Abstract
Propionic acid and its salts are widely used as food and feed preservative. Currently, these compounds are chemically produced, which is more profitable compared to biotechnological production using bacteria of the Propionibacterium genus. Appropriate steps can enable reducing the production costs; for example, cheap industrial byproducts can be used as culture media. One such cost-effective raw material is apple pomace, a low-value byproduct from the food industry. It contains sugars such as glucose and fructose which can serve as potential carbon sources for microorganisms. This paper discusses the possibility of using apple pomace in the production of propionic acid and presents an economic analysis of the production process. The tested strain produced 8.01 g/L of propionic acid (yield 0.40 g/g) and 2.29 g/L of acetic acid (yield 0.11 g/g) from apple pomace extract. The economic analysis showed that the production of 1 kg of propionic acid (considering only waste) from 1000 kg of apple pomace would cost approximately 1.25 USD. The manufacturing cost (consumables, including feedstock, labor, and utilities) would be approximately 2.35 USD/kg, and the total cost including taxes would be approximately 3.05 USD/kg. From the economic point of view, it is necessary to improve the production of propionic acid from apple pomace, to increase the yield of fermentation and thus decrease the total production costs. This can be achieved, for example, using industrial byproducts as nitrogen and vitamin sources, instead of high-cost substrates such as yeast extract or peptone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Propionic acid is synthesized by a petrochemical process involving hydrocarboxylation of ethylene with a catalyzer (e.g., carbonyl or rhodium nickel), which causes extensive damage to the natural environment (Stowers et al. 2014). However, the chemical production of this compound is more profitable than the biotechnological route using bacteria of the Propionibacterium genus. The cost of synthetic propionic acid is 1000 USD/1000 kg, while 1000 kg of natural propionic acid produced by Propionibacterium bacteria costs 2000 USD. Propionic acid is globally used as feed and cereal grain preservative, food preservative (propionate salts suppress the growth of mold and some bacteria, e.g., Listeria monocytogenes—when propionic acid is combined with lactic and acetic acids), and herbicide, as well as in the production of cellulose acetate (Wemmenhove et al. 2016; Piwowarek et al. 2018). In the pharmaceutical industry, sodium propionate is applied in animal therapy for treating wound infections and as a component of antiarthritic medicines (Hebert and Hebert 2017). The total production of propionic acid is estimated at 450,000 tons per year with an annual increase of 2.7% (2014–2020). The highest producer of propionic acid is BASF, the yield of which covers approximately 31% of the global market (Baumann and Westermann 2016; Gonzalez-Garcia et al. 2017). The global market of propionic acid accounted for 1.2 billion USD in 2018 and is predicted to generate 1.6 billion USD by 2026. The market is projected to grow at a CAGR (Compound Annual Growth Rate) of 3.5% from 2019 to 2026 (Global Opportunity Analysis and Industry Forecast 2019–2026). This progress can be achieved by the developing countries of Africa and Asia, which are becoming increasingly popular for trade. Natural preservatives were introduced by developed countries, with an aim of consuming and selling high-quality products without the addition of synthetic preservatives (clean-label products). The rise in the use of natural preservatives (including propionic acid) was facilitated by changes in lifestyle and diet and rapid growth of the food industry. Organic acids are a group of chemical compounds used in the food industry as preservatives. Their global market is expected to reach 12.54 billion USD by 2026 (Kim et al. 2018).
Due to the deficiency of resources, fluctuation of petroleum prices, environmental damage caused by chemical production, increasing costs associated with the utilization of organic waste, and use of natural and ecological edible products, the concept of propionic acid production using microbial technology is gaining importance (Ekman and Börjesson 2011; Ali et al. 2021). The industrial use of fermentation technology for the production of propionate is limited due to the low yield and complexity of the process. These drawbacks are related to the negative feedback mechanism of the acids produced in the process. Therefore, metabolic, genetic engineering, and co-cultivation systems (co-culture of propionic acid bacteria (PAB) and Saccharomyces cerevisiae or lactic acid bacteria) were applied to increase the effectiveness of propionic acid production (Suwannakham et al. 2006; Liu et al. 2012; Zhu et al. 2012; Zhuge et al. 2014; Guan et al. 2015; Wang et al. 2015; Xie et al. 2019). Because the use of genetically modified organisms is still considered to be controversial in society, studies have been conducted to naturally increase the yield of propionic acid, without any interference in the genome of the target organisms. The best producer of propionic acid—Acidipropionibacterium acidipropionici (formerly Propionibacterium acidipropionici)—can give a yield of 0.78 or 0.86 g/g (with glycerol as carbon source) and productivity of 0.32–2.10 g/L⋅h (Liu et al. 2011; Dishisha et al. 2012; Turgay et al. 2020). However, a further improvement of yield and reduction in cost are required for the fermentation process to be more competitive than the chemical method (Rodriguez et al. 2014; Stowers et al. 2014). Carbon and nitrogen sources account for the largest part (30%) of the total costs of propionic acid production (Tufvesson et al. 2013). Hence, currently, there is a search for waste materials that could be a cheaper alternative to conventional C and N sources (Kot et al. 2020). Several products are considered excellent carbon sources, including glycerol, corncob molasses, corn stover, cane molasses, beet molasses, wheat flour, sugarcane bagasse, Jerusalem artichoke, cheese whey, wheat bran, and even effluent from animal feed production (Zhang and Yang 2009a, 2009b; Feng et al. 2011; Zhu et al. 2012; Wang and Yang 2013; Dishisha et al. 2015; Wang et al. 2017, 2020; Teles et al. 2019; Xie et al. 2019). However, many of these require expensive pretreatments and enzymatic hydrolysis, which account for a significant portion of the final product cost. Apple pomace contains sugars such as glucose, fructose, saccharose, and sorbitol, which are carbon sources that can be directly assimilated and fermented by a wide group of microorganisms, including bacteria of the Propionibacterium genus.
