Abstract
Sensitive and effective phytoplasma DNA amplification in symptomatic rose cultivars is a long unresolved problem. In the present study, improvement in standardization for PCR assay for phytoplasma detection was established with rose samples by selection of various combinations of nested primer pairs of 16S ribosomal gene and secA gene. CTAB DNA extraction method was slightly modified by adding 2% polyvinyl pyrrolidone and increased the isopropanol volume which yielded better quality DNA. Best amplification results were achieved in nested PCR assay employing P1/P7, R16mF2/R16mR2 and R16F2n/R16R2, P1/P7 and R16mF2/R16mR2, and R16mF2/R16mR2 and fU5/rU3 primer pairs. Besides, a multiplex PCR assay was also developed and optimized for consistent identification of phytoplasma in rose samples by employing primer pairs of 16S rRNA and secA genes together in a single PCR reaction by optimizing annealing temperature at 55 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rose (Rosa × hybrida L.; family Rosaceae) is the most important commercial cut flower; comprises of more than 150 species and occupies the first position in international as well as domestic trade. The Dutch cut flowers trade accounted for roughly 2.6 billion Euros, whereas garden plants had a turnover of over 400 million Euros. The revenue of cut flower sales of roses is leading worldwide with a value of 707 million Euros (Royal FloraHolland 2020). In India, total area under rose cultivation is 29.41 thousand hectares with a production of 301.95 thousand MT (NHB Database 2017). Diseases are the main cause of low productivity of ornamentals including rose, and phytoplasmas are one of the most emerging plant pathogens in twenty-first century and major causes of many ornamental diseases. About 90 species of ornamental plants reported to be associated with phytoplasmas belonging to 14 different 16S ribosomal groups worldwide (Bellardi et al. 2018). Among the diseases, phytoplasma diseases associated with different maladies in rose cause serious loss in flower production and effects on flower marketability (Madhupriya et al. 2017). Three different groups (16SrI, 16SrII and 16SrV) of phytoplasma are reported worldwide on rose (Kaminska et al. 2001; Madhupriya et al. 2017; Bellardi et al. 2018).
Detection of phytoplasma in plants is still very crucial and no standard protocol has been established so far universally, which could be used for diagnosis for all types of plant tissues and species. Timely detection of a phytoplasma disease is very important in the prevention and management of the disease. Since, symptoms caused by phytoplasma are not always very clear at all the growth stages in rose cultivars in the fields, only molecular diagnosis would be the authentic and reliable option available for the phytoplasma diagnosis in rose samples. But it is always tedious and unpredictable to extract good quality DNA and its routine successful amplification with universal phytoplasma specific primers from the rose samples because of presence of excessive phenolic compounds and other secondary metabolites (Friar 2005; Madhupriya et al. 2017).
The advent of nucleic acid-based technologies has allowed improving sensitivity to limits below the potential pathogenic thresholds solving some of the challenges posed by the need for specific and sensitive detection of phytoplasmas (Lopez et al. 2009; Bertaccini et al. 2019). To implement an efficient phytoplasma disease control measure in rose crops, detection of the phytoplasma is crucial and requires the use of a suitable molecular detection protocol involving better quality and phytoplasma enriched DNA extraction, selection of effective set of primer pairs and standardization of PCR amplification cycles. PCR assays are generally used for identification of phytoplasmas with different round of nested PCR assays and different sets of primer pairs. But it is potentially expensive, time taking and resource intensive (Bertaccini et al. 2019). However, the duplex and multiplex PCR (d-PCR and m-PCR) assays, which incorporates different sets of specific primers for two or more DNA targets in one reaction tube and enables simultaneous amplification of different target nucleic acid length in a single PCR test (Pallás et al. 2018). Application of d-PCR and m-PCR are quick, reliable, and cost-effective methods that have been used successfully for detecting phytoplasmas in several plant species (Biswas et al. 2013; Swarnalatha and Reddy 2014; Malandraki et al. 2015; Reddy and Rao 2019).
We have been facing a regular problem in sensitive phytoplasma DNA amplifications from suspected symptomatic rose cultivars in our lab. Therefore, we attempted to develop an effective DNA enrichment extraction method along with selection of suitable primer pairs and standardization of nested PCR assay for consistent good target amplification of phytoplasmas DNA in rose samples. We also attempted to develop a multiplex PCR assay by employing different sets of ribosomal gene and secA gene specific primer pairs together for a successful and consistent amplification of phytoplasma DNA associated with rose samples in a single PCR reaction.
