Abstract
Although, the C2H2 zinc finger (ZF) family of plant transcription factors have been implicated in multiple biological processes, they are yet to be characterized in the economically important chilli pepper (Capsicum annuum). In this study, a total of 79 C2H2 ZF genes were identified in the pepper genome. Phylogenetic analysis categorized the pepper C2H2 ZF (CaZF) members into five subfamilies each with unique conserved domains and functions. Genomic organization revealed that CaZF genes have variable number of introns consistent with the characteristics defined by the evolutionary analysis. Segmental duplication-based purifying selection contributed to the expansion of CaZF genes in pepper. Additionally, 11 CaZF genes were identified as targets for 38 miRNAs indicating their role in post-transcriptional silencing-mediated genetic regulation. Gene expression analysis revealed that 18 CaZF genes were differentially expressed post-infection with the anthrocnose pathogen Colletotrichum truncatum, uncovering their potential function in pepper response to biotic stresses. Moreover, CaZFs were significantly induced post-treatment with methyl jasmonate and ethylene indicating their role in defense signaling. Notably, the MeJA responsive cis-elements were detected in the promoter regions of majority of CaZF genes, suggesting that CaZFs may be implicated in defense-responsive signal cross talking. Additionally, 18 CaZF genes were differentially expressed under drought and heat treatment, indicating their involvement in plant response to abiotic stresses. Overall, a comprehensive analysis of CaZF gene family in pepper provided significant insights into the understanding of C2H2 ZF-mediated stress regulation network, which would benefit the genetic improvement of pepper and other allied plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sessile nature and autotrophic lifestyle of the plants makes them exposed to various environmental stresses including extreme temperatures, drought, salinity, toxicity to hazardous materials and pathogenic infections leading to significant losses in yield and quality (Jones and Dangl 2006). To combat against such threats, plants have evolved an array of adaptive defense mechanisms including oxidative burst, callose deposition and regulation of signal transduction pathways (Muthamilarasan and Prasad 2013). The rapid activation of plant defense response is facilitated by concerted effort of multiple signal molecules such as ethylene, jasmonates and salicylic acid which triggers a variety of biochemical pathways converging the information into the nucleus. These signaling informations leads to the production of reactive oxygen species (ROS), biosynthesis of secondary metabolites and transcriptional activation of a large number of defense related genes (Ng et al. 2018). Regulatory expression of defense genes is interceded by the differential activity of the DNA binding transcription factors (TFs) which functions as molecular controls during biological processes including stress responses (Seo and Choi 2015). Atleast seven plant TFs including basic leucine zipper (bZIP), APETALA2/Ethylene response factor (AP2/ERF), myloblastosis related (MYB), basic helix loop helix (bHLH), WRKY, NAC (no apical meristem, ATAF1 and cup-shaped cotyledon) and zinc finger proteins (ZFPs) have been implicated in biotic and abiotic stress responses (Ng et al. 2018). Among them, the ZFPs are the largest group of TFs accounting for nearly 15% of the total TFs in model plant genomes. These are characterized by a two stranded antiparallel β-sheet and a α-helix forming a compact three-dimensional finger structure made up of cysteine and/or histidine residues coordinated by a zinc ion (Kielbowicz-Matuk 2011). Based on the type and order of the zinc coordinated residues, the ZFPs are categorised as C2H2, C2HC, C2HC5, C2C2, C3H, C3HC4, C4, C4HC3, C6, and C8 (Kielbowicz-Matuk 2011). Since the discovery of first plant-specific ZFPs in 1998, they have been shown to play fundamental roles in numerous biological processes (Han et al. 2020).
The C2H2 type ZFPs, also known as the Kruppel-like zinc finger proteins with a typical X2-Cys-X(2–4)-Cys-X12-His-X(3–5)-His signature motif are the most extensive and widely studied groups of transcriptional factors in eukaryotes (Kielbowicz-Matuk 2011). Compared to other eukaryotic organisms, the plant C2H2 ZFPs are characterized by long and diverse space between tandem zinc fingers and presence of a conserved QALGGH sequence in the ZF domain of the α-helix. C2H2-ZFP family has been comprehensively defined in several plant genomes including 179 in Arabidopsis thaliana (Englbrecht et al. 2004), 189 in rice (Agrawal et al. 2007), 122 in wheat (Faraji et al. 2018), 124 in foxtail millet (Muthamilarasan et al. 2014), 109 in poplar (Liu et al. 2015) and 104 in tomato (Hu et al. 2019). C2H2 ZFPs exhibit extensive structural and functional variations including transcriptional regulation, RNA metabolism and protein–protein interactions. Emerging evidences indicate that C2H2 ZFPs are implicated in a wide range of stress responses in many plant species (Han et al. 2020). For instance, in Arabidopsis thaliana, AZF1 and AZF3 positively regulate cold tolerance (Sakamoto et al. 2004), over-expression of ZAT7 improved salinity tolerance by activating defense genes (Cifti-Yilmaz et al. 2007), RHL41 is associated with light stress (Kodaira et al. 2011) and upregulated expression of ZAT18 mediated drought tolerance (Yin et al. 2017). In rice, the over-expression of Oryza sativa zinc finger protein 245 (OsZFP245) led to increased tolerance to cold, drought and oxidative stress (Huang et al. 2009). Also, the over-expression of Ponicrus trifoliate zinc finger protein (PtrZPT2-1) in tobacco showed enhanced tolerance to salt, cold and drought through decreased accumulation of hydrogen peroxide and increased accumulation of osmolytes (Liu et al. 2017). Likewise, C2H2 ZFPs are also involved in other developmental processes including trichome differentiation (Zhou et al. 2013), fruit ripening (Weng et al. 2015), senescence (Moreau et al. 2018), floral morphogenesis (Lyu and Cao 2018), seed germination (Feurtado et al. 2011) and miRNA biogenesis (Yang et al. 2006). All these reports clearly indicate that C2H2 ZFPs are prominent transcriptional activators of downstream genes involved in multi-physiological and stress signal transduction pathways in multitudes of plant species. However, a complete characterization and functional exploration of C2H2 ZFPs in chilli pepper (Capsicum annuum L.) is yet ascertained.
Chilli pepper is one of the most cultivated solanaceous vegetable crop that acts as an essential spice in the gourmandises all over the world. It is a major source of vitamin A and C besides having several pharmaceutical properties attributed to the presence of capsaicin and capsanthin (Srinivasan et al. 2016). However, a significant decline in chilli productivity has been realized in the recent time owing to several biotic factors, the prominent being the anthracnose disease caused by Colletotrichum species complex (Mishra et al. 2018a, b). Although, C2H2 ZFPs play essential roles in multiple biological processes, their involvement in pathogen defense is exploited only to a limited extent. Interestingly, CaZFP1from Capsicum annuum is the first C2H2 ZFP that has been incriminated in enhanced resistance to both bacterial and fungal pathogen (Kim et al. 2004). Recently, plant immunity has been induced by upregulated expression of C2H2 ZFPs in tobacco (Zhang et al. 2016), Vitis vinifera (Yu et al. 2016) and potato (Lawrence et al. 2019). Most recently, the expression profiling of C2H2 ZFPs in Cucumis sativus has demonstrated contrasting response of two genes against pathogen infection. While Csa7G071440 genes promoted defence response in Nicotiana benthaminana post-infection with Phytophthora infestans, Csa1G085390 gene induced pathogenicity (Yin et al. 2020). A recent study from our lab related to dynamic defense transcriptome profiling of C. annuum post-infection with Colletotrichum truncatum, revea trled that the difference between susceptibility and resistance were linked to the magnitude of expression changes in 23 C2H2 ZF transcription factors (unpublished data). Based on this study, we hypothesize that specialized C2H2 ZFPs may act as potential master regulators of chilli pepper immunity against anthracnose pathogen.
In the present work, we performed a systemic genome-wide profiling and gene expression analysis to identify and explore stress responsive C2H2 ZF genes in pepper. A total of 79 C2H2 ZF genes were identified from pepper genome and subsequently analyzed for their chromosomal location, gene duplication, phylogenetic relationship, gene structures, conserved domains, and cis-acting elements in the promoter regions. Further, the expression pattern of CanZFPs was explored in two contrasting cultivars of chilli pepper (resistant: Punjab Lal, PL; and susceptible: Arka Lohit, AL) post-inoculation with C. truncatum and treatment with signaling molecules (MeJA, ET, SA and ABA) to interpret their functional role in the defense signaling pathways governing pepper–anthracnose interaction.
