-DM4 supplementation significantly reduced the increased levels of glucose, insulin, total cholesterol (TC), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in HFD-fed mice. In addition, C-DM4 extract inhibited lipid droplet accumulation in liver tissues of obese mice, hyperplasia of pancreatic islets and enlargement of adipocytes in adipose tissues (Jung et al. 2021). These results of the present study support the earlier findings that a combination of herbs or seeds offers better anti-hyperglycemic properties in comparison to individual herb or seeds.
Effect of PF supplementation on blood glucose level (mg/dL) in mice for weeks 1–6 (A) and specifically on the 6th week (B). Values represent mean ± SEM (n = 6). **p < 0.001 and ***p < 0.01 as compared with DIO control. $$$p < 0.01 as compared with the control. Data were analyzed with one-way ANOVA followed by post hoc analysis (Dunnett’s multiple comparison test). PF polyherbal formulation
Effect of PF on body weight
Consumption of more dietary fat may be related to increased fat accumulation and thus increased body weight (Yang et al. 2014; Lee et al. 2019; El-Zayat et al. 2019). Mice fed with HFD showed a significant increase in body weight compared to normal diet, as HFD consumption is considered the obesity hallmark (Meriga et al. 2017). Obesity is the main modifiable risk for T2DM. In the present study, the body weight of PF-administered animal group showed a significant (p < 0.001) reduction in the body weight at a dose of 250 and 500 mg/kg respectively, when compared with the HFD-fed animal group (Fig. 5). Furthermore, at lower dose (125 mg/kg) PF extract showed non-significant effect on the body weight. The finding of this study is in line with previous reports which stated that herbal formulation control weight gain in HFD-fed mice. Tongbi San, a formulation comprising a combination of three herbs (Cyperus rotundus L., Citrus unshiu Markovich and Poria cocos) was found to exhibit anti-obesity potential in HFD-fed mice when supplemented for 11 weeks (Park et al. 2019). Another anti-obesity formulation named LI85008F (Moringa oleifera, Murraya koenigii and curcuma longa) prevented body fat accumulation and altered lipid metabolism in HFD-fed mice when supplemented for 13 weeks (Choi et al. 2021). Previous studies have reported that herbal formulation extract supplementation may prevent or improve obesity and insulin resistance by modulating lipid metabolism and suppressing appetite (Lee et al. 2016; Yimam et al. 2016).
Effect on lipid profile
Lipid profile is crucial in the diagnosis and treatment of several diseases including T2DM. Diabetes mellitus-induced hyperlipidemia occurs as a result of the excess mobilization of fats from the adipose tissue due to the underutilization of glucose (Akpan et al. 2012). The elevated total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-c) and low levels of high-density lipoprotein cholesterol (HDL-c) in diabetic conditions imply diabetic dyslipidemia, which is caused due to insulin resistance leading to impairment of the key enzymes and pathways involved in lipid metabolism (Table 2) (Gao et al. 2009; Ozder 2014). There is a strong link between lipid metabolism and glucose metabolism and the inability to maintain lipid levels is a major cause of chronic complications in T2DM (Song et al., 2021a, b). In addition to this, the majority of T2DM patients exhibit dyslipidemia which is important in mediating the cardiovascular risk in T2DM (Parhofer 2015). Therefore, regulation of lipid metabolism should be considered as an important criterion along with glucose-regulating function in T2DM patients (Song et al., 2021ab). In the present study, the serum lipid profile was examined at the end of the study. A significant increment in lipid profiles of HFD-fed diabetic mice was observed including TC, TG and LDL-c, all this combined with a decrease in HDL-c. Middle and higher doses of PF extract (250 and 500 mg/kg) markedly improved lipid profile alterations induced by HFD, compared to low doses (125 mg/kg). Thus, it can be said that the anti-hyperlipidemic activity of PF extract was dose dependent. However, HDL-c level was not significantly different among the treated groups. This finding of the present study is in line with earlier reports which demonstrated that the TG, TC and LDL-c level reducing potential of herbal extracts might be due to the presence of polyphenolic compound, which plays a role in the prevention of advanced glycation end product (AGE) formation in diabetic mice (Jia et al. 2009). It has been reported that the extract of herbal formulations may delay the absorption of glucose and fatty acids, thus providing few substrates for triglyceride production. In addition, the PF extract might have inhibitory effects on pancreatic lipase as demonstrated by OLTT, which may contribute to its anti-hyperlipidemic activities (Subhasree et al., 2015; Kifle and Belayneh 2020). The results of the present study were found to be similar to the earlier studied anti-obesity PF, Yangkyuksanwha-tang. The supplementation of Yangkyuksanwha-tang to HFD-fed mice for 6 weeks resulted in lower TC, TG and LDL-c levels with a reduction in body weight (Koh et al. 2019). Furthermore, a study demonstrated that herbal extract and glibenclamide might act on reserved fats and inhibit the release of free fatty acids, decreasing the TC and TG levels and increasing HDL levels and similar findings were observed in the present study (Rahman et al. 2021).
Liver histology
Liver is the vital organ that regulates the metabolism of glucose, lipid and proteins (Park et al. 2015). Obesity due to HFD leads to the accumulation of lipid in liver resulting in fatty liver and other health complications including T2DM. In the present study, histological changes in the liver were studied to understand the effect of PF on the liver of untreated and treated mice (Fig. 6). Administration of PF extract resulted in a reduction of lipid droplets accumulation and restoration of hepatic architecture in mice as evidenced by histological examination of the liver tissue. Liver of untreated mice appeared normal without inflammation and lipid accumulation. The results of histological study showed that PF administration reduced excessive lipid deposition in liver tissues by accelerating lipid metabolism in T2DM mice and maintained the normal lipid metabolism, which played an effective protective role for the liver. This finding of the study is important because it has been reported that lipids and glucose play an important role in energy metabolism and both are regulated by the liver, thus exhibiting a close relationship between glucose and lipid metabolism (Parhofer 2015).
