Abstract
Circular RNA (circRNA) has been well studied in many diseases, whereas their role in patients with postoperative cognitive dysfunction (POCD) remains largely unclear. Here, we investigated the therapeutic effects of dexmedetomidine (Dex) on POCD and analyzed the role of circRNA as well as the pathways that may be involved. The Morris water maze test demonstrated that POCD rats have a longer incubation period than the normal group, but the latency of POCD rats was significantly lower after Dex treatment. Moreover, HE staining showed that Dex improved hippocampal pathological changes. RNA sequencing showed 164 differentially expressed circRNAs between POCD and Dex groups; 74 were upregulated and 90 were downregulated in the Dex group. A total of 20,790 target genes for differentially expressed circRNAs were observed in RNAhybrid and Miranda databases. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses showed that the target genes of differentially expressed circRNAs are mainly focused on positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage, negative regulation of cell adhesion mediated by integrin, and response to cytokines and other function of life activities and involved in the P53 signaling pathway and nuclear factor kappa B (NF-κB) signaling pathway. Furthermore, the expression of five candidate circRNAs (circ-Shank3, circ-Cdc42bpa, circ-chrx-24658, cir-chr17-3642 and circ-Sgsm1) and target genes were consistent with the RNA sequencing results, which was verified by quantitative real-time polymerase chain reaction (qRT-PCR). These results indicate that circ-Shank3 participate in the process of Dex improved POCD through regulating the P53 and NF-κB signaling pathways and may potentially facilitate POCD treatment through the development of clinical drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD) is a degenerative neurological disorder, especially common in the elderly population (Millan et al. 2012). POCD symptoms are similar to those of Alzheimer’s disease (AD), and include persistent impairment of memory, language retrieval or comprehension, attention focusing, abstract thinking and executive function (Fodale et al. 2010; Li et al. 2019). Approximate 12–26% of patients over 60 years of age have been affected by POCD after surgery with anesthesia (Goettel et al. 2017; Glumac et al. 2018). The pathological mechanisms of POCD involve a complex process caused by various factors, including multiple cells and molecules, which are mainly related to the pathogenesis of inflammatory responses in the central nervous system, oxidative stress, free radical damage, and metabolic disorder of neuroprotective factors (Zhang et al. 2015b). The major predisposing factors of POCD include advanced age combined with hypertension, coronary heart disease, and other chronic diseases, as well as exposure to anesthetics and length of surgery (Shoair et al. 2015). Currently, the treatment methods for POCD include electroacupuncture, used to strengthen cognitive function by activating the a7-nicotinic acetylcholine receptor and suppressing neuroinflammation (Liu et al. 2017). MiR-410-3p is induced and thereby exhibits a neuroprotective effect on POCD by targeting the CXCR5 via the PI3K/Akt signaling pathway. This has shown some improvement in POCD along with some undesired effects.
Dexmedetomidine (Dex) is an intravenous central sympatholytic drug and an effective alpha-2 (α2) adrenergic receptor agonist approved for conscious sedation in the intensive care unit (Kontak et al. 2013). Within the central nervous system, Dex induces an α2 adrenoceptor blockade of sympathetic nerve activity (SNA) in multiple tissues and vascular beds (Marcus et al. 2004). Dex might also be useful in the setting of cocaine overdose, as low doses of Dex can eliminate cocaine-induced increases in skin SNA, mean arterial pressure (MAP), and heart rate (HR) (Menon et al. 2007). However, the clinical applicability of the data was limited by exclusively studying cocaine-naïve subjects receiving a low-dose cocaine (2 mg/kg) challenge, which is much lower than doses typically used by cocaine addicts (Vongpatanasin et al. 1999). The use of Dex in other studies have been described as follows: Dex in conjunction with remifentanil may be a safe option that provides excellent patient satisfaction while potentially attenuating postprocedural pain (Park et al. 2019); Dex as an adjunct in thoracic paravertebral blockade (TPVB) in a prior study provided effective pain relief and significantly reduced opioid requirement in video-assisted thoracic surgery (VATS) (Hong et al. 2019). A study described the impact of Dex on long-term outcomes after non-cardiac surgery in the elderly: a 3-year follow-up of a randomized controlled trial (Riquelme et al. 2016). Perioperative infusion of Dex effectively reduced both the incidence and severity of acute kidney injury (AKI) and improved outcomes in patients undergoing valvular heart surgery without untoward hemodynamic side effects (Cho et al. 2016).