Materials and methods
Twenty leaf samples from two symptomatic rose cultivars ‘Deepak’ and ‘MS Randhawa’ (ten samples of each cultivar from ten different plants in the field) showing typical phytoplasma symptoms and asymptomatic plant samples were collected during a survey in the experimental fields of Division of Floriculture and Landscaping, ICAR-IARI, New Delhi, India during July to August 2019. Total genomic DNA was extracted from leaf midrib by CTAB method (Ahrens and Seemuller 1992) with slight modifications.
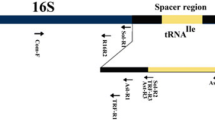
The concentration of the extracted DNA was adjusted to 50 ng/μl and used as a template for amplifications in six different PCR runs with separate combination of first and nested primer pairs for 16S rRNA gene and secA genes (Table 1). In the first and second set of PCR run, the most commonly used universal phytoplasma specific, first round and nested PCR primers, P1/P7 and R16F2n/R16R2 and P1/P7 and3F/3R were used (Table 1). Since, there were no amplifications of phytoplasma with any of the two most commonly used nested universal primer pairs, another set of first and nested primer pairs combinations were attempted using P1/P7 as the first primer pair followed by two nested round of primer pairs, R16mF2/R16mR2 and R16F2n/R16R2 (PCR run 3). In the fourth PCR run, a combination of P1/P7 in first round followed by R16mF2/R16mR2 as nested primers and the fifth PCR run consisted of R16mF2/R16mR2 as first round followed by fU5/rU3 as nested primers were used. However, the sixth PCR run used the universal set of first round secAfor1/secArev3 followed by nested primer pair secAfor2/secArev3 for secA gene (Table 1). PCR reactions were performed in a Master-cycler (Eppendorf, Germany). The PCR cycling parameters for the normal PCR were used as described earlier for the above mentioned primer pair combinations (Dickinson and Hodgetts 2013; Rao et al. 2014; Omar 2016).The details of primers and their sequences are listed in Table 1. Each PCR run with different set of primer pairs was repeated three times with all the 20 rose samples (from both the test cultivars) to confirm and validate the amplification results.
In another set of experiment, a multiplex PCR assays was established by employing four primer pairs, P1/P7, R16mF2/R16mR2, R16F2n/R16R2 (PCR run 3) and secAfor2/secArev3 for 16S rRNA gene and secA genes in a single PCR reaction. The PCR reaction was set up in a 50 μl reaction mixture as suggested earlier (Reddy and Rao 2019) with a slight modification containing DNA (~ 50 ng, 2 μl), 44 μl of PCR mastermix (GoTaq® Green Master Mix 2X by Promega Biotech India Pvt. Ltd.), 1.25 μl of primer pair P1/P7 (10 μM/μl), 1 μl of primer pair R16mF2 and R16mR2, 1 μl of primer pair R16F2n and R16R2 and 0.5 μl of primer pair secAfor2 and secArev3 and 0.25 μl nuclease free water to make up the complete reaction volume. Different primer combinations and PCR programme cycles were tested with varying annealing temperatures ranging from 54 to 56 °C (data not shown). The PCR cycling parameters for the multiplex PCR were used as 94 °C, 4 min (1X) followed by 35 cycles of 94 °C, 45 s; 55 °C, 1 min; 72 °C, 2 min and a final extension at 72 °C, 10 min.
Five amplified 16S rRNA and secA gene fragments from nested and multiplex PCR assays were purified using the Wizard® SV Gel and PCR Clean-up System (Promega, Madison, USA). The purified products were sequenced in both directions at Eurofins Genomics India Pvt. Ltd. Karnataka, India. The contig of forward and reverse sequences of the phytoplasma thus obtained was assembled using software CAP contig assembly program and aligned using Clustal W method of BioEdit7 software (Thompson et al. 1994). The 16S rRNA gene sequences generated from regular and multiplex PCR assays in the present study and the reference phytoplasma strains sequences retrieved from GenBank were used to construct phylogeny through MEGA 7.0 version software employing the neighbor joining method for identification of phytoplasma associated with rose cultivars (Kumar et al. 2016).