Materials and methods
Identification of C2H2 Zinc finger genes in chilli pepper
The pepper genome sequences were downloaded from the Pepper Genome Database (PGD) (Kim et al. 2014). The hidden Markov Model (HMM) profile of the C2H2 Zinc Finger (ZF) domain (PF00096) downloaded from the Protein family (Pfam) database (http://pfam.sanger.ac.uk) was used as query sequence to search for putative C2H2 ZF genes in the pepper genome using HMMER 3.0 software (Fin et al. 2011) with an E value cut-off of < 1 × 10–10. The corresponding C2H2 ZF protein sequences of Arabidopsis thaliana and Oryza sativa were obtained from the plant transcription factor database (http://planttfdb.cbi.edu.cn/) and also used as query sequence to identify all the pepper C2H2 ZF encoded proteins by searching against pepper proteome sequences and eliminating similar sequences from all the search results. Candidate C2H2 ZF genes were further examined by the SMART (http://smart.emblheidelberg.de/) domain analysis tool and the conserved domain database (CDD; http://www.ncbi.nlm.nih.gov/cdd/) with the default parameters and distinctive C2H2 ZF domain containing sequences were only identified for further analysis. Physicochemical properties including the molecular weights, isoelectric points (pI) and hydropathy values of the pepper C2H2 ZF proteins were estimated using the ExPaSy ProtParam tool (http://web.expasy.org/protparam/). The subcellular localization of the pepper C2H2 ZF proteins were predicted using the WoLFPSORT (http://wolfsort.org/) and mGOASVM (plant V2) web server (http://bioinfo.eie.polyu.edu.hk/mGoaSvmServer2/mGOASVM_plant/) with default parameters.
Chromosomal localization and duplication of C2H2 ZF genes
The C2H2 zinc finger genes were located on 12 pepper chromosomes based on their location data retrieved from the GFF genome files of the pepper genome database. The distribution of pepper C2H2 ZF genes was mapped for each chromosome using the MapChart v.2.0 (Voorrips 2002). The duplicated genes were identified based on the criteria that the similarity of aligned region is greater than 70% and the sequence coverage is greater than 80% of the longer sequence (Wei et al. 2016).The synonymous (Ks) and non-synonymous substitution rates of the C2H2 ZF genes were estimated using the DnaSP software ver. 5.10 (Librado et al. 2009). Paralogs were considered as tandemly duplicated if two or more pepper C2H2 ZF genes were ordered within a region of 100 kb distance (Fan et al. 2017). The segmental duplication events were confirmed when the coparalogs were located on duplicated chromosomal blocks in the same or different chromosomes.
Gene structure, conserved motif and gene ontology analyses
The intro/exon organization of the identified C2H2 ZF genes was obtained by alignment of the genomic DNAs and the corresponding coding sequences using the Gene Structure Display Server (GSDS 2.0; http://gsds.cbi.pku.edu.cn/). Structural motif annotation of the C2H2 ZFs was performed using the Multiple Expectation Maximization for Motif Elucidation (MEME) system (Ver 5.0.3; http://meme.nbcr.net/meme/) with the following set parameters: maximum number of predicted motifs-10; any number of repetitions, optimum motif with between 10 and 200 residues and E value for motif retention- ≤ 1 × 10–10. Further, the functional annotation of the C2H2 ZF genes was performed using the Blast2GO program (Conesa and Gotz 2008) and the functions were cross validated using the DeepGO function prediction tool (Kulmanov and Hoehndorf 2018). Gene ontology associated the C2H2 ZF gene with GO terms that were classified into three categories: biological processes, cellular components and molecular functions.
Phylogenetic analysis of C2H2 zinc finger proteins
The predicted pepper C2H2 ZFs and the protein sequences from A. thaliana and O. sativa were subjected to multiple sequence alignment using Clustal Omega with default parameters. The aligned sequences were used for phylogenetic analysis using the Molecular Evolutionary Genetic Analysis (MEGA v 10.1) package (Kumar et al. 2018). An unrooted phylogenetic tree was developed using the neighbor-joining (NJ) algorithm with the parameters as follows: substitution–Poisson model, data subset-p-distance and pairwise deletion; bootstrapping-1000 bootstrap replications.
Promoter analysis of pepper C2H2 ZF genes
Genomic sequence of about 2 Kb upstream of the translation start site of the pepper C2H2 ZF gene sequences were downloaded from PGD. The putative promoter regions were analyzed for the presence of cis-acting regulatory elements including for hormone signaling, biotic and abiotic stresses using the Plant CARE database (Lescot et al. 2002).
Plant materials, pathogen inoculation and stress treatments
Two chilli pepper cultivars (cv.) Punjab Lal (PL) and Arka Lohit (AL) with variable response to the anthracnose pathogen C. truncatum (Cot) were used in the present study. A super virulent C. truncatum strain MTCC-3414 (obtained from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India) was used for plant inoculation in a temperature controlled growth chamber. Cot inoculation of the chilli pepper mature red ripened fruits was performed as described previously (Mishra et al. 2018a, b). Briefly, the conidial suspension was prepared from 10-day-old culture by flooding the potato dextrose agar (PDA) plates with distilled water and scrapping the surface to collect the conidia. Three replications of the samples each containing 25 fruits of the red ripened stage of both the genotypes were harvested and washed in distilled water. Sterilized fruits were punctured at the distal and proximal end using a 1 mm syringe needle and 2.0 μl of the conidial suspension (5 × 105 conidia/ml) was injected into the wounds. Fruits were inoculated in moisture boxes and maintained at 28 °C, optimum daylight conditions (12 h light/12 h dark) and relative humidity of 95% for 9 days (Mishra et al. 2016). Whole fruits of control and treated plants were harvested at 0, 3, 5, 7 and 9 days after inoculation (DAI), snap frozen with liquid nitrogen and stored at − 80 °C for RNA isolation.
For hormonal treatment, 20 days old chilli pepper seedlings were sprayed with 100 μM methyl jasmonate (MeJA) dissolved in 1% ethanol, 3% Ethephon dissolved in 1% ethanol, 500 μM salicylic acid (SA) dissolved in water and 100 μM abscisic acid (ABA) dissolved in water. Control plants were treated with sterile distilled water for SA and ABA and 1% ethanol for MeJA and Ethephon. For drought stress simulation, 20 days old seedlings were treated with 20% (m/v) polyethylene glycol (PEG 6000). Heat stress was imposed by subjecting the seedlings to 42 °C for the duration of experiments. Control and treated samples were harvested at 0, 6, 12, 24 and 48 h after inoculation (HAI), frozen in liquid nitrogen and stored at − 80 °C deep freezer until use.
RNA isolation and RT-qPCR analysis
A set of five fruits from each time points were collected for RNA extraction. Total RNA was extracted from the frozen control and treated samples using the Trizol reagent (Invitrogen, Darmsradt, Germany) and purified with DNAse I (Promega, Madison, WI, USA) in line with the manufacturer’s instructions. The quality and concentration of RNA were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, USA). RNA samples with 260/280 nm ratio between 2.0 and 2.1 were used for further analysis. The first strand cDNA was synthesized by transcribing 2 μg RNA using the high capacity cDNA synthesis kit (Life Technologies, Burlington, ON, CA) as per manufacturer’s instructions.