Circular RNAs (circRNAs), a novel class of widespread and diverse endogenous non-coding RNAs generated from non-canonical back-splicing events, have emerged to play key roles in many biological processes (Fu et al. 2019; Chen et al. 2019b). CircRNAs, generated by a back-splicing event of one or two exons, are emerging as a large class with specific expression patterns (Aufiero et al. 2019). CircRNAs have been characterized in various cell lines and brain tissues and found to participate in multiple biological processes implicated in the pathogenesis of various diseases, including diabetes and many vascular diseases (Jin et al. 2019; Xie et al. 2019). Moreover, circRNAs were reported to be involved in the neuromodulation system (Xie et al. 2019). Recent studies discovered that most circRNAs are involved in sequestration of proteins or microRNAs, transcriptional regulation, interference with splicing, and translation to produce polypeptides at the molecular level (Li et al. 2018a; Wilusz 2018). Additionally, circRNA is less abundant in sporadic Alzheimer’s disease CA1 hippocampal regions relative to age-matched controls (Lukiw 2013). However, the mechanisms by which circRNAs work in the regulation and development of POCD remain underexplored.
In the current study, the POCD rat model was established and treated with Dex. The underlying mechanism of Dex regulating POCD was investigated by circRNA sequencing between Dex and POCD groups.
Materials and methods
Animals
This study was approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Nanchang University. All animal experiments were performed in accordance with the Guides for the Care and Use of Laboratory Animals. Sprague–Dawley (SD) rats (male, 18 months of age) were purchased from Chengdu Dashuo Laboratory Animal Co., Ltd., China. The animals were housed at room temperature (22 ± 1 °C) with a 12 h light–dark cycle and access to food and water freely accessible. All patients fasted for 6 h prior to the preoperative.
Model construct and drug treatment
A splenectomy was performed to establish a POCD model in aged rats of POCD and Dex groups. A total of 16 male SD rats were assigned into four in vivo model groups (n = 4 rats per group): (A) control; (B) sham operation; (C) POCD and (D) Dex. Rats of the control group were intraperitoneally injected (IP) with 2 ml of normal saline 30 min before surgery; Sham operation group rats received surgery without splenectomy, and IP with 2 ml normal saline 30 min before surgery and 2% sodium pentobarbital (50 mg/kg) during the operation. The rats of the POCD group were IP with 2 ml normal saline 30 min before surgery, 2% sodium pentobarbital during the operation, and received a splenectomy under anesthesia. We disinfected the skin with iodophors at the surgical incision and made a small transverse incision along the lower edge of the left rib about 1 cm. The length of the incision was about 2 cm. The subcutaneous tissue was bluntly separated layer by layer, and then the abdominal cavity entered to freely ligate the spleen-related blood vessels. The splenectomy was performed, and the abdomen was sutured without bleeding. The wound was disinfected with iodophor, and the operation was ensured aseptic throughout the procedure. The Dex group received IP Dex (20 μg/kg) 30 min before surgery in addition to the same splenectomy procedure as the POCD group. The Morris water maze test was performed on the first, third, seventh, and fourteenth days following after surgery.
Morris water maze test
The identification software of the Morris water maze (XR-XM101, Shanghai New Soft Information Technology Co., Ltd.) was filled with water and temperature controlled at 25 ± 1 °C. The diameter of the pool was 1.6 m and the height was 40 cm. The sample size for these experiments was defined in 4 animals in each model. A platform was hidden 1 cm below the water surface. We brought the animals into the laboratory and familiarized them with the environment before the experiment. During the procedure, the curtains were closed around the maze to reduce the possibility of distal cues. In the first stage (positioning navigation), rats were placed in the four-quadrant water maze in turn against the wall (all rats needed to switch to the next quadrant when they were done). If the animal boarded the platform area within 90 s, the recording ended. If the animal did not board the platform area within 90 s, the stick was used to guide the rat to the platform and kept there for 30 s. Each rat was trained 4 times a day for 5 days. The interval between trials could vary by 2 min. The starting platform was 10 × 10 cm and the reduced platform was 5 × 5 cm. In the second stage (space exploration), all the first to fourth quadrants had been completed, the platform of water maze was removed, and the rats were placed in the same quadrant against the wall to observe the action track of the animals for 90 s.
Hematoxylin and eosin staining
Hematoxylin and eosin (HE) staining was used to detect the pathological changes of the rats’ hippocampal neurons. The hippocampal neuron tissues were fixed in 10% formaldehyde (China national Pharmaceutical Group, Shanghai) solution for 48 h and were washed with running water; the ethanol of different concentrations was dehydrated. The tissue was then immersed in wax, embedded in paraffin (China national Pharmaceutical Group, Shanghai), and sliced to a thickness of 4–7 μm. The slides were placed in a 65 °C constant temperature oven for 30 min, xylene I for 15 min and then xylene II (China national Pharmaceutical Group, Shanghai) for 15 min. The dewaxed sections were soaked in ethanol of different concentrations for 5 min and rinsed with tap water for 10 min. The sections were then stained with HE (BASO), respectively. The cell nucleus was stained with hematoxylin (BASO) for 5 min, and the cytoplasm was stained using eosin (BASO) for 1–2 min. The stained sections were dehydrated with pure alcohol and examined under a light microscope (ECLIPSE Ni, Nikon USA).