Results and discussion
Rose cultivars ‘Deepak’ and ‘MS Randhawa’ showing phytoplasma related symptoms of phyllody (Fig. 1a) and flower malformation (Fig. 1b) were observed in rose fields at IARI, New Delhi, during a survey in July–August 2019.
Best DNA quality was achieved in rose samples with ~ 1.8 ratio of absorbance at 260/280 nm and 2 ratio at 260/280 nm absorbance in NanoDrop™ spectrophotometer by CTAB extraction method with slight modifications by adding 2% polyvinyl pyrrolidone to the CTAB buffer before incubation and increased the isopropanol from 0.8 to 1 volume ratio in aqueous phase to precipitate the better quality nucleic acid. No amplification of phytoplasma DNA was achieved in first round of PCR assay using P1/P7 primer pairs in any of the symptomatic rose cultivars in the study (data not shown). Further, no amplification was observed in nested PCR assay using P1/P7 and R16F2n/R16R2, and P1/P7 and 3F/3R set of nested primer pairs (PCR run 1 and 2) in any of the tested symptomatic rose cultivars. However, these set of nested primers are commonly used for the routine amplification of phytoplasma DNA from most of the plants and insects’ sample worldwide (Bertaccini et al. 2019). However, the phytoplasma amplification of ~ 1.25 kb using primer pair P1/P7, R16mF2/R16mR2 and R16F2n/R16R2 (PCR run 3) was achieved consistently in all the twenty rose samples of two rose cultivars ‘Deepak’ and ‘MS Randhawa’ (Table 1).The amplifications of ~ 1.4 kb product were achieved using primer pair P1/P7 and R16mF2/R16mR2 (PCR run 4) and ~ 880 bp in PCR run 5 using primer pairs R16mF2 /R16mR2 and fU5/rU3 (Table 1; Fig. 2a–c). In PCR run 6, amplification of ~ 480 bp product of secA gene was obtained in all the twenty symptomatic samples of both the rose cultivars using secAfor1/secArev3 and secAfor2/secArev3 primer pair (Fig. 2a–c) (Table 1). No amplification was achieved with the asymptomatic plants with any of the primer pairs used in PCR run 1 to 6.
Gel images showing results of amplifications by different sets of primer pairs: a P1/P7 and R16mF2/R16mR2, lane 1: positive control, lane 2: ‘Deepak’, lane 3: ‘MS Randhawa’, lane 4: negative control and lane M: marker; b P1/P7, R16mF2/R16mR2 and R16F2n/R16R2, lane M: marker, lane 1: positive control, lane 2 ‘Deepak’, lane 3: ‘MS Randhawa’ and lane 4: negative control; c R16mF2/R16mR2 and fU5/rU3, lane M: marker, lane 1: positive control, lane 2: ‘Deepak’, lane 3: ‘MS Randhawa’ and lane 4: negative control; d lane M: marker, lane 1: R16F2n/R16R2, lane 2: secAfor2/secArev3, lane 3 and lane 4: multiplex PCR assay using P1/P7, R16mF2/R16mR2, R16F2n/R16R2 and secAfor2/secArev3 of ‘Deepak’ and ‘MS Randhawa’, respectively
In multiplex PCR assays, expected PCR amplicons of ~ 1.25 kb for 16Sr RNA gene and ~ 480 bp for secA gene were consistently obtained using four set of primer pairs (P1/P7, R16mF2/R16mR2, R16F2n/R16R2 and secAfor2/secArev3) in a single reaction in two rose cultivars ‘Deepak’ and ‘MS Randhawa’(Fig. 2d). This successful amplification of 16S rRNA and secA genes in a single PCR multiplex assay was achieved by manipulation of increasing the PCR buffer (44 µl) and reducing the primer quantity from 2 µl (in normal PCR) to 1.25 µl in P1/P7 primer pair, 1 µl in R16mF2/R16mR2 primer pair and R16F2n/R16R2 primer pair and 0.5 µl secAfor2/secArev3 primer pair. Three different annealing temperatures (54–56 °C) were tested for amplifications of 16S rRNA gene/secA genes products and the most distinct and intensified amplified products were achieved at 55 °C annealing temperature in the multiplex PCR assay (Fig. 2d). In multiplex PCR assay, attempts were made with primer pairs of 16S rRNA gene used in PCR run 4 and 5 along with secA gene specific primer. But no amplification was achieved in either of the combinations of PCR run 4 and 5 along with secA gene primer. Our results confirmed that only the primer used in PCR run 3 along with secA gene specific primer are capable of amplifying rose phytoplasma strains. These sets were repeated at least three times and very good amplification was achieved consistently indicating the suitability and validity of these four set of primer pairs.