The first stand cDNA was diluted ten times and used as template for quantitative real time PCR (qRT-PCR). qRT-PCR was performed using an unique set of gene specific primers designed from 25 pepper C2H2 ZF genes (Table S1). Prior to qPCR, the specificity of the designed primers were confirmed through RT-PCR and separation of cDNA amplified product through agarose gel electrophoresis. Each real-time RT-PCR reaction was set in a total volume of 10 mL reaction mixture comprising 5 μL of Kappa Biosystem’s FASTSYBR Green mix (D Mark, Toronto, ON, CA), 1 μL each of forward and reverse primers (5 μM), 1 μL of 5 ng of reverse transcribed cDNA and 2 μL nuclease free water. The reactions were subjected to fast qPCR on a Stepone Plus real time PCR system (Life Technologies, Burlington, ON, Canada) with the following thermo cyclic conditions: initial denaturation of 95 °C for 1 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. To validate the specificity of the PCR product, a melt curve analysis was carried out using a cycle of 65 °C for 15 s followed by a slow temperature increase to 95 °C at the rate of 0.2 °C/s. Ct values for each samples were estimated from nine reactions including three biological replicates with each biological replicates having three technical replicates. C. annuum Ubiquitin containing enzyme 3 (CaUBC3) and glyceraldehyde-3-phosphate dehydrogenase (CaGADPH) were used as endogenous control. The transcript accumulation in the control sample was set to 1 and the threshold cycle values were mathematically transformed into the relative expression levels of the genes using the comparative 2−ΔΔCt method (Livak and Schmittgen 2001). The statistical significance of the qRT-PCR results were analyzed with two-way analysis of variance (ANOVA) and multiple comparisons were done using uncorrected Fischer’s LSD test. The statistical significance in the difference of the mean values was score at p < 0.05.
Results
Identification and characterization of C2H2 ZF genes in Capsicum annuum
Comprehensive search of the pepper genome database using the HMM profile of the Pfam C2H2 ZF domain (PF00096) resulted in the identification of 86 pepper genome sequences that were annotated as encoding C2H2 ZFPs. Manual analysis of the candidate sequences using SMART and CDD tools detected seven sequences without a complete C2H2 ZF domain and were removed. The remaining 79 sequences were assigned as pepper C2H2 ZF genes. For convenience, the 79 proteins were named as CanZFP1 to CanZFP79 (C2H2 ZFPs of Capsicum annuum). Analysis of physicochemical and molecular properties revealed that the length of the encoded proteins varied between 120 aa (CanZF22) to 683 aa (CanZF7) with an average of 391 aa. Consequently, the computed molecular weights ranged from 139.14 to 775.16 kDa and the predicted pI values varied from 4.71 (CanZF71) to 9.61 (CanZF59) indicating acidic and alkaline proteins. The predictions of subcellular localization using WolF PSORT analysis showed that 70 (88.6%) members of C. annuum C2H2-ZFPs were predicted as nuclear proteins, six as cytoplasmic proteins while the remaining three C2H2-ZFPs were localized in the endoplasmic reticulum. The details of other informations about the DNA and protein sequences of C2H2-ZF genes are shown in Table S2.
Chromosomal distribution and duplication of C. annuum C2H2 ZFs
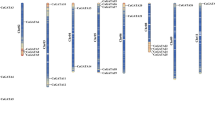
The predicted C2H2-ZFPs were mapped across the C. annuum genome to determine their chromosomal locations. The in silico mapping demonstrated that all the 79 CanZFs were unevenly distributed across the 12 chromosomes (Fig. 1). Among them, both the chromosomes 5 and 6 had the highest number each with 12 C2H2-ZFs, followed by nine genes in chromosome 1, seven genes in chromosome 8, five genes on each of chromosome 7 and 11, four genes in each of chromosome 9 and 12 and three genes in each of chromosome 2, 4 and 10 (Fig. 1). The gene duplication event of the C2H2-ZFPs was analyzed to understand their contribution in the genomic expansion and realignment of pepper genome. Based on the alignment of sequence length and similarity of the aligned regions, we identified two tandemly duplicated gene pairs (CanZF72/CanZF76 and CanZF43/CanZF78) that were mapped within a distance of less than 5 Kb on chromosome 5 and 8, respectively, (Table S3). On the other hand, 34 gene pairs exhibited segmental duplication suggesting that both inter and intra-chromosomal duplication events have contributed to the expansion of this gene family in chilli pepper. Further, the non-synonymous substitution (Ka), synonymous substitution (Ks) and Ka/Ks ratio were estimated for the homologous gene pair in C. annuum to explore the evolutionary divergence of C2H2-ZFs. The Ka/Ks ratio of the 36 paralogous C2H2-ZF gene pairs ranged between 0.096 and 0.408 with an average of 0.228 (Table S3) suggesting that all the gene pairs have evolved under the influence of purifying selection and their mutations had the disadvantageous effect.
Chromosome distribution and duplication event coordinates of pepper C2H2 ZF genes. The chromosome number (Chr01–Chr12) is indicated at the top of each chromosome. Gene name is indicated on the right side while the physical position (Mb) of each mapped gene is represented in the left side of each chromosome. Segmental duplications are denoted by red lines while the tandem duplications are indicated by green boxes. The scale on the left represent the physical map distance (Mb) among genes within the chromosomes
Phylogeny, gene structure and conserved domain analysis of chilli pepper C2H2 ZFs
A comprehensive phylogenetic analysis using the neighbour joining method was performed with 1000 bootstrap replicates to determine the evolutionary relationship among the C2H2-ZFPs of C. annuum. An unrooted phylogenetic tree distributed the 79 CanC2H2-ZFPs into five groups (I, II, III, IV and V) (Fig. 2a). Group I had the highest number (24) of C2H2-ZFPs, followed by group V (19), group IV (14) and both group II and III each with 11 proteins. This classification is similar to that observed for C2H2-ZF genes in other plant species. Based on the phylogenetic relationship, 36 pairs of genes demonstrated significantly strong bootstrap support (> 96%) and were categorized as paralogous sister pairs (Table S3).
Phylogenetic relationship and gene structure of pepper C2H2 ZF genes. a Phylogenetic tree developed with MEGA 10 using the amino acid sequences of 79 C2H2 ZF genes. An unrooted tree was developed from the aligned protein sequences obtained from Clustal Omega using the neighbor-joining (NJ) algorithm with the parameters as follows: substitution–Poisson model, data subset-p-distance and pairwise deletion; bootstrapping-1000 bootstrap replications. The percentage bootstrap scores are indicated on the nodes. The tree shows five major subgroups (I–V) represented in different colours. b Organization of exon/intron structures of pepper C2H2 ZF genes. The alignment of the genomic DNAs and the corresponding coding sequences was performed using the Gene Structure Display Server (GSDS 2.0; http://gsds.cbi.pku.edu.cn/). Red boxes represent exons, black lines represents introns and blue boxes indicate the untranslated regions (UTRs). The length of the protein can be estimated by the scale at the bottom
To understand the structural diversity of pepper C2H2-ZF genes, we developed the intron/exon organization by comparing the full length cDNA sequence with the corresponding genomic DNA sequence of individual C2H2-ZF genes (Fig. 2b). The alignment of genomic and cDNA sequences showed that 36 (45.5%) of the total 79 CanZF genes had only 1 exon, 12 genes (15.18%) had 2 exons, 13 genes (16.4%) had 3 exons, six genes (7.6%) had 4 exons while the remaining 12 genes had more than 5 exons with the highest of nine exons in CanZF10. Interestingly, the closely related C2H2-ZF genes from the same group depicted similar organization in terms of number and lengths of introns and exons consistent with the characteristics defined by the evolutionary analysis. For instance, all members of group I and II contains one or two exons, while those in group IV and V had 3 to 9 exons with a few exceptions. Contrary to this, group III represented significant variation in the intron/exon structure across all its members.