Western blot
Total protein was extracted, and the concentration of the protein sample was detected by a bicinchoninic acid (BCA) kit (Pierce Biotechnology, Inc., Rockford, IL, USA). After the sample buffer was added, the samples were boiled at 95 °C for 10 min. Then, the proteins (30 μg /well) were separated with sodium dodecyl sulfate--polyacrylamide gels (SDS-PAGE). After electrophoresis, the protein was transferred onto a polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). After blocking with 5% skim milk, the membrane was washed three times for 10 min followed by overnight incubation at 4 °C with specific primary antibodies (1:2000; bs-18047R, Bioss), and GAPDH (1:1000; 60004–1-Lg, Proteintech) diluted in the blocking buffer. After three 10 min washes with TBST, blots were incubated by goat anti-rabbit IgG-HRP for 1 h. GAPDH was used as an internal control. Proteins of interest were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, MA) under a Chemi Doc MP system (Bio-Rad, Hercules, CA, USA). ImageJ software (version 1.8.0-112; National Institutes of Health) was used to calculate the intensity of the search bands.
RNA sequencing
The hippocampal tissue of three POCD rats and three Dex rats was detected by RNA sequencing. Total RNA was initially extracted using Trizol reagent (Invitrogen, USA). After measurement with RNA NanoDrop, we characterized circRNA transcripts using RNA sequencing (RNA-seq) analysis of ribosomal RNA depleted. CircRNA molecules can be specifically enriched for sequencing; a sequencing library was constructed using an RNA library construction kit (NEB, USA). The operation steps are as follows: 3′ and 5′ adaptors were sequentially attached to the total RNA. A mix of ten different calibrator oligoribonucleotides with known sequences and concentrations was added in the 3′ ligation step as an internal parameter. The Agilent 2100 (Agilent Technologies) evaluated the quality of RNA library construction. Subsequently, the library was sequenced by Illumina Hiseq 2000 (Illumina, USA).
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated from the hippocampus using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA of 1 μg was reverse transcribed to cDNA via reverse transcription kit (Thermo #K1622). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in accordance with the manufacturer’s protocol, using the 2 × Master Mix (Roche) and examined by ABI QuantStudio 6 Flex (Applied Biosystems Inc., USA). The levels of target genes were calculated based on the cycle threshold (Ct) values compared to a reference gene using the formula 2−ΔΔCt. GAPDH mRNA was used as references for mRNA and linear cricRNA, respectively. The detailed primer information is shown in Table 1.
Bioinformatic analysis
The raw sequencing data were analyzed by FAST-QC. Firstly, circRNA expression was obtained through interrelated clean reading and the circRbase database. The amplified sequences, compared to circRNA mintbase databases, and the corresponding suggestions were stated. The differentially expressed circRNAs that were obtained by analyzing the expression of circRNA from POCD and Dex rat hippocampal tissues were restricted to the following criteria: |log2FC|> 2 and P-value less than 0.05 (|log2FC|> 2, P < 0.05) and the false discovery rate (FDR) threshold (FDR < 0.05). Target genes of differential circRNAs were predicted by Miranda and RNA hybrid. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of target genes were used to initially analyze the functions of circRNA with a different expression. A circRNA-miRNA-mRNA ceRNA network was constructed, using Cytoscape 3.7.2 software.
Statistical analysis
The results were presented as the means ± standard deviation of at least three independent experiments. Comparisons were performed using Student's t-test as indicated between two groups. One-way ANOVA with post hoc Tukey test was used for multiple comparisons. The GraphPad Prism 8.0 software (San Diego, CA, USA) was used for graphing. P˂0.05 was considered to represent a statistically significant difference.
Results
Dex improves cognitive ability and pathology of POCD rats
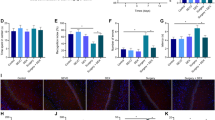
HE staining was used to examine pathological changes in hippocampal neurons. The results showed that the neurons were clear in the hippocampus of normal and sham-operated rats, and the nucleus was large and round, located in the center of the cell. The hippocampal neuron of the POCD model group initially showed nuclear condensation, vacuolation, and inflammatory cell infiltration. Dex treatment was shown to significantly improve nuclear condensation and vacuolation in hippocampal neurons (Fig. 1a). We further explored the treatment effect of Dex to POCD rats. The old rats in these four categories studied received splenectomies, and Dex was injected intraperitoneally to verify the therapeutic effect. We found that splenectomy successfully induced cognitive dysfunction in the rats and prolonged arrival time to the platform. The Morris water maze test indicated that Dex can reduce the latency of POCD rats and reduce platform crossover, suggesting that Dex anesthesia improved cognitive dysfunction in POCD rats (Fig. 1b). Moreover, the escape latency of the POCD model group and the Dex treatment group was higher than that of the control group and the sham operation group on day 1. On day 3, the latency of the Dex treatment group was significantly shortened. On day 7 and day 14, the latency of the POCD model group was significantly higher than that of the control group and the sham operation group. However, the latency of the Dex treatment group was significantly lower than that of the POCD model group (Fig. 1c).