The amplified PCR products (each of 16S rRNA and secA from each of the test cultivars) obtained in the normal nested PCR and multiplex PCR assays for 16S rRNA and secA genes were sequenced in both directions and were deposited in the GenBank. Since, all the 20 rose phytoplasma sequences from the two rose cultivars showed 100% sequence identity among themselves for 16S rRNA gene and secA genes, only two sequences each of the two isolates each of rose cultivar ‘Deepak’ and ‘MS Randhawa’ were submitted to GenBank for 16S rRNA gene (GenBank Acc. Nos. MW309813 and MW309814) and secA gene (GenBank Acc. Nos. MW323441 and MW323442). BLAST and pair wise sequence comparison analysis of 16S rRNA gene sequences of rose phytoplasma isolates obtained from the normal nested PCR and multiplex PCR assays (GenBank Acc. Nos. MW334949 and MW334950) showed 100% sequence identity among themselves and 99.7% with ‘Candidatus Phytoplasma asteris’ related strains of Plumbago auriculata leaf yellowing (GenBank Acc. Nos. MN239504) and Echinacea purpurea aster yellows (GenBank Acc. Nos. MK992774). Similarly, the secA gene sequences of the two rose phytoplasma strains obtained from the normal nested PCR and multiplex PCR assays (GenBank Acc. Nos. MW362159 and MW362160) showed 100% sequence identity among themselves and shared 99.3% identity with other previously identified ‘Candidatus Phytoplasma asteris’ related strains of sesame phyllody (GenBank Acc. Nos. MG761718) and periwinkle phyllody (GenBank Acc. Nos. KY689743). Phylogenetic analysis based on 16S rRNA gene and secA gene sequences of rose phytoplasma strains of the two representative cultivars ‘Deepak’ and ‘MS Randhawa’ showed clustering with 16SrI phytoplasma group (Figs. 3, 4). Our results confirmed association of Ca. P. asteris strains in the symptomatic rose cultivars based on sequence analysis of 16S rRNA and secA genes. This confirms the validity of multiplex PCR assay for authentic and reliable identification of phytoplasma strains associated with rose cultivars in the present study. Our results confirmed that the identity of phytoplasma strains associated with rose cultivars was same with amplified products achieved in normal and multiplex PCR assays. This also confirms the validity of the newly developed multiplex PCR assays utilizing both the 16S rRNA and secA genes specific primers.
Erratic distribution and low titre of phytoplasma especially in infected woody plants like rose was reported and because of the presence of phenols and secondary metabolites which creates problem in the successful amplification of phytoplasma DNA in the PCR reaction (Friar 2005; Manimekalai et al. 2016). Therefore, addition of 2% polyvinyl pyrrolidone to the CTAB buffer was suggested (Xu et al. 2004) which also provided better removal of polyphenols in the DNA extraction protocol in rose samples in the present study and yielded better DNA quality. Direct PCR assays using P1/P7 or nested PCR assay using P1/P7 and R16F2n/R16R2 and/ P1/P7 and 3F/3R primer pairs failed to yield desired amplifications in any of the symptomatic rose samples in the present study. But a consistent desired target DNA amplifications of phytoplasmas was achieved using combination of other nested primer pairs P1/P7 and R16mF2/R16mR2 (~ 1.4 kb), nested PCR assays using P1/P7, R16mF2/R16mR2 and R16F2n/R16R2 (~ 1.25 kb) and R16mF2/R16mR2 and fU5/rU3 (~ 880 bp).
