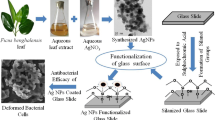
Abstract
In this study, Ocimum basilicum (a proven broad spectrum medicinal plant for broad-spectrum pharmacological activities) leaf extract was used as conjugates for the fabrication of silver nanoparticles (AgNP). Color change of the reaction mixture and UV–Visible spectrophotometry indicated the fabrication of silver nanoparticles, further X-ray diffraction (XRD) crystallography, scanning electron microscopy (SEM), transmission electron microscopic images (TEM), and Selected area electron diffraction (SAED) confirms the purity, monodispersity, and morphology including size (22.4 nm) and conjugated functional group of Ocimum basilicum. The conjugation of functional OH, N–O, and C=O groups was confirmed by Fourier-transform infrared spectroscopy (FT-IR). The engineered AgNP have shown significantly efficient antibacterial and antibiofilm activities (92.7% biofilm inhibition) on diverse clinical strains and thus showed its potential for use in clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal nanoparticles have important roles in biomedical applications which include drug delivery, diagnostics, imaging, sensing, gene delivery, artificial implants, bio-labelling, sensors, and tissue engineering applications (Malapermal et al. 2017; Kumar et al. 2020; Zou et al. 2020). Usually, conjugation of additional active ingredients could not be possible with chemical synthesis protocol for silver nanoparticle (AgNP) and thus might not show efficient bioactivity but green synthesized AgNP could solve this as being less toxic (Roy et al. 2013; Shah et al. 2015; Mladenova et al. 2018; Behravan et al. 2019; Ahmed and Mustafa 2020). To overcome the toxicity and bioactivity/delivery challenges, researchers started using environmentally benign and cost-effective biological methods as alternative—using bacteria, fungi, and plant (Bharde et al. 2008; Singh et al. 2011; Sivaranjani and Meenakshisundaram 2013; Iravani et al. 2014). Interestingly, medicinal plants are of more interest in the biogenic synthesis of nanoparticle due to the presence of rich bioactive secondary metabolites, which act as reducing agents and play a major role in the reduction of metal ions and capping of AgNPs. It can also be noted that the quantity and quality of phytochemicals present in plants differ even among intra-genus and intraspecies, which ultimately affect the morphological features of the nanoparticles (Sharma et al. 2020; Vijayakumar et al. 2020a, b). The biological activity of different plants (inter or intraspecies) is related to the difference in bioactive compounds distribution, which are more frequent in some plants than in others. The herbal plant Eclipta albe from Asteraceace family is richest source of phenols, carotenoids, vitamins, and flavonoids which act as a reducing agent for nanoparticle preparation. The presence of the phenolic group in this plant extract acts a quenching the free radicals by hydrogen donation (Sinha and Raghuwanshi 2016). It can also be noted that conjugation of plant extracts with rajat bhasmas—a proved nanosized drug used since Ayurvedic era (Sharma and Prajapati 2016; Mukkavalli et al. 2017)—is well explored in the ayurvedic medicine system (Kumar et al. 2006). The previous studies indicated that those metal nanoparticles derived from plant sources may have excellent killing efficacy of biological pathogens including antioxidant, antibacterial, anti-diabetic and anticancerous activities. Among the noble biosynthesized metal nanoparticles, AgNPs are of more interest to scientists due to its better physiochemical stability, and bioactivities (Dos Santos et al. 2014). Different biological methods are gaining recognition for AgNP synthesis due to the multiple applications of biosynthesized AgNPs. The use of plants in the green synthesis of nanoparticles emerges as a cost-effective and eco-friendly approach (Pal et al. 2019). The discovery and development of antibiotics, antimicrobial agent, antimicrobial peptides are the most powerful and successful achievements of modern science and technology for the control of biofilm-oriented diseases, and biosynthesized nanoparticles could be the game-changer in this. Ocimum basilicum is a medicinal plant well explored for its pharmaceutical and bio-activities (Jayapriya and Lalitha 2013) and widely used as tonic, digestive, for treating skin infections, insect stings, antibacterial, antifungal, and antiviral medicine (Kaya et al. 2008; Dambolena et al. 2010; Romeilah et al. 2010; Azam and Irshad 2016). However, Ocimum basilicum is least explored for the AgNP synthesis and analyzing the pharmaceutical-/bio-activities. In this study, we intend to synthesize AgNP by using Ocimum basilicum leaf extract as a reducing agent and further the same has been studied for antibacterial, and antibiofilm activity of clinical pathogens.