The 79 C2H2-ZF genes from pepper were subjected to MEME analysis to determine the diversification of conserved motifs among the related sequences. Ten conserved motifs were identified out of which motif 1, 3, 4 and 6 represented different types of C2H2-ZF domains (Fig. 3, Table S4). While the conserved motif 1 (CEICNKGFQSGQALGGHKRGHR) was found in all the sequences, motif 3 (CKTCNKVFSSFQALGGHRASH) was found in all members of group I, II and III and only two members (CanZF12, CanZF22) of group IV. Motif 6 (CKLCGKKFPSGKALGGHMRCH) was found in the N-terminal region of group I and II proteins while motif 4 (CPEPSCVHHDPSRALGDLTGIKKHFSRKH) was restricted to the C-terminal region of C2H2-ZFPs from group V. Additionally, motif 8 (DLNLPPP) representing a hexapeptide amphiphilic repressor domain was found in the C-terminal region of 41 C2H2-ZFPs suggesting their possible involvement in transcriptional inactivation. Apart from this, four observed motifs had unidentified conserved structure including motif 9 (LPWKLKQRNKKEVK) represented in only four sequences implying the possible functional divergence of these C2H2-ZFPs in pepper. Further, the protein sequences of the identified genes were aligned to confirm the characteristics of the C2H2-ZF domains in pepper. Multiple alignment showed that 59 (74.6%) C2H2-ZFPs contained the classical conserved consensus Cys-X(2)-Cys-X(12)-His-X(3)-His sequence, 16 (20.2%) C2H2-ZFPs contained the Cys-X(2)-Cys-X(12)-His-X(5)-His sequence while the remaining four had the Cys-X(2)-Cys-X(12)-His-X(4)-His sequence. Based on the previous pattern of classification of plant C2H2-ZFPs (Alam et al. 2019), 37 pepper C2H2-ZFPs were characterized as Q-type due to the presence of a plant-specific conserved domain ‘QALGGH’. Fifteen C2H2-ZFPs were designated as M-type with defined modification of the Q-type conserved domain, 20 sequences with no conserved motif within the ZF domain were categorized as C-type and the remaining sequences with conserved motifs within and in the flanking regions of the C-type ZFs were labelled as Z-type. The number of ZF domains ranged from 1 to 5 among the pepper C2H2-ZFPs (Table S5). Two proteins (CanZF1 and CanZF2) have 5 ZF domains all of which were C-type. Nine proteins consisted of four ZF domains including one (CanZF3) with all C-type ZFs and eight ZFPs both Q-type and M-types ZF domains. Majority of pepper ZFPs (33) contained 3 ZF domains which included 5 ZFPs with only C-type and 2 ZFPs with only Q-type ZF domains. Also, 27 sequences were two fingered proteins among which, 18 proteins contained two Q-type domains and 7 proteins contained two C-type domains.
Conserved motif analysis of pepper C2H2 ZF proteins. Motif analysis was performed using MEME 5 software with set parameters as follows: maximum number of predicted motifs-10; any number of repetitions, optimum motif with between 10 and 200 residues and E value for motif retention- ≤ 1 × 10–10. a Ten conserved motifs were identified as represented in different colored boxes while the length of the proteins are indicated by black lines. b Sequence of motif 1, 3, 4 and 6 corresponding to the conserved C2H2 ZF domain found across the pepper C2H2 ZF proteins. The detailed motif sequences are represented in Table S4
Comparative phylogeny of C2H2 ZFs genes in chilli pepper, Arabidopsis and rice
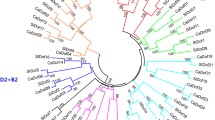
To verify the evolutionary pattern of development in the C2H2 ZF family of pepper, a phylogenetic tree was constructed using the sequence alignment 79 pepper ZFPs, 28 rice ZFPs and 13 Arabidopsis ZFPs (Fig. 4). The ZFPs were broadly grouped into five prominent clusters supported by significant bootstrap values. Group I and II together consisted of 36 pepper ZFPs, all of which carried a Q-type or a modified M-type ZF domain. Likewise, Group III comprised of 18 C-type ZFPs and group IV categorised five Z-type pepper ZFPs along with similar proteins from A. thaliana and O. sativa. Notably, group V (shaded in blue) contained 19 pepper ZFPs all of which had both the C-type as well as the Z-type ZF domains. Negating a few exceptions, the phylogenetic tree revealed that the plants ZFPs generally exhibited a species bias distribution suggesting that different organisms exhibit independent sequence diversification to satisfy differential adaptability and functional attributes.
Phylogenetic tree of C2H2 ZF proteins from pepper, Arabidopsis and rice. Seventy nine pepper ZFPs, 28 rice ZFPs and 13 Arabidopsis ZFPs were aligned to generate the phylogenetic tree using the neighbor-joining (NJ) method with 1000 bootstrap replicates. The numbers in the branches indicate the percentage of bootstrap replications. Pepper, rice and Arabidopsis C2H2 ZF proteins were marked with different colored dots and squares. The five groups (I–V) of C2H2 ZFPs are indicated in different colors
Cis-regulatory element analysis and functional annotation of C2H2 ZFs
To explore the transcriptional regulatory activity of the pepper ZF genes, their promoter sequences (1.5 Kb upstream region) were search for the presence of cis-element using the PlantCARE database. The in silico analysis revealed 13 prominent cis-acting elements conferring responsiveness to phytohormones, biotic and abiotic stresses including responsive elements were found including TC-rich motif (defense response); T/G-box, CGTCA-element and TGACG-element all responsive to MeJA; ERE (ethylene response element), ABRE (Abscisic acid response element), TCA-element (responsive to SA) and MBS (MYB element for drought inducibility), W-box (WRKY stress responsive element); HSE (heat shock element); TGA-element (responsive to auxins); TCT-element (responsive to light) and RY-element for seed specific regulation (Table S6). CanZF64, 71 and 78 possessed the maximum types of cis-elements (12) while CanZF 14 and 26 have only two cis-elements (Table S7). Further, CanZF46 has the most number of cis-elements (39) including, 12 responsive to MeJA, ten defense related, six ethylene related, four each of drought and ABA related and two responsive to IAA (Table S7). Likewise, CanZF40 consisted of 36 cis-element consisting of 12 MeJA related, 10 defense related, four ET and heat related and two drought, ABA and IAA related. Notably, 32 CanZF genes (40.5%) have TC-rich repeats (GTTTTCTTAC) in the promoter regions implying their possible regulatory role in pathogen defense. The maximum number of TC-rich repeats (9) were identified in CanZF40 and 46 followed by 6 elements in CanZF 39, 4 elements each in CanZF68 and 74 and 3 repeat elements in CanZF10 (Table S7).
The GO term enrichment analysis using Blast2GO assigned 14 functional groups to the 79 pepper C2H2 ZFPs distributed within the molecular function (4), biological process (7) and cellular component (3) categories (Table S8). While various functional annotations were allocated to the same genes, majority of pepper C2H2 ZF genes were represented by biological process domain followed by cellular component and molecular function. Among the biological process categories, ‘cellular process’ provided the highest abundance of pepper C2H2 ZF genes (40; 50.6%), trailed by ‘metabolic process’ (18; 22.7%) and response to stimulus (9; 11.3%). Within the cellular component category, the GO term ‘cell’ was the largest group (50 genes; 63.2%) followed by ‘cell part’ (16 genes; 20.2%) and ‘organelle’ (12 genes; 15.2%). Similarly, in the molecular function domain, the highly represented categories were ‘nucleic acid binding’ (32 genes; 40.5%) and ‘ion binding’ (29 genes; 36.7%).
CanZF genes as targets for pepper miRNAs
Based on the previous report that pepper miRNAs also target C2H2 ZF transcripts, we searched for miRNA target sites in all the CanZF genes of chilli pepper using psRNATarget web server with default parameters. Alignment of pepper ZF transcripts with miRNA sequences resulted in the identification of 38 miRNA binding sites for 11 pepper miRNAs (Table S9). These miRNAs included can-miR164, canmiR168, can-miR171, can-miR390 and can-miR396. The miRNAs with most abundant targets were can-miR390 (11), can-miR168 (6), can-miR171 (4) and can-miR396 (3). Among the putative CanZF targets, CanZF24 was targeted by three pepper miRNAs (Can-miR168, can-miR390 and can-miR9476).
Differential response of pepper C2H2 ZFs in response to anthracnose infection
To explore the differential response of pepper C2H2 ZF genes to pathogenic infection, qRT-PCR of 25 selected pepper C2H2 ZF genes from different groups was performed to determine the relative abundance of pepper C2H2 ZF genes in the control and treated plant samples of PL and AL genotypes at different time points post-infection with Colletotrichum truncatum (Cot). The genes induced or repressed by more than twofold were considered significantly differentially expressed. qPCR analysis revealed that 18 pepper C2H2 ZF genes were induced in response to anthracnose infection while the remaining seven genes showed no precise change in their expression pattern post-treatment with Cot (Figs. 5 and 6). These 18 genes could be categorized into three classes based on a significant change in their transcript accumulation pattern upon Cot infection. Eight genes (CanZF10, 11, 16, 39, 40, 46, 68 and 74) were specifically induced in response to Cot infection in PL genotypes but mostly remained unresponsive or downregulated in AL genotypes and, therefore, classified as having a positive role in pepper resistance to Cot. Under incompatible interaction in the resistant PL genotype, all the eight genes had a gradual increase in transcript level, significantly upregulated by 5 DAI, reached the peak at 7 DAI and subsequently decreased by 9 DAI. Among them, CanZF46 demonstrated the most significant expression which was induced 6.76, 25.22, 36.82 and 30.51 folds at 3, 5, 7 and 9 DAI, respectively. Likewise, CanZF40 was induced 4.32, 12.37, 16.83 and 13.69 folds at 3, 5, 7 and 9 DAI, respectively. In contrast, four genes (CanZF5, 12, 71 and 79) showed upregulated expression in AL genotype but no obvious change or reduced expression in PL genotype and were categorized as favourably responsive to Cot infection. CanZF71 demonstrated the most significant expression in the AL genotype with 6.32, 11.37, 14.56 and 9.93 folds transcript accumulation at 3, 5, 7 and 9 DAI, respectively. The remaining six genes (CanZF3, 19, 26, 36, 56 and 78) demonstrated increased expression in both PL and AL genotypes upon Cot infection and were considered as basal responsive genes involved in pepper–anthracnose interaction. However, their expression was still significantly lower as compared to expression of C2H2 ZF genes with unique expression in either PL or AL genotype. CanZF19 was the most expressive gene in this category with transcript accumulation of 4.56, 5.37, 8.37 and 5.32 folds in PL and 3.83, 5.17, 7.68 and 4.67 folds in AL genotypes at 3, 5, 7 and 9 DAI respectively.