Histological examination of liver tissue (magnification, × 100) after hematoxylin and eosin staining. In the control group, HFD group and other treated groups lipid droplets are indicated by yellow-colored arrows, whereas hepatocytes in all the groups are indicated by white-colored arrows. HFD high-fat diet-induced obese diabetic mice, PF polyherbal formulation
Different studies have shown that herbal formulation consisting of three or more herbs improve fatty liver condition in DIO mice. A herbal formula named Yin Zhi Huang consisting of four herbs (Artemisia scoparia, Gardeniae fructus, Scutellaria baicalensis Georgi and Lonicerae japonicae flos), used as food in Asia, was found to be effective in controlling hepatic steatosis. The formulation was fed to C57BL/6 male mice for 16 weeks along with HFD, which resulted in a reduction of body weight, alleviation of hepatic lipid accumulation and restoration of plasma levels of TG and TC. The study suggested that supplementation of the formulation ameliorates diet-induced obesity and hepatic steatosis by decreasing AMPK/SREBP-1 pathway-mediated de novo lipogenesis and increasing AMPK/ACC/CPT1A pathway-mediated mitochondrial fatty acid β oxidation (Yao et al. 2020). Another herbal composition GGEx18, composed of Laminaria japonica, Rheum palmatum and Ephedra sinica, was able to prevent hepatic steatosis and hyperlipidemia at a dose of 250 and 500 mg/kg b.wt. in HFD-fed C57BL/6 mice by activating hepatic PPARα (Shin and Yoon 2012). Similar to our study, the lower dose of GGEx18 (125 mg/kg b.wt.) did not show any significant changes in liver of HFD-fed mice, proving that doses of 250 and 500 mg/kg b.wt. were effective in controlling hepatic steatosis. In another study, C57BL/6 male mice were fed HFD for 16 weeks and then treated with a herbal formula, MIT (Ephedra sinica, Panax ginseng and Alisma orientale), for 8 weeks. The results of the study suggested that MIT has the potential to prevent and treat obesity-related non-alcoholic fatty liver disease via regulating the levels of serum glucose and free fatty acids, inflammation, lipid accumulation and ROS-mediated liver damage (Ahn et al. 2020). The results of our study are in agreement with the above-mentioned studies that herbal formulation improves fatty liver by effectively reversing metabolic and histological changes associated with HFD consumption.
Kidney histology
HFD supplementation has been reported to cause alteration in kidney lipid metabolism, leading to renal damage including glomerulosclerosis, interstitial fibrosis, albuminuria and increased oxidative stress ultimately causing T2DM and its complications (Deji et al. 2009; Garcia et al. 2018). In the present study, the control group showed normal kidney architecture (Fig. 7). In HFD-fed obese and diabetic mice, kidneys showed increased Bowman’s space, mild swelling of the glomerulus and tubular degeneration. Administration of PF extracts to HFD + diabetic mice for 6 weeks resulted in an alleviation of glomerulus swelling and normal glomerulus architecture. Similar findings were reported for a potent anti-obesity formulation 18KHT01 when studied on HFD-fed C57BL/6 male mice. The formulation consisted of Quercus acutissima, Camellia sinensis and Geranium thunbergii, along with Citrus limon (fruit juice). Supplementation of 18KHT01 ameliorated histological alterations of kidney, which included mild infiltration of macrophages and enlargement of Bowman’s space of glomeruli (Pandeya et al. 2021). The findings of the present study may provide information on renal changes in HFD-fed mice, as very few herbal formulations have been studied for their effect on renal changes in HFD-fed mice.
Pancreas histology
In an excessive nutritional state, as seen in obesity, hyperglycemia and hyperlipidemia are often noticed, favoring insulin resistance and chronic inflammation. The differences in genetic susceptibility of β-cells result in toxic pressures including inflammation, inflammatory stress, ER stress, and metabolic, oxidative and amyloid stress, with the potential of ultimately leading to the loss of islet integrity (Fraulob et al. 2010; Galicia-Garcia et al. 2020). Our results corroborate these findings and showed that HFD supplementation caused loss of islet integrity, which was indicated by the irregular shape of pancreatic islet. After administration of PF for 6 weeks, improvement in the pancreatic tissues was observed, which is evidenced by more integrated cell structure in the pancreatic islets, with normal shape and size (Fig. 8). Apart from this, no major changes were observed in HFD-fed mice and this finding is in agreement with earlier finding (Lu et al. 2020). The PF showed dose-dependent improvement in tissue architecture. The standard drug (glibenclamide) used for the study has been reported to improve the architectural form of the islets of Langerhans and enhance the insulin secretion from the pancreatic beta cells (Asgary et al.2012; Parasuraman et al. 2019). The histological findings of this study were in accordance with earlier studies which suggested that the pancreas protective potential of PF could be due to reduction of oxidative stress (mediated by phenolic compounds present in PF), thus preserving pancreatic β-cell integrity and leading to insulinotrophic action (Taghizadeh et al. 2015). Earlier studies have shown that a combination of grape pomade and omija fruit preserved pancreatic islets’ architecture with increased pancreatic expressions of insulin and glucagon in HFD-fed mice (Cho et al. 2015). Relating earlier reports to our current findings, it can be said that the pancreas protective effect of PF can help in combating T2DM and its secondary complications. The anti-diabetic and anti-hyperlipidemic potential of PF observed in this study could be due to the increased amount of phytochemical constituents resulting from combining the three seeds in an optimized ratio.