Materials and methods
Material collection
Ocimum basilicum (Genovese) herb plant leaves were collected from the local area of Krishnan koil region, (latitude and longitude coordinates: 9.5747° N, 77.6798° E) Srivilliputhur, Tamilnadu, India; which was identified and confirmed by trained botanist. Microbial cultures were collected from the stock culture maintained by the Department of Microbial Technology, Madurai Kamaraj University,Madurai, TamilNadu, India. Silver nitrate (99% purity) was indented from Sigma- Aldrich (Merck), Bengaluru, India. The LB media and the antibiotics Ampicillin were procured from Hi-Media, Mumbai, India. All reagents supplied were of analytical grade and were used without further purification. Double deionized (DI) water (with a measured resistivity of 18.2 MΩ cm−1) was used, throughout the experiments.
Preparation of Ocimum basillicum leaf extracts
Ocimum basillicum (OB) was grounded thoroughly after double wash using water followed by ethanol and the paste was then dissolved in 200 mL of ethanol with 72 h of continuous shaking. The extract was stored at 4 °C after proper filtration and ethanol removal.
Synthesis of silver nanoparticles
100 mg of Ocimum bacilicum ethanolic crude extract of (OBET) was dissolved in 1 mL MilliQ® water. Further, 1 mL of the solution was gently mixed to 50 mL of 1 mM AgNO3 solution and kept in a magnetic stirrer for 12 h at 37 °C at pH 7.0. The Initial observation of brown color change indicated the reduction of Ag+ ion (Selvam et al. 2019), further color changes and the stability confirm the formation of AgNPs, which was then centrifuged at 7000 rpm for 20 min and the collected AgNPs are re-dispersed in ethanol and washed with sterile DI water, repeatedly thrice. The purity, size, crystalline nature, and conjugation of the functional group of the obtained AgNPs were confirmed by further characterization studies.
Characterization
The collective oscillation of conduction band electrons in AgNPs was analyzed at 400–450 nm by the UV–Vis spectrophotometer (Shimadzu-100, Kyoto, Japan) in the wavelength range of 200–800 nm. About 0.2 gm of AgNPs were pelletized with KBr for the FT-IR analysis using a SHIMADZU-IRTracer-100, Shimadzu, Kyoto Japan in the range of 4000–500 cm−1 in reflection mode. XRD patterns of the AgNPs were recorded using D8 Advance Eco (Bruker, Billerica, Massachusetts, United States). The particle size and shape were analysed and confirmed by SEM (Zeiss 018 SEM. JSM-7100F, JEOL Ltd., Tokyo, Japan) and TEM (Hitachi T-4500 TEM, Chiyoda City, Tokyo, Japan) instrumentation. It can be noted that the protocol for characterization has been followed as per the earlier reported protocols for UV–Vis spectrophotometer (Dasgupta et al. 2016), FTIR (Singhal et al. 2011), XRD (Paulkumar et al. 2014), SEM (Balaji et al. 2017), and TEM (Tammina et al. 2017).
Antimicrobial activity
The antibacterial efficiency of the newly synthesized AgNPs was analyzed by the disc diffusion method (Panácek et al. 2016). Briefly, the overnight grown Gram positive and Gram-negative clinical cultures (Bacillus subtilis, E. coli Pseudomonus aureginosa, Enterobactesr sp., Staphylococcus aureus) (1 × 10–5 colony forming unit) were uniformly spread on the agar plate. The different concentrations of AgNPs (25, 50, 75 and 100 mg/mL) were placed on the plates and incubated for 24 h at 37 °C. The clinical pathogen susceptibility was measured by the zone of clearance (mm).
Antibiofilm activity
5 μL of overnight grown Psuedomonas aeruginosa culture (0.05 OD at 600 nm) was added into 1.5 mL of Mueller Hinton broth in 24 well microtiter plates. The different concentrations of AgNPs from 50 to 250 μg/mL was added into well with the pathogen and kept at 37 °C overnight under static conditions. Further, the biofilm was washed thrice with sterile PBS and washed again with 1% crystal violet. Further, the wells were washed with 200 μL ethanol and the absorbance was recorded at 570 nm. Notably, the experimental results were compared with untreated AgNPs considered as negative control. Further, the AgNP efficacy with biofilm formation was evaluated by using SEM (Kannan et al. 2019). Briefly, the sterile glass cover slip was immersed in a 2 mL MHI broth containing 6 well plates and 5 μL of pathogens were incubated in the absence and presence of AgNPs for 24 h. The cover slip was washed with sterile PBS and fixed with 2% glutaraldehyde and further degraded with ethyl alcohol and the biofilm formation was visualized by VEGA3 TESCAN (Brno–Kohoutovice Czech Republic).