Dexmedetomidine (Dex) attenuates neuronal damage in Postoperative cognitive dysfunction (POCD) rats induced by splenectomy. a Hematoxylin and eosin (HE) staining showed the pathological change of rat hippocampal tissue in the treatment group was improved. Note: The image-taking multiple is 200 times. b Morris water maze test analyzed the rat's crawling track, showing improved cognitive performance after Dex treatment. c The bar diagram shows the escape latency of rats in the control group, the sham operation group, the POCD group, and the Dex group. Note: Control, normal rats without surgery group; Sham, rats undergoing surgery without splenectomy; POCD, rats undergoing splenectomy under anesthesia; Dex, rats undergoing splenectomy under Dex and anesthesia. Representative HE stain of rat hippocampal tissues after intraperitoneal injection or control. Data are presented as mean standard deviation, n = 4/group. (*P < 0.05, **0.001 < P < 0.01.)
Reads filtering and mapping
Greater than 96% of clean reads were generated in each sample library. After filtering, on average, 91,981,830, 81,534,914, 94,301,368, 86,052,988, 86,734,256, and 84,936,714 clean reads were obtained for 3 samples each of Dex1, Dex2, Dex3, POCD1, POCD2, and POCD3, respectively, and more than 90% were mapped to the circRNA database (Table 2). These results suggested that the data of RNA sequencing were credible.
According to the results of RNA sequencing analysis, the largest number of simultaneous overlays of an exon was 1054 circRNAs, most circRNAs contained 1–5 exons. Except for circRNAs containing two exons, the number of exons in all circRNAs showed a decreasing trend. The more the exons contained, the smaller the number of circRNAs (Fig. S1A). Chromosome 1 was enriched with up to 740 circRNAs, and the chromosome y enriched circRNA was the least with 4. They were widely distributed in all chromosomes (Fig. S1B).
Differentially expressed circRNAs in rat hippocampal tissues of Dex group
To identify cricRNAs that are differentially regulated after a Dex treatment, we performed a differential expression analysis between the hippocampal tissue of the Dex group and the POCD group. A total of 164 differentially expressed cricRNAs between the Dex group and the POCD group were identified by RNA sequencing. Among the 164 differentially expressed circRNAs, 74 were upregulated and 90 were downregulated in the Dex group compared to the POCD group (Fig. 2a). Heatmaps of differentially expressed circRNAs in the comparison of Dex vs POCD suggested that the samples of Dex and POCD with the same cognitive stages were distinguished by clustering (Fig. 2b). Together, these results showed the different cricRNA expressions between POCD rats and Dex-anesthetized POCD rats, suggesting that Dex-anesthetized POCD rats can regulate unique circRNAs that may be associated with cognitive impairment.
Differential circular RNAs (circRNAs) expression analysis between Dex and POCD group of rat hippocampal tissue. a The volcano map describes the number, significance, and reliability of the differential expression of circRNAs between the Dex group and the POCD group. The abscissa is log2 (FC value) and the ordinate is −log10 (P-value). Each dot represents one circRNA, the red dots represent upregulated genes, and the green dots represent downregulated genes in the Dex group, while the gray dots represent genes that are not differentially expressed between two groups. b The clustering heatmap of differentially expressed circRNAs between Dex and POCD groups. Expression values are depicted in line with the color scale. The difference enhancement increased from green to red. Each column represents one sample, and each row indicates a transcript. Red represents upregulated genes; green represent downregulated genes
GO and KEGG analysis of the differentially expressed cricRNAs
To further elucidate the target genes of the differentially expressed cricRNAs, we employed Miranda and RNAhybrid analysis. The results showed a total of 20,790 target genes for differentially expressed cricRNAs were observed in both databases. Among them, 19,198 and 5947 target genes were observed in the RNAhybrid and Miranda databases, respectively. There were 4355 target genes differentially expressing cricRNAs overlapping in two databases, accounting for 20.95% of the total database (Fig. 3a). To understand the main functions of the differentially expressed circRNAs, GO analyses were performed. We revealed that these target genes were mainly focused on positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage, negative regulation of cell adhesion mediated by integrin, and response to cytokines and other life activities (Fig. 3b). Kyoto Encyclopedia for Genes and Genomes (KEGG) enrichment analysis revealed that the target gene was involved in the regulation of 140 signaling pathways, including 10 significantly enriched pathways, while 130 enriched pathways were not significant. CricRNAs target genes were significantly involved in the P53 signaling pathway, NF-kappa B (NF-κB) signaling pathway, leukocyte transendothelial migration, and other signaling pathways (Fig. 3c).