HPTLC
HPTLC analysis of three individual seeds and PF was conducted to identify the phytochemicals of therapeutic importance. The presence of flavonoid (rutin) was confirmed in N.nucifera and PF with Rf values of 0.37 after derivatization with natural product reagent under UV light at 254 and 366 nm and in white light (Figs. 9, 10, 11). Rutin is known to exhibit various health-beneficial properties such as antioxidant, anti-inflammatory, anti-carcinogenic and anti-hyperglycemic effects (Kamalakkannan and Prince, 2006; Jadhav and Puchchakayala, 2012; Hunyadi et al. 2012; Panche et al. 2016; Prasad and Prasad 2019). Different unknown compounds were found in the seed extract of S.hispanica and C.quinoa. Studies have found that flavonoids not only possess lipase inhibitory potential, but also α-glucosidase inhibitory activity, which can prevent hyperglycemia, alleviate hyperinsulinemia, increase glucose tolerance and prevent and treat obesity (Liu et al. 2020b, a). Flavonoids from N.nucifera leaf showed anti-hyperglycemic effect in diabetic mice (Zhou et al. 2009). Flavonoids are also considered as α-amylase inhibitors (Najafian et al, 2010). Furthermore, studies have shown that HPTLC analysis is useful in identifying phytochemicals including flavonoids in various seeds and PF which exhibit anti-hyperglycemic effects (Cao et al. 2003; Parimala and Shoba, 2014; Salunke et al. 2015; Bhardwaj and Modi, 2017; Kumar et al. 2021). The outcomes of this study were in line with the formerly published studies which reported that the possible anti-hyperglycemic and anti-hyperlipidemic action of flavonoid in single and combinatorial herbal formulation extract in HFD-fed rats was found to be upregulation of hepatic superoxide dismutase activity, reduction of hepatic malondialdehyde content, downregulation of hepatic CYP2E1 expression and increase of glucose transporter 4 expressions in skeletal muscle of the treatment-receiving rats (Ma et al. 2012; Ojiaco et al. 2016). The developed PF could effectively reduce postprandial free fatty acid which could be attributed to its phenolic constituents as these are reported to inhibit pancreatic lipase by competitively binding to the enzyme active site (Yoshikawa et al. 2002; Martinez-Gonzalez et al. 2017; Goncalves and Romano, 2017). Furthermore, studies have also reported that rutin could preserve the intact functional β-cells and protect them from further deterioration which is necessary for insulin production (Arora et al., 2021). The pancreas protective effect of PF observed in this study was due to presence of flavonoids in the PF (De la Garza et al. 2014). Also, flavonoids are reported to exert hypolipidemic effect by suppressing HMG–CoA (hexamethyl glucose–coenzyme A) as reported in earlier study (De et al. 2019). It can be said that due to the presence of flavonoids in the PF extract, the PF exhibited glucose and lipid-regulatory potential and the same was confirmed by oral starch and sucrose tolerance test, oral lipid tolerance test, and anti-diabetic and anti-hyperlipidemic assays in DIO mice.
Conclusion
The current study highlights the anti-diabetic and anti-hyperlipidemic potential of methanolic extract of a developed and optimized PF in the DIO mice model for the first time. The study demonstrated that the PF is an effective anti-diabetic agent with multiple therapeutic effects including improved glucose tolerance and TG levels, while preventing metabolic syndrome and lifestyle-related diseases caused by excess consumption of HFD. The PF improved the histological architecture of the pancreatic islets, liver and kidney. These therapeutic activities of PF could be due to the presence of flavonoids as revealed by HPTLC. These results may provide a mechanistic basis for the use of PF as a potent natural functional food, drug or add-on therapy for achieving proper control on obesity-associated diabetes and preventing or delaying diabetic complications. Multicomponent synergism is suggested to be responsible for the observed therapeutic effects. However, further studies need to be carried out to explore the mechanism of action of the individual active phytochemical present in each seed extract along with their combinatorial interactions.
References
Ahn SH, Yang ES, Cho HR, Lee SO, Ha KT, Kim K (2020) Herbal formulation MIT ameliorates high-fat diet-induced non-alcoholic fatty liver disease. Integr Med Res 9:1–10. https://doi.org/10.1016/j.imr.2020.100422
Akpan EJ, Okokon JE, Offong E (2012) Antidiabetic and hypolipidemic activities of ethanolic leaf extract and fractions of Melanthera scandens. Asian Pac J Trop Biomed 2:523–527. https://doi.org/10.1016/S2221-1691(12)60089-6
Angeli V, Silva PM, Massuela DC, Khan MW, Hamar A, Khajehei F, Grae-Hönninger S, Piatti C (2020) Quinoa (Chenopodium quinoa Willd.): an overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 9:1–31. https://doi.org/10.3390/foods9020216
Araújo TG, De Oliveira AG, Vecina JF, Marin RM, Franco ES et al (2016) Parkinsonia aculeata (Caesalpineaceae) improves high-fat diet-induced insulin resistance in mice through the enhancement of insulin signaling and mitochondrial biogenesis. J Ethnopharmacol 183:95–102. https://doi.org/10.1016/j.jep.2016.02.048
Arora SK, Verma PR, Itankar PR, Prasad SK, Nakhate KT (2021) Evaluation of pancreatic regeneration activity of Tephrosia purpurea leaves in rats with streptozotocin-induced diabetes. J Tradit Complement Med 5:435–445. https://doi.org/10.1016/j.jtcme.2021.03.001
Asgary S, Rahimi P, Mahzouni P, Madani H (2012) Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan induced diabetic rats. J Res Med Sci 17:386–392
