Abstract
Expansin, a cell wall-modifying gene family, has been well characterized and its role in biotic and abiotic stress resistance has been proven in many monocots, but not yet studied in banana, a unique model crop. Banana is one of the staple food crops in developing countries and its production is highly influenced by various biotic and abiotic factors. Characterizing the expansin genes of the ancestor genome (M. acuminata and M. balbisiana) of present day cultivated banana will enlighten their role in growth and development, and stress responses. In the present study, 58 (MaEXPs) and 55 (MbaEXPs) putative expansin genes were identified in A and B genome, respectively, and were grouped in four subfamilies based on phylogenetic analysis. Gene structure and its duplications revealed that EXPA genes are highly conserved and are under negative selection whereas the presence of more number of introns in other subfamilies revealed that they are diversifying. Expression profiling of expansin genes showed a distinct expression pattern for biotic and abiotic stress conditions. This study revealed that among the expansin subfamilies, EXPAs contributed significantly towards stress-resistant mechanism. The differential expression of MaEXPA18 and MaEXPA26 under drought stress conditions in the contrasting cultivar suggested their role in drought-tolerant mechanism. Most of the MaEXPA genes are differentially expressed in the root lesion nematode contrasting cultivars which speculated that this expansin subfamily might be the susceptible factor. The downregulation of MaEXPLA6 in resistant cultivar during Sigatoka leaf spot infection suggested that by suppressing this gene, resistance may be enhanced in susceptible cultivar. Further, in-depth studies of these genes will lead to gain insight into their role in various stress conditions in banana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Banana and plantain represent a significant source of economic growth, income, food security, and nutrition and around 99% of production are from developing countries (FAO 2020). The banana production is greatly affected by biotic (corm weevil, root lesion and root knot nematodes, Fusarium and bacterial wilt, bunchy top virus disease, etc.) and abiotic stresses (salinity, heat, drought) which affects the livelihood of smallholding banana farmers (Nansamba et al. 2020). Thus, for sustainable banana production, the cultivation of resistant/tolerant accessions becomes the key factor (Blomme et al. 2011). Incorporation of resistance, without co-inheritance of undesirable genes through conventional breeding approach is complex owing to its poor seed setting nature. This co-inheritance of undesirable genes can be avoided by implementing the gene manipulation techniques either through over expression or knockdown of target genes with functions associated with resistance/tolerance or susceptibility phenotypes (Datta 2013). The success of the genetic engineering/genome-editing techniques depends on identifying the target genes that need to be manipulated. The mechanism of tolerance/resistance in bananas has been well studied for various abiotic and biotic stresses (Wang et al. 2012; Li et al. 2013; Backiyarani et al. 2014; Saravanakumar et al. 2016; Muthusamy et al. 2016), and there is clear evidence of involvement of genes pertaining to signaling perception and transduction, transcription factors, disease resistant proteins, plant hormones and cell wall organization. Plant cell wall not only provides a structural framework for plant growth; but also act as a barrier to protect the cells in response to biotic and abiotic stresses (Tucker and Koltunow 2014) through remodeling and disassembly of the cell wall (Marowa et al. 2016). In banana, it has been evidenced that cell wall-modifying genes are associated with biotic stress resistance (Van den berg et al. 2007) and drought tolerance (Muthusamy et al. 2016; Cheng et al. 2016; Wang et al. 2017).
These cell wall modification are achieved through the action of various proteins, of which expansin, a structural protein, is secreted into the cell wall space and plays a major role in expansion (Fukuda 2014). Cosgrove (2000) and Bashline et al. (2014) reported that expansin act as cell wall loosening agents and like a zipper, breaks the hydrogen bonds between cellulose micro fibrils and matrix polysaccharides. This expansin mediated cell wall modification is associated with environmental stress tolerance such as drought (Zhao et al. 2011; Li et al. 2011b) heat (Zhao et al. 2011), salt (Lüet al. 2013; Zorbet al. 2015), and oxidative stress tolerance (Han et al. 2017). Similarly, Abuqamar et al. (2013) evidenced that tolerant level has been enhanced for the necrotrophic fungi through suppressing the expansin genes and up regulation of expansin gene was observed during cyst nematode infestation in tomato (Fudaliet al. 2008) and soybean roots (Ithal et al. 2007; Klink et al. 2007). The differential expression of expansin genes in different tissues such as leaf (Pien et al. 2001a, b), stem (Santiago et al. 2018), root (Lee et al. 2003), pollen (Pezzottiet al. 2002), fruit (Brummell et al. 1999a, b), and seed (Yan et al. 2014) revealed that they have diverse functions and differential expression in various tissues, development stages, and stress conditions. In banana, the whole-genome sequencing of the ancestors of A and B genome have been completed by D’Hont et al. (2012) and Davey et al. (2013), and the comparative genome analysis of banana with maize, rice, sorghum, date palm, Brachypodium and Arabidopsis proteomes revealed that enzymes of cell wall biosynthesis of Musa are forming a specific clusters (D’Hont et al. 2012). This emphasized that in-depth study on expansin, a cell wall protein, is warranted to understand their role under various physiological stages of banana. This emphasized that a more comprehensive understanding of the expansin classes and their possible differential expression patterns in banana tissues and stress conditions are essential and useful for dissecting their role in bananas. This can be achieved through comparative analysis of gene families from the expression profile of the contrasting cultivars under challenged and unchallenged conditions (Kaliyappan et al. 2016). To date, the role of banana expansins has been studied only for fruit ripening (Trivedi and Nath 2004; Asha et al. 2007; Asif et al. 2014) and their role on biotic and abiotic stress resistance are yet to be studied. Thus, the present study has been carried out for a systemic analysis on expansins in A and B genome of Musa, the ancestors of the present day commercial cultivars, through genome-wide identification, characterization, and their expression in the contrasting cultivars during biotic (Pseudocercospora eumusae, Pratylenchus coffeae) and drought stresses.
Materials and methods
Identification of expansin family members in banana
The nucleotide and protein sequences of expansin genes of A and B genome were downloaded from the Banana genome hub database (https://banana-genome-hub.southgreen.fr/). The genome IDs of expansins were arranged according to their location in chromosomes from Chr01 to Chr11. Expansin IDs were assigned for each subfamily as EXPA, EXPB, EXPLA, and EXPLB suffixed with Ma and Mb for A and B genomes, respectively (Supplementary file 1). The expansin sequences of the model crop, Arabidopsis thaliana, and Oryza sativa were obtained from Phytozome v12.1 (https://phytozome.jgi.doe.gov/pz/portal.html) and used as query sequences to perform the blast searches against A and B genome with a 1 × 10–5 cutoff e value. Protein sequences of putative expansins were examined by PFAM (http://pfam.xfam.org/) to confirm the existence of the conserved domains (DPBB_1 domain and Pollen_allerg_1 domain) and candidate genes not having the characteristic domains were discarded for further study. Hidden Markov Model (HMM) with PFAM numbers PF03330 and PF01357 were used to search putative expansin genes in the protein dataset using HMMSEARCH with a threshold of e values < 10–5 (Finn et al. 2010). The molecular weight (MW) and isoelectric point (pI) were acquired from the ExPaSy (https://web.expasy.org/compute_pi/) for each protein sequence. Subcellular localization of the expansin proteins was predicted by online software WoLF PSORT II (https://www.genscript.com/wolf-psort.html?src=leftbar).
Phylogenetic tree construction
Multiple sequence alignment was done to explore the evolutionary relationships of expansins between Musa (A, B genome), rice, and Arabidopsis with default parameters using MUSCLE v.3.8.31 (Larkin et al. 2007). MEGA10.0 was performed to construct a maximum likelihood (ML) phylogenetic tree using all sites with bootstrap analysis (1000 replicates) (Kumar et al. 2018).