Statistical analysis
All statistical analysis was performed and expressed as mean of parallel duplicates of ANOVA correlation. P < 0.05 was considered as statistically significant.
Results and discussion
Characterization of AgNPs
Though Oscimum basilicum is well explored for its bio-/pharmaceutical- activities but not much explored for its role in the biosynthesis of nanoparticles. Although, many researchers have approached green synthesized silver and other nanoparticles using different plant extract (Huang and Yang 2004; Ahmed et al. 2016; Ishwarya et al. 2018) but this is first of its kind study highlighting the AgNP biosynthesized and decorated using active ingredients from Oscimum basilicum L. (Tulsi)—a potential medicinal plant. Further, detailed experimentation of the antibacterial and antibiofim activity has been performed on selected clinical microbes, which enables it to be a good resource for biomedical application, after detailed clinical studies.
The initial confirmation of AgNPs synthesis was confirmed by colour changes to reddish-brown indicates the AgNP formation, due to the presence of secondary metabolites in Ocimum basilicum L. leaf extract, which can reduce Ag+ ion in AgNO3 to Ago ion and further AgNP. Due to the effects of surface plasmon resonance the AgNO3 reduced AgNPs. Figure 1, indicates the initial color change after 12 h of reaction time in continuous shaking. The AgNP formation in the presence of plant extract is the indication that the extract is acting as a reducing and capping agent, which was further confirmed with sophisticated instrumentation for characterization, the initial observations are in agreement with the earlier reports (Chandran et al. 2006; Ramteke et al. 2013). The formation of AgNPs was further confirmed by UV–Vis spectrum, in ethanolic (solvent) (Fig. 2) with a sharp peak at 450 nm due to the surface plasmon resonance of AgNPs. The similar observation of sharp peak at 450 nm due the surface Plasmon resonance of AgNP is also reported by various earlier researches (Pirtarighat et al. 2019). It can be noted that the absorbance band also depends upon time and concentrations of AgNO3 as well as the size and shape of the silver nanoparticles (Dasgupta et al. 2016).
Fourier-transform infrared spectroscopy (FTIR) analysis
The plant extract containing secondary metabolites is responsible for the shifting of the vibrational band after the reaction of a plant extract with AgNO3. FTIR analysis indicates that the stretching band appeared at 3400 cm−1 corresponding to the alcoholic and phenolic OH groups in the plant extract, (Fig. 3). The other peaks at 1703 and 1510 cm−1 corresponds to the C=O and N–O asymmetric nitro compounds, respectively in the plant extract. Moreover, the low-intensity peak at 2940 cm−1 and the broader band at 3315 cm−1 are attributed to the presence of –CH2 and the –OH groups, respectively. The above-depicted compounds could be responsible for the reducing as well as capping agents during the process of AgNP fabrication. Notably, the earlier researches also confirmed that the presence of phytochemicals, phenols, tannins, flavonoids, alkaloids act as a capping and reducing agent for synthesizing Cd-AgNPs (Prabu et al. 2019). Earlier researchers also confirmed similar secondary metabolite—with key functional groups like aldehyde, ketone and carboxyl—are responsible for AgNPs generation in the presence of Ocimum basicilicum (Jain and Mehata 2017; Pirtarighat et al. 2019). Due to the presence of the pharmaceutically active compound in O. bacilicum leave extracts—methyl chavicol, linalool, methyl cinnamate, methyleugenol, eugenol, and geraniol (Runyoro et al. 2010)—it shows the important medicinal properties like antimicrobial, anti-fungal, anti-inflammatory, anti-tumour, and anti-viral (Açıkgöz 2020). These active ingredients could be the capping agent in the presently reported study and thus could be hypothesized to give the synergistic impact of active ingredients of O. bacilicum as well as AgNPs for antibacterial and antibiofilm activities.