Apples are a source of many products; they are consumed directly as a fruit, used to prepare juices (either alone or in combination with other fruits), ciders, or as dried pieces. Every year, a large number of apples are pressed, which results in a huge quantity of skin, pulp, and seeds, which are collectively called apple pomace (Grigoras et al. 2013; Magyar et al. 2016). It should be noted that the use of Propionibacterium bacteria would enable decreasing the environmental pollution caused by the disposal of this difficult-to-eliminate waste. Apple pomace is mostly considered as waste and disposed of in landfills. Currently, the applications of apple pomace are limited. For example, due to the low level of protein, this waste is not deemed ideal for use as animal feed. Furthermore, isolation of pectin from apple pomace is quite expensive and unprofitable. Hence, new applications are sought for apple pomace. The large annual production (10 billion tons all over the world), composition (e.g., sugars, organic acids, fibers, minerals, vitamins) (Piwowarek et al. 2019), and low cost suggest that it might be favorable to transform apple pomace to its microbiological metabolites (Zhang et al. 2020). Two studies have analyzed the utilization of apple pomace by PAB. Piwowarek et al. (2016) used apple pomace for the production of propionic acid by Propionibacterium freudenreichii, but the production process turned out to be inefficient. In 2019, the same group (Piwowarek et al. 2019) optimized this process, but only on the flask scale. Therefore, the present study aimed to evaluate the possibility of using apple pomace for the production of propionic acid in scale-up fermentation in a bioreactor. In addition, an economic analysis of propionic acid production from apple pomace extract (APE) was performed to check whether the use of waste materials may allow decreasing the cost of propionic acid production, thus making the biosynthesis approach cheaper and more profitable compared to the use of other waste materials and chemical methods. This article presents the economic analysis based on the obtained results, and also shows several hypothetical variants (based on literature data) depicting the possibility of improving the biotechnological utilization of apple pomace.
Materials and methods
Biological material
Propionibacterium freudenreichii T82 strain was obtained from the collection of the Department of Food Biotechnology and Microbiology, Warsaw University of Life Sciences. The bacterial culture was stored in the VL medium at a temperature of 4 °C.
The apple pomace used in this work was obtained from the same series as in the study of Piwowarek et al. (2019). It was derived from different Polish varieties of apples and was stored at a temperature of − 20 °C without washing, drying, or milling.
Media
Inoculum medium
The inoculum was prepared in a liquid VL (BTL) medium consisting of yeast extract (5 g/L), peptone (5 g/L), sodium chloride (5 g/L), meat extract (3 g/L), L-cysteine hydrochloride (0.4 g/L—to create an anaerobic condition), and glucose (0.5 g/L) (pH 7.0). The pH of the medium was adjusted to 7.0 by adding 20% NaOH. The medium was sterilized in an autoclave for 15 min at 117 °C.
Media with pure sugars (control media)—glucose and/or fructose
The concentration of sugars in the control media was chosen such that it substitutes the concentration of the total sugars present in APE. The control media contained: (I) 2.5 g/L of glucose, (II) 2.5 g/L of fructose, or (III) 1.25 g/L of glucose and 1.25 g/L of fructose. Each medium (I, II, and III) was also supplemented with peptone (5 g/L), yeast extract (10 g/L), potassium hydrogen phosphate (1.5 g/L), dipotassium hydrogen phosphate (2.5 g/L), and L-cysteine hydrochloride (0.4 g/L—to create an anaerobic condition) (pH 7.0). The pH of the media was adjusted to 7.0 by adding 20% NaOH. The C/N molar ratio of the media was approximately 6.82:1. The media were sterilized in an autoclave for 15 min at 117 °C.
Apple pomace medium
The media containing apple pomace were prepared as follows. To 1000 g of apple pomace, 1000 mL of distilled water was added, and the mixture was heated for 30 min at 70–75 °C (above 80 °C, the extraction rate and efficiency are lowered due to the conversion of protopectin to pectin). After heating, the insoluble materials were removed by pressing and filtration, and the supernatant (extract) was used to prepare the experimental substrates (Piwowarek et al. 2019).
The production medium contained APE as a carbon source (approximately 2.5 g/L of sugars after diluting), with the following growth-supporting components added according to Piwowarek et al. (2019): peptone (5 g/L), yeast extract (10 g/L), L-cysteine hydrochloride (0.4 g/L—to create an anaerobic condition), potassium hydrogen phosphate (1.5 g/L), dipotassium hydrogen phosphate (2.5 g/L), and biotin (0.2 mg/L). Bioreactor with the medium was sterilized in an autoclave for 15 min at 117 °C. The C/N molar ratio of the medium was approximately 4.70:1 (Piwowarek et al. 2019). Biotin was added to the medium after sterilization through sterile disposable syringe filters. The composition of this substrate was chosen based on a study that optimized the composition of the apple pomace medium (Piwowarek et al. 2019).
Culture conditions
Inoculum culture
The inoculum cultures were grown in Erlenmeyer flasks containing 50 mL of the medium at 30 °C. The incubation was continued until the optical density of the suspension reached 2.0 (λ = 550 nm). Then, the cultures were centrifuged (25 mL, 10 min at 10,000 rpm; Centrifuge 5804R, Eppendorf). The resulting supernatant was decanted, and the biomass was suspended in the control media (25 mL) or production medium (400 mL). The inoculation cultures were added to flasks or bioreactors containing the control media (225 mL) and production medium (3600 mL).
Flask cultures (control media—with glucose and/or fructose)
The volume of the control media was 250 mL (to keep the volume of media constant, for each analyzed hour of fermentation different flasks were used for the process—0, 24, 48, 72, 96, and 120 h). The inoculum constituted 10% of the production medium. The pH of the media during the fermentation process was adjusted to 7.0 by adding 20% NaOH every 24 h. All the fermentation processes were carried out in static flasks under anaerobic conditions at 37 °C.
Bioreactor cultures (medium with apple pomace)
Cultures with APE were carried out in a bioreactor (BIOFLO 300; New Brunswick Scientific, USA) containing 4 L of fluid culture medium. The inoculum constituted 10% of the production medium. Cultures were carried out for 120 h with agitation at 100 rpm at 37 °C and a pH range of 5.5–7.0. The active acidity of the medium during the fermentation process was adjusted automatically to pH 7.0 by adding 20% NaOH. Samples were collected for analysis at 0, 24, 48, 72, and 120 h of the process.