Gene structure, cis-regulatory elements and genomic distribution of expansin genes in banana
The structure (exon–intron) of the expansin genes was drawn by the Biosequence Structure Illustrator program of the TBtools software v. 1.077 (Chen et al. 2020). MEME tool was used to identify the conserved motifs (http://meme-suite.org/tools/meme). TBtools was used for mapping the expansin genes on the Musa A and B genome. Quick MCScanX Wrapper program of TBtools was used to identify the tandem and segmental duplication gene pairs. Upstream sequences (2 kb) of each expansin genes were analyzed to identify cis-regulatory elements using Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Synteny and gene duplication analysis
Duplication events of the expansins were analyzed using MCScanX (Wang et al. 2012). Advanced Circos was used to create collinear analysis diagrams for both Musa A and B genome (Chen et al. 2020). Multiple synteny plot was drawn between Musa A, B genome with A. thaliana and O. sativa using TBtools.
Ka/Ks analysis and estimated divergence time for the duplicated Musa expansins
Gene duplication events were analyzed by calculating non-synonymous substitution rate and synonymous replacement rate using PAML program (Yn00 package) (Xu and Yang 2013; Nei and Gojobori 1986). As Ks of duplication genes are expected to be similar over time in a molecular clock (Shiu et al. 2004), Ks values for each gene pair were used to calculate the approximate date of the duplication time (T) (million years ago, Mya) using the formula: T = Ks/2λ × 10−6 where λ = clock-like substitution rate (Lynch and Conery 2000) and λ for banana = 4.5 × 10–9) (Lescot et al. 2008).
Expression analysis of expansins
The differential expression value (log2 fold change) of expansins during P. eumusae, P. coffeae, and drought stress conditions in the respective contrasting cultivars were retrieved from the database maintained at ICAR-NRCB (http://nrcb.res.in/nrcbbio/about.html). Significant log2 fold change values of the contrasting expressed expansins were used to draw a heat map by TBtools.
Plant materials and stress treatments
The pot cultured plants of P. eumusae-resistant (cv. Manoranjitham, AAA) and -susceptible (cv. Grand Nain, AAA) cultivars were inoculated by spraying P. eumusae spores on both surfaces of leaves as per the protocol followed by Saravanakumar et al. (2016). The suckers of P. coffeae-resistant (YKM 5, AAA) and -susceptible (cv. Nendran, AAB) cultivars were pared and treated with fungicides, nematicides, and pot cultured. Four weeks after planting, mixed life stages of root lesion nematode (P. coffeae) were inoculated by following the methodology developed by De Schutter et al. (2001). Roots were collected at 0 and fifth day after inoculation. Uniform size suckers of drought-tolerant (cv. Saba, ABB) and -susceptible (cv. Grand Nain, AAA) cultivars were pot cultured. Five months old plants were subjected to water-deficit stress (Ravi and Uma 2011) and leaf samples were collected at 0 and 20 days. Leaf and root samples collected at different time intervals under different stresses were snap-frozen in liquid nitrogen and stored at − 80 °C for further use.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from challenged and unchallenged contrasting cultivars for various stresses independently using Sigma RNA isolation kit (Sigma, USA) following manufactures protocol. One microgram of RNA of each sample was used for cDNA synthesis (cDNA synthesis kit, Thermo Scientific, USA). Synthesized cDNA was diluted (1:10) with nuclease free water and used as a template. Specific gene primers were designed using Musa acuminata ssp. malaccensis (DH Pahang) sequences by the Primer3Plus tool which is used for expression studies. The expression of unchallenged resistant and susceptible cultivars under each stress was considered as a control to calculate the fold change in their respective challenged cultivars. Ribosomal protein S2 (RPS) was used as the internal control. The total experiment was conducted in the Roche LightCycler® 480 System with LightCycler® 480 Software Version 2.0 (Table S3).
Interaction network of expansin proteins
Protein–protein interaction (PPI) was studied using STRING v11.0 (Szklarczyk et al. 2019) for expansin genes of A and B genome independently. The PPI network was constructed using the A. thaliana as a reference. The minimum required interaction score parameters were set at the medium confidence level.
Results
Identification of banana expansin genes
A total of 58 and 55 expansin gene sequences of M. acuminate ssp. malaccensis (DH-Pahang) and M. balbisiana (DH-PKW), respectively, were retrieved from the banana genome hub (Droc, 2013). The subfamilies of expansin genes (EXPA, EXPB, EXLA, and EXLB) were identified based on the conserved domains, double-psi β-barrel (DPBB) and the β-sandwich (D2 ± pollen allergen domain) and found that maximum genes belong to the EXPA subfamily (36 and 33) followed by EXPB (12, 15), EXPLA (6, 6) and EXPLB (3, 1) in A and B genome, respectively. Of which only 83 and 64% of the genes present in A and B genome, respectively, had signal peptides. Except few, all the members of EXPA and EXPLA had a pI value above 7.0. However, the pI values of all EXPBs and EXPLBs were below 7.0, except for six members (Supplementary file 2).
Gene structure and phylogenetic analysis
The structure of expansin genes revealed that they are diverse among and between the members of the subfamilies (Fig. 1a, b) which might be due to loss or gain of introns. In A genome, 30 genes had 3 exons, 14 had 4 exons, 8 had 5 exons, 5 had 2 exons, and 1 (MaEXPB2) had only 1 exon (Fig. 1a), while 1 to 6 exons were found in expansin genes of B genome. It was observed that 2 had 6 exons, 5 had 5 exons, 11 had 4 exons, 25 had 3 exons, 10 had 2 exons, and 1 (MbaEXPB3) had only 1 exon (Fig. 1b). The EXPA members contained mostly three exons except for a few genes, but all EXPB, EXLA, and EXLB genes contained more than three exons in both the genomes.
a Phylogenetic relationship, motif compositions and gene structure of expansin genes in Musa A genome. A Phylogenetic relationships of 58 expansins in Musa A genome, They are classified in four groups: α-expansin (EXPA), β-expansin (EXPB), expansin-like A (EXLA) and expansin-like B (EXLB); B different motif compositions of expansins in Musa A genome. The conserved motifs are represented by boxes with different colors. C Gene structure organization of expansin genes of Musa A genome. b Phylogenetic relationship, motif compositions and gene structure of expansin genes in Musa B genome. A Phylogenetic relationships of 55 expansins in Musa B genome, They are classified in four groups: α-expansin (EXPA), β-expansin (EXPB), expansin-like A (EXLA) and expansin-like B (EXLB); B different motif compositions of expansins in Musa B genome. The conserved motifs are represented by boxes with different colors. C Gene structure organization of expansin genes of Musa B genome
In total, 20 conserved motifs were identified in banana expansin genes (Fig. 1a, b) (Supplementary file 3). Among them, motif 4, 6, 7, 14 and 3, 4, 6, 10 were highly conserved in all the subfamilies in both A and B genome, respectively. In the EXPA group, motifs 0, 3, 4, 6, 9, 15 constitute the DBPP domain, while motifs 1, 5, 7 constitute the Pollen_allerg domain. Motifs 3, 6, 8 are commonly present in EXPB, EXPLA, and EXPLB subfamilies whereas motif 0 is common to EXPB and EXLA alone and constitute the DBPP domain, while motifs 5, 10, 12 are common to EXPB and EXPLB whereas motifs 5, 11, 16 are present in EXPLA and constitute the Pollen_allerg domain in A genome. In the case of B genome, motifs 0, 2, 4, 6, and 12 constitute the DBPP domain, while motifs 1, 5, 7 constitute the Pollen_allerg domain in EXPA. The EXPB and EXLA had motifs 0, 2, 4, 15 while, EXLB had motifs 2,4,13 and constitute the DBPP domain, whereas motifs 5, 8, 11, 16, motifs 8, 10, 17 and motifs 8, 11, 15, respectively, constitute the Pollen_allerg domain. Phylogenetic analysis grouped these expansin genes into above-mentioned four major classes (Fig. 2) and found that EXPA was the largest class in both the genomes.