Bansal P, Paul P, Mudgal J, Nayak GP, Thomas Pannakal S, Priyadarsini KI et al (2012) Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol 64:651–658. https://doi.org/10.1016/j.etp.2010.12.009
Bazargani YT, de Boer A, Leufkens HGM, Mantel-Teeuwisse AK (2014) Selection of essential medicines for diabetes in low and middle income countries: a survey of 32 national essential medicines lists. PLoS ONE 9:1–8. https://doi.org/10.1371/journal.pone.0106072
Belayneh YM, Birru EM (2018) Antidiabetic activities of hydromethanolic leaf extract of calpurnia aurea (ait.) benth. subspecies aurea (Fabaceae) in mice. Evid Based Complement Altern Med 2018:1–10. https://doi.org/10.1155/2018/3509073
Ben Salem M, Kolsi RBA, Dhouibi R, Ksouda K, Charfi S, Yaich M et al (2017) Protective effects of Cynara scolymus leaves extract on metabolic disorders and oxidative stress in alloxan-diabetic rats. BMC Complement Altern Med 17:1–19. https://doi.org/10.1186/s12906-017-1835-8
Bhardwaj A, Modi KP (2017) Pharmacognostical screening and determination of antioxidant activity of Nelumbo nucifera gaertn ethanol seed extract by different in vitro models. Int J Pharm Pharm Sci 9:64–70. https://doi.org/10.22159/ijpps.2017v9i3.16362
Brend Y, Galili L, Badani H, Hovav R, Galili S (2012) Total phenolic content and antioxidant activity of red and yellow quinoa (Chenopodium quinoa Willd.) seeds as affected by baking and cooking conditions. Food Nutr Sci 03:1150–1155. https://doi.org/10.4236/fns.2012.38151
Cao QH, Qu WJ, Deng YX, Zhang ZC, Niu W, Pan YF (2003) Effects of flavonoids from the seed and fruit residue of Hippophae rhamnoides L. on glycometabolism in mice. J Chin Med Mater 26:735–737
Chandran R, Parimelazhagan T, George BP (2017) Antihyperglycemic activity of the bark methanolic extract of Syzygium mundagam in diabetic rats. Alexandria J Med 53:317–324. https://doi.org/10.1016/j.ajme.2016.12.001
Chang CLT, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC (2013) Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid-Based Complement Altern Med 2013:1–33. https://doi.org/10.1155/2013/378657
Chen X, He X, Sun J, Wang Z (2022) Phytochemical composition, antioxidant activity, α-glucosidase and acetylcholinesterase inhibitory activity of quinoa extract and its fractions. Molecules 27:1–18. https://doi.org/10.3390/molecules27082420
Cho S-J, Jung UJ, Kim H-J, Kim YJ, Han Y, Moon BS et al (2015) Mixture of ethanol extract of grape pomade and omija of prevents hyperglycemia and alleviates oxidative stress in mice fed an obesogenic diet. J Diabetes Metab 6:1–8. https://doi.org/10.4172/2155-6156.1000562
Choi HJ, Kim HY, Park KS (2021) Antiobesity effect of a novel herbal formulation LI85008F in high-fat diet-induced obese mice. Evid Based Complement Altern Med 2021:1–8. https://doi.org/10.1155/2021/6612996
da Marineli R S, Moraes ÉA, Lenquiste SA, Godoy AT, Eberlin MN, Maróstica MR (2014) Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci Technol 59:1304–1310. https://doi.org/10.1016/j.lwt.2014.04.014
Dakam W, Biyegue CFN, Fannang SV, Oben JE (2021) Leaf extracts of Glyphaea brevis attenuate high blood glucose and lipids in diabetic rats induced with streptozotocin. Pharmacogn Res 13:82–88. https://doi.org/10.4103/pr.pr_99_20
De Cassia LA, Corgozinho MLMV, Alves LV, Martins SR, Duarte RCF, Cardoso CN et al (2022) Effect of the use of Chia (Salvia Hispanica L.) seeds on antioxidant status and anthropometric parameters in obese, type 2 diabetics and/or hypertensive patients. Res Soc Dev 11:1–10. https://doi.org/10.33448/rsd-v11i4.27432
De B, Bhandari K, Katakam P, Goswami TK (2019) Development of a standardized combined plant extract containing nutraceutical formulation ameliorating metabolic syndrome components. SN App Sci 1:1–12. https://doi.org/10.1007/s42452-019-1518-9
De la Garza AL, Etxeberria U, Palacios-Ortega S, Haslberger AG, Aumueller E, Milagro FI et al (2014) Modulation of hyperglycemia and TNF α-mediated inflammation by helichrysum and grapefruit extracts in diabetic db/db mice. Food Funct 5:2120–2128. https://doi.org/10.1039/c4fo00154k
Deji N, Kume S, Araki SI, Soumura M, Sugimoto T, Isshiki K et al (2009) Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Ren Physiol 296:118–126. https://doi.org/10.1152/ajprenal.00110.2008
Del Hierro JN, Reglero G, Martin D (2020) Chemical characterization and bioaccessibility of bioactive compounds from saponin-rich extracts and their acid-hydrolysates obtained from fenugreek and quinoa. Foods 9:1–24. https://doi.org/10.3390/foods9091159
Del Rosario FM, Oliva ME, Aiassa V, D’Alessandro ME (2020) Salvia hispanica L. (chia) seed improves skeletal muscle lipotoxicity and insulin sensitivity in rats fed a sucrose-rich diet by modulating intramuscular lipid metabolism. J Funct Foods 66:1–9. https://doi.org/10.1016/j.jff.2019.103775
Drew BS, Dixon AF, Dixon JB (2007) Obesity management: update on orlistat. Vasc Health Risk Manag 3:817–821
El-Zayat SR, Sibaii H, El-Shamy KA (2019) Physiological process of fat loss. Bull Natl Res Cent 43:1–15. https://doi.org/10.1186/s42269-019-0238-z
Enciso-Roca EC, Aguilar-Felices EJ, Tinco-Jayo JA, Arroyo-Acevedo JL, Herrera-Calderon O (2021) Biomolecules with antioxidant capacity from the seeds and sprouts of 20 varieties of Chenopodium quinoa Willd. (Quinoa). Plants 10:1–21. https://doi.org/10.3390/plants10112417
Enes BN, de Paula Dias Moreira L, Toledo RCL, Moraes EA, de Castro Moreira ME, Hermsdorff HHM et al (2020) Effect of different fractions of chia (Salvia hispanica L.) on glucose metabolism, in vivo and in vitro. J Funct Foods 71:1–11. https://doi.org/10.1016/j.jff.2020.104026
Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA (2010) A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr 46:212–223. https://doi.org/10.3164/jcbn.09-83
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB et al (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21:1–34. https://doi.org/10.3390/ijms21176275
Gao D, Li Q, Li Y, Liu Z, Fan Y, Liu Z et al (2009) Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother Res 23:1257–1262. https://doi.org/10.1002/ptr.2603
Gao Y, Niu Y-F, Wang F, Hai P, Wang F, F Y-D (2016) Clerodane diterpenoids with anti-hyperglycemic activity from Tinospora crispa. Nat Prod Bioprospect. 6:247–255. https://doi.org/10.1007/s13659-016-0109-3
Garcia IJP, Cezar JS, Lemos BS, Silva LN, Ribeiro MDA et al (2018) Effects of high fat diet on kidney lipid content and the Na, K-ATPase activity. Braz J Pharm Sci 54:1–13. https://doi.org/10.1590/s2175-97902018000117165
García-Parra M, Roa-Acosta D, García-Londoño V, Moreno-Medina B, Bravo-Gomez J (2021) Structural characterization and antioxidant capacity of quinoa cultivars using techniques of FT-MIR and UHPLC/ESI-Orbitrap MS spectroscopy. Plants 10:1–15. https://doi.org/10.3390/plants10102159
Ghelani H, Razmovski-Naumovski V, Nammi S (2017) Chronic treatment of (R)-α-lipoic acid reduces blood glucose and lipid levels in high-fat diet and low-dose streptozotocin-induced metabolic syndrome and type 2 diabetes in sprague-dawley rats. Pharmacol Res Perspect 5:1–12. https://doi.org/10.1002/prp2.306
Goncalves S, Romano A (2017) Inhibitory properties of phenolic compounds against enzymes linked with human diseases. Phenolic compounds-Biologial activity (Chapter 6). IntechOpen, London, pp 99–118
Graf BL, Poulev A, Kuhn P, Grace MH, Lila MA, Raskin I (2014) Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem 163:178–185. https://doi.org/10.1016/j.foodchem.2014.04.088
Hunyadi A, Martins A, Hsieh T-J, Seres A, Zupko I (2012) Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 7:1–6. https://doi.org/10.1371/journal.pone.0050619
Jadhav R, Puchchakayala G (2012) Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int J Pharm Pharm Sci 4:251–256
Jang J, Park Y, Yoon M (2018) Effects of Gyeongshingangjeehwan 18 on pancreatic fibroinflammation in high-fat diet-fed obese C57BL/6J mice. Biomed Sci Lett 24:341–348. https://doi.org/10.15616/bsl.2018.24.4.341
Jia Q, Liu X, Wu X et al (2009) Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine 16:744–750. https://doi.org/10.1016/j.phymed.2008.12.012
Jung SM, Kwon SE, Kang SY, Kim SJ, Jung HW, Park Y-K (2021) Anti-obesity and anti-diabetic effects of a polyherbal extract consisting of Coptidis rhizoma, Salviae miltiorrhizae radix, and Cinnamomi cortex in high fat diet-induced obesity mice. J Korean Med Obes Res 21:59–68. https://doi.org/10.15429/jkomor.2021.21.2.59
Kakimoto PA, Kowaltowski AJ (2016) Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol 8:216–225. https://doi.org/10.1016/j.redox.2016.01.009
Kamalakkannan N, Prince PSM (2006) Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol 98:97–103. https://doi.org/10.1111/j.1742-7843.2006.pto_241.x
Kiani Z, Hassanpour-Fard M, Asghari Z, Hosseini M (2018) Experimental evaluation of a polyherbal formulation (Tetraherbs): antidiabetic efficacy in rats. Comp Clin Pathol 2018:1–9. https://doi.org/10.1007/s00580-018-2755-9
Kifle ZD, Belayneh YM (2020) Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of Hagenia abyssinica (Roasaceae) leaves in streptozotocin-induced diabetic mice. Diabetes Metab Syndr Obes: Targets Ther 13:4085–4094. https://doi.org/10.2147/DMSO.S279475
Kim M-J, Shin H-S (2012) Antioxidative effect of lotus seed and seedpod extracts. Food Sci Biotechnol 21:1761–1766. https://doi.org/10.1007/s10068-012-0234-7
Kim MO, Seo JH, Bin KE, Kang MJ, Lee SU, Moon DO et al (2018) Aceriphyllum rossii exerts lipid-lowering action in both normal and hyperlipidemic mice. Nat Prod Commun 13:471–474. https://doi.org/10.1177/1934578x1801300423
Koh YM, Jang SW, Ahn TW (2019) Anti-obesity effect of Yangkyuksanwha-tang in high-fat diet-induced obese mice. BMC Complement Altern Med 19:1–12. https://doi.org/10.1186/s12906-019-2669-3
Kopp W (2019) How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes, Metab Syndr Obes Targets Ther 12:2221–2236. https://doi.org/10.2147/DMSO.S216791
Kulczy´nski B, Kobus-Cisowska J, Taczanowski M, Kmiecik D, Gramza-Michałowska A (2019) The chemical composition and nutritional value of chia seeds- current state of knowledge. Nutrients 11(1):16. https://doi.org/10.3390/nu11061242
Kumar N, Pratibha N, Sharma S (2020) Effect of solvents on physiochemical properties of freeze-dried pomegranate seed (cv. Bhagwa). Int J Fruit Sci 20:S590–S604. https://doi.org/10.1080/15538362.2020.1747042
Kumar TS, Muthusamy P, Radha R, Ilango K (2021) Formulation and evaluation of in vitro antidiabetic polyherbal tablets form some traditional used herbs. J Phytopharmcol 10:173–179. https://doi.org/10.31254/phyto.2021.1035
Kustrin SA, Hettiarachchi CG (2014) Quantitative high performance thin layer chromatography for the analysis of herbal medicines: problems and advantages. Mod Chem App 2:1–2. https://doi.org/10.4172/2329-6798.1000e118
Lee YH, Jin B, Lee SH, Song M, Bae HH, Min BJ et al (2016) Herbal formula HT048 attenuates diet-induced obesity by improving hepatic lipid metabolism and insulin resistance in obese rats. Molecules 2016:1–13. https://doi.org/10.3390/molecules21111424
Lee M, Kim J, Choi J, Park J, Kim H, Song B et al (2019) (2019) Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp Ther Med 17:2185–2193. https://doi.org/10.3892/etm.2019.7191