Expression analysis of 12 selected pepper C2H2 ZF genes in the resistant cv. Punjab Lal (PL) and the susceptible cv. Arka Lohit (AL) upon C. truncatum infection. RNA was extracted from a set of five fruits collected after 0, 3, 5, 7 and 9 days after infection (DAI). mRNA level was normalized to that in untreated sensitive line Arka Lohit (0 h). At 0 h the relative expression is equal to 1. The housekeeping genes were GAPDH and UBI3. Error bars show standard deviations for three independent experiments in real-time PCR. * and ** indicates the significant difference at p value < 0.05 and < 0.01, respectively, between infected and mock samples identified through two-way ANOVA test
Expression analysis of 13 selected pepper C2H2 ZF genes in the resistant cv. Punjab Lal (PL) and the susceptible cv. Arka Lohit (AL) upon C. truncatum infection. RNA was extracted from a set of five fruits collected after 0, 3, 5, 7 and 9 days after infection (DAI). mRNA level was normalized to that in untreated sensitive line Arka Lohit (0 h). At 0 h the relative expression is equal to 1. The housekeeping genes were GAPDH and UBI3. Error bars show standard deviations for three independent experiments in real-time PCR. * and ** indicates the significant difference at p value < 0.05 and < 0.01, respectively, between infected and mock samples identified through two-way ANOVA test
Expression patterns of pepper C2H2 ZF genes to treatment with signaling molecules
Established on the theory that, plants response to biotic stresses are systemically orchestrated by phytohormones and stress-related signal molecules, the transcript levels of the 25 selected pepper C2H2 ZF genes were analyzed using qRT-PCR following temporal exogenous application of MeJA, SA, ET and ABA to explore their role in defense signaling during chilli pepper–anthracnose interaction. qPCR analysis revealed that the most significant changes in the expression of pepper C2H2 ZF genes was concomitant to JA and ET defense signaling during compatible and incompatible interaction (Fig. 7). CanZF36, 40 and 61 were significantly upregulated in the PL genotype after treatment with MeJA and ET with highest transcript accumulation reported in CanZF36 (16.23 folds for MeJA and 11.43 folds for ET) followed by CanZF40 (12.67 fold for MeJA and 13.62 fold for ET) at 24 hpi. CanZF46, 56 and 74 were specifically upregulated in PL genotype post-treatment with JA (CanZF46: 19.39 fold at 24 hpi; CanZF56: 15.21 at 12 hpi; CanZF74: 14.63 fold at 24 hpi) but showed no change in expression with ET, ABA and SA treatment. Likewise, the transcript of four genes (CanZF12, 14, 39 and 68) were accumulated as early as 6 hpi (CanZF39: 4.83 fold; CanZF12: 3.93 fold; CanZF14: 3.27 fold and CanZF68: 2.96 fold) and remained significantly higher during the entire period of treatment with ET in the PL genotype. However, no change in expression of the four genes could be observed in both the genotypes post-treatment with JA, SA and ABA. Two C2H2 ZF genes demonstrated transcript accumulation at 24 hpi (CanZF25: 4.43 fold; CanZF29: 4.01 fold) only in the PL genotype post-treatment with SA but the expression was significantly lower compared to other pepper C2H2 ZF genes post-treatment with JA and ET. Similarly, the transcript levels of three genes increased significantly in plants of PL genotypes treated with ABA and reached its peak as early as 12 hpi (CanZF3: 13.21 fold; CanZF10:9.26 fold and CanZF16: 11.23 fold) but showed no response to treatment with SA, JA and ET. Additionally, the transcript levels of ten genes (CanZF5, 6, 11, 19, 26, 48, 64, 71, 78 and 79) were significantly inferior in both the genotypes post-treatment with JA, SA, ET and ABA. In other words, the expression of these genes largely remained unchanged or reduced in response to the treatment with all four defense signaling molecules under both compatible and incompatible interactions.
Differential expression pattern of pepper C2H2 ZF genes in the resistant cv. Punjab Lal (PL) and the susceptible cv. Arka Lohit (AL) post-treatment with defense-responsive signal molecules using qRT-PCR. RNA was extracted from a set of five fruits from chilli pepper cv PL and AL collected after 0, 6, 12, 24 and 48 h after treatment. mRNA level was normalized to that in untreated sensitive line Arka Lohit (0 h). Red shading indicates a positive correlation while blue shading indicates a negative correlation between pepper C2H2 ZF genes and a particular hormone response. JA Jasmonic acid, SA salicylic acid, ET ethylene, ABA abscisic acid. The expression data were normalized using the heatmapper program
Expression of pepper C2H2 ZF genes under drought and heat stress
Given that the promoter region in the majority of the pepper C2H2 ZF genes was characterized by the presence of drought responsive MBS element and heat responsive HSE element, we analyzed the expression profiles of the selected 25 ZF genes following temporal exogenous application of PEG and heat stress to explore their potential roles in drought and heat tolerance. qPCR analysis demonstrated that 18 genes were induced, 1 gene (CanZF78) was repressed while the expression of 6 genes (CanZF3, 25,29, 48, 68 and 79) largely remained unchanged under drought stress in both the chilli cultivars (Fig. 8). Among the induced genes, the transcript accumulation was realized as early as 6 hpi which gradually increased and reached the peak at 24 hpi before decreasing at 48 hpi. CanZF61 showed the highest transcript accumulation (16.03 fold at 24 hpi) followed by CanZF40 (15.67 fold at 24 hpi) and CanZF46 (14.39 fold at 24 hpi) under drought stress. Under high temperature treatment, 11 genes (CanZF48, 68 and others) were upregulated, 3 genes (CanZF3, 78 and 79) were downregulated whereas the transcript accumulation in another 11 genes (CanZF10, 39 and others) was highly insignificant. Although, the expression level of genes was identical in both the cultivars, the PL genotype had a relatively higher transcript accumulation over the Al genotypes. CanZF48 (16.23 fold at 24 hpi) and CanZF25 (14.63 fold at 24 hpi) were the most induced genes under heat stress. Likewise, CanZF25, 29, 48 and 68 were significantly induced at all-time points. Taken together, the expression of most of the candidate C2H2 ZF genes was significantly altered post-treatment with PEG and heat, signifying that they might be involved in plants response to multiple abiotic stresses.
Expression analysis of pepper C2H2 ZF genes under drought and heat stress using qRT-PCR. RNA was extracted from a set of five fruits from chilli pepper cv Punjab Lal (PL) and Arka Lohit (AL) collected after 0, 6, 12, 24 and 48 h after treatment. mRNA level was normalized to that in untreated sensitive line Arka Lohit (0 h). The expression data were normalized and visualized using the heatmapper program. Red shading indicates a positive correlation while blue shading indicates a negative correlation between pepper C2H2 ZF genes and a particular hormone response
Discussion
Chilli pepper is one of the most cultivated spice-cum vegetable crop across the tropical and sub-tropical regions of the world for its culinary and pharmaceutical properties (Kim et al. 2014). Sustainability of chilli-based agriculture is threatened by several biotic factors, the most prominent being the chilli anthracnose caused by Colletotrichum species complex (Mishra et al. 2018a, b). Increasing reports in the recent times indicates that C2H2 ZFPs are significantly involved in multiple biological processes as has been demonstrated in many plant species (Han et al. 2020). Hence, the detection of differentially expressed C2H2 ZFPs in response to anthracnose pathogen will offer a stage for delineating their regulatory networks in the resistance of chilli pepper to C. truncatum infection.