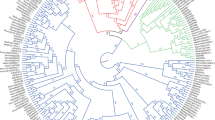
Phylogenetic tree of expansins from Musa A & B genome, Arabidopsis and Oryza. MEGA10.0 was used to construct a Maximum Likelihood phylogenetic tree with 1000 bootstrap replications. Triangles, circles, squares and diamond shapes represent the expansins of Musa A, B, rice, Arabidopsis, respectively. Bootstrap values are shown on the tree
Chromosomal location and synteny analysis
The expansin genes were unevenly mapped on 11 chromosomes and the distribution was almost similar in both the genomes. Chromosome 02 had 13 expansins in both the genome, while chromosome 03 in A genome and 08 in both the genomes had no expansin genes (Fig. 3a, b). The collinear relationship of MaEXP and MbaEXP was studied and the results indicated that the expansion of the expansin gene family was due to tandem, segmental or whole-genome duplication (WGD) (Fig. 4a, b). Most of the tandem and segmental duplication have occurred in chr 02 of both the genomes. Synteny relationship of expansin genes was studied to understand the evolutionary events that had taken place among the orthologous from O. sativa (56 genes), A. thaliana (36 genes), and Musa (A and B genome). Most of expansin genes showed a one-to-one corresponding relationship between A and B genome and with O. sativa than with A. thaliana (Fig. 5) (Table S2).
a Distribution of expansin genes on chromosomes in Musa A genome. The gene density is displayed in the form of heatmaps on the chromosomes, high and low densities are represented by red and blue. b Distribution of expansin genes on chromosomes in Musa B genome. The gene density is displayed in the form of heatmaps on the chromosomes, high and low densities are represented by red and blue
a Collinearity mapping of expansin genes in Musa A genome. Different chromosomes are shown in yellow color. The gene density is displayed in the form of histogram. The inner colored lines represent the collinearity relationships of EXPA, EXPB and EXPLA subfamilies, respectively. A total of 13 pairs of Tandem and 19 pairs of segmented duplication genes were detected in the Musa A genome. b Collinearity mapping of expansin genes in Musa B genome. Different chromosomes are shown in yellow color. The gene density is displayed in the form of histogram. The inner colored lines represent the collinearity relationships of EXPA, EXPB and EXPLA subfamilies, respectively. A total of 16 pairs of Tandem and 14 pairs of segmented duplication genes were detected in the Musa B genome
Ka/Ks analysis and estimated divergence time for the duplicated expansins
A maximum number of paralogues was observed for EXPA (A genome 90%, B genome 66%) followed by EXPB (A genome 10%, B genome 34%), whereas no paralogues were observed for EXPLA and EXPLB subfamilies. The paralogues harboring similar gene structures had a more similar function in both the genome (Supplementary file 4). For example, MaEXPA16 and 14 having similar exon and intron structures showed similar putative biological function (C: extracellular region; C: cell wall; P: plant-type cell wall organization; C: integral component of membrane) (Jinet et al. 2020). Variation in gene structure and function between paralogues has been suggested to be a consequence of the evolutionary pressure that favors the retention or loss of the mutated alleles from a population (Lan et al. 2009). Analysis of selection pressure among the duplicated MaEXP and MbEXP gene pairs revealed that 17 duplicated pairs of both the genome were under purifying or negative selection; Three of A genome and one of B genome duplicated pairs resulting from the positive selection or accelerated evolution and 3 neutral selection in B genome (Supplementary file 4). The duplication events of MaEXP and MbEXP paralogues are suggested to have occurred between 1.08 and 40.59 million years ago (Mya) in A genome and 1.07 to 122.94 Mya in the case of B genome, respectively (Supplementary file 4). Among the duplication events, 6 and 7 of the 20 and 21 pairs are suggested to be more recently duplicated, of which, the paralogues of EXPB subfamily members were recently evolved in both the genomes but found more in B genome.
Cis-elements
Cis-elements in the expansin promoter were identified using the PlantCARE database (Fig. 6a, b), (Supplementary file 5). Three types of cis-elements related to development, hormone, and abiotic stresses were identified. Cis-elements related to development include light-response, metabolism regulation, and meristem expression; hormone stress include methyl jasmonate (MeJA), abscisic acid (ABA)-responsive relate element (ABRE), gibberellin (GA)-responsive and auxin-responsive; abiotic stresses include anoxic-specific inducible element GC-motif, drought-inducible cis-element MBS and low-temperature responsive (LTR) were identified. Among these, MeJA, ABRE and drought-responsive cis-elements were abundant. A total of 51, 47 MeJA; 52, 48 ABREs; and 35, 37 drought-responsive elements were found in the promoter regions of both the genomes of banana, respectively. Among them MaEXPA7 had 12 ABREs response elements, MaEXPA7 and MaEXPA10 had 7 MeJA stress response elements, and MaEXPA22, MaEXPA23, and MaEXPB8 had a maximum of three drought-responsive elements in A genome. MbaEXPB8 had 12 ABREs, MbaEXPA11 had 9MeJA stress response elements and MbaEXPA22, MbaEXPB15, and MbaEXPLA2 had three drought-responsive elements in B genome (Fig. 7a, b).
a Predicted cis-elements of expansin gene promotors in Musa A genome. Promoter regions 2000 bp upstream of 58 EXPs analyzed by PlantCARE. Different-colored rectangles represented different cis-elements, and anaerobic induction response elements are highlighted in red ellipses. b The number of promoter elements in the MaEXPs’ promoter regions. Five major cis-elements of MaEXPs are indicated by different-colored boxes with numbers
a Predicted cis-elements of expansin gene promotors in Musa B genome. Promoter regions 2000 bp upstream of 55 EXPs analyzed by PlantCARE. Different-colored rectangles represented different cis-elements, and MeJA response elements are highlighted in green ellipses. b The number of promoter elements in the MbaEXPs’ promoter regions. Five major cis-elements of MbaEXPs are indicated by different-colored boxes with numbers
Differential expression of expansins under various stresses and validation through qRT-PCR analysis
The expression profile of expansins was analyzed from the challenged and unchallenged transcriptome data of resistant and susceptible cultivars under biotic and abiotic stress conditions (Fig. 8). It is interesting to note that 27.5% of genes (16 numbers) were not expressed in the samples (root/leaf) taken for this study. Among non-expressed genes in each subfamily, 70% belong to EXPB followed by EXPLB (66%). Out of 72.5% of expressed genes, 13 (MaEXPA2, MaEXPA4, MaEXPA8, MaEXPA10, MaEXPA11, MaEXPA14, MaEXPA15, MaEXPA21, MaEXPA23, MaEXPA28, MaEXPA32, MaEXPA35, MaEXPB8) were expressed only in root whereas one (MaEXPA33) was expressed only in leaf tissue (Supplementary file 6).
A graphical representation of expression details of expansin genes in biotic and abiotic stresses in Musa cultivars. The heat map was drawn using log2 logarithmic transformed expression values. Red to green represents high and low expression levels, respectively. Based on the expression, the expansin genes were hierarchically clustered and divided into various gene clusters in the figure
Among the expressed expansin genes, a maximum number of expansins were differentially expressed under nematode infection (43%) followed by drought (28%) and Sigatoka infection (24%). None of the MaEXPLB genes was significantly differentially expressed in the contrasting cultivars for all the stresses except MaEXPLB1 which was upregulated 3.57-fold upon challenge with Sigatoka in the susceptible cultivar. While maximum members of MaEXPLA were significantly differentially expressed in all the contrasting cultivars for all the stresses. In general, under drought, more genes were downregulated in susceptible (62%) compared to tolerant cultivar (46%). Among the 13 members of MaEXPB, only one (MaEXPB13) was highly upregulated in tolerant cultivar whereas no variation was observed in the susceptible cultivar upon drought.