X-ray diffraction analysis of AgNPs
The crystallinity of AgNPs was confirmed by the X-ray diffraction analysis (Fig. 4) i.e. the lattice planes of the crystalline structure of AgNPs are obtained; the major broad peak obtained and the XRD pattern of AgNP obtained by the organic content of Tulsi leaves shows the crystalline nature. The sharp peaks at various 2θ values of 31.910, 45.870, 54.530, 57.210, and 76.470 corresponds to (200), (111), (222), and (311) were observed. The observed strong peaks indicate the crystalline nature of AgNPs, which is also clear evident from the earlier literature (Guirguis and Moselhey 2012; Ramteke et al. 2013). It can be noted that earlier Huang and team have also engineered the size range of 20–60 nm with spherical shape of silver and gold nanoparticles which act as a novel biocidal agent and 55–80 nm size of the spherical and triangular size of gold nanoparticle was synthesized by using different plant extracts e.g. (i) Cinnamomum camphora (Huang et al. 2007) (ii) lixivium of sundried Cinnamomum camphora leaf in tubular microreactors (Huang et al. 2008). In another study, researchers have used Aloe vera extract as a reducing agent for synthesizing spherical and triangular shape of silver nanoparticles (Chandran et al. 2006). Ghosh and co-workers have also reported that the biosynthesized Au core Ag shell nanoparticle using Dioscorea bulbifera which is a potential antibiofilm an antileishmanial agent (Ghosh et al. 2015). The observed peaks in the reported study confirm the AgNPs are crystalline in nature. The JCPDS records indicate that the AgNPs intense peak 2θ values are 38.2, 44.3, 62.8, 70.3. The size of the AgNP was calculated by the Scherrer Debby equation and the average calculated size was 22.4 nm.
X-ray diffraction patterns of biosynthesized OBET-AgNPs by O. basilicum L. extract. XRD indicates the lattice planes of crystalline structure of AgNPs and the size calculated is in the range of 22 nm. In Fig. 1 of XRD pattern is shown typically peaks at 38.1° and 77.2° corresponding to the (111) and (311) diffractions for face centered cubic (fcc) silver phase (JCPS 04-0783), that coexists with the cubic phase of AgCl at 27.9°, 32.3°, 46.3°, 55.0°, 57.6°, 67.6°, 74.6°, 76.9°, and 85.7° and that corresponds to the (111), (200), (220), (311), (222), (400), (420), and (422) planes (JCPDS file: 31-1238) of Ag/AgCl nanoparticles synthesis
Electron microscopic characterization of AgNP
The presence of nanoparticles could be observed in SEM, but few places agglomeration, as well as self-assembly in flower like structure, was also observed. The size range variation was observed in between 20 and 25 nm, and the same was also confirmed by the particle size distribution curve (Fig. 5) The HR-TEM images also confirms the crystalline form of spherical shaped AgNPs of the size distribution (Fig. 6); which are in good agreement with SEM micrographs and particle size distribution curve analysis and shown in (Fig. 5), though these nanoparticles underwent aggregation too It can be noted that other research groups have also similar observations using different plants e.g. (i) Terminalia chebula extract (Edison and Sethuraman 2012), (ii) Momordica charantia leaf (Ajitha et al. 2015), (iii) Actinidia deliciosa (Naraginti and Li 2017), (iv) Acacia nilotica (Arya et al. 2018), (v) Dillenia indica fruit extract (Swargiary et al. 2019) (vi) Urtica dioica (Linn.) (Jyoti et al. 2016) (vii) geranium leaf extracts (Pelargonium graveolens) (Bharathi et al. 2018).
SEM images of biosynthesized AgNPS at different magnification (a–c) and particles size distribution curve analysis of AgNPs (d). SEM confirmed the nanosized morphology of biosynthesized AgNP, but few places agglomeration as well as self-assembly in flower-like structure was also observed. The size range variation was observed between 20 and 25 nm
Antimicrobial activity of AgNPs
The antimicrobial activity of AgNPs against Gram positive and Gram-negative bacteria is illustrated in Fig. 7. The herb Ocimum basilicum (which is well explored potent antimicrobial agent for controlling clinical pathogens) been used in this study for the biological synthesis the AgNP and the synergistic effect of the capping agent (from plant extract) as well as AgNP is showing improved zone of inhibition. For the treatment of 100 mg/mL AgNPs, the zone of inhibition was observed maximum against Gram positive Bacillus subtilis (12 mm) followed by Staphylococcus aureus (9 mm), Gram-negative E. coli (15 mm), Enterobacter sp. (10 mm), and Pseudomonous aureginosa (15 mm). Earlier reports also indicated that the euginol compound present in the Ocimum family of herbal plants is the factor behind the enhanced antibacterial activity against the clinical pathogen (Prakash and Gupta 2005; Kousik and Baldev 2012). Additionally, it has also been reported that AgNPs also have improved antibacterial activity against clinical pathogen. Few scientific reports hypothesized that the metal nanoparticles are having an increased surface to volume ratio and ability to bind easily with sulphur and phosphorus-containing groups and thus enhances the antibacterial and antibiofilm activities. The size, orientation, and physical properties of nanoparticles have reportedly been shown to change the performance of any material (Bose and Chatterjee 2016).