Target gene analysis of differentially expressed circRNAs. a Venn diagrams illustrating the target genes of 164 differentially expressed circRNAs between the RNAhybrid and Miranda databases. The 19,198 and 5947 target genes were observed in the RNAhybrid and Miranda databases, respectively. b The bubble chart displays Gene Ontology (GO) analysis of differentially expressed circRNAs in the Dex group and POCD group of rat hippocampal tissues. The change of bubbles from green to red means that the degree of gene function is increased, and the large bubbles indicated that the number of genes is enriched. c Column chart display significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of differentially expressed circRNAs between the Dex group and POCD group of rat hippocampal tissues. Red bars indicate significant enrichment (P < 0.05)
Validation of the key circRNA
To verify the RNA-seq results, we selected five candidate circRNAs with high differential expression multiples and high abundance. We validated these candidate circRNAs in the hippocampal tissue of POCD and Dex rats by qRT-PCR. The qRT-PCR results are shown in Fig. 4a. The expression of circ-Shank3 (P < 0.01), circ-Cdc42bpa (P < 0.001), and circ-chrx-24658 (P < 0.05) was significantly higher in the POCD group compared to the Dex group; circ-Shank3 had a larger difference multiple, compared with other circRNAs. Furthermore, cir-chr17-3642 and circ-Sgsm1 were non-significant in the Dex group compared to the POCD group (Fig. 4a). For the circ-Shank3, we confirmed that it was circular by divergent and convergent RT-PCR (Fig. 4b, top). Sequencing results were compared with circBase to find its splice junction site. We further analyzed circ-Shank3 by agarose gel electrophoresis (Fig. 4b, bottom).
Differential expression of circRNAs and target genes was verified. a qRT-PCR showed that the expressions of circ-shank3, circ-cdc42bpa, and circ-chrx-24658 in the hippocampal tissues of the Dex group were significantly lower than those in the POCD group. (*P < 0.05, ** 0.001 < P < 0.01, ***P < 0.001.) b The circular characteristic of circ-Shank3 was successfully verified by divergent and convergent primers. The top red arrow points to the splice junction. The bottom red arrow refers to divergent primer verification, and the bottom gray arrow refers to convergent primer verification. gDNA, genomic DNA; cDNA, complementary DNA
Verification of circ-shank3 target gene
To initially explore the regulatory mechanism of circ-Shank3, we constructed a circRNA-miRNA-mRNA ceRNA regulatory network of circ-Shank3. Circ-Shank3 may regulate target genes Icam1, Cdkn1a, C1qb, Lbp, etc., through miR-3552, miR-615, miR-666-3p, and miR-1956-5p. In addition, target genes of circ-Shank3 can be significantly enriched into the P53 signaling pathway, NF-κB signaling pathway, Leukocyte transendothelial migration, natural killer cell-mediated cytotoxicity, and other pathways (Fig. 5a). We also screened five differentially expressed target genes (C1qb, Fkbp5, Icam1, Lbp, Lrrc32) of circ-Shank3. qRT-PCR showed that Fkbp5 and Lrrc32 were significantly down-regulated in the Dex group, whereas the other target genes showed no significant difference (Fig. 5b).
Verification of the target gene of circ-shank3. a The networks of circRNAs and miRNA with the corresponding co-expression mRNAs in each comparison. Purple indicates the key circRNA, red indicates the associated miRNA, blue indicates the target gene mRNA, and green indicates the pathway in which the target gene is involved. b Histogram showed that differentially expressed target genes Fkbp5 and Lrrc32 of circ-Shank3 were significantly down-regulated in the Dex group. (** 0.001 < P < 0.01)
Discussion
POCD may cause serious mental disorders, anxiety, personality changes, and memory impairment (Wang et al. 2019). The first international POCD study reported that the incidence of POCD in patients aged 60 years or older was 25.8% and 9.9%, 1 week and 3 months following major non-cardiac surgery, respectively (Moller et al. 1998). Currently, no effective treatment for POCD has been reported. Westholm JO discovered that circRNAs are dominantly enriched in the nervous system and increase with age by genome-wide analysis of Drosophila circRNA (Westholm et al. 2014). However, the study of circRNA has been rarely reported in POCD. In this study, we found that Dex can improve cognitive impairment and inflammation of POCD rats. RNA-seq results revealed differentially expressed circRNAs between the Dex and POCD groups. The target genes of circ-Shank3 are mainly involved in inflammation-related pathways, such as the P53 signaling pathway and the NF-κB signaling pathway.