Lin M, Han P, Li Y, Wang W, Lai D, Zhou L (2019) Quinoa secondary metabolites and their biological activities or functions. Molecules 24:1–47. https://doi.org/10.3390/molecules24132512
Liu M, Zhu K, Yao Y, Chen Y, Guo H et al (2020a) Antioxidant, anti-inflammatory and antitumor activities of phenolic compounds from white, red and black Chenopodium quinoa seeds. Cereal Chem 97:703–713. https://doi.org/10.1002/cche.10286
Liu T-T, Liu X-T, Chen Q-X, Shi Y (2020b) Lipase inhibitors for obesity: a review. Biomed Pharmacother 128:1–9. https://doi.org/10.1016/j.biopha.2020.110314
Lu HF, Lai YH, Huang HC, Lee IJ, Lin LC, Liu HK et al (2020) Ginseng-plus-Bai-Hu-Tang ameliorates diet-induced obesity, hepatic steatosis, and insulin resistance in mice. J Ginseng Res 44:238–246. https://doi.org/10.1016/j.jgr.2018.10.005
Ma D-Q, Jiang Z-J, Xu S-Q, Yu X, Hu X-M, Pan H-Y (2012) Effects of flavonoids in Morus indica on blood lipids and glucose in hyperlipidemia-diabetic rats. Chin Herb Med 4:314–318. https://doi.org/10.3969/j.issn.1674-6348.2012.04.008
Martínez-Cruz O, Paredes-López O (2014) Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J Chromatogr A 1346:43–48. https://doi.org/10.1016/j.chroma.2014.04.007
Martinez-Gonzalez AI, Alvarez-Parrilla E, Diaz-Sanchez AG, De la Rosa LA, Nunez-Gastelum JA, Vazquez-Flores AA, Gonzalez-Aguila GA (2017) In vitro inhibition of pancreatic lipase by polyphenols: a kinetic, fluorescence spectroscopy and molecular docking study. Food Technol Biotechnol 55:519–530. https://doi.org/10.17113/ftb.55.04.17.5138
Meriga B, Parim B, Chunduri VR, Naik RR, Nemani H, Suresh P, Uddandrao VS (2017) Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab 14:72. https://doi.org/10.1186/s12986-017-0228-9
Mudgal J, Shetty P, Reddy ND, Akhila HS, Gourishetti K, Mathew G et al (2016) In vivo evaluation of two Thiazolidin-4-one derivatives in high sucrose diet fed pre-diabetic mice and their modulatory effect on AMPK, Akt and p38 MAP Kinase in L6 Cells. Front Pharmacol 7:1–10. https://doi.org/10.3389/fphar.2016.00381
Najafian M, Ebrahim-Habibi A, Yaghmaei P, Parivar K, Larijani B (2010) Core structure of flavonoids precursor as an anti-hyperglycemic and anti-hyperlipidemic agent: an in vivo study in rats. Acta Biochim Pol 57:553–560
Ochiai M (2020) Evaluating the appropriate oral lipid tolerance test model for investigating plasma triglyceride elevation in mice. PLoS ONE 15:1–17. https://doi.org/10.1371/journal.pone.0235875
OECD guideline for testing of chemicals (2001) Guideline 423: Acute oral toxicity-Acute oral toxic class method 2001. Paris
Ojiaco OA, Chikezie PC, Ogbuji AC (2016) Blood glucose level and lipid profile of alloxan-induced hyperglycemic rats treated with single and combinatorial herbal formulations. J Tradit Complement Med 6:184–192. https://doi.org/10.1016/j.jtcme.2014.12.005
Oliva ME, del Rosario FM, Joubert MBV, D’Alessandro ME (2021) Salvia hispanica L. (chia) seed promotes body fat depletion and modulates adipocyte lipid handling in sucrose-rich diet-fed rats. Food Res Int 2021:1–10. https://doi.org/10.1016/j.foodres.2020.109842
Oluwagunwa OA, Alashi AM, Aluko RE (2021) Inhibition of the in vitro activities of α-amylase and pancreatic lipase by aqueous extracts of Amaranthus viridis, Solanum macrocarpon and Telfairia occidentalis leaves. Front Nutr 8:1–17. https://doi.org/10.3389/fnut.2021.772903
Ozder A (2014) Lipid profile abnormalities seen in T2DM patients in primary healthcare in Turkey: a cross-sectional study. Lipids Health Dis 13:1–6. https://doi.org/10.1186/1476-511X-13-183
Pan Y, Cai B, Wang K, Wang S, Zhou S, Yu X et al (2009) Neferine enhances insulin sensitivity in insulin resistant rats. J Ethnopharmacol 124:98–102. https://doi.org/10.1016/j.jep.2009.04.008
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:1–15. https://doi.org/10.1017/jns.2016.41
Pandeya PR, Lamichhane R, Lamichhane G, Lee K-H, Lee HK, Rhee S-J (2021) 18KHT01, a potent anti-obesity polyherbal formulation. Front Pharmacol 12:1–19. https://doi.org/10.3389/fphar.2021.807081
Parasuraman S, Thing GS, Dhanaraj SA (2014) Polyherbal formulation: concept of ayurveda. Pharmacogn Rev 8:73–80
Parasuraman S, Ching TH, Leong CH, Banik U (2019) Antidiabetic and antihyperlipidemic effects of a methanolic extract of Mimosa pudica (Fabaceae) in diabetic rats. Egypt J Basic Appl Sci 6:137–148. https://doi.org/10.1080/2314808X.2019.1681660
Parhofer GK (2015) Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J 39:353–362. https://doi.org/10.4093/dmj.2015.39.5.353
Parimala M, Shoba FG (2014) In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali burm F. BMC Complement Altern Med 14:1–9. https://doi.org/10.1186/1472-6882-14-361
Park H, Hwang YH, Kim DG, Jeon J, Ma JY (2015) Hepatoprotective effect of herb formula KIOM2012H against nonalcoholic fatty liver disease. Nutrients 7:2440–2455. https://doi.org/10.3390/nu7042440
Park JH, Lee YJ, Kim YH, Yoon KS (2017) Antioxidant and antimicrobial activities of quinoa (Chenopodium quinoa Willd.) seeds cultivated in Korea. Prev Nutr Food Sci 22:195–202. https://doi.org/10.3746/pnf.2017.22.3.195
Park YJ, Lee GS, Cheon SY, Cha YY, An HJ (2019) The anti-obesity effects of Tongbi-san in a high-fat diet-induced obese mouse model. BMC Complement Altern Med 19:1–14. https://doi.org/10.1186/s12906-018-2420-5
Pérez Gutiérrez RM, Muñiz-Ramirez A, Garcia-Campoy AH, Flores JMM (2021) Evaluation of the antidiabetic potential of extracts of Urtica dioica, Apium graveolens, and Zingiber officinale in mice, Zebrafish, and pancreatic β-Cell. Plants 10:1–17. https://doi.org/10.3390/plants10071438