Analysis of fermentation broth
The following parameters of fermentation broth were analyzed: content of sugars, total protein, pH, and content of propionic and acetic acids. Sugars were determined using the Miller method (1959), while total protein was determined by the Kjeldahl method PN-EN ISO 5983–1(2006). Potentiometric method was used for determining the pH of the media (Conbest CP-501).
Propionic and acetic acids produced during the fermentation process were analyzed by gas chromatography equipped with a flame ionization detector. Before the extraction of the acids, 25% sulfuric acid (VI) was added to the media to release free organic acids from sodium propionate and sodium acetate (resulting from pH control). In addition, carboxylic acid was extracted from the media using a mixture of hexane (Chempur) and diethyl ether (Chempur) (1/1, v/v). The chromatographic separation was carried out on a ZB-WAX plus column (30 m × 0.25 mm × 0.25 μm). Qualitative and quantitative calculations were made by comparing the retention times of the tested samples with those of the standards and internal standard (undecanoic acid—C11:0; Sigma Aldrich). Correction factors were used to calculate the concentrations of acids.
The acids production was analyzed by the following parameters:
Yield = \(\frac{\mathrm{product concentration measured in the aqueous phase }[\mathrm{g}]}{\mathrm{carbon source concentration measured in the aqueous phase }[\mathrm{g}]}\) [g/g].
Productivity = \(\frac{\mathrm{product concentration measured in the aqueous phase }[\mathrm{g}]}{\mathrm{volume of the fermenting medium }\left[\mathrm{L}\right]\cdot \mathrm{ time }[\mathrm{h}]}\) [g/L·h].
P/A ratio = \(\frac{\mathrm{propionic acid concentration measured in the aqueous phase }[\mathrm{g}]}{\mathrm{acetic acid concentration measured in the aqueous phase}[\mathrm{g}]}\)
Measurement of bacterial biomass
Cell dry weight (d.w.) was measured throughout the fermentation process, by analyzing the changes in biomass. A total of 25 mL of the culture medium was centrifuged for 10 min at 10,000 rpm (Centrifuge 5804R, Eppendorf). Then, the supernatant was removed, and the biomass was washed with deionized water and centrifuged again (10 min at 10,000 rpm; Centrifuge 5804R, Eppendorf). The wet cellular biomass was dried at 85 °C (SML 32/250 Zelmed, Poland) until a constant weight was reached.
Economic analysis
Economic analysis was carried out for the production of calcium and sodium propionate from APE in a few variants (Table 1). Based on the study of Yang et al. (2018), the following were used as the baseline for a 1000-MT plant in the analysis: propionic acid concentration = 50 g/L, propionic acid yield = 0.50 g/g (theoretical yield), and productivity = 1 g/L⋅h. For four variants, a yield of 0.40 g/g was used (practical yield obtained in this study). The unit costs of raw materials and utilities were assumed based on the current market prices, whereas major equipment costs and capital investments were assumed based on the actual costs of a fermentation plant with 1000-MT capacity (Tufvesson et al. 2013; Cheng et al. 2017; Yang et al. 2018). After performing membrane filtration to separate the cells for recycling, the fermentation broth containing 10% (w/v) total solids (approximately 50% propionic acid, 20% other acids, 20% calcium, and 10% others) was concentrated to 50% total solids via evaporation, and then spray-dried to a powder product containing 63.5% calcium/sodium propionate or 50% propionic acid and less than 5% (w/w) water (Yang et al. 2018). Parameters such as equipment operation/maintenance, utilities, depreciation, labor, financial cost, facilities and administrative costs, tax, total capital investment, and revenues were standardized (Yang et al. 2018) for all analyzed variants for data clarity.
Statistical analysis
All experiments were carried out in triplicates. Mathematical and statistical calculations were performed using Excel 2013 for Windows 10 and Statistica 10.0 (StatSoft Inc.). Normality of the data was tested using the Shapiro–Wilk test, and variance homogeneity using the Levene test. To determine the significance of differences between the mean values of different experimental groups, a single-variant analysis of variance and Tukey’s test were carried out. All analyses were performed at a significance level of α = 0.05.
Results and discussion
Flask cultures in media with pure glucose and/or fructose
The first stage of this work aimed to evaluate the fermentation process of apple pomace by P. freudenreichii T82 strain. As preliminary research, the culture was carried out in flasks in media containing pure sugars (i.e., glucose and fructose) that are present in apple pomace. A similar concept was applied by Feng et al. (2011), Liang et al. (2012), and Yang et al. (2018). They studied propionic acid fermentation of sugars contained in, respectively, sugarcane (glucose, fructose, and saccharose), Jerusalem artichoke (glucose and fructose), and soy molasses (glucose, fructose, galactose, and raffinose).
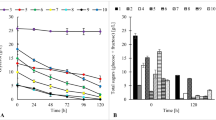
The present study showed that in the medium containing glucose, the sugar was completely utilized at 96 h of fermentation (24.19 g/L). In the media containing fructose and both glucose and fructose, the complete depletion of carbon sources by bacteria was observed at 120 h of the process (24.46 and 24.97 g/L, respectively). The total growth of P. freudenreichii T82 strain was similar in each medium (> 4.3 g d.w./L), but the most rapid growth was noticed in the media containing glucose as the only carbon source. Furthermore, in this culture variant, a biomass yield of more than 4 g d.w./L was obtained at 48 h (Fig. 1).
The process of propionic acid fermentation in culture media is described in Fig. 1 and Table 2. Propionic acid was the main product obtained in each medium. In the medium containing glucose as the only carbon source (I), the maximum production of propionic and acetic acids amounting to 9.04 and 3.97 g/L was achieved at 96 h of fermentation, with a yield of 0.37 and 0.17 g/g, respectively. In the culture media containing fructose (II), as well as glucose and fructose (III), the maximum production of acids was observed 24 h later. In the medium containing fructose, the maximum production of propionic and acetic acids by bacteria was, respectively, 9.31 (yield 0.38 g/g) and 3.52 g/L (yield 0.14 g/g), while in the medium containing both sugars it was 9.16 (yield 0.37 g/g) and 3.62 g/L (yield 0.14 g/g), respectively (Table 2).