Although, the whole genome sequencing and assembly has promoted the characterization of several pepper gene families (Kim et al. 2014, 2017), only one pepper C2H2 ZF gene have been implicated in specific networks related to defense response (Kim et al. 2004). In the present study, a systematic analysis resulted in the identification of 79 C2H2 ZF genes consisting of 206 C2H2 ZF domains in C. annuum genome. The length of the identified sequences varied between 120 and 683 aa residues signifying greater complexity among the pepper C2H2 ZFPs. Majority of the pepper C2H2 ZFPs were predicted to be localized in the nucleus, confirming their primary functional role as transcription factors in regulating the expression of genes related to growth and development (Xu et al. 2012). Contrary to this, thirteen genes were predicted to encode cytoplasmic proteins suggesting that these groups of ZFPs have specific and divergent functions. The number of exons varied from one (45% of pepper ZF genes) to nine (CanZF10) among the pepper ZF genes. Notably, 36 genes (45.5%) have no introns while another 26 genes (32.9%) had only 2 or 3 introns of shorter length. Previous reports have shown that genes with no introns or shorter introns are inclined to be retained in plants and are rapidly activated in response to environmental challenges (Li and Liu 2019). Consequently, pepper ZFPs with shorter gene structure may have significant involvement in responding to biotic and abiotic stresses as has been demonstrated in other plant species (Hu et al. 2019; Alam et al. 2019; Yuan et al. 2018). Interestingly, CanZF4 and CanZF71 with only one exon were also grouped with other genes in the same phylogenetic group with six to nine exons (Fig. 2). This indicates that structural variation in genes due to integration and realignment of genetic fragments is critical to the evolution of gene families (Xu et al. 2012). The 79 C2H2 ZFPs were subdivided into five major groups based on the conservation pattern of the ZF domain (Cys-x(2–4)-Cys-x(12)-His-x(3–5)-His) and variation in the plant-specific conserved motif ‘QALGGH’ (Fig. 3). With only a few exception, this distribution was well supported by the phylogenetic analysis (Fig. 4). Thirty six pepper C2H2 ZF genes were characterized by the presence of a perfect or slightly modified ‘QALGGH’ motif and were classified as Q-type ZFPs. These ZFPs are not found in any other living organisms and have been previously reported to be specifically involved in plant-specific growth and development (Han et al. 2020). Also, four proteins (CanZF64, 69, 70 and 71) specifically contained the motif 9 suggesting that they may have special functions. Overall, the difference in gene structure and motif composition among different members from the same or different groups supported the phylogenetic classification and would be helpful for further functional characterization of these biologically important group of transcription factors in pepper.
Tandem and segmental gene duplication along with transposition events acts as major force of evolutionary mechanisms responsible for realignment and expansion of organism’s genome (Kong et al. 2007). Duplication of genes has been reported in other transcription factor gene families of pepper including GRASs and WRKYs (Liu et al. 2018; Zheng et al. 2019). In our analysis, we found that 34 pepper C2H2 ZF gene pairs were preferentially distributed in duplicated blocks through segmental duplication while only two pairs of genes appeared to have undergone tandem duplication. This result was similar to the duplication of WRKY and GRAS TF genes in C. annuum (Liu et al. 2018; Zheng et al. 2019) suggesting that segmental duplication contribute significantly to the expansion of pepper C2H2 ZF gene family. Further, the Ka/Ks ratios for the duplicated gene pairs ranged from 0.096 to 0.408 suggesting the involvement of a strong purifying selection pressure during species evolution. Previous report has shown that C. annuum has undergone two rounds of divergence including its separation from Solanaceae about 19 million years ago (mya) and a Capsicum specific divergence about 1.1 mya (Kim et al. 2017). By estimating the duplication dates of the paralogous pairs, we found that the segmental duplication of gene pairs occurred between 0.3 and 0.5 mya while the two tandem duplication pairs were formed 0.89 and 0.93 mya, respectively. This suggests that the segmental duplications of pepper C2H2 ZF genes are relatively new and have occurred after the formation of the tandem duplication.
It is evident from previous reports that cis-elements are central to the transcriptional regulation of genes involved in biotic, abiotic and hormonal responses (Sheshadri et al. 2016). All the pepper C2H2 ZF genes identified in the present study were characterized by the presence of multiple stress responsive (TC-rich repeats, MBS, W-box and HSE) and hormone responsive (CGTCA-motif, TGACG-motif, ERE, ABRE and other) cis-elements in their promoter region (Table S7). CanZF40, 46, 64, 71 and 78 accounted for maximum number of cis-elements inferring that these genes could be involved in the process of plant response to multiple stresses. Particularly, the defense-responsive TC-rich repeats (GTTTTCTTAC) were found in 32 genes accounting for 17.6% of all the cis-elements. Such sequence has been previously described in tobacco and grapes as cis-element involved in defense against fungal phytopathogens (Zhang et al. 2016; Yu et al. 2016). Therefore, the results from the present study will significantly contribute to further understand the vital function of C2H2 ZF genes towards resistance to C. truncatum, the most belligerent fungal phytopathogen infecting chilli pepper. Moreover, the evaluation of miRNAs targeting pepper C2H2 ZF transcripts revealed that 24 genes were targeted by 11 pepper miRNAs (Table S8). Can-miR396 targeting CanZF46 and 47, can-miR164 targeting CanZF64 and 69 and can-miR168 targeting CanZF24 and 31 have been previously reported to exhibit differential response to anthracnose infection (Mishra et al. 2018b). Although this provides a primary clue on the post-transcriptional regulation of pepper C2H2 ZF, a detailed exploration is required to understand the role of pepper C2H2 ZF in the regulation of post-transcriptional gene silencing mechanism towards chilli pepper immunity to C. truncatum infection.
Previous studies have reported divergent expression of C2H2 ZF genes in response to various abiotic stresses (Liu et al. 2015; Hu et al. 2019; Alam et al. 2019). Considering this, we explored the expression pattern of selected pepper ZF genes after drought (PEG) and heat stress (Fig. 8). qPCR analysis demonstrated that 18 of these genes had a significant response to one or both of these stresses. Liu et al. (2015), also reported similar findings in one of their study in P. trichocarpa. Similarly, 18 SlZFs were differentially expressed in tomato after heat stress compared to control seedlings (Hu et al. 2019). Moreover, in cucumber, an increased expression of seven CsZFs was reported in response to PEG (Chen et al. 2020). Taken together, these finding show the potential role of CanZFs in the regulation of abiotic stresses.
Plants growth and development is largely affected by multiple environmental stresses. Previous studies have reported that C2H2 ZFPs are involved in various biological processes and play broad spectrum regulatory roles in plant response to multiple stresses including pathogenic infection and hormone signaling (Han et al. 2020). In light of this, pepper plants were exposed to a super virulent strain of the anthracnose pathogen C truncatum under compatible and incompatible interactions in the present study to explore their effect on the expression levels of representative 25 CanZFPs from each group. The qRT-PCR analysis confirmed that the expression of eight genes (CanZF46, CanZF40 and others) was induced in PL genotype with reduced or no significant response in compatible AL genotype while four other proteins were specifically induced in the susceptible AL genotype with no significant change in resistant PL plants (Figs. 5 and 6). Our result corroborate with previous studies on the inducibility of C2H2 ZF homologs. In Nicotiana benthaminana, over-expression of NbCZF1 demonstrated induced resistance against Phytophthora nicotianae coupled with accumulation of reactive oxygen species (ROS) and nitric oxide (NO) in the guard cells (Zhang et al. 2016). In Vitis vinifera, expression of VvZFP11 revealed significant response to infection by the fungal pathogen Erisiphe necator (Yu et al. 2016). More recently, the over-expression of a Q-type StZFP2 increased the resistance to late blight infection in potato (Lawrence et al. 2019). An earlier study has also shown that one C. annuum C2H2 ZF gene, CaZFP1 was significantly induced in response to bacterial pathogen Xanthomonas campestris pv vesicatoria and fungal pathogen Colletotrichum coccodes (Kim et al. 2004). Interestingly, the multiple alignment of amino acid sequences revealed that both CanZF46 and CanZF40 exhibited greater than 80% amino acid identity with the previously identified CaZFP1 from pepper and StZFP2 from potato (Fig. S1). This suggests that CanZF40 and CanZF46 could be orthologs of defense-responsive ZFPs and might function in a similar fashion in inducing anthracnose resistance in chilli pepper. Overall, the CanZFPs induced by C. truncatum in PL genotype in the present study might represent a preformed resistance mechanism directly attributed to the high degree of resistance of PL to anthracnose infection. However, more detailed investigation through the characterization of over-expression and silencing lines of these genes is required to decipher the exact molecular mechanism involved in the C2H2 ZF-mediated chilli defense signaling in response to C. truncatum infection.