Antibiofilm activity of AgNPs
Herbal plant-based silver nanoparticles are not only preventing bacterial infection also acts as an antibiofilm agent (Liu et al. 2014). The bacteria formed colony on the medical devices via bacterial quorum sensing and to promote the biofilm development on the surface of urinary catheters, bone joints, heart valves, dental implants, prostheses, contact lenses, and endotracheal tubes (Khatoon et al. 2018). Different methods are available for controlling biofilm formation in medical devices as well as in food industries e.g. membrane potential disruption of biofilm–embedded cells, interruption of quorum sensing systems, controlling of EPS production, inhibition of alarmone system, gene regulation control for biofilm formation and transport of binding protein (Yasir et al. 2018).
The biofilm inhibition efficiency of AgNPs were evaluated by crystal violet assay and SEM microscopic images. The interaction between AgNPs and the bacterial cell membrane was confirmed by the SEM. The capping agents from O. bacillicum on the surface of biosynthesized AgNPs further get attached to the bacterial cell wall and alter the membrane function and prevent the bacterial respiration which ultimately lead to bactericidal activity. The role of O. bacillicum active compounds on antibiofilm been studied earlier by Grayer et al. (1996). It is also reported earlier that AgNP interacts with bacterial DNA and ROS generation which leads to prevent the bacterial replications and ultimately to bactericidal activity (Dasgupta and Ramalingam 2016). Based on these studies it can be stated that, biosynthesized AgNP could potentially show the synergistic impacts of its own as well as capping agents (Pirtarighat et al. 2019).
In this study, we analysed the antibiofilm efficiency of silver nanoparticles synthesized from traditional medicinal plant O. bacilicum leaf extract (Fig. 8a) which shows its efficient antiobiofilm activity for the Pseudomonas aureginosa rod-shaped bacteria interconnected with overspread clumping of biofilm. The Fig. 8 shows the efficiency of AgNPs for inhibiting the biofilm formation up to 92.7% for 250 µg/mL of AgNPs, which means that the biofilm attachment of 250 µg/mL of AgNPs shows only 7.3%. Figure 9a indicates the biofilm attachment and biofilm inhibition studies. The synthesized silver nanoparticles decrease the growth of Pseudomonas aureginosa, drastically and prevent the quorum-sensing signals and thus prevent the biofilm formations (Hammer and Bassler 2003).
Recently the herbal plant-derived metal nanoparticles were studied for the uropathogens E. Coli, Pseudomonas sp. and Klebsiella sp. The AgNPs are effectively interacting to the membrane and inhibit the normal growth to drastically reduce the number of colonies as well as the antibiofilm formation (Kannan et al. 2019). The bimetallic nanoparticles, Au core Ag shell NPs showed highest biofilm inhibition up to 83.68 ± 0.09% against A. baumannii followed by P. aeruginosa, E. coli, and S. aureus, 18.93 ± 1.94%, 22.33 ± 0.56%, and 30.70 ± 1.33%, respectively (Ghosh et al. 2015). Hamed et al. (2020) also reported the efficient antibiofilm activity of biologically synthesized AgNPs (the culture filtrate extracts, including the bacterial supernatants and cell filtrate of the two actinomycete strains, were used as the reducing agent for the biosynthesis) have shown potential efficacy as a biocidal agent, antibiofilm agent.
Conclusion
The eco-friendly, nontoxic, and medicinal plant O. bacilicum are used as a reducing agent for synthesizing AgNPs at room temperature. The secondary metabolite present in the selected herbal plants is responsible for the reduction of monodispersed biologically synthesized AgNP of 20–25 nm size range along with organic capping agent has been obtained. The morphological analysis clearly indicated the controlled sizes of nanoparticles were generated during their reduction process. An enhanced antibacterial and antibiofilm activity of AgNPs was demonstrated and the synthesized AgNPs could have the future application for the urgent needs for controlling the pathogen survival in the clinical and food processing industries. From the antimicrobial and the antibiofilm studies it can be concluded that the synthesized AgNPs are effective against the Gram positive and Gram-negative pathogens. The future application of thus fabricated AgNPs could be in antimicrobial therapy and many other applications for biomedical and food industries. Biofilms are causing major disadvantages in clinically, especially catheters when the pathogens are deposited on the surface of biomedical equipment easily and form bacterial complex easily. This work could be further prototyped as a potential coating to prevent the surface of biomedical instruments and equipment/tools from the food sector.