Dex is an anti-inflammatory and analgesic drug approved for use in severe clinical patients (Kontak et al. 2013). Xiong B suggested that Dex may improve cognitive functioning in aged rats by restraining relaxin-3 and c-fos expression to inhibited neural over-excitability (Xiong et al. 2016). Dex has also been identified in recent studies to alleviate the hippocampus neuronal apoptosis of young rats after isoflurane anesthesia, through the amelioration of the stress reaction and the release of cytokines to ameliorate the POCD in elderly patients (Sanders et al. 2009; Hofer et al. 2009); alleviate the neurotoxicity caused by isoflurane through inhibiting the TLR2/NF-κB signaling pathway (Pang et al. 2020); and attenuate pancreatic injury and inflammatory response of pancreatitis mice by inhibiting NLRP3 inflammasome (Li et al. 2018b). Zhang et al. (2015a) showed that Dex can down-regulate inflammatory factor levels in septic rats by inhibiting the NF-κB pathway in septic rats and suggested that Dex may be beneficial in the treatment of sepsis. However, the effect of Dex on the POCD rat model is still unclear. We believed that Dex can improve cognitive impairment and tissue inflammatory cell infiltration in POCD rats.
CircRNAs are involved in neuronal development, aging, and cardiovascular disease (Lei et al. 2018), and circRNA plays a significant role in the disease development of diabetes mellitus (Jin et al. 2019), AD (Idda et al. 2018) and cancer progression (Zhang et al. 2013). CircRNA is present in the hippocampal region of sporadic AD CA1 rather than age-matched controls (Lukiw 2013). Gao et al. (2019) revealed differentially expressed circRNAs using microarray assay, suggesting their potential involvement in POCD pathogenesis. Recently, studies have implied that dysregulation of circRNAs and other ncRNAs is involved in DEX-induced cytotoxicity in human osteoblasts (Zhu et al. 2019; Zhang et al. 2018). Nevertheless, circRNA’s role is still unclear in POCD. Therefore, we need to further identify cricRNA as a key regulatory factor in many mental illness processes and the function of cricRNAs in POCD. In this study, we identified 164 differentially expressed circRNAs induced by Dex, which mainly enriched the P53 and NF-κB signaling pathway. NF-κB is a major regulator of inflammation and immune responses (Kalogeris et al. 2012). Peng Kong suggested that circ-Sirt1 exerts synergistic anti-inflammatory effects by inhibiting the activation of NF-κB in vascular smooth muscle cells (Kong et al. 2019). CircLRP6 regulates hyper glucose-induced oxidative stress and inflammation in mesangial cells via sponging miR-205, activating TLR4/NF-κB pathway (Chen et al. 2019a). Additionally, P53 is a major tumor suppressor, whose diverse activities serve to inhibit neoplastic processes. Recent understanding of the p53 pathway reveals a broad interaction with inflammatory elements, such as cytokines, infectious agents, and major immune-regulatory pathways like NF-κB (Cooks et al. 2014). Dex induced changes in the expression of circRNA, and these differential circRNAs are mainly involved in the P53 signaling pathway and NF-κB signaling pathway, suggesting that Dex may improve POCD by mediating circRNA leading to regulation of P53 and NF-κB pathways.
Conclusion
In summary, Dex administration significantly improved cognitive impairment caused by splenectomy in a clinically relevant model of POCD. Bioinformatics analysis showed that the target genes of circRNA significantly enriched the P53 signaling pathway and the NF-κB signaling pathway. We presented a hypothesis, which will be verified in the future, that circ-Shank3 may regulate the P53 and NF-κB signaling pathways to participate in the process of Dex improving POCD by targeting Fkbp5 and Lrrc32.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aufiero S, Reckman YJ, Pinto YM, Creemers EE (2019) Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol 16(8):503–514. https://doi.org/10.1038/s41569-019-0185-2
Chen B, Li Y, Liu Y, Xu Z (2019a) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol 234:21249–21259
Chen L, Wang F, Bruggeman EC, Li C, Yao B (2019b) circMeta: a unified computational framework for genomic feature annotation and differential expression analysis of circular RNAs. Bioinform 36:539–545
Cho JS, Shim JK, Soh S, Kim MK, Kwak YL (2016) Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int 89(3):693–700. https://doi.org/10.1038/ki.2015.306
Cooks T, Harris CC, Oren M (2014) Caught in the cross fire: p53 in inflammation. Carcinogenesis 35(8):1680–1690. https://doi.org/10.1093/carcin/bgu134