Phytohormones like SA, MeJA and ET orchestrate plants response to biotic and abiotic stresses through specialized signal transduction pathways (Yang et al. 2019). While SA intercede defense to biotropic pathogens through activation of PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI), JA and ET play a major role in defense responses against the necrotrophs (Yang et al. 2019). In contrast, the classical phytohormone ABA is adopted to fine-tune the plant defense response (Ton et al. 2009). Hence, we extended our study to investigate the expression analysis of 24 representative CanZFPs by exposing the pepper plants to SA, MeJA, ET and ABA induced stresses. The results revealed that ten CanZFs genes were induced either by MeJA or ET alone or simultaneously by both the hormones in the PL genotype but downregulated or showed no significant response in the AL genotype. Likewise, two genes had upregulated expression post-treatment with SA albeit at a very low level as compared to MeJA and ET. Gourcilleau et al. (2011) reported a similar finding in which PtaZFP1 and PtaZFP2 from P. trichocarpa were induced by both ET and MeJA stress. Meanwhile, a study in V. vinifera reported that VvZFP11 was upregulated by SA and MeJA stress (Yu et al. 2016). ET induced induction of a Q-type C2H2 ZF has already been reported in C. annuum (Kim et al. 2004). Concurrent induction of multiple pepper C2H2 ZFPs post-treatment with MeJA, ET and SA together with their pathogen induced expression is suggestive of a synergistic role of these phytohormones towards anthracnose resistance in chilli pepper.
Conclusions
In this study, a comprehensive genome-wide analysis led to the identification of 79 C2H2 ZF transcription factors in C. annuum. Analysis of the phylogenetic relationships, chromosomal location and conserved motifs classified them into five major subgroups. The genes were indiscriminately distributed across the 12 chromosomes and the expansion of pepper C2H2 ZF gene family was attributed to segmental duplication events. A total of 38 miRNA target sites were predicted in 24 pepper C2H2 ZF genes for 11 pepper miRNAs. Cis-acting elements in the promoter regions of pepper ZF genes presented evidences about their possible functional expression in response to biotic and abiotic stresses. The significantly induced expression of multiple pepper C2H2 ZF genes in response to phytohormones in the resistant genotypes signified a coordinated response of JA/ET and SA mediated signaling pathways during chilli resistance to anthracnose pathogen. Furthermore, the expression levels of eight selected C2H2 ZF genes and particularly CanZF40 and CanZF46 was significantly upregulated in the resistant pepper genotype post-inoculation suggesting their specific involvement in the direct defence mechanism of chilli pepper against the anthracnose pathogen. We have recently initiated a comparative expressional analysis of pepper C2H2 ZF genes through virus-induced gene silencing of CanZF40 and CnaZF46 in the resistant PL genotype and over-expression in the susceptible AL genotype to generate more information about the molecular dynamics of these genes under compatible and incompatible interaction between chilli pepper and C. truncatum. Nevertheless, the data presented in this study will provide fundamental information for better understanding of the signalling pathways involved in C2H2 ZF-mediated regulation of C. truncatum resistance in chilli pepper.
References
Agarwal P, Arora R, Ray S et al (2007) Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol Biol 65:467–485. https://doi.org/10.1007/s11103-007-9199-y
Alam K, Batool DL, Cui YQ et al (2019) Comprehensive genomic survey, structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS ONE 14:e0216071. https://doi.org/10.1371/journal.pone.0216071
Chen Y, Wang G, Pan J et al (2020) Comprehensive genomic analysis and expression profiling of the C2H2 zinc finger protein family under abiotic stresses in cucumber (Cucumis sativus L.). Genes 11:171. https://doi.org/10.3390/genes11020171
Ciftci-Yilmaz S, Morsy MR, Song L et al (2007) The EAR-motif of the Cys2/His2 zinc finger protein Zat and plays a key role in the defense response of Arabidopsis to salinity stress. J Biol Chem 282(12):9280–9268. https://doi.org/10.1074/jbc.M611093200
Conesa S, Götz E (2008) Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genom 2008:619832. https://doi.org/10.1155/2008/619832
Englbrecht CC, Schoof H, Böhm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom 5:39. https://doi.org/10.1186/1471-2164-5-39
Fan F, Yang X, Cheng Y et al (2017) The DnaJ gene family in pepper (Capsicum annuum L.): comprehensive identification, characterization and expression profiles. Front Plant Sci 8:689. https://doi.org/10.3389/fpls.2017.00689
Faraji S, Rasouli SH, Kazemitabar SK (2018) Genome-wide exploration of C2H2 zinc finger family in durum wheat (Triticum turgidum ssp. Durum): insights into the roles in biological processes especially stress response. Biometals 10:1007. https://doi.org/10.3390/genes11020171
Feurtado JA, Huang D, Wicki-Stordeur L et al (2011) The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell 23:772–1794. https://doi.org/10.1105/tpc.111.085134
Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:W29-37. https://doi.org/10.1093/nar/gkr367
Gourcilleau D, Lenne C, Armenise C et al (2011) Phylogenetic study of plant Q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic, cold and mechanical stresses. DNA Res 18:77–92. https://doi.org/10.1093/dnares/dsr001
Han G, Lu C, Guo J et al (2020) C2H2 zinc finger proteins: master regulators of abiotic stress responses in plants. Front Plant Sci 11:115. https://doi.org/10.3389/fpls.2020.00115
Hu X, Zhu L, Zhang Y et al (2019) Genome-wide identification of C2H2 zinc-finger genes and their expression patterns under heat stress in tomato (Solanum lycopersicum L.). PeerJ 7:e7929. https://doi.org/10.7717/peerj.7929
Huang J, Sun SJ, Xu DQ et al (2009) Increased tolerance of rice to cold, drought and oxidative stresses mediated by the overexpression of a gene that encodes the zinc finger protein ZFP245. Biochem Biophys Res Commun 389:556–561. https://doi.org/10.1016/j.bbrc.2009.09.032
Jones JDG, Dangl L (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Kiełbowicz-Matuk A (2011) Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci 186:78–85. https://doi.org/10.1016/j.plantsci.2011.11.015
Kim SH, Hong JK, Lee SC (2004) CAZFP1, a Cys2/His2-type zinc-finger transcription factor gene functions as a pathogen-induced early-defense gene in Capsicum annuum. Plant Mol Biol 55:883–904. https://doi.org/10.1007/s11103-004-2151-5
Kim S, Park M, Yeom SI et al (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46:270–278. https://doi.org/10.1038/ng.2877
Kim S, Park J, Yeom SI et al (2017) New reference genome sequences of hot pepper reveal the massive evolution of plant disease-resistance genes by retroduplication. Genome Biol 18:210. https://doi.org/10.1186/s13059-017-1341-9
Kodaira KS, Qin F, Tran LSP (2011) Arabidopsis C2H2 zinc-finger proteins AZF1 and AZF2 negatively regulate ABA-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol 111:182683. https://doi.org/10.1104/pp.111.182683
Kong H, Landherr LL, Frohlich MW et al (2007) Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J 50:873–885. https://doi.org/10.1111/j.1365-313X.2007.03097.x
Kulmanov M, Hoehndorf R (2018) DeepGOPlus: improved protein function prediction from sequence. BioRxiv. https://doi.org/10.1101/615260
Kumar S, Stecher G, Li M (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lawrence SD, Novak NG, Perez FG et al (2019) Over expression of the Q-type ZFP StZFP2 in potato increases resistance to potato late blight (Phytophthora infestans) infection. J Plant Interact 14:129–136. https://doi.org/10.1080/17429145.2018.1562109