References
Açıkgöz MA (2020) Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2020.112278
Ahmed RH, Mustafa DE (2020) Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int Nano Lett 10:1–14. https://doi.org/10.1007/s40089-019-00291-9
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28
Ajitha B, Reddy YAK, Reddy PS (2015) Biosynthesis of silver nanoparticles using Momordica charantia leaf broth: evaluation of their innate antimicrobial and catalytic activities. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2015.02.017
Arya G, Kumari RM, Sharma N et al (2018) Evaluation of antibiofilm and catalytic activity of biogenic silver nanoparticles synthesized from Acacia nilotica leaf extract. Adv Nat Sci Nanosci Nanotechnol. https://doi.org/10.1088/2043-6254/aae989
Azam M, Irshad S (2016) Phytochemical screening and antibacterial activities of essential oil, ethanolic and methanolic extracts of Ocimum basilicum L. Pak J Biochem Mol Biol 49:36–39
Balaji S, Mandal BK, Ranjan S et al (2017) Nano-zirconia—evaluation of its antioxidant and anticancer activity. J Photochem Photobiol B Biol 170:125–133. https://doi.org/10.1016/j.jphotobiol.2017.04.004
Behravan M, Panahi AH, Naghizadeh A et al (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154. https://doi.org/10.1016/j.ijbiomac.2018.11.101
Bharathi D, Josebin MD, Vasantharaj S, Bhuvaneshwari V (2018) Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J Nanostructure Chem. https://doi.org/10.1007/s40097-018-0256-7
Bharde AA, Parikh RY, Baidakova M et al (2008) Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir. https://doi.org/10.1021/la704019p
Bose D, Chatterjee S (2016) Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl Nanosci. https://doi.org/10.1007/s13204-015-0496-5
Chandran SP, Chaudhary M, Pasricha R et al (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. https://doi.org/10.1021/bp0501423
Dambolena JS, Zunino MP, López AG et al (2010) Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effects on growth and fumonisin production by Fusarium verticillioides. Innov Food Sci Emerg Technol 11:410–414. https://doi.org/10.1016/j.ifset.2009.08.005
Dasgupta N, Ramalingam C (2016) Silver nanoparticle antimicrobial activity explained by membrane rupture and reactive oxygen generation. Environ Chem Lett 14:477–485. https://doi.org/10.1007/s10311-016-0583-1
Dasgupta N, Ranjan S, Rajendran B et al (2016a) Thermal co-reduction approach to vary size of silver nanoparticle: its microbial and cellular toxicology. Environ Sci Pollut Res Int 23:4149–4163. https://doi.org/10.1007/s11356-015-4570-z
Dos Santos CA, Seckler MM, Ingle AP et al (2014) Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci 103:1931–1944. https://doi.org/10.1002/jps.24001
Edison TJI, Sethuraman MG (2012) Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. https://doi.org/10.1016/j.procbio.2012.04.025
Ghosh S, Jagtap S, More P et al (2015) Dioscorea bulbifera mediated synthesis of novel AucoreAgshell nanoparticles with potent antibiofilm and antileishmanial activity. J Nanomater. https://doi.org/10.1155/2015/562938
Grayer RJ, Kite GC, Goldstone FJ et al (1996) Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry. https://doi.org/10.1016/S0031-9422(96)00429-3
Guirguis OW, Moselhey MTH (2012) Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat Sci. https://doi.org/10.4236/ns.2012.41009
Hamed AA, Kabary H, Khedr M, Emam AN (2020) Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Adv. https://doi.org/10.1039/c9ra11021f
Hammer BK, Bassler BL (2003) Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50:101–104. https://doi.org/10.1046/j.1365-2958.2003.03688.x
Huang H, Yang X (2004) Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr Res. https://doi.org/10.1016/j.carres.2004.08.005
Huang J, Li Q, Sun D et al (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:105104. https://doi.org/10.1088/0957-4484/18/10/105104
Huang J, Lin L, Li Q et al (2008) Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried Cinnamomum camphora leaf in tubular microreactors. Ind Eng Chem Res. https://doi.org/10.1021/ie701698e
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385