Among the members of MaEXPLB and MaEXPB subfamilies, MaEXPB8 alone showed > twofold downregulation in resistant cultivar upon nematode infection. This speculated that MaEXPB8 may play a major role in nematode resistance. Three members of MaEXPLA subfamily (MaEXPLA4,-5,-6) were significantly upregulated only in a susceptible cultivar with > 1.5-fold whereas no variation was observed in resistant cultivar. This revealed that MaEXPLA members might be the susceptible factors as it favors nematodes by loosening the host cell and facilitates easy feeding. Similarly out of 20 members of MaEXPA subfamily, 50% of the members were significantly downregulated (> 1.5-fold) in resistant, whereas in susceptible cultivar except for MaEXPA5 and - 3, all other members were either upregulated or no variation in the expression.
Eight expansin genes were selected based on RNA-seq data and validated through qRT-PCR in the contrasting cultivars for each stress (Fig. 9). MaEXPA26 and MaEXPB13 were differentially expressed in the contrasting cultivars upon drought stress. Most of the MaEXPs showed significant downregulation in resistant cultivar during P. eumusae infection. qRT-PCR data for nematode stress are in concordance with transcriptome data, and three genes, MaEXPA36, MaEXPLA1, and MaEXPLA6 showed significant downregulation upon P. coffeae infection in resistant cultivar.
Interaction network of expansin proteins
The interaction network of the expansin proteins was constructed based on the interaction relationship of the homologous expansins proteins in Arabidopsis and this will help in understanding the biological phenomena in which expansins are involved in banana. The banana expansin proteins corresponded with the Arabidopsis expansin proteins are listed below them (Fig. 10a, b). The result showed that EXPA7 and EXPA18 from both the genomes were predicted as the core nodes in the network and interact with other proteins such as RHS12, PRP3, RHS19, RHS13, and MOP10, which suggested that they might participate in diverse functions by interacting with other proteins.
Discussion
Expansins participate in pH-dependent cell wall loosening during cell growth and are involved in plant growth, development and responses to abiotic and biotic stresses (Chen et al 2020) as shown in previous studies (Table S4). Among monocots, expansin genes were characterized in rice (Shin et al. 2005), wheat (Han et al. 2015), maize (Wu et al. 2001), and sugarcane (Santiago et al. 2018) but detailed studies on other monocots are warranted. Expansin superfamilies in banana are largely unexplored. Being a model crop for polyploidy and parthenocarpy, identification and characterization of genes in banana is paramount important which can be achieved with the availability of whole-genome sequencing of A and B genome (D’Hont et al. 2012; Davey et al. 2013). In this study, we performed a comprehensive analysis with both A and B genomes, which constitute all edible bananas, for the identification of expansin gene groups through in silico analyses. Further to gain more insight into their biological functions, the expression profiling of MusaEXPs (MEXPs) under drought, Sigatoka and nematode stresses were analyzed. In addition, key domains, promoter motifs, their evolutionary dynamics and structural syntenies were identified.
Herein, we identified 58 and 55 EXPs in M. acuminata and M. balbisiana, respectively, which are higher than rice, maize, sugarcane and this may be due to lineage specific duplication either by tandem and segmental duplication or transposition (Santiago et al. 2018; Han et al. 2019). In bananas, the presence of all the four subfamilies of expansin in banana indicated that this gene has emerged before the differentiation of the plant species. Though the expansin members of the same subfamily had similar properties, the significant difference at sequence level observed among the subfamilies revealed that expansin genes adapt to functional requirements by changing their properties (Han et al. 2019). Further, the presence of more members in the EXPA subfamily revealed their expansion and its function in growth and development (Lv et al. 2020) and under various stresses (Santiago et al. 2018). The intron–exon structure and motif composition were similar within the expansin subfamily suggesting that the genes belonging to the same subfamily are highly conserved, confirming their close evolutionary relationships (Han et al. 2019; Lv et al. 2020). The structure of expansin genes varies within the subfamily due to intron loss/gain events but the basic genetic structure is a 3-exon/2-intron pattern and similar results have also been reported in the case of tomato expansins (Lu et al. 2016). Insertion of introns might have occurred due to a type of transposable element which had splice sites or by the duplication of exonic sequences which had a splice site or due to mutate introns of non-classical types which probably do behave as self-inserting elements (Rogers 1990). The presence of additional introns is likely useful in increased gene expression (Jo and Choi 2015).
The evolutionary relationships between MaEXPs and MbEXPs showed that the subfamily members of both the genome are highly similar at sequence levels as they are grouped as a single cluster. This close relationship among the subgroups can be in feed from the fact that all essential genic regions were present in both A and B genomes (Davey et al. 2013). The high collinearity of the expansins between the A and B genomes in banana indicated that they might have similar functions (Lv et al. 2020). The banana expansin genes clustered with the respective candidate members of rice and Arabidopsis suggesting these genes perform the same function as the model plants and might have evolved before the divergence of Musa ~ 4.6 Mya (Lescot et al. 2008). Uneven distribution of expansin genes was observed in banana as in the case of many monocots and dicot plants (Zhang et al. 2014; Guimaraes et al. 2017a, b; Li et al 2016a, b). Clustering of genes belonging to the same subfamily, for example, EXPB in chr02, suggested that it might be due to tandem and segmental duplication, which lead to major variation in family size and distribution (Cannon et al. 2004; Lv et al. 2020).
The key cis-regulatory elements found in EXP promoters are associated with light and hormonal regulations, notably with response to MeJA. Light responsive promoter motifs are key in determining the molecular changes and biochemical constitutions of multiple biological processes in plants. In addition, previous studies demonstrated that light-response motifs of EXPs are participating in the leaf and shoot growth (Dornbusch et al. 2014; Han et al. 2019). The regulation of EXPs in Stellaria longipes was shown to be responsible for shade avoidance (Sasidharan et al. 2008). The presence of cis-elements responsive to hormones suggested that the expression of expansins can be influenced by hormones such as cytokinins (Downes and Crowell 1998), ethylene (Belfield et al. 2005), and auxins (McQueen-Mason et al 1992; Zhao 2012). MeJA and ABRE responsive elements are predominant promoter motifs in EXPs although their interactive relationships in growth and development are yet to be known clearly (Han et al. 2019). The other cis-element related to low-temperature responsive, drought inducibility MBS (CAACTG) and cis-elements related to other abiotic stresses were also present in EXP promoter motifs suggesting their possible role in salt, drought and cold stresses (Chen et al. 2016, 2017; Feng et al. 2019; Ren et al. 2018). Overall, drought-responsive elements were higher in expansin genes of B genome which is known to harbor genes for various abiotic and biotic stresses (Davey et al. 2013). Meristem responsive cis-elements were also present in some of the promoters which confirm its role in leaf and stem initiation and growth (Kuluev et al. 2012; Sampedro et al. 2015).
EXPLA and EXPLB have no paralogues in both the genomes of banana which suggested that these might have occurred recently whereas EXPA and EXPB exist before the divergence of vascular plants (Sampedro et al. 2006). Purifying selection eliminates the deleterious alleles or genetic polymorphism that arises through mutations to maintain the natural fitness of the organism whereas positive selection fixes advantageous mutations and usually leads to species divergence (Yuan et al. 2015). The occurrence of more tandem and segmental duplication in both genomes might be due to negative selection which reveals that this gene family is conserved. Duplication events in Musa have occurred recently than the progenitors as a consequence of whole-genome duplication reflecting a conserved and slowly evolving banana expansin gene family (D’Hont et al. 2012) and similar results have been reported for the CCCH-ZFP gene family in banana (Mazumdar et al. 2017). The recent evolution of expansin genes in both the genomes suggested that it is co-evolving with the pathogens (Cosgrove et al. 2015). The occurrence of more paralogues of EXPB in B genome speculated that EXPB is highly evolving and this may contribute to resistant nature of B genome (Davey et al. 2013). A similar kind of expansion of EXPB subfamily was reported in other monocots such as rice, maize and sugarcane (Santiago et al. 2018). Sampedro et al. (2006) suggested that the divergent EXPB proteins have evolved to substitute xylans such as (glucurono) arabinoxylans which allows more efficient roles in the cell wall mechanics.