Perumal N, Nallappan M, Shohaimi S, Kassim NK, Tee TT, Cheah YH (2022) Synergistic antidiabetic activity of Taraxacum officinale (L.) Weber ex F.H. Wigg and Momordica charantia L. polyherbal combination. Biomed Pharmacother 145:1–13. https://doi.org/10.1016/j.biopha.2021.112401
Pettersson US, Walden TB, Carsson P-O, Jansson L, Philipson M (2012) Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0046057
Prasad R, Prasad SB (2019) A review on the chemistry and biological properties of Rutin, a promising neutraceutical agent. Asian J Pharm Pharm 5:1–20. https://doi.org/10.31024/ajpp.2019.5.s1.1
Raajeswari PA, Meenakshi C (2017) Antioxidant and hepatoprotective activity of Lotus (Nelumbo nucifera) seed extract. Food Sci Nutr Technol 2:1–7
Rahman JM, de Camargo AC, Shahidi F (2017) Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods 35:622–634. https://doi.org/10.1016/j.jff.2017.06.044
Rahman S, Jan G, Jan FG, Ur Rahim H (2021) Phytochemical screening and antidiabetic, antihyperlipidemic, and antioxidant effects of Leptopus cordifolius decne in diabetic mice. Front Pharmacol 12:1–11. https://doi.org/10.3389/fphar.2021.643242
Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK (2006) Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol 104:322–327. https://doi.org/10.1016/j.jep.2005.09.025
Rawat S, Kumar N, Kothiyal P (2013) Evaluate the anti-diabetic activity of myrica esculenta in streptozotocin induced rats. Int J Univ Pharm Bio Sci 2:510–525
Rubavathi S, Ayyappadasan G, Sangeetha N, Harini T, Saranya D, Harshapradha P (2020) Studies on antioxidant and anti-obesity activity of Salvia hispanica (Chia) seed extracts. J Drug Del Ther 10:98–106. https://doi.org/10.22270/jddt.v10i3-s.4169
Sahu MK, Singh VK, Rao SP (2018) Development and evaluation of antidiabetic potential of polyherbal formulation in streptozotocin induced animal model. Int J Cell Sci Mol Biol 5:29–37. https://doi.org/10.19080/IJCSMB.2018.05.555656
Salunke S, Pande V, Kendre P, Vibhute S (2015) Effect of standardized polyherbal formulations on blood glucose, body weight, food and water consumption of rat. Pharm Sci 2015:57–64. https://doi.org/10.15171/PS.2015.18
Sampaio SL, Fernandes A, Pereira C, Calhelha RC, Sokovic M, Santos-Buelga C et al (2020) Nutritional value, physicochemical characterization and bioactive properties of the Brazilian quinoa BRS Piabiru. Food Funct 2020:1–9. https://doi.org/10.1039/d0fo00055h
Sarega N, Imam MU, Md Esa N, Zawawi N, Ismail M (2016) Effects of phenol lic-rich extracts of Clinacanthus nutans on high fat and high cholesterol diet-induced insulin resistance. BMC Complement Altern Med 16:1–11. https://doi.org/10.1186/s12906-016-1049-5
Shang Y, Khafipour E, Derakhshani H, Sarna LK, Woo CW et al (2017) Short term high fat diet induces obesity-enhancing changes in mouse gut microbiota that are partially reversed by cessation of the high fat diet. Lipids 2017:1–13. https://doi.org/10.1007/s11745-017-4253-2
Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC (2014) Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bull Fac Pharm Cairo Univ 52:211–217. https://doi.org/10.1016/j.bfopcu.2014.09.003
Shin SS, Yoon M (2012) The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits high-fat diet-induced hepatic steatosis via hepatic PPARα activation. Pharm Biol 50:1261–1268. https://doi.org/10.3109/13880209.2012.666982
Shinde JS, Khurde SS, Suchita SL, Chavan SS, Hulmajge SB (2016) Need of polyherbal formulations and its standardization: a review. World J Pharm Pharm Sci 5:526–533
Singh J, Parasuraman S, Kathiresan S (2018) Antioxidant and antidiabetic activities of methanolic extract of Cinnamomum cassia. Pharmacogn Res 10:237–242
Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH (2003) Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine 10:165–169. https://doi.org/10.1078/094471103321659889
Solares-Pascasio JI, Ceballos G, Calzada F, Barbosa E, Velazquez C (2021) Antihyperglycemic and lipid profile effects of Salvia amarissima Ortega on streptozocin-induced type 2 diabetic mice. Molecules 26:1–16. https://doi.org/10.3390/molecules26040947
Song C, Lv W, Li Y, Nie P, Lu J et al (2021a) Alleviating effect of quinoa and underlying mechanism on hepatic steatosis in high fat diet fed rat. Nutr Metab 18:1–16. https://doi.org/10.1186/s12986-021-00631-7
Song X, Dong H, Zang Z, Wu W, ZhuZang WH et al (2021b) Kudzu resistant starch: an effective regulator of type 2 diabetes mellitus. Oxid Med Cell Longev 2021:1–15. https://doi.org/10.1155/2021/4448048
Subhasree N, Kamella A, Kaliappan I, Agrawal A, Dubey GP (2015) Antidiabetic and antihyperlipidemic activities of a novel polyherbal formulation in high fat diet/streptozotocin induced diabetic rat model Ind. J Pharmacol 47(509):513. https://doi.org/10.4103/0253-7613.165200
Subramanian R, Asmawi MZ, Sadikun A (2008) In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol 55:391–398. https://doi.org/10.18388/abp.2008_3087
Suvarna R, Shenoy RP, Hadapad BS, Nayak AV (2021) Effectiveness of polyherbal formulations for the treatment of type 2 diabetes mellitus - a systematic review and meta-analysis. J Ayurveda Integr Med 12:213–222. https://doi.org/10.1016/j.jaim.2020.11.002
Taghizadeh M, Rashidi AA, Taherian AA, Vakili Z, Sajadian MS, Ghardashi M (2015) Antidiabetic and antihyperlipidemic effects of ethanol extract of Rosa canina L. fruit on diabetic rats: An experimental study with histopathological evaluations. J Evid Based Complement Altern Med 21:NP25-30. https://doi.org/10.1177/2156587215612626
Tamiru W, Engidawork E, Asres K (2012) Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complemet Altern Med 12:1–7. https://doi.org/10.1186/1472-6882-12-151