Lescot M, Déhais P, Thijs G (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327. https://doi.org/10.1093/nar/30.1.325
Li J, Liu X (2019) Genome-wide identification and expression profile analysis of the Hsp20 gene family in Barley (Hordeum vulgare L.). PeerJ 7:e6832. https://doi.org/10.7717/peerj.6832
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Liu Q, Wang Z, Xu X (2015) Genome-wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in Poplar (Populus trichocarpa). PLoS ONE 10:e0134753. https://doi.org/10.1371/journal.pone.0134753
Liu D, Yang L, Luo M et al (2017) Molecular cloning and characterization of PtrZPT2-1, a ZPT2 family gene encoding a Cys2/His2-type zinc finger protein from trifoliate orange (Poncirus trifoliata (L.) Raf.) that enhances plant tolerance to multiple abiotic stresses. Plant Sci 263:66–78. https://doi.org/10.1016/j.plantsci.2017.07.012
Liu B, Sun Y, Xue J et al (2018) Genome-wide characterization and expression analysis of GRAS gene family in pepper (Capsicum annuum L.). PeerJ 6:e4796. https://doi.org/10.7717/peerj.4796
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lyu T, Cao J (2018) Cys2/His2 zinc-finger proteins in transcriptional regulation of flower development. Int J Mol Sci 19(9):2589. https://doi.org/10.3390/ijms19092589
Mishra R, Nanda S, Rout E, Chand SK, Mohanty JN, Joshi RK (2016) Differential expression of defense-related genes in chilli pepper infected with anthracnose pathogen Colletotrichum truncatum. Physiol Mol Plant Pathol 97:1–10. https://doi.org/10.1016/j.pmpp.2016.11.001
Mishra R, Mohanty JN, Chand SK et al (2018a) Can-miRn37a mediated suppression of ethylene response factors enhances the resistance of chilli against anthracnose pathogen Colletotrichum truncatum L. Plant Sci 267:135–147. https://doi.org/10.1016/j.plantsci.2017.12.001
Mishra R, Rout E, Joshi RK (2018b) Identification of resistant sources against anthracnose disease caused by Colletotrichum truncatum and Colletotrichum gloeosporioides in Capsicum annuum L. Proc Natl Acad Sci India Sec B Biol Sci 89:517–524. https://doi.org/10.1007/s40011-018-0965-1
Moreau C, Hofer JMI, Eléouët M et al (2018) Identification of stipules reduced, a leaf morphology gene in pea (Pisum sativum). New Phytol 220:288–299. https://doi.org/10.1111/nph.15286
Muthamilarasan M, Prasad M (2013) Plant innate immunity: an updated insight into defense mechanism. J Biosci 38:433–449. https://doi.org/10.1007/s12038-013-9302-2
Muthamilarasan M, Bonthala VS, Mishra AK et al (2014) C2H2 type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Funct Int Genom 14:531–543. https://doi.org/10.1007/s10142-014-0383-2
Ng DWK, Abeysinghe JK, Kamali M (2018) Regulating the regulators: the control of transcription factors in plant defense signaling. Int J Mol Sci 19:3737. https://doi.org/10.3390/ijms19123737
Sakamoto H, Maruyama K, Sakuma Y (2004) Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol 136:2734–2746. https://doi.org/10.1104/pp.104.046599
Seo E, Choi D (2015) Functional studies of transcription factors involved in plant defenses in the genomics era. Brief Funct Genom 14:260–267. https://doi.org/10.1093/bfgp/elv011
Sheshadri SA, Nishanth MJ, Simon B (2016) Stress-mediated cis-element transcription factor interactions iterconnecting primary and specialized metabolism in planta. Front Plant Sci 25:1725. https://doi.org/10.3389/fpls.2016.01725
Srinivasan K (2016) Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr 56:1488–1500. https://doi.org/10.1080/10408398.2013.772090
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14:310–317. https://doi.org/10.1016/j.tplants.2009.03.006
Voorrips RE (2020) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Wei Y, Wan H, Wu Z (2016) A comprehensive analysis of carotenoid cleavage dioxygenases genes in Solanum lycopersicum. Plant Mol Biol Rep 34:512–523. https://doi.org/10.1007/s11105-015-0943-1
Weng L, Zhao F, Li R et al (2015) The zinc finger transcription factor SlZFP2 negatively regulates abscisic acid biosynthesis and fruit ripening in tomato. Plant Physiol 167:931–949. https://doi.org/10.1104/pp.114.255174
Xu G, Guo C, Shan H, Kong H et al (2012) Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA 109:1187–1192. https://doi.org/10.1073/pnas.1109047109
Yang L, Liu Z, Lu F et al (2006) SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J 47:841–850. https://doi.org/10.1111/j.1365-313X.2006.02835.x
Yang J, Duan G, Li C et al (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front Plant Sci 10:1349. https://doi.org/10.3389/fpls.2019.01349
Yin M, Wang Y, Zhang L et al (2017) The Arabidopsis Cys2/His2 zinc finger transcription factor ZAT18 is a positive regulator of plant tolerance to drought stress. J Exp Bot 68:2991–3005. https://doi.org/10.1093/jxb/erx157
Yin J, Wang L, Zhao J et al (2020) Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol 20:359. https://doi.org/10.1186/s12870-020-02575-1
Yu Y, Li X, Wu Z et al (2016) VvZFP11, a Cys2His2-type zinc finger transcription factpr is involved in defense responses in Vitis vinifera. Biol Plant 60:292–298. https://doi.org/10.1007/s10535-016-0598-2
Yuan S, Li X, Li R et al (2018) Genome-wide identification and classification of soybean C2H2 zinc finger proteins and their expression analysis in legume-Rhizobium Symbiosis. Front Microbiol 9:126. https://doi.org/10.3389/fmicb.2018.00126
Zhang H, Zhao T, Zhuang P et al (2016) NbCZF1, a Novel C2H2-type zinc finger protein, as a new regulator of SsCut-induced plant immunity in Nicotiana benthamiana. Plant Cell Physiol 57:2472–2484. https://doi.org/10.1093/pcp/pcw160
Zheng J, Liu F, Zhu C et al (2019) Identification, expression, alternative splicing and functional analysis of pepper WRKY gene family in response to biotic and abiotic stresses. PLoS ONE 14:e0219775. https://doi.org/10.1371/journal.pone.0219775
Zhou Z, Sun L, Zhao Y et al (2013) Zinc finger protein 6 (ZFP6) regulates trichome initiation by integrating gibberellin and cytokinin signaling in Arabidopsis thaliana. New Phytol 198:699–708. https://doi.org/10.1111/nph.12211
Acknowledgements
RKJ is thankful to Dept. of Science Technology, Govt. of India for infrastructure support through the CURIE program. RM is also thankful to the President, Centurion University of Technology and Management for his encouragement and support.
Funding
Research work in the laboratory is supported by grants from Science and Engineering Research Board (SERB) (Grant no. EMR/2016/005234) and Dept. of Biotechnology (Grant no. BT/PR23412/BPA/118/284/2017), Govt. of India. BM is thankful for a Junior Research Fellowship from DBT, Govt. of India (Grant no. BT/PR23412/BPA/118/284/2017).
Author information
Authors and Affiliations
Contributions
RM and RKJ conceived and designed the research work. RS and BM conducted the research experiments including data curation, functional analysis, gene prediction and stress treatment. RM performed the qPCR validation of the genes and statistical analysis. RS, BM and RM wrote the manuscript and RKJ critically reviewed the manuscript. All the authors have read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest to influence the work reported in this paper. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
13205_2020_2601_MOESM10_ESM.jpg
Supplementary file10 Multiple protein sequence alignment of CanZF40, CanZF46, CaZFP1 from pepper and StZFP2 from potato. The alignment was performed with ClustalOmega tool using default parameters. (JPG 290 KB)
Rights and permissions
About this article
Cite this article
Sharma, R., Mahanty, B., Mishra, R. et al. Genome wide identification and expression analysis of pepper C2H2 zinc finger transcription factors in response to anthracnose pathogen Colletotrichum truncatum. 3 Biotech 11, 118 (2021). https://doi.org/10.1007/s13205-020-02601-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02601-x