Protein–protein interaction network revealed that expansin proteins form functional complexes, mediating the expression of other genes involved in cell wall modification, cell wall structure in root hairs and other root hair specific genes, removal of H2O2, oxidation of toxic reductants, biosynthesis, and degradation of lignin and suberization in banana (Lin et al. 2016). Muthusamy et al. (2020) also proved that the overexpression of expansin influences the expression of an interacting gene, the cell wall-plasma membrane linker protein-encoding gene. This suggested that apart from its role in cell wall development and cytoskeleton formation, expansins are also involved in protecting the plant tissues during the stress conditions by preventing oxidative stress which occurred during various biotic and abiotic stress conditions which indicates that expansin has potential of resistance to wide stresses.
Mining the transcriptome profile under specific physiological conditions will facilitate the identification of the best candidate gene/s. Study on the expression of expansin genes in the Musa transcriptome data set revealed that the expression of expansins is varying under different stress conditions. In general, the expression pattern of contrasting cultivars is the same except MaEXPA18 (− 4.5-fold) and MaEXPA26 (− 5.3-fold) under drought stress condition. As these genes are significantly downregulated in susceptible cultivar during drought stress, it is speculated that these genes may play a major role in drought tolerance by improving osmotic adjustment (Chen et al. 2016), water retention, and elevating the antioxidant enzymes (Yang et al. 2020). Sampedro and Cosgrove (2005) hypothesized that overexpression of EXPA alter the microtubule arrays, cellulose deposition, and cell wall thickening which are essential for stomatal guard cells and their adjacent cells during stomatal morphogenesis and thereby enhance the drought tolerance by modifying the leaf growth (Lü et al. 2013). It was observed that MaEXPB13 is highly upregulated in drought-tolerant cultivar. This is in concordance with the result of Zhang et al. (2019) who reported that significant upregulation of EXPB during drought stress leads to altering the cell walls and thereby reducing the negative effects of drought stress. Li et al. (2011a) and Muthusamy et al. (2020) proved that overexpression of EXPB subfamily induces drought tolerance under water-deficit stage. The presence of cis-elements induced by drought in their promoters again reiterates that the over expression of these expansin genes resulted in drought tolerance. Li et al. (2013) also proved that the efficiency of drought tolerance can be improved by over expression of expansin by manipulating the promoter which driven the expansin gene. Wu et al. (1996) has reported the overall involvement of expansins under drought in various other plant species.
Out of 20 significantly expressed EXP genes, 50% of the genes were downregulated in nematode-resistant cultivar while these genes were either upregulated or no expression variation was observed in the susceptible cultivar upon nematode infection. Upregulation of EXP genesin-susceptible cultivar suggested that these genes might act as the susceptible factors as it favors nematodes by loosening the host cell and facilitating easy feeding. A similar kind of upregulation of several expansin genes in syncytia induced by H. schachtii in in A. thaliana roots was observed by Wieczorek et al. (2006). Cosgrove (2000) also stated that these expansin proteins cause the extension of the cell wall through turgor-driven slippage of cellulose microfibrils in the susceptible cultivar and thereby loosen the cell wall of the host root system which indirectly helps the nematode feeding site. This study suggested that by suppressing the differentially expressed MaEXPA genes, the resistance can be improved in susceptible cultivar as it may lead to minimizing the modification and/or restructuring of cell walls in the feeding sites.
A similar kind of expression pattern was observed for all the differentially expressed EXP genes in both the cultivars under Sigatoka stress condition, except for MaEXPLA1 and MaEXPLA6. But their expression pattern was different in the contrasting cultivar, i.e., MaEXPLA1 was upregulated and MaEXPLA6 was downregulated in resistant cultivar. In-depth study on the role of these EXPLAs in Sigatoka resistant reaction is warranted. Downregulation of MaEXPA and MaEXPLA upon P. eumusae infection corroborates with the findings of Abuqamar et al. (2013) who proved that absence or downregulation of one of the EXPLA subfamily members led to increased resistance to Botrytis cinerea. Chen et al. (2018) reported overexpression of expansin negatively regulates the Pseudomonas syringae DC3000 and Tobacco mosaic virus resistance in tobacco. Muthusamy et al. (2020) also demonstrated that the significant reduction in expression under various biotic stresses such as Turnip mosaic virus, Pectobacterium carotovorum and club root diseases alters resistance in Brassica rapa. But in-depth study on MaEXPLA genes in Sigatoka leaf spot disease resistance is obligatory to understand their contribution to various resistant reactions.
Conclusion
Genome-wide analysis of the expansin gene family was performed in A and B genome of banana and its expression under various biotic and abiotic stresses were studied. Our finding provides new insight into the structure, evolution, divergence, and function of banana expansins. The RNA-seq data revealed that the expression pattern of expansin genes is varied for biotic and abiotic stresses. The differential expression pattern of MaEXPA18 and MaEXPA26 in the contrasting cultivars for drought stress revealed that these might be candidate genes for drought-tolerant mechanisms. Differential expression of EXPA gene members among the P. coffeae-resistant (down) and -susceptible (up) cultivars emphasized their role in nematode-resistant reaction. The downregulation of MaEXPLA6 in P. eumusae-resistant cultivar under challenged condition reiterate their involvement in resistant mechanism. Further in-depth study on members of these expansin subfamilies will pave way for confirming their candidature for stress resistance in bananas.
Abbreviations
- EXP:
-
Expansin
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- FAO:
-
Food and Agriculture Organization
- HMM:
-
Hidden Markov model
- PPI:
-
Protein–protein interaction
- MEME:
-
Multiple Em for motif elucidation
- MEGA:
-
Molecular evolutionary genetics analysis
- MeJA:
-
Methyl jasmonate
References
Abbasi A, Malekpour M, Sobhanverdi S (2021) The Arabidopsis expansin gene (AtEXPA18) is capable to ameliorate drought stress tolerance in transgenic tobacco plants. Mol Biol Rep 48(8):5913–5922. https://doi.org/10.1007/s11033-021-06589-2
Abuqamar S, Ajeb S, Sham A, Enan MR, Iratni R (2013) A mutation in the expansin-like A2 gene enhances resistance to necrotrophic fungi and hypersensitivity to abiotic stress in Arabidopsis thaliana. Mol Plant Pathol 14:813–827
Asha SVA, Sane AP, Nath P (2007) Multiple forms of α-expansin genes are expressed during banana fruit ripening and development. Postharvest Biol Technol 45(2):184–192
Asif MH, Lakhwani D, Pathak S et al (2014) Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol 14:316. https://doi.org/10.1186/s12870-014-0316-1
Backiyarani S, Uma S, Arunkumar G, Saraswathi MS, Sundararaju P (2014) Differentially expressed genes in incompatible interactions of Pratylenchus coffeae with Musa using suppression subtractive hybridization. Physiol Mol Plant Pathol 86:11–18