Feng et al. (2011) used P. freudenreichii CCTCC M207015 strain and found that it can assimilate and ferment glucose, fructose, and saccharose. The complete utilization of carbon sources was observed only in the media with glucose, after 120 h of the process. The highest amount of propionic acid was produced in the media with glucose—14.58 g/L. The best propionic acid/acetic acid (P/A) ratio (5.34:1) was observed in the fructose medium. Liang et al. (2012) used A. acidipropionici ATCC 4875 strain to produce propionic acid from glucose and fructose. The primary concentration of carbon sources in the media was 60 g/L. Both glucose and fructose were used by the tested strain. In the medium containing fructose as the only carbon source, lower acetic acid production was observed than that in the glucose medium. The highest yield of propionic acid was obtained through simultaneous fermentation of glucose and fructose.
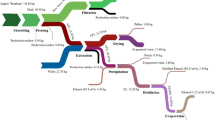
Pyruvate is an important compound of PAB metabolism, from which propionic acid, acetic acid, and biomass are produced (Fig. 2). Propionibacterium bacteria can produce pyruvate (and from pyruvate—propionic acid) through two pathways: EMP (Embden–Meyerhof–Parnas) (Piwowarek et al. 2020) (Online Resource 1) (Kanehisa and Goto 2000) and HMP (Pentose Phosphate Pathway) (Piwowarek et al. 2020) (Online Resource 2) (Kanehisa and Goto 2000). Glucose can be used in both pathways, while fructose is used only in the EMP pathway, which has one lesser enzymatic reaction than that involving glucose as a substrate. In the HMP pathway, 11 mol of NAD+ are reduced to 11 mol of NADH, with 5 mol of pyruvate and 5 mol of ATP produced. This pathway is mainly used to synthesize ATP, which is required for the growth of bacteria. In turn, in the EMP pathway, 2 mol of pyruvate and NADH and 2 mol of ATP are produced. PAB require 4 mol of pyruvate, 33.7 mol of ATP, and 5.75 mol of NADH for their growth (Wang and Yang 2013), which can be provided rapidly by the HMP pathway. During biomass production, 5.75 mol of NAD+ are produced. This hampers the intracellular redox balance, resulting in a higher synthesis of acetic acid and reduction of NAD+ to NADH, which can be used for propionic acid synthesis. Hence, faster growth of bacteria and more intense synthesis of acetic acid by P. freudenreichii T82 strain were observed in the media containing only glucose as a carbon source (Fig. 1). Moreover, faster total utilization of sugar was found, which led to a faster fermentation process. This probably resulted from a greater amount of ATP created during the HMP pathway. Simultaneous fermentation of glucose and fructose caused an increase in the P/A ratio compared to that observed when glucose alone was used in the medium (Table 2).
The route of propionic acid production by Propionibacterium freudenreichii T82 (Wang and Yang 2013; Piwowarek et al. 2018). As a complement to figure 1, the scheme of EMP and HMP pathways obtained on the basis of bioinformatic analysis of the genome of the P. freudenreichii T82 strain (Piwowarek et al. 2020) are included - Online Resource 1 and Online Resource 2
The results obtained by Feng et al. (2011), Liang et al. (2012), and Yang et al. (2018) are quite similar to those of the present study. The higher concentration and yield of propionic acid production in the cited papers might be related to the higher concentrations of carbon sources (4–6 g/L), higher scale of the experiment, or the use of a more efficient strain (e.g., A. acidipropionici). However, all these findings (Feng et al. 2011; Liang et al. 2012; Yang et al. 2018) indicate the lower production of acetic acid and similar or higher production of propionic acid with the use of fructose or glucose and fructose as carbon sources compared to glucose alone. The obtained data suggest that P. freudenreichii T82 strain can grow and produce propionic acid in a culture medium consisting of apple pomace, as APE mainly contains glucose and fructose as sugars (Piwowarek et al. 2016, 2019). In the next step, the fermentation process was carried out in a bioreactor environment with the medium containing waste in the form of apple pomace.
Bioreactor cultures in media with apple pomace extract
The present study evaluated propionic acid fermentation with APE using P. freudenreichii T82 strain in a bioreactor. The culture was carried out in a medium, the composition of which was based on Piwowarek et al. (2019). The temperature of the medium was maintained at 37 °C and pH at 5.5–7.0. It was assumed that the use of a bioreactor should enable higher production of propionic acid from APE if the optimal pH of the medium was maintained throughout the process.
Figure 3 shows the fermentation of APE in a bioreactor environment. The results showed that the tested strain utilized all the reducing sugars contained in the medium (20.05 g/L). The complete utilization of these carbon sources was observed at 96 h of the process. After 120 h of fermentation, the use of saccharose was not found. Piwowarek et al. (2019) used the same medium for fermenting propionic acid on a flask scale. Compared to their results (0.17 g/L⋅h), the rate of sugar consumption in this study was higher (0.21 g/L⋅h), which might be because the fermentation took place for up to 96 h instead of 120 h.
Propionic acid fermentation of APE medium in bioreactor environment. *Protein can be considered as nitrogen as well (to obtain nitrogen concentration divide the protein content by 6.25—according to the Kjeldahl method). a, b, c, d, e—designated homogeneous groups of the influence of fermentation time on the use of sugar. a, b, c, d, e—designated homogeneous groups of the influence of fermentation time on the use of protein. a, b, c, d, e—designated homogeneous groups of the influence of fermentation time on the bacterial growth. a, b, c, d, e—designated homogeneous groups of the influence of fermentation time on production of propionic acid. a, b, c, d, e—designated homogeneous groups of the influence of fermentation time on production of acetic acid All analysis were carried out using the Tukey’s test (a one-way analysis was carried out variance)
The total biomass yield of Propionibacterium freudenreichii T82 strain within the first 48 h of culture was 2.7 g d.w./L, whereas from 48 to 120 h, it was 0.42 g d.w./L. Statistically significant growth was observed up to 96 h, during which the highest growth of the tested strain was noted (3.34 g d.w./L). In the flask, the maximum biomass yield of P. freudenreichii T82 was recorded at 120 h—3.09 g d.w./L (Piwowarek et al. 2019). In turn, this yield of cellular biomass was achieved after 3 days of cultivation in the bioreactor. The lethal phase did not occur in the 5 days of fermentation. The ability of PAB to survive for a longer time in the culture medium, especially in the log phase, is related to the fact that this bacterial species can produce glycogen, trehalose, and polyP (Falentin et al. 2010), which provide energy for the microorganisms to survive in stressful conditions (nutrient deficiency or presence of acids) (Cardoso et al. 2007).