Fodale V, Santamaria LB, Schifilliti D, Mandal PK (2010) Anaesthetics and postoperative cognitive dysfunction: a pathological mechanism mimicking Alzheimer's disease. Anaesthesia 65(4):388–395. https://doi.org/10.1111/j.1365-2044.2010.06244.x
Fu H-W, Lin X, Zhu Y-X, Lan X, Kuang Y, Wang Y-Z, Ke Z-G, Yuan T, Chen P (2019) Circ-IGF1R has pro-proliferative and anti-apoptotic effects in HCC by activating the PI3K/AKT pathway. Gene 716:144031
Gao R, Li M, Wang Q, Chen H, Yu H, Liu J, Zhu T, Chen C (2019) Identification of the potential key circrnas in elderly patients with postoperative cognitive dysfunction. http://www.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2019&index=10&absnum=1799
Glumac S, Kardum G, Karanovic N (2018) A Prospective cohort evaluation of the cortisol response to cardiac surgery with occurrence of early postoperative cognitive decline. Med Sci Monit 24:977–986. https://doi.org/10.12659/msm.908251
Goettel N, Burkhart CS, Rossi A, Cabella BC, Berres M, Monsch AU, Czosnyka M, Steiner LA (2017) Associations between impaired cerebral blood flow autoregulation, cerebral oxygenation, and biomarkers of brain injury and postoperative cognitive dysfunction in elderly patients after major non-cardiac surgery. Anesth Analg 124(3):934–942
Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, Graf BM, Bierhaus A, Weigand MA (2009) Central sympatholytics prolong survival in experimental sepsis. Crit Care 13(1):R11. https://doi.org/10.1186/cc7709
Hong B, Lim C, Kang H, Eom H, Kim Y, Cho HJ, Han W, Lee S, Chung W, Kim YH (2019) Thoracic Paravertebral Block with Adjuvant Dexmedetomidine in Video-Assisted Thoracoscopic Surgery: A Randomized, Double-Blind Study. J Clin Med. https://doi.org/10.3390/jcm8030352
Idda ML, Munk R, Abdelmohsen K, Gorospe M (2018) Noncoding RNAs in Alzheimer's disease. Wiley Interdiscip Rev RNA. https://doi.org/10.1002/wrna.1463
Jin G, Wang Q, Hu X, Li X, Pei X, Xu E, Li M (2019) Profiling and functional analysis of differentially expressed circular RNAs in high glucose-induced human umbilical vein endothelial cells. FEBS Open Biol 9(9):1640–1651. https://doi.org/10.1002/2211-5463.12709
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Kong P, Yu Y, Wang L, Dou Y-Q, Zhang X-H, Cui Y, Wang H-Y, Yong Y-T, Liu Y-B, Hu H-J (2019) circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res 47(7):3580–3593
Kontak AC, Victor RG, Vongpatanasin W (2013) Dexmedetomidine as a novel countermeasure for cocaine-induced central sympathoexcitation in cocaine-addicted humans. Hypertension 61(2):388–394
Lei K, Bai H, Wei Z, Xie C, Wang J, Li J, Chen Q (2018) The mechanism and function of circular RNAs in human diseases. Exp Cell Res 368(2):147–158. https://doi.org/10.1016/j.yexcr.2018.05.002
Li X, Yang L, Chen LL (2018a) The biogenesis, functions, and challenges of circular RNAs. Mol Cell 71(3):428–442. https://doi.org/10.1016/j.molcel.2018.06.034
Li Y, Pan Y, Gao L, Lu G, Zhang J, Xie X, Tong Z, Li B, Li G, Li W (2018b) Dexmedetomidine attenuates pancreatic injury and inflammatory response in mice with pancreatitis by possible reduction of NLRP3 activation and up-regulation of NET expression. Biochem Biophys Res Commun 495(4):2439–2447. https://doi.org/10.1016/j.bbrc.2017.12.090
Li PJ, Guo YQ, Ding PY, Liu RB, Deng F, Feng XX, Yan WJ (2019) Neuroprotective effects of a Smoothened receptor agonist against postoperative cognitive dysfunction by promoting autophagy in the dentate gyrus of aged rats. Neurol Res 41(10):867–874. https://doi.org/10.1080/01616412.2019.1628411
Liu P-R, Zhou Y, Zhang Y, Diao S (2017) Electroacupuncture alleviates surgery-induced cognitive dysfunction by increasing α7-nAChR expression and inhibiting inflammatory pathway in aged rats. Neurosci Lett 659:1–6
Lukiw WJ (2013) Circular RNA (circRNA) in Alzheimer's disease (AD). Front Genet 4:307. https://doi.org/10.3389/fgene.2013.00307
Marcus MT, Walker T, Swint JM, Smith BP, Brown C, Busen N, Edwards T, Liehr P, Taylor WC, Williams D, von Sternberg K (2004) Community-based participatory research to prevent substance abuse and HIV/AIDS in African-American adolescents. J Interprof Care 18(4):347–359. https://doi.org/10.1080/13561820400011776
Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li J-L, Victor RG, Vongpatanasin W (2007) Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am Coll Cardiol 50(7):626–633
Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joels M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11(2):141–168. https://doi.org/10.1038/nrd3628