Ishwarya R, Vaseeharan B, Kalyani S et al (2018) Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2017.11.006
Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. https://doi.org/10.1038/s41598-017-15724-8
Jayapriya E, Lalitha P (2013) Synthesis of silver nanoparticles using leaf aqueous extract of Ocimum basilicum (L.). Int J ChemTech Res 5:2985–2992
Jyoti K, Baunthiyal M, Singh A (2016) Characterization of silver nanoparticles synthesized using Urtica dioica Linn leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. https://doi.org/10.1016/j.jrras.2015.10.002
Kannan S, Sathasivam G, Marudhamuthu M (2019) Decrease of growth, biofilm and secreted virulence in opportunistic nosocomial Pseudomonas aeruginosa ATCC 25619 by glycyrrhetinic acid. Microb Pathog. https://doi.org/10.1016/j.micpath.2018.11.026
Kaya I, Yigit N, Benli M (2008) Antimicrobial activity of various extracts of Ocimum basilicum L. and observation of the inhibition effect on bacterial cells by use of scanning electron microscopy. African J Tradit Complement Altern Med 5:363–369. https://doi.org/10.4314/ajtcam.v5i4.31291
Khatoon Z, McTiernan CD, Suuronen EJ et al (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Kousik DM, Baldev K (2012) A review on therapeutic uses of Ocimum sanctum Linn (Tulsi) with its pharmacological actions. Int J Res Ayurveda Pharm 3:645–647
Kumar A, Nair AGC, Reddy AVR, Garg AN (2006) Availability of essential elements in bhasmas: analysis of ayurvedic metallic preparations by INAA. J Radioanal Nucl Chem 270:173–180. https://doi.org/10.1007/s10967-006-0326-z
Kumar R, Aadil KR, Shivendu R, Kumar VB (2020) Advances of nanotechnology and nanomaterials based strategies for neural tissue engineering. J Drug Deliv Sci Technol 57:101617. https://doi.org/10.1016/j.jddst.2020.101617
Liu X, Tang B, Gu Q, Yu X (2014) Elimination of the formation of biofilm in industrial pipes using enzyme cleaning technique. MethodsX 1:130–136. https://doi.org/10.1016/j.mex.2014.08.008
Malapermal V, Botha I, Krishna SBN, Mbatha JN (2017) Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci 24:1294–1305. https://doi.org/10.1016/j.sjbs.2015.06.026
Mladenova B, Diankov S, Karsheva M et al (2018) Plant mediated synthesis of silver nanoparticles using extracts from Tilia cordata, Matricaria chamomilla, Calendula officinalis and Lavandula angustifolia flowers. J Chem Technol Metall 53:623–630
Mukkavalli S, Chalivendra V, Singh BR (2017) Physico-chemical analysis of herbally prepared silver nanoparticles and its potential as a drug bioenhancer. OpenNano 2:19–27. https://doi.org/10.1016/j.onano.2017.01.001
Naraginti S, Li Y (2017) Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2017.03.023
Pal G, Rai P, Pandey A (2019) Green synthesis of nanoparticles a greener approach for a cleaner future. In: Green synthesis, characterization and applications of nanoparticles. Elsevier, Amsterdam
Panácek A, Smékalová M, Kilianová M et al (2016) Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 21:E26. https://doi.org/10.3390/molecules21010026
Paulkumar K, Gnanajobitha G, Vanaja M et al (2014) Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J. https://doi.org/10.1155/2014/829894
Pirtarighat S, Ghannadnia M, Baghshahi S (2019) Biosynthesis of silver nanoparticles using Ocimum basilicum cultured under controlled conditions for bactericidal application. Mater Sci Eng C 98:250–255. https://doi.org/10.1016/j.msec.2018.12.090
Prabu K, Rajasekaran A, Bharathi D, Ramalakshmi S (2019) Anti-oxidant activity, phytochemical screening and HPLC profile of rare endemic Cordia diffusa. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2018.04.025
Prakash P, Gupta N (2005) Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 49:125–131
Ramteke C, Chakrabarti T, Sarangi BK, Pandey RA (2013) Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem. https://doi.org/10.1155/2013/278925
Romeilah RM, Fayed SA, Mahmoud GI (2010) Chemical compositions, antiviral and antioxidant activities of seven essential oils. J Appl Sci Res 6:50–62
Roy N, Gaur A, Jain A et al (2013) Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environ Toxicol Pharmacol 36:807–812. https://doi.org/10.1016/j.etap.2013.07.005