The maximum production of propionic acid (8.01 g/L) was observed at 96 h, while that of acetic acid (2.34 g/L) at 120 h of fermentation. In the bioreactor environment, within 4 days of fermentation, the tested strain was able to produce more propionic acid compared to culture in the flask after 120 h (7.65 g/L) (Piwowarek et al. 2019). The use of bioreactor instead of flask allowed obtaining more propionic acid in a shorter time, which may be important considering the profitability of propionic acid production from apple pomace. Table 3 shows the parameters of propionic acid fermentation. At the beginning of fermentation, the yield of propionic acid was 0.26 g/g at 24 h and 0.29 g/g at 48 h. The total yield was 0.40 g/g. The highest yield of acetic acid (0.16 g/g) was observed at 24 h. The low yield of propionic acid at the beginning was probably due to the intensive growth of bacteria in the initial phase of culture, during which bacterial metabolism is directed toward cell division and growth (log phase). On entering the stationary phase, the production of propionic acid by the tested strain increased. The higher yield of acetic acid at the beginning of fermentation compared to the total yield (0.11 g/g) might be related to the regulation of redox potential. The growth of bacterial biomass caused the oxidation of NADH to NAD+. As a result, higher production of compensating metabolite (acetic acid) was required, during which NAD+ was reduced to NADH (Fig. 2).
The results obtained in bioreactor and their comparison with the results obtained in flasks, as well as the results of Piwowarek et al. (2019), show that fermentation in the bioreactor leads to faster consumption of carbon sources, intensified growth of bacteria, and higher yield of propionic acid production and higher P/A ratio. This may be because of the following reason. In the bioreactor, fermentation was carried out in a culture medium with constant pH at 5.5–7.0 (optimal range according to the literature data). The use of a bioreactor enabled automatic regulation of pH. When the pH of the culture medium decreased to 5.5, 20% NaOH was added automatically (to stabilize active acidity and bring the pH to the neutral level). Thus, the pH of the culture medium was maintained stable at the optimal level for PAB throughout the fermentation process. The culture medium in the flask scale was neutralized at 24-h intervals (Piwowarek et al. 2019), due to which the pH of the culture environment decreased below 5.0. The pH of the culture is the crucial factor for the dissociation of acids produced during fermentation. Undissociated acids show hydrophobic characteristics and can easily pass through a cell, where they exhibit strong toxicity. The active acidity of the cytoplasm is maintained at neutral pH, which prevents the degradation of cellular components that are sensitive to acids and bases. After the acids enter a cell, they are dissociated due to the neutral pH of the cytoplasm. An excess of H+ or anion concentration in the cytoplasm inhibits metabolism and disturbs the energy balance of the cell. It is likely that due to constant neutralization of the culture medium, undissociated acids that could permeate the cells were not present in the culture environment. Furthermore, H+ ions did not penetrate the cells because of the cell structure; thus, the expulsion of protons by H+ATPase was not required. Consequently, more ATP was used by bacteria for their growth (Budin-Verneuil et al. 2005).
Feng et al. (2011) obtained 11.98 g/L of propionic acid from cane molasses. The yield of the fermentation process was 0.38 g/g. The initial sugar concentration was 40 g/L (4.18 g/L glucose, 7.01 g/L fructose, and 28.81 g/L sucrose). The tested strain (P. freudenreichii CCTCC M207015) consumed 82.80% of sugars. Liang et al. (2012) used Jerusalem artichoke (20 g/L glucose and 40 g/L fructose) as a carbon source for A. acidipropionici ATCC 4875 strain which produced 22.9 g/L of propionic acid with a yield of 0.42 g/g and consumed sugar at a level of 91.17%. Soy molasses have been proved to be the best among the wastes. Yang et al. (2018) showed that A. acidipropionici ACT-1 strain produced 21.1 g/L of propionic acid from hydrolysate soy molasses with a yield of 0.46 g/g and total utilization of sugars (42.2 g/L). In fibrous-bed bioreactor (FBB), the yield was even higher—0.51 g/g. As shown in the above-cited studies, P. freudenreichii T82 strain was found to synthesize propionic acid from waste with a similar yield (0.40 g/g). To achieve this efficiency, APE was supplemented with nitrogen sources and biotin (Piwowarek et al. 2019). On the other hand, the majority of industrial byproducts that were applied to produce propionic acid in the laboratory required pretreatment (chemical or enzymatic hydrolysis) and/or supplementation. Feng et al. (2011) and Yang et al. (2018) used waste Propionibacterium cells and corn steep liquor, respectively, which suggested that expensive yeast extract can be replaced with lower cost nitrogen sources such as industrial waste materials. The higher yield of propionic acid production in the studies of Liang et al. (2012) and Yang et al. (2018) likely resulted from the fact that they used a better producer of propionic acid—A. acidipropionici. In the case of the same species—P. freudenreichii—the yield was similar, regardless of the media (pure sugars, cane molasses, APE) (0.37–0.40 g/g). However, the highest yield was obtained in the medium with APE, which may be due to the addition of biotin, use of better nitrogen sources such as yeast extract and peptone, or differences in the applied culture conditions (temperature, pH control, volume of culture, C/N molar ratio).