The experiments were repeated three times with all the tested rose samples to validate the consistency of amplification with different sets of nested primer pairs used and verified with similar results. Our results suggested that either of these nested primer pairs could be used for successful amplifications of phytoplasmas associated with rose samples. Earlier workers have also utilized different set of nested primer pairs (P1/P7 or R16mF2/R16mR1 with R16F2n/R16R2 and P1/P7 and fU5/rU3) for successful amplification of phytoplasmas associated with woody plant species with higher amount of phenols and secondary metabolites (Abou-Jawdah et al. 2002; Verdin et al. 2003). Our study suggests that the selection of specific primer pair for target gene amplification in identification of phytoplasma strain is very important and needs further standardization for the improvement in diagnosis of phytoplasma in different woody plant host samples.
In the present study, a suitable and reliable multiplex PCR assay has been developed to amplify the phytoplasma associated with rose samples utilizing 16S rRNA and secA genes specific primer pairs. Multiplex PCR assay were also developed earlier to detect phytoplasma strains either belonging to two different phytoplasma groups or using different gene specific primers in a single PCR reaction (Clair et al. 2003; Reddy and Rao 2019; Gholami et al. 2020). To save time and resources, multiplex PCR assays developed in the present study would have a great significance in reliable diagnosis of rose phytoplasmas by amplifying both the 16S rRNA and secA genes in a single PCR reaction using multiset primer pairs. Multiplex PCR assay can also overcome the constraints of individual PCR assay and has the potential to produce accurate, reliable and sensitive results with less effort and resources.
The standardized PCR with different sets of primers and multiplex PCR assay protocol could have applied significance for detecting phytoplasmas in rose and other woody plant tissue samples. Besides, similar multiplex PCR assay may be developed with other combinations of ribosomal and multilocus genes specific primers for finer group/subgroup characterization of phytoplasma strains in rose and other woody plant hosts.
References
Abou-Jawdah Y, Karakashian A, Sobh H, Martini M, Lee IM (2002) An epidemic of almond witches’-broom in Lebanon: classification and phylogenetic relationships of the associated phytoplasma. Pl Dis 86(5):477–484
Ahrens U, Seemüller E (1992) Detection of DNA of plant pathogenic mycoplasma like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82:828–832
Bellardi MG, Bertaccini A, Madhupriya, Rao GP (2018) Phytoplasma diseases in ornamental crops. In: Rao GP, Bertaccini A, Fiore N, Leiftwing LW (eds) Phytoplasmas: plant pathogenic bacteria-I. Springer, Singapore, pp 191–233
Bertaccini A, Fiore N, Zamorano A, Tiwari AK, Rao GP (2019) Molecular and serological approaches in detection of phytoplasmas in plant and insects. In: Bertaccini A, Oshima A, Kube M, Rao GP (eds) Phytoplasmas: plant pathogenic bacteria-III. Springer, Singapore, pp 105–136
Biswas C, Dey P, Satpathy S (2013) A multiplex nested PCR assay for simultaneous detection of Corchorus golden mosaic virus and a phytoplasma in white jute (Corchorus capsularis L.). Lett Appl Microbiol 56(5):373–378
Clair D, Larrue J, Aubert G, Gillet J, Cloquemin G, Boudon-Padieu E (2003) A multiplex nested-PCR assay for sensitive and simultaneous detection and direct identification of phytoplasma in the Elm yellows group and Stolbur group and its use in survey of grapevine yellows in France. Vitis 42(3):151–157
Deng S, Hiruki C (1991) Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. J Microbiol Methods 14:53–61
Dickinson M, Hodgetts J (2013) PCR analysis of phytoplasma based on the secA gene. In: Dickinson M, Hodgetts J (eds) Phytoplasma: methods and protocols. Springer, New York, pp 205–216
Friar EA (2005) Isolation of DNA from plants with large amount of secondary metabolites. Methods Enzymol 395:3–14
Gholami J, Bahar M, Talebi M (2020) Simultaneous detection and direct identification of phytoplasmas in the aster yellows (16SrI), clover proliferation (16SrVI) and stolbur (16SrXII) groups using a multiplex nested PCR assay in potato plants. Potato Res 63:403–415