Runyoro D, Ngassapa O, Vagionas K et al (2010) Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chem. https://doi.org/10.1016/j.foodchem.2009.06.028
Selvam P, Vijayakumar T, Wadhwani A, Muthulakshmi L (2019) Bioreduction of silver nanoparticles from aerial parts of Euphorbia hirta L. (EH-ET) and its potent anticancer activities against neuroblastoma cell lines. Indian J Biochem Biophys 56:132–136
Shah M, Fawcett D, Sharma S et al (2015) Green synthesis of metallic nanoparticles via biological entities. Materials (Basel) 8:7278–7308. https://doi.org/10.3390/ma8115377
Sharma R, Prajapati PK (2016) Nanotechnology in medicine: leads from ayurveda. J Pharm Bioallied Sci 8:80–81. https://doi.org/10.4103/0975-7406.171730
Sharma K, Guleria S, Razdan VK (2020) Green synthesis of silver nanoparticles using Ocimum gratissimum leaf extract: characterization, antimicrobial activity and toxicity analysis. J Plant Biochem Biotechnol 29:213–224. https://doi.org/10.1007/s13562-019-00522-2
Singh A, Shukla R, Hassan S et al (2011) Cytotoxicity and cellular internalization studies of biogenic gold nanotriangles in animal cell lines. Int J Green Nanotechnol Biomed. https://doi.org/10.1080/19430892.2011.633479
Singhal G, Bhavesh R, Kasariya K et al (2011) Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanoparticle Res. https://doi.org/10.1007/s11051-010-0193-y
Sinha S, Raghuwanshi R (2016) Phytochemical screening and antioxidant potential of Eclipta prostrata (L.) L-A valuable herb. Int J Pharm Pharm Sci 8:255–260
Sivaranjani K, Meenakshisundaram M (2013) Biological synthesis of silver nanoparticles using Ocimum basillicum leaf extract and their antimicrobial activity. Int Res J Pharm 4:225–229
Swargiary M, Mitra A, Halder D, Kumar S (2019) Fruit extract capped colloidal silver nanoparticles and their application in the reduction of methylene blue dye. Biocatal Biotransformation. https://doi.org/10.1080/10242422.2018.1508283
Tammina SK, Mandal BK, Ranjan S, Dasgupta N (2017) Cytotoxicity study of Piper nigrum seed mediated synthesized SnO2 nanoparticles towards colorectal (HCT116) and lung cancer (A549) cell lines. J Photochem Photobiol B Biol 166:158–168. https://doi.org/10.1016/j.jphotobiol.2016.11.017
Vijayakumar S, Divya M, Vaseeharan B et al (2020a) Biological compound capping of silver nanoparticle with the seed extracts of black cumin (Nigella sativa): a potential antibacterial, antidiabetic, anti-inflammatory, and antioxidant. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01713-4
Vijayakumar S, Divya M, Vaseeharan B et al (2020b) Biogenic preparation and characterization of ZnO nanoparticles from natural polysaccharide Azadirachta indica L. (neem gum) and its clinical implications. J Clust Sci. https://doi.org/10.1007/s10876-020-01863-y
Yasir M, Willcox MDP, Dutta D (2018) Action of antimicrobial peptides against bacterial biofilms. Materials (Basel) 11:2468. https://doi.org/10.3390/ma11122468
Zou W, Sastry M, Gooding JJ et al (2020) Recent advances and a roadmap to wearable UV sensor technologies. Adv Mater Technol. https://doi.org/10.1002/admt.201901036
Acknowledgements
Lakshmanan Muthulakshmi acknowledges the Department of Science and Technology–Science and Engineering Research Board (PDF/2017/001574), Government of India, for the award of a National Postdoctoral Fellowship. The authors acknowledge the financial assistance of DST-PURSE –MKU for the SEM facility. SR and ND acknowledge the University of Johannesburg, South Africa to provide the research facilities.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally in study design, experiments, and manuscript writing.
Corresponding authors
Ethics declarations
Ethical statements
All the ethical guidelines have been strictly followed, as required. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Muthulakshmi, L., Vijayakumar, T., Selvam, P. et al. Strong and nonspecific synergistic antibacterial/antibiofilm impact of nano-silver biosynthesized and decorated with active ingredients of Oscimum basilicum L.. 3 Biotech 11, 153 (2021). https://doi.org/10.1007/s13205-021-02687-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02687-x