Bashline L, Lei L, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7(4):586–600
Belfield EJ, Ruperti B, Roberts JA, McQueen-Mason S (2005) Changes in expansin activity and gene expression during ethylene-promoted leaflet abscission in Sambucus nigra. J Exp Bot 56:817–823. https://doi.org/10.1093/jxb/eri076
Blomme G, Eden-Green S, Mustaffa M, Nwauzoma B, Thangavelu R (2011) Major diseases of banana. In: Pillay M, Tenkouano A (eds) Banana breeding: progress and challenges. CRC Press, Boca Raton, pp 85–119
Boron AK, Van Loock B, Suslov D, Markakis MN, Verbelen JP, Vissenberg K (2015) Over-expression of AtEXLA2 alters etiolated Arabidopsis hypocotyl growth. Ann Bot 115(1):67–80
Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P (1999a) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11:2203–2216
Brummell D, Harpster M, Civello P, Palys J, Bennett A, Dunsmuir P (1999b) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11(11):2203–2216
Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biology 4(1):10. https://doi.org/10.1186/1471-2229-4-10
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Chen F, Dahal P, Bradford KJ (2001) Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol 127(3):928–936
Chen Y, Han Y, Zhang M, Zhou S, Kong X, Wang W (2016) Overexpression of the wheat expansin gene TaEXPA2 improved seed production and drought tolerance in transgenic tobacco plants. PLoS ONE 11(4):e0153494. https://doi.org/10.1371/journal.pone.0153494
Chen Y, Han Y, Kong X, Kang H, Ren Y, Wang W (2017) Ectopic expression of wheat expansin gene TaEXPA2 improved the salt tolerance of transgenic tobacco by regulating Na+/K+ and antioxidant competence. Physiol Plant 159:161–177
Chen L, Zou W, Fei C, Wu G, Li X, Lin H, Xi D (2018) α-Expansin EXPA4 positively regulates abiotic stress tolerance but negatively regulates pathogen resistance in Nicotiana tabacum. Plant Cell Physiol 59:2317–2330
Chen C, Chen H, Zhang Y, Thomas HR, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202
Cheng L, Wang Y, He Q, Li H, Zhang X, Zhang F (2016) Comparative proteomics illustrates the complexity of drought resistance mechanisms in two wheat (Triticum aestivum L.) cultivars under dehydration and rehydration. BMC Plant Biol 16:188. https://doi.org/10.1186/s12870-016-0871-8
Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14(12):3237–3253
Cho HT, Kende H (1997) Expression of expansin genes is correlated with growth in deep water rice. Plant Cell 9(9):1661–1671
Civello PM, Powell AL, Sabehat A, Bennett AB (1999) An expansin gene expressed in ripening strawberry fruit. Plant Physiol 121(4):1273–1280
Cosgrove DJ (2000) New genes and new biological roles for expansins. Curr Opin Plant Biol 3(1):73–78
Cosgrove DJ (2015) Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol 25:162–172. https://doi.org/10.1016/j.pbi.2015.05.014
D’Hont A, Denoeud F, Aury JM et al (2012) The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488:213–217. https://doi.org/10.1038/nature11241
Dai F, Zhang C, Jiang X, Kang M, Yin X, Lu P, Gao J (2012) RhNAC2 and RhEXPA4 are involved in regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol 160:2064–2082
Datta A (2013) Genetic engineering for improving quality and productivity of crops. Agric Food Secur 2:15. https://doi.org/10.1186/2048-7010-2-15
Davey MW, Gudimella R, Harikrishna JA et al (2013) A draft Musa balbisiana genome sequence for molecular genetics in polyploid, inter- and intra-specific Musa hybrids. BMC Genom 14:683. https://doi.org/10.1186/1471-2164-14-683
De Schutter B, Speijer PR, Dochez C, Tenkouano A, De Waele D (2001) Evaluating host plant reaction of Musa germplasm to Radopholus similis by inoculation of single primary roots. Nematropica 31(2):295–299
Dornbusch T, Michaud O, Xenarios I, Fankhauser C (2014) Differentially phased leaf growth and movements in Arabidopsis depend on coordinated circadian and light regulation. Plant Cell 26:3911–3921. https://doi.org/10.1105/tpc.114.129031
Downes BP, Crowell DN (1998) Cytokinin regulates the expression of a soybean β-expansin gene by a posttranscriptional mechanism. Plant Mol Biol 37:437–444
Droc G, Larivière D, Guignon V, Yahiaoui N, This D, Garsmeur O, Dereeper A, Hamelin C, Argout X et al (2013) The banana genome Hub. Database 2013:bat35. https://doi.org/10.1093/database/bat035
FAO (2020) Medium-term Outlook: Prospects for global production and trade in bananas and tropical fruits 2019 to 2028. Rome
Feng X, Xu Y, Peng L, Yu X, Zhao Q, Feng S, Zhao Z, Li F, Hu B (2019) TaEXPB7-B, abeta-expansin gene involved in low-temperature stress and abscisic acid responses, promotes growth and cold resistance in Arabidopsis thaliana. J Plant Physiol 240:153004
Finn RD, Mistry J, Tate J, Coggill P, Heger A et al (2010) The Pfam protein families database. Nucleic Acids Res 38:D211–D222
Fudali S, Sobczak M, Janakowski S, Griesser M, Grundler FM, Golinowski W (2008) Expansins are among plant cell wall modifying agents specifically expressed during development of nematode-induced syncytia. Plant Signal Behav 3(11):969–971
Fukuda H (2014) Plant cell wall patterning and cell shape. Wiley, Hoboken
Guimaraes LA, Mota APZ, Araujo ACG et al (2017a) Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol Biol 94:79–96. https://doi.org/10.1007/s11103-017-0594-8
Guimaraes LA, Mota APZ, Araujo de Alencar ACG, Figueiredo LF, Pereira BM, de Passos Saraiva MA et al (2017b) Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol Biol l94(1–2):79–96
Guo W, Zhao J, Li X, Qin L, Yan X, Liao H (2011) A soybean bexpansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66(3):541–552
Han Y, Chen YH, Yin SH, Zhang M, Wang W (2015) Over-expression of TaEXPB23, a wheat expansin gene, improves oxidative stress tolerance in transgenic tobacco plants. J Plant Physiol 173:62–71
Han QH, Huang B, Ding CB, Zhang ZW, Chen YE, Hu C, Zhou LJ, Huang Y, Liao JQ, Yuan S (2017) Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front Plant Sci 8:785
Han Z, Liu Y, Deng X, Liu D, Liu Y, Hu Y, Yan Y (2019) Genome-wide identification and expression analysis of expansin gene family in common wheat (Triticum aestivum L.). BMC Genom 20(1):101. https://doi.org/10.1186/s12864-019-5455-1
Hemalatha N, Rajesh MK, Narayanan NK (2011) Genome-wide analysis and identification of genes related to expansin gene family in indica rice. Int J Bioinform Res Appl 7(2):162–167. https://doi.org/10.1504/IJBRA.2011.040094
Ithal N, Recknor J, Nettleston D, Nettleton D, Maier T, Baum TJ, Mitchum MG (2007) Parallel genome-wide expression profiling of host and pthaogen during soybean cyst nematode infection of soybean. Mol Plant Microbe Interact 20:510–525
Jin KM, Zhuo RY, Xu D, Wang YJ, Fan HJ, Huang BY, Qiao GR (2020) Genome-wide identification of the expansin gene family and its potential association with drought stress in moso bamboo. Int J MolSci 21(24):9491. https://doi.org/10.3390/ijms21249491 (PMID: 33327419)
Jo BS, Choi SS (2015) Introns: the functional benefits of introns in genomes. Genom Inform 13(4):112–118. https://doi.org/10.5808/GI.2015.13.4.112
Kaliyappan R, Viswanathan S, Suthanthiram B, Subbaraya U, Marimuthu Somasundram S, Muthu M (2016) Evolutionary expansion of WRKY gene family in banana and its expression profile during the infection of root lesion nematode, Pratylenchus coffeae. PLoS ONE 11(9):e0162013. https://doi.org/10.1371/journal.pone.0162013