Economic analysis
The cost of the substrate is an important component of the final product cost (Rodriguez et al. 2014). Corn dextrose containing 95% of glucose costs 0.45 USD/kg, and the yield of propionic acid production is 0.50 g/g (theoretical yield); thus, it allows producing 1 kg of this acid at a cost of 1.03 USD. The cost of production of 1 kg of propionic acid from corn is 0.46 USD; from soy molasses, 0.22 USD; and from glycerol, 0.88 USD (theoretical yield 0.65 g/g) (Table 4). With the use of apple pomace (yield 0.40 g/g) and 4% of sugars, the cost of production of 1 kg propionic acid is 1.25 USD, while with 5% of sugars it is 1 USD/kg. It should be noted that this calculation considers only the carbon sources used. Raw material processing (e.g., hydrolysis) is associated with additional expenses such as the purchase of enzymes and overall technology. Apart from the carbon source, other compounds are required for the synthesis of propionic acid. With regard to the nitrogen source, yeast extract is the most expensive, but it is the best source of nitrogen and vitamins for PAB. Thus, the use of 1 kg of this substrate for producing 1 kg of propionic acid is more expensive, which is approximately 0.81 USD (Table 4).
The efficient production of propionate through the biotechnological approach is limited mainly by the negative feedback mechanism of acids (Guan et al. 2013; Ali et al. 2020). Acids substantially decrease the pH of the culture environment and thus restrict the growth of bacteria and their metabolism (Piwowarek et al. 2018). The easiest method of alkalization of culture media involves the use of appropriate alkalizers such as NaOH or CaO; the cheapest among them is CaO, while the most expensive one is ammonia. When NaOH is used, the production cost of 1 kg of propionic acid is 0.38 USD.
Apart from carbon and nitrogen sources, pH of the culture environment, and other compounds, a very important component of the cost of propionic acid production is energy and water consumption. Due to this, it is necessary to obtain a huge amount of propionic acid in a short time (productivity of fermentation). Energy and water are needed at each stage of the production process, from the preparation of culture media, fermentation, separation of biomass, thickening of the fermentation broth, and separation of propionic acid, to the drying of propionate to a powder form (Yang et al. 2018). Because it is difficult to separate propionic acid from acetic acid, lower synthesis of acetic acid results in a lower cost of production of pure propionic acid from the fermentation broth (Yang et al. 2007). Currently, the production of propionic acid through the biotechnological approach cannot compete with the chemical method using crude oil, where the total production cost of 1 kg of propionate is 1.3 USD. Considering the expenses alone, it is difficult and almost impossible to change the method of industrial production of propionic acid (Tufvesson et al. 2013; Stowers et al. 2014).
Table 5 shows the estimated economics of propionic acid production from APE in a few variants (Table 1). The process involves the initial processing of material, fermentation process, separation of bacterial cells from media, evaporation to thicken the media, and drying to obtain the final product in a powder form (Yang et al. 2018). The estimated cost of propionic acid production from APE, yeast extract, and NaOH used as an alkalizer with a production yield of 0.40 g/g (conditions applied in this research) is 3.05 USD/kg (Table 5). The main component of expenses is substrates—52%. For dextrose, raw materials are the primary component of the product cost—47%. When soy molasses and corn molasses are used, the costs of substrates account for 17.6% and 24.4% of the total cost, respectively (Yang et al. 2018). According to Yang et al. (2018), the total cost of obtaining 1 kg of propionic acid (with a production yield of 0.50 g/g) from dextrose and yeast extract is 2.12 USD (payback period: 38.3 years); from corn and corn-starch liquor (CSL)—1.49 USD (payback period: 8.46 years); and from soy molasses and CSL—1.37 USD (payback period: 7.35 years). At 3.0 USD/kg of the selling price, 15% tax rate on revenues, and estimated capital investment of 6.85 million USD, the propionic acid production from APE, yeast extract, and NaOH is unprofitable—there would be no payback and profits (without payback) (Table 5). To obtain profits, the selling price of the product would have to be approximately 3.5–4 USD/kg (payback period: 15.15 and 7.19 years, respectively). However, due to such a price, propionic acid production using apple pomace in the biotechnological route is still not competitive to the petrochemical method. On the other hand, the added values of the biotechnological method, which cannot be measured in terms of financial aspects, are diversification of waste, a substantial decrease of environmental pollution, and safety of the obtained products, which is associated with the GRAS (Generally Recognized as Safe) and QPS (Qualified Presumption of Safety) status of PAB. In the present study, yeast extract was used as a nitrogen source and NaOH was added for pH control. Their high price led to a huge increase in production cost. Therefore, these substrates need to be replaced with a cheap alternative like industrial wastes, such as potato wastewater, CSL, and CaO (Dishisha et al. 2013; Zhang et al. 2015b; Yang et al. 2018; Kot et al. 2020). Product cost can also be lowered by increasing the yield of propionic acid production due to the decrease in depreciation cost. Increasing the concentration of propionic acid in the fermentation broth to more than 50 g/L can reduce the total liquid volume, and thus the amount of steam and electricity used in the process (Yang et al. 2018). If APE, potato wastewater, and NaOH (4% of sugars, yield 0.40 g/g) were used, the total cost of propionic acid production would be 2.64 USD/kg (payback period: 19.18 years), but if APE, potato wastewater, and CaO (4% of sugars; yield 0.50 g/g) were used it would be 2.38 USD/kg (payback period: 11.01 years), which is approximately 0.70 USD/kg lesser compared to the variant of the medium used in this study (VII). The lowest cost of propionic acid production will be attained when all the sugars are consumed by bacteria (5%)—2.28 USD/kg (payback period: 9.48 years) (APE, potato wastewater, CaO; yield 0.50 g/g). Higher yield of propionic acid production and consumption of sugars by bacteria may be achieved using the best propionic acid producer—A. acidipropionici (Guan et al. 2018).