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351(9106):857–861. https://doi.org/10.1016/s0140-6736(97)07382-0
Pang X, Zhang P, Zhou Y, Zhao J, Liu H (2020) Dexmedetomidine pretreatment attenuates isoflurane-induced neurotoxicity via inhibiting the TLR2/NF-κB signalling pathway in neonatal rats. Exp Mol Pathol 112:104328
Park J-H, Soh S, Kwak Y-L, Kim B, Choi S, Shim J-K (2019) Anesthetic efficacy of dexmedetomidine versus midazolam when combined with remifentanil for percutaneous transluminal angioplasty in patients with peripheral artery disease. J Clin Med 8(4):472
Riquelme JA, Westermeier F, Hall AR, Vicencio JM, Pedrozo Z, Ibacache M, Fuenzalida B, Sobrevia L, Davidson SM, Yellon DM, Sanchez G, Lavandero S (2016) Dexmedetomidine protects the heart against ischemia-reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol Res 103:318–327. https://doi.org/10.1016/j.phrs.2015.11.004
Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M (2009) Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 110(5):1077–1085. https://doi.org/10.1097/ALN.0b013e31819daedd
Shoair OA, Grasso Ii MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW (2015) Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major non-cardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol 31(1):30–36. https://doi.org/10.4103/0970-9185.150530
Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG (1999) Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation 100(5):497–502. https://doi.org/10.1161/01.cir.100.5.497
Wang M, Su P, Liu Y, Zhang X, Yan J, An X, Wang X, Gu S (2019) Abnormal expression of circRNA_089763 in the plasma exosomes of patients with post-operative cognitive dysfunction after coronary artery bypass grafting. Mol Med Rep 20(3):2549–2562
Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC (2014) Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 9(5):1966–1980
Wilusz JE (2018) A 360 view of circular RNAs: from biogenesis to functions. Wiley Interdiscip Rev RNA 9(4):e1478
Xie F, Zhao Y, Wang SD, Ma J, Wang X, Qian LJ (2019) Identification, characterization, and functional investigation of circular RNAs in subventricular zone of adult rat brain. J Cell Biochem 120(3):3428–3437. https://doi.org/10.1002/jcb.27614
Xiong B, Shi Q, Fang H (2016) Dexmedetomidine alleviates postoperative cognitive dysfunction by inhibiting neuron excitation in aged rats. Am J Transl Res 8(1):70–80
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL (2013) Circular intronic long noncoding RNAs. Mol Cell 51(6):792–806. https://doi.org/10.1016/j.molcel.2013.08.017
Zhang J, Wang Z, Wang Y, Zhou G, Li H (2015a) The effect of dexmedetomidine on inflammatory response of septic rats. BMC Anesthesiol 15:68. https://doi.org/10.1186/s12871-015-0042-8
Zhang QB, Xiao-Feng LI, Neurology DO (2015b) Research advances in the pathogenesis of postoperative cognitive dysfunction. Med Recapitul
Zhang X-Y, Shan H-J, Zhang P, She C, Zhou X-Z (2018) LncRNA EPIC1 protects human osteoblasts from dexamethasone-induced cell death. Biochem Biophys Res Commun 503(4):2255–2262
Zhu C-Y, Yao C, Zhu L-Q, She C, Zhou X-Z (2019) Dexamethasone-induced cytotoxicity in human osteoblasts is associated with circular RNA HIPK3 downregulation. Biochem Biophys Res Commun 516(3):645–652
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YH contributed to conceptualization and funding acquisition: YH. Data curation: CC and FD. CC and FD were involved in formal analysis. CC and FD were involved in investigation. CC and FD were involved in methodology.CC and FD contributed to writing—original draft. CC and FD contributed to writing— review and editing. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Nanchang University. All animal experiments were performed in accordance with the Guides for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2020_2163_MOESM1_ESM.tif
Supplementary file1 Figure S1. The expression pattern of circRNAs was examined by RNA sequencing during the Dex treatment of POCD rats. The results showed that the higher the number of exons covered, the fewer the circRNAs. (A) The statistical chart of exon number count covered by circRNAs. (B) The number of circRNAs distributed on each chromosome number. Among them, the number of circRNAs on chromosomes 1, 2, and 6 were all greater than 500.
Rights and permissions
About this article
Cite this article
Cao, C., Deng, F. & Hu, Y. Dexmedetomidine alleviates postoperative cognitive dysfunction through circular RNA in aged rats. 3 Biotech 10, 176 (2020). https://doi.org/10.1007/s13205-020-2163-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2163-0