Gundersen DE, Lee I-M (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol Mediterr 35:144–151
Hodgetts J, Boonham N, Mumford R, Harrison N, Dickinson M (2008) Phytoplasma phylogenetics based on analysis of secA and 23S rRNA gene sequences for improved resolution of candidate species of ‘Candidatus Phytoplasma’. Intl J Syst Evol Microbiol 58(8):1826–1837
Kaminska M, Dziekanowska D, Rudzinâ Ska-langwald A (2001) Detection of phytoplasma infection in rose, with degeneration symptoms. J Phytopathol 149:3–10
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
López MM, Llop P, Olmos A, Marco-Noales E, Cambra M, Bertolini E (2009) Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr Issues Mol Biol 11:13–45
Lorenz KH, Schneider B, Ahrens U, Seemüller E (1995) Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and non ribosomal DNA. Phytopathology 85(7):771–776
Madhupriya, Banyal N, Raju DVS, Manimekalai R, Rao GP, Paul Khurana SM (2017) Association of different groups of phytoplasma in flower malformation, phyllody, foliar yellowing, and little leaf disease of rose (Rosa sp.). J Hort Sci Biotech 92(4):424–431
Malandraki I, Varveri C, Olmos A, Vassilakos N (2015) One-step multiplex quantitative RT-PCR for the simultaneous detection of viroids and phytoplasmas of pome fruit trees. J Virol Methods 213:12–17
Manimekalai R, Soumya VP, Sathish KR, Selvarajan R, Reddy K, Thomas GV, Sasikala M, Rajeev G, Baranwal VK (2010) Molecular detection of 16SrXI group phytoplasma associated with root (wilt) disease of coconut (Cocos nucifera) in India. Pl Dis 94:636
Manimekalai R, Soumya VP, Nair S, Rajeeve GR, Rao GP (2016) PCR detection of phytoplasmas from different tissues of root (wilt) diseased coconut palms. Phytopathogenic Mollicutes 6(1):23–28
NHB Database (2017) Indian horticulture database. NHB, Ministry of Agriculture, Government of India
Omar AF (2016) Association of ‘Candidatus Phytoplasma cynodontis’ with Bermuda grass white leaf disease and its new hosts in Qassim province, Saudi Arabia. J Plant Interact 11(1):101–107
Pallás V, Sánchez-Navarro JA, James D (2018) Recent advances on the multiplex molecular detection of plant viruses and viroids. Front Microbiol 9:2087
Rao GP, Tiwari AK, Kumar S, Baranwal VK (2014) Identification of sugarcane grassy shoots-associated phytoplasma and one of its putative vectors in India. Phytoparasitica 42:349–354
Reddy MG, Rao GP (2019) Development of a multiplex PCR assay for detection and identification of a chickpea phytoplasma strain utilizing 16S rRNA and imp genes specific primers. Phytopathogenic Mollicutes 9(2):257–262
Royal Floraholland (2020) Facts and figures. http://www.statista.com
Swarnalatha P, Reddy MK (2014) Duplex PCR for simultaneous detection of Begomovirus and Phytoplasma from naturally infected tomato. Pest Manag Hortic Ecosyst 20:59–68
Thompson JD, Higgins DG, Gibson TJ (1994) Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput Appl Biosci 10(1):19–29
Verdin E, Salar P, Danet JL, Choueiri E, Jreijiri F, El Zammar S, Gelie B, Bove JM, Garnier M (2003) ‘Candidatus Phytoplasma phoenicium’ sp. nov., a novel phytoplasma associated with an emerging lethal disease of almond trees in Lebanon and Iran. Int J Sys Evol Microbiol 53(3):833–838
Xu Q, Wen X, Deng X (2004) A simple protocol for isolating genomic DNA from chestnut rose (Rosa roxburghii Tratt) for RFLP and PCR analyses. Plant Mol Biol Rep 22(3):301
Author information
Authors and Affiliations
Contributions
The authors conceived idea, analysed data and drafted manuscript for publication. The first author, TR did all the experiments in the field and lab and also contributed significantly in preparation of draft of the manuscript. N, KPS, MKS and AT contributed equally in preparation and finalization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there was no conflict of interest.
GenBank submission
All the 16S rRNA and secA gene sequences are submitted to GenBank.
Rights and permissions
About this article
Cite this article
Rihne, T., Namita, Singh, K.P. et al. Improvement in molecular detection of phytoplasma associated with rose by selection of suitable primers and development of a multiplex PCR assay. 3 Biotech 11, 190 (2021). https://doi.org/10.1007/s13205-021-02713-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02713-y