Previous studies have shown that propionic acid yield can be increased using appropriate cofactors, such as biotin (Dishasia et al. 2015, Piwowarek et al. 2019). In addition, the crucial components of propionate synthesis are cobalamin, nicotinic acid, and pantothenic acid. As pure vitamins are expensive, industrial wastes containing them can be used to decrease cost, at an amount that ensures safe excess in the medium during the fermentation process. A disadvantage of apple pomace is the lower content of carbon sources compared to other waste raw materials, including soy molasses, beet molasses, and glycerol. Therefore, to obtain a similar concentration of propionic acid with a similar production yield, higher amounts of apple pomace need to be used as compared to other materials. Apple pomace is not an ideal medium for culturing PAB, but it can be used in combination with other waste materials such as glycerol and/or potato wastewater. In such a culture medium, apple pomace could serve as the source of carbon and vitamins (Piwowarek et al. 2019), while potato wastewater can provide nitrogen and vitamins (Dishasia et al. 2015). Moreover, glycerol can limit acetic acid production, thus ensuring higher efficiency of propionic acid synthesis (Wang and Yang 2013). In future studies focusing on the utilization of apple pomace, CaO or CaCO3 should be used as an alkalizer instead of the more expensive NaOH (Zhang et al. 2015a). Propionate production could also be improved using appropriate fermentation techniques such as immobilization, using FBB, or by enabling cell adaptation to a higher concentration of propionic acid and to increase cell density and viability in the fermentation (Chen et al. 2012; Eş et al. 2017; Gonzalez-Garcia et al. 2017).
Conclusion
The obtained results show that apple pomace could be used in the production of propionic acid with bacteria of the Propionibacterium genus. During 96 h of culture, PAB produced 8.01 g/L of propionate with a yield of 0.40 g/g of substrate. The economic analysis showed that apple pomace should be used as one of the waste components of the medium, and not alone. It ought to be emphasized that to make propionic acid production from apple pomace more profitable than chemical production, further studies are necessary to increase the efficiency of propionic acid fermentation and simultaneously decrease the cost of this process. It may be achieved by applying a continuous culture in a medium consisting of only waste materials (e.g., apple pomace, potato wastewater, glycerol), which are low-cost sources of carbon, nitrogen, and vitamins instead of expensive traditional compounds such as yeast extract, peptone, or pure vitamins. A more profitable fermentation process may enable the natural production of propionic acid and replace the chemical synthesis of this compound. At the same time, it will contribute to improving the environment through the microbiological utilization of waste materials.
References
Ali R, Saravia F, Hille-Reichel A, Gescher J, Horn H (2021) Propionic acid production from food waste in batch reactors: Effect of pH, types of inoculum, and thermal pre-treatment. Bioresour Technol 319:124166. https://doi.org/10.1016/j.biortech.2020.124166
Baumann I, Westermann P (2016) Microbial production of short chain fatty acids from lignocellulosic biomass: current processes and market. BioMed Res Int 1–15. https://doi.org/https://doi.org/10.1155/2016/8469357
Budin-Verneuil A, Pichereau V, Auffray Y, Ehrlich DS, Maguin E (2005) Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 5:4794–4807
Cardoso FS, Castro RF, Borges N, Santos H (2007) Biochemical and genetic characterization of the pathways for trehalose metabolism in Propionibacterium freudenreichii, and their role in stress response. Microbiology 153:270–280
Chen F, Feng XH, Xu H, Zhang D, Ouyang PK (2012) Propionic acid production in a plant fibrous-bed bioreactor with immobilized Propionibacterium freudenreichii CCTCC M207015. J Biotechnol 164:202–210. https://doi.org/10.1016/j.jbiotec.2012.08.025
Cheng C, Zhou Y, Lin M, Wei P, Yang ST (2017) Polymalic acid fermentation by Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: fermentation kinetics and economic analysis. Bioresour Technol 223:166–174
Dishisha T, Alvarez MT, Hatti-Kaul R (2012) Batch- and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici. Bioresour Technol 118:553–562. https://doi.org/10.1016/j.biortech.2012.05.079
Dishisha T, Ståhl A, Lundmark S, Hatti-Kaul R (2013) An economical biorefinery process for propionic acid production from glycerol and potato juice using high cell density fermentation. Bioresour Technol 135:504–512. https://doi.org/10.1016/j.biortech.2012.08.098
Dishisha T, Ibrahim MHA, Cavero VH, Alvarez MT, Hatti-Kaul R (2015) Improved propionic acid production from glycerol: combining cyclic batch and sequential batch fermentations with optimal nutrient composition. Bioresour Technol 176:80–87. https://doi.org/10.1016/j.biortech.2014.11.013
Ekman A, Börjesson P (2011) Environmental assessment of propionic acid produced in an agricultural biomass-based biorefinery system. J Clean Prod 19:1257–1265. https://doi.org/10.1016/j.jclepro.2011.03.008
Eş I, Khaneghah AM, Hashemi SMB, Koubaa M (2017) Current advances in biological production of propionic acid. Biotechnol Lett 39:635–645. https://doi.org/10.1007/s10529-017-2293-6
Falentin H, Deutsch SM, Jan G, Loux V, Thierry A, Parayre S, Maillard MB, Dherbécourt J, Cousin FJ, Jardin J, Siguier P, Couloux A, Barbe V, Vacherie B, Wincker P, Gibrat JF, Gaillardin C, Lortal S (2010) The complete genome of Propionibacterium freudenreichii CIRM-BIA1T, a hardy actinobacterium with food and probiotic applications. PLoS ONE 5:1–12. https://doi.org/10.1371/journal.pone.0011748
Feng X, Chen F, Xu H, Wu B, Li H, Li S, Ouyang P (2011) Green and economical production of propionic acid by Propionibacterium freudenreichii CCTCC M207015 in plant fibrous-bed bioreactor. Bioresour Technol 102:6141–6146. https://doi.org/10.1016/j.biortech.2011.02.087