Klink VP, Overall CC, Alkharouf N, MacDonald MH, Matthews BF (2007) A time-course comparative microarray analysis of an incompatible and compatible response by Glycine max (soybean) to Heterodera glycines (soybean cyst nematode). Planta 226:1423–1447
Krishnamurthy P, Muthusamy M, Kim JA et al (2019) Brassica rapa expansin-like B1 gene (BrEXLB1) regulate growth and development in transgenic Arabidopsis and elicits response to abiotic stresses. J Plant Biochem Biotechnol 28:437–446
Kuluev BR, Knyazev AB, Lebedev YP, Chemeris AV (2012) Morphological and physiological characteristics of transgenic tobacco plants expressing expansin genes: AtEXP10 from Arabidopsis and PnEXPA1 from poplar. Russ J Plant Physiol 59(1):97–104
Kuluev BR, Safiullina MG, Knyazev AV, Chemeris AV (2013) Effect of ectopic expression of NtEXPA5 gene on cell size and growth of organs of transgenic tobacco plants. Russ J Dev Biol 44(1):28–34
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kwon YR, Lee HJ, Kim KH, Hong SW, Lee SJ, Lee H (2008) Ectopic expression of expansin3 or expansin beta1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnol Lett 30(7):1281–1288
Lan T, Yang ZL, Yang X, LiuYJ WXR, Zeng QY (2009) Extensive functional diversification of the populus glutathione S-transferase supergene family. Plant Cell 21:3749–3766
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lee Y, Kende H (2001) Expression of beta-expansins is correlated with inter nodal elongation in deep water rice. Plant Physiol 127(2):645–654
Lee HW, Kim J (2013) EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol 54(10):1600–1611
Lee DK, Ahn JH, Song SK, Do CY, Lee JS (2003) Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol 131(3):985–997
Lescot M, Piffanelli P, Ciampi AY, Ruiz M, Blanc G, Leebens-Mack J, da Silva FR, Santos CM, Dhont A, Garsmeur O, Vilarinhos AD (2008) Insights into the Musa genome: syntenic relationships to rice and between Musa species. BMC Genom 9:58
Li F, Xing SC, Guo QF, Zhao MR, Zhang J, Gao Q et al (2011a) Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J Plant Physiol 168:960–966
Li Z, Zhang L, Yu Y, Quan R, Zhang Z, Zhang H et al (2011b) The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68:88–89
Li F, Han Y, Feng Y, Xing S, Zhao M, Chen Y, Wang W (2013) Expression of wheat expansin driven by the RD29 promoter in tobacco confers water-stress tolerance without impacting growth and development. J Biotechnol 163(3):281–291
Li AX, Han YY, Wang X, Chen YH, Zhao MR, Zhou SM et al (2015) Root-specific expression of wheat expansin gene TaEXPB23 enhances root growth and water stress tolerance in tobacco. Environ Exp Bot 110:73–84
Li Y, Tu L, Pettolino FA, Ji S, Hao J, Yuan D, Deng F, Tan J, Hu H, Wang Q, Llewellyn DJ, Zhang X (2016ab) GbEXPATR, a species-specific expansin, enhances cotton fibre elongation through cell wall restructuring. Plant Biotechnol J 14(3):951–963. https://doi.org/10.1111/pbi.12450
Li NN, Pu YY, Gong YC, Yu YL, Ding HF (2016ba) Genomic location and expression analysis of expansin gene family reveals the evolutionary and functional significance in Triticum aestivum. Genes Genom 38(11):1021–1030
Lin L, Cheng YB, Pu YQ, Sun S, Li X, Jin MJ, Pierson EA, Gross DC, Dale BE, Dai SY, Ragauskas AJ, Yuan S (2016) Systems biology-guided biodesign of consolidated lignin conversion. Green Chem 18:5536–5547. https://doi.org/10.1039/C6GC01131D
Lü P, Kang M, Jiang X et al (2013) RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta 237:1547–1559. https://doi.org/10.1007/s00425-013-1867-3
Lu Y, Liu L, Wang X, Han Z, Ouyang B, Zhang J, Li H (2016) Genome-wide identification and expression analysis of the expansin gene family in tomato. Mol Gene Genom 291(2):597–608. https://doi.org/10.1007/s00438-015-1133-4
Lv L, Zuo D, Wang X, Cheng H, Zhang Y, Wang Q, Song G, Ma Z (2020) Genome-wide identification of the expansin gene family reveals that expansin genes are involved in fibre cell growth in cotton. BMC Plant Biol. https://doi.org/10.1186/s12870-020-02362-y
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155
Ma N, Wang Y, Qiu S, Kang Z, Che S, Wang G, Huang J (2013) Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 8(10):e75997
Marowa P, Ding A, Kong Y (2016) Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep 35:949–965. https://doi.org/10.1007/s00299-016-1948-4
Mazumdar P, Lau SE, Wee WY, Singh P, Harikrishna JA (2017) Genome-wide analysis of the CCCH zinc-finger gene family in banana (Musa acuminata): an insight in to motif and gene structure arrangement, evolution and salt stress responses. Trop Plant Biol 10(4):177–193. https://doi.org/10.1007/s12042-017-9196-5
McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4:1425–1433
Minoia S, Boualem A, Marcel F, Troadec C, Quemener B, Cellini F, Bendahmane A (2015) Induced mutations in tomato SlExp1 alter cell wall metabolism and delay fruit softening. Plant Sci 242:1–8
Mollet JC, Leroux C, Dardelle F, Lehner A (2013) Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2(1):107–147
Muthusamy M, Uma S, Backiyarani S, Saraswathi MS, Chandrasekar A (2016) Transcriptomic changes of drought-tolerant and sensitive banana cultivars exposed to drought stress. Front Plant Sci 7:1609. https://doi.org/10.3389/fpls.2016.01609
Muthusamy M, Kim JY, Yoon EK, Kim JA, Lee SI (2020) BrEXLB1, a Brassica rapa expansin-like B1 gene is associated with root development, drought stress response, and seed germination. Genes (basel) 11(4):404. https://doi.org/10.3390/genes11040404
Nansamba M, Sibiya J, Tumuhimbise R, Karamura D, Kubiriba J, Karamura E (2020) Breeding banana (Musa spp.) for drought tolerance: a review. Plant Breed 139(4):685–696. https://doi.org/10.1111/pbr.12812
Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426
Palapol Y, Kunyamee S, Thongkhum M, Ketsa S, Ferguson IB, VanDoorn WG (2015) Expression of expansin genes in the pulp andthe dehiscence zone of ripening durian (Durio zibethinus) fruit. J Plant Physiol 182:33–39
Pérez-Vicente L, Carreel F, Roussel V, Carlier J, Abadie C (2021) Pseudocercospora guidelines for the evaluation of resistance to leaf spots of banana. In: Dita M (ed) Practical guidelines for early screening and field evaluation of banana against Fusarium wilt, Pseudocercospora leaf spots and drought. Bioversity International, Montpellier (France), p 83
Pezzotti M, Feron R, Mariani C (2002) Pollination modulates expression of the PPAL gene, a pistil-specific β-expansin. Plant Mol Biol 49:187–197
Pien S, Wyrzykowska J, Mcqueenmason S, Smart C, Fleming A (2001a) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98:11812–11817
Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001b) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98(20):11812–11817
Ravi I, Uma S (2011) Phenotyping bananas and plantains for adaptation to drought. In: Philippeand M, Jean-Marcel R (eds) Drought phenotyping in crops: from theory to practice. CGIAR Generation Challenge Programme/CIMMYT, Texcoco
Ren Y, Chen Y, An J, Zhao Z, Zhang G, Wang Y, Wang W (2018) Wheat expansin gene TaEXPA2 is involved in conferring plant tolerance to Cd toxicity. Plant Sci 270:245
Rogers JH (1990) The role of introns in evolution. FEBS Lett 268(2):339–343. https://doi.org/10.1016/0014-5793(90)81282-s
Rose JK, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc Natl Acad Sci USA 94(11):5955–5960
Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB (2000) Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol 123(4):1583–1592
Sampedro J, Cosgrove DJ (2005) The expansin superfamily. Genome Biol 6:242
Sampedro J, Carey RE, Cosgrove DJ (2006) Genome histories clarify evolution of the expansin superfamily: new insights from the poplar genome and pine ESTs. J Plant Res 119(1):11–21. https://doi.org/10.1007/s10265-005-0253-z