Tan BL, Norhaizan ME, Liew W (2018) Review article nutrients and oxidative stress : friend or foe ? Oxid Med Cell Longev 2018:1–24. https://doi.org/10.1155/2018/9719584
Tang Y, Zhang B, Li X, Chen PX, Zhang H, Liu R et al (2016) Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.5b05761
Tang D, Liu L, Ajiakber D, Ye J, Xu J, Xin X et al (2018) Anti-diabetic effect of Punica granatum flower polyphenols extract in type 2 diabetic rats: activation of Akt/GSK-3b and inhibition of IRE1a-XBP1 pathways. Front Endocrinol 9:1–11. https://doi.org/10.3389/fendo.2018.00586
Tanisha MM (2019) Optimization of an antihyperglycemic triherbal formulation using response surface methodology. J Sci Ind Res 78:39–45
Tran N, Pham B, Le L (2020) Bioactive compounds in anti-diabetic plants : from herbal medicine to modern drug discovery. Biology 9:1–31. https://doi.org/10.3390/biology9090252
Truong D-H, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC (2019) Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Quality 2019:1–10. https://doi.org/10.1155/2019/8178294
Varkey IC, Kasthuri GM (2016) HPTLC analysis of stem bark extracts of Terminalia chebula Retz. for alkaloid profile. Asian J Biochem 11:97–103. https://doi.org/10.3923/ajb.2016.97.103
Virk JK, Kalia AN, Gauttam VK, Mukhija M, Rath G (2020) Development and characterization of spheroidal antidiabetic polyherbal formulation from fresh vegetable juice: a novel approach. J Food Biochem 2020:1–13. https://doi.org/10.1111/jfbc.13290
Vuksan V, Choleva L, Jovanovski E, Jenkins AL, Au-Yeung F, Dias AG et al (2017a) Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: a randomized, controlled, crossover study. Eur J Clin Nutr 71:234–238. https://doi.org/10.1038/ejcn.2016.148
Vuksan V, Jenkins AL, Brissette C, Choleva L, Jovanovski E, Gibbs AL et al (2017b) Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: a double-blind randomized controlled trial. Nutr Metab Cardiovasc Dis 27:138–146. https://doi.org/10.1016/j.numecd.2016.11.124
Wang Z, Hu J, Hamzah SS, Ge S, Lin Y, Zheng B et al (2019) N-Butanol extract of lotus seeds exerts antiobesity effects in 3T3-L1 preadipocytes and high-fat diet-fed mice via activating adenosine monophosphate-activated protein kinase. J Agric Food Chem 67:1092–1103. https://doi.org/10.1021/acs.jafc.8b05281
Yang Y, Smith DL, Keating KD, Allison DB, Nagy TR (2014) Variations in body weight, food Intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 22:2147–2155. https://doi.org/10.1002/oby.20811
Yao Q, Li S, Cheng X, Zou Y, Shen Y, Zhang S (2020) Yin Zhi a traditional Chinese herbal formula, ameliorates diet-induced obesity and hepatic steatosis by activating the AMPK/SREBP-1 and the AMPK/ACC/CPT1A pathways. Ann Transl Med. 8:1–13. https://doi.org/10.21037/atm.2020.01.31
Ye F, Shen Z, Xie M (2002) Alpha-glucosidase inhibition from a Chinese medical herb (Ramulus mori) in normal and diabetic rats and mice. Phytomedicine 9:161–166. https://doi.org/10.1078/0944-7113-00065
Yimam M, Jiao P, Hong M, Brownell L, Lee YC, Hyun EJ et al (2016) Appetite suppression and antiobesity effect of a botanical composition composed of Morus alba, Yerba mate, and Magnolia officinalis. J Obes 2016:1–12. https://doi.org/10.1155/2016/4670818
Yoshikawa M, Shimoda H, Nishida N, Takada M, Matsuda H (2002) Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lipolytic activities have mild antiobesity effects in rats. J Nutr 132:1819–1824. https://doi.org/10.1093/jn/132.7.1819
You JS, Lee YJ, Kim KS, Kim SH, Chang KJ (2014) Anti-obesity and hypolipidaemic effects of Nelumbo nucifera seed ethanol extract in human pre-adipocytes and rats fed a high-fat diet. J Sci Food Agric 294:568–575. https://doi.org/10.1002/jsfa.6297
Zhang J, Kang M-J, Kim M-J, Kim M-E, Song J-H, Lee Y-M et al (2008) Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr Res Pract 2:200–203. https://doi.org/10.4162/nrp.2008.2.4.200
Zhang Y, Feng F, Chen T, Li Z, Shen QW (2016) Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J Ethnopharmacol 2016:1–32. https://doi.org/10.1016/j.jep.2016.07.002
Zhou T, Luo D, Li X, Luo Y (2009) Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J Med Plants Res 3:290–293
Zhu M, Liu T, Zhang C, Guo M (2017) Flavonoids of Lotus (Nelumbo nucifera) seed embryos and their antioxidant potential. J Food Sci 82:1834–1841. https://doi.org/10.1111/1750-3841.13784
Acknowledgements
This work was supported by JAIN (Deemed-to-be University), Bangalore, India. The authors sincerely thank the University Grants Commission for providing research grant to conduct the research. The authors are grateful to the Regional Ayurveda Research Institute for Metabolic Disorders, Bengaluru, India, for authentication of seed samples. The authors are also grateful to In Vivo Biosciences, Bengaluru, India, for providing the animal facility to carry out the experiment.
Funding
University Grants Commission—South Eastern Regional Office, F1-17.1/2013-14/RGNF-2013-14-SC-BIH-36937, Tanisha.
Author information
Authors and Affiliations
Contributions
Tanisha performed the collection of the samples, extraction, designing and performing the experiment. SV guided the first author in performing animal study. MM designed the concept, corrections and drafting of the manuscript. The authors have read and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanisha, Venkategowda, S. & Majumdar, M. Amelioration of hyperglycemia and hyperlipidemia in a high-fat diet-fed mice by supplementation of a developed optimized polyherbal formulation. 3 Biotech 12, 251 (2022). https://doi.org/10.1007/s13205-022-03309-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03309-w