Sampedro J, Guttman M, Li LC, Cosgrove DJ (2015) Evolutionary divergence of β-expansin structure and function in grasses parallels emergence of distinctive primary cell wall traits. Plant J 81(2015):108–120
Santiago TR, Pereira VM, de Souza WR, Steindorff AS, Cunha BADB, Gaspar M et al (2018) Genome-wide identification, characterization and expression profile analysis of expansins gene family in sugarcane (Saccharum spp.). PLoS ONE 13(1):e0191081. https://doi.org/10.1371/journal.pone.0191081
Santini L, MunhozCde F, Bonfim MF Jr, Brandão MM, Inomoto MM, Vieira ML (2016) Host transcriptional profiling at early and later stages of the compatible interaction between Phaseolus vulgaris and Meloidogyne incognita. Phytopathology 106(3):282–294. https://doi.org/10.1094/PHYTO-07-15-0160-R
Saravanakumar AS, Uma S, Thangavelu R, Backiyarani S, Saraswathi MS, Sriram V (2016) Preliminary analysis on the transcripts involved in resistance responses to eumusae leaf spot disease of banana caused by Mycosphaerella eumusae, a recent add-on of the sigatoka disease complex. Turk J Bot 40:461–471
Sasidharan R, Chinnappa CC, Voesenek LA, Pierik R (2008) The regulation of cell wall extensibility during shade avoidance: a study using two contrasting ecotypes of Stellaria longipes. Plant Physiol 148:1557–1569. https://doi.org/10.1104/pp.108.125518
Shin JH, Jeong DH, Park MC, An G (2005) Characterization and transcriptional expression of the alpha-expansin gene family in rice. Mol Cells 20(2):210–218
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16:1220–1234
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J et al (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Tirumalaraju SV, Jain M, Gallo M (2011) Differential gene expression in roots of nematode-resistant and -susceptible peanut (Arachis hypogaea) cultivars in response to early stages of peanut root-knot nematode (Meloidogyne arenaria) parasitization. J Plant Physiol 168:481–492
Trivedi PK, Nath P (2004) MaExp1, an ethylene-induced expansin from ripening banana fruit. Plant Sci 167(6):1351–1358. https://doi.org/10.1016/j.plantsci.2004.07.005
Tucker MR, Koltunow AM (2014) Traffic monitors at the cell periphery: the role of cell walls during early female reproductive cell differentiation in plants. Curr Opin Plant Biol 17:137–145. https://doi.org/10.1016/j.pbi.2013.11.0
Van Den Berg N, Berger DK, Hein I, Birch PR, Wingfield MJ, Viljoen A (2007) Tolerance in banana to Fusarium wilt is associated with early up-regulation of cell wall-strengthening genes in the roots. Mol Plant Pathol 8(3):333–341. https://doi.org/10.1111/j.1364-3703.2007.00389.x
Wang RK, Li LL, Cao ZH, Zhao Q, Li M, Zhang LY, Hao YJ (2012) Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol Biol 79(1–2):123–135
Wang Y, Xia Q, Wang G, Zhang H, Lu X, Sun J, Zhang X (2017) Differential gene expression in banana roots in response to Fusarium wilt. Can J Plant Pathol 39(2):163–175. https://doi.org/10.1080/07060661.2017.1342693
Wei PC, Zhang XQ, Zhao P, Wang XC (2011) Regulation of stomatal opening by the guard cell expansin AtEXPA1. Plant Signal Behav 6:740–742. https://doi.org/10.4161/psb.6.5.15144
Wieczorek K, Golecki B, Gerdes L, Heinen P, Szakasits D, Durachko DM, Cosgrove DJ, Kreil DP, Puzio PS, Bohlmann H, Grundler FM (2006) Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J 48(1):98–112. https://doi.org/10.1111/j.1365-313X.2006.02856.x
Won S, Choi S, Kumari S, Cho M, Lee SH, Cho H (2010) Root hair specific EXPANSIN B genes have been selected for Graminaceae root hairs. Mol Cells 30(4):369–376
Wu YJ, Sharp RE, Durachko DM, Cosgrove DJ (1996) Growth maintenance of the maize primary root at low water potential involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiol 111:765–772
Wu YJ, Thorne ET, Sharp RE, Cosgrove DJ (2001) Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol 126:1471–1479
Xu B, Yang Z (2013) PAMLX: a graphical user interface for PAML. Mol Biol Evol 30(12):2723–2724. https://doi.org/10.1093/molbev/mst179
Xu Q, Xu X, Shi Y, Xu J, Huang B (2014) Transgenic tobacco plants overexpressing a grass PpEXP1 gene exhibit enhanced tolerance to heat stress. PLoS ONE 9:e100792
Yan A, Wu M, Yan L, Hu R, Ali I, Gan Y (2014) AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. PLoS ONE 9:e85208
Yang L, Wang S, Lu H, Liu L, Sa R (2020) Effects of dissociation water retention on pore structure disintegration in hydrate sediments. Front Energy Res 8:599542. https://doi.org/10.3389/fenrg.2020.599542
Yu Z, Kang B, He X, Lv S, Bai Y, Ding W, Wu P (2011) Root hair specific expansins modulate root hair elongation in rice. Plant J 66(5):725–734
Yuan M-L, Zhang Q-L, Guo Z-L, Wang J, Shen Y-Y, Shao R (2015) The complete mitochondrial genome of corizus tetraspilus (Hemiptera: Rhopalidae) and phylogenetic analysis of pentatomomorpha. PLOS ONE 10(6):e0129003. https://doi.org/10.1371/journal.pone.0129003
Zhang W, Yan H, Chen W, Liu J, Jiang C, Jiang H et al (2014) Genome-wide identification and characterization of maize expansin genes expressed in endosperm. Mol Genet Genom 289(6):1061–1074
Zhang H, Liu H, Yang R, Xu X, Liu X, Xu J (2019) Over-expression of PttEXPA8 gene showed various resistances to diverse stresses. Int J Biol Macromol 130:50–57
Zhao Y (2012) Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant 5:334–338. https://doi.org/10.1093/mp/ssr104
Zhao MR, Li F, Fang Y, Gao Q, Wang W (2011) Expansin-regulated cell elongation is involved in the drought tolerance in wheat. Protoplasma 248(2):313–323
Zhou J, Xie J, Liao H, Wang X (2014) Overexpression of b-expansin gene GmEXPB2 improves phosphorus efficiency in soybean. Physiol Plant 150(2):194–204
Zhou S, Han Y, Chen Y, Kong X, Wang W (2015) The involvement of expansins in response to water stress during leaf development in wheat. J Plant Physiol 183:64–74
Zorb C, Muhling KH, Kutschera U, Geilfus CM (2015) Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: is the epidermis growth-restricting? PLoS ONE 10:e0118406. https://doi.org/10.1371/journal.pone.0118406
Acknowledgements
This study was supported by Indian Council of Agricultural Research (ICAR), India under the project NPFGGM-Functional Genomics (3020). We express our sincere gratitude to Director, ICAR-National Research Centre for Banana, India for the facilities provided for this project.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, resources, writing, and overall monitoring: SBR. Draft preparation, review and editing: CA. Sample preparation and analysis: RT, PG, and MM. Methodology, in silico analysis, work design, and formatting: ACS. qRT-PCR work and data analysis: BR and PSK. Supervision, conceptualization, and project administration: SU.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
13205_2021_3106_MOESM5_ESM.xlsx
Supplementary file5 Supplementary file 5 Cis acting elements from Expansin gene family promoters of banana A & B genome (XLSX 58 KB)
13205_2021_3106_MOESM6_ESM.xlsx
Supplementary file6 Supplementary file 6 Digital Gene Expression details of MaEXPs between resistant and susceptible cultivars under Sigatoka & Nematode and Drought stress. (XLSX 19 KB)
Rights and permissions
About this article
Cite this article
Backiyarani, S., Anuradha, C., Thangavelu, R. et al. Genome-wide identification, characterization of expansin gene family of banana and their expression pattern under various stresses. 3 Biotech 12, 101 (2022). https://doi.org/10.1007/s13205-021-03106-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-03106-x