Gonzalez-Garcia RA, McCubbin T, Navone L, Stowers S, Nielsen LK, Marcellin E (2017) Microbial Propionic Acid Production Fermentation 3(2):1–20. https://doi.org/10.3390/fermentation3020021
Grigoras CG, Destandau E, Fougere L, Elfakir C (2013) Evaluation of apple pomace extracts as a source of bioactive compounds. Ind Crop Prod 49:794–804. https://doi.org/10.1016/j.indcrop.2013.06.026
Guan N, Liu L, H-d S, Chen RR, Zhang J, Li J, Du G, Shi Z, Chen J (2013) Systems-level understanding how Propionibacterium acidipropionici respond to propionic acid stress at the microenvironment levels: mechanism and application. J Biotechnol 167:56–63. https://doi.org/10.1016/j.jbiotec.2013.06.008
Guan N, Zhuge X, Li J (2015) Engineering propionibacteria as versatile cell factories for the production of industrially important chemicals: advances, challenges, and prospects. Appl Microbiol Biotechnol 99:585–600. https://doi.org/10.1007/s00253-014-6228-z
Guan N, Du B, Li J, Shin H, Chen RR, Du G, Chen J, Liu L (2018) Comparative genomics and transcriptomics analysis-guided metabolic engineering of Propionibacterium acidipropionici for improved propionic acid production. Biotechnol Bioeng 115:483–494. https://doi.org/10.1002/bit.26478
Hebert RF, Hebert Sam-EL (2017) Stable Indole-3-Propionate Salts of S-adenosyl-L-Methionine. U.S. Patent 9534010, 3 January 2017. https://www.google.com/patents/US9534010. Accessed 04 October 2020
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kim HM, Park JH, Choi IS, Wi SG, Ha S, Chun HH, Hwang IM, Chang JY, Hoi HJ, Kim JC, Park HW (2018) Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PLoS ONE 13:e0207801. https://doi.org/10.1371/journal.pone.0207801
Kot A, Pobiega K, Piwowarek K, Kieliszek M, Błażejak S, Gniewosz M, Kieliszek M (2020) Biotechnological methods of management and utilization of potato industry waste—a review. Potato Res 63:431–444. https://doi.org/10.1007/s11540-019-09449-6
Liang ZX, Li L, Shuang Li, Cai YH, Yang ST, Wang JF (2012) Enhanced propionic acid production from Jerusalem artichoke hydrolysate by immobilized Propionibacterium acidipropionici in a fibrous-bed bioreactor. Bioprocess Biosyst Eng 35:915–921. https://doi.org/10.1007/s00449-011-0676-y
Liu Y, Zhang YG, Zhang RB, Zhang F, Zhu J (2011) Glycerol/Glucose CoFermentation: one more proficient process to produce propionic acid by Propionibacterium acidipropionici. Curr Microbiol 62:152–158
Liu Y, He JL, Zhao JH, Wei MB, Yang XP, Zheng SJ (2012) Enhanaced propionic acid production by mixed culture of Propionibacterium acidipropionici and Saccharomyces cerevisiae. Adv Mater Res 550–553:1424–1428. https://doi.org/10.4028/www.scientific.net/AMR.550-553.1424
Magyar M, da Costa SL, Jin M, Sarks C, Balan V (2016) Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl Microbiol Biotechnol 100:7349–7358. https://doi.org/10.1007/s00253-016-7665-7
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem 31:426-. https://doi.org/https://doi.org/10.1021/ac60147a030
Piwowarek K, Lipińska E, Hać-Szymańczuk E (2016) Possibility of using apple pomace in the process of propionic-acetic fermentation. Electron J Biotechn 23:1–6
Piwowarek K, Lipińska E, Hać-Szymańczuk E, Kieliszek M, Ścibisz I (2018) Propionibacterium spp.-source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl Microbiol Biotechnol 102:515–538. https://doi.org/10.1007/s00253-017-8616-7
Piwowarek K, Lipińska E, Hać-Szymańczuk E, Rudziak A, Kieliszek M (2019) Optimisation of propionic acid production in apple pomace extract with Propionibacterium freudenreichii. Prep Biochem Biotech 49(10):974–986. https://doi.org/10.1080/10826068.2019.1650376
Piwowarek K, Lipińska E, Hać-Szymańczuk E, Kieliszek M, Kot A (2020) Sequencing and analysis of the genome of propionibacterium freudenreichii T82 strain: importance for industry. Biomolecules 10(2):348. https://doi.org/10.3390/biom10020348
PN-EN ISO 5983–1: 2006 (2006) Feed—determination of nitrogen content and calculation of total protein content - Part 1: Kjeldahl method
Rodriguez BA, Stowers CC, Cox BM (2014) The production of propionic acid, propanol, propylene via sugar fermentation: an industrial perspective on the progress, technical challenges and future outlook. Green Chem 16:1066–1076
Stowers CC, Cox BM, Rodriguez BA (2014) Development of an industrializable fermentation process for propionic acid production. J Ind Microbiol Biotechnol 41:837–852
Suwannakham S, Huang Y, Yang ST (2006) Construction and characterization of ack knock-out mutants of Propionibacterium acidipropionici for enhanced propionic acid fermentation. Biotechnol Bioeng 94:383–395
Teles JC, Stolle EM, Koloda SA, Barana AC (2019) Production of propionic acid by Propionibacterium acidipropionici from Agroindustrial effluents. Braz Arch Biol Technol 62:e19180550. https://doi.org/10.1590/1678-4324-2019180550
Tufvesson P, Ekman A, Sardari RRR, Engdahl K, Tufvesson L (2013) Economic and environmental assessment of propionic acid production by fermentation using different renewable raw materials. Bioresour Technol 149:556–564
Turgay M, Bachmann HP, Irmler S, von Ah U, Frö Hlich-Wyder MT, Falentin H, Deutsch SM, Jan G, Thierry A (2020) Propionibacterium spp. and Acidipropionibacterium spp. In: Reference Module in Food Science. Elsevier, Amsterdam. https://https://doi.org/10.1016/B978-0-08-100596-5.23016-3
Wang Z, Yang ST (2013) Propionic acid production in glycerol/glucose co-fermentation by Propionibacterium freudenreichii subsp. shermanii. Bioresour Technol 137:116–123. https://doi.org/10.1016/j.biortech.2013.03.012
Wang Z, Lin M, Wang L, Ammar EM, Yang ST (2015) Metabolic engineering of Propionibacterium freudenreichii subsp. shermanii for enhanced propionic acid fermentation: effects of overexpressing three biotin-dependent carboxylases. Process Biochem 50:194–204
Wang X, Salvachua D, Sanches NV, Michener W, Bratis A, Dorgan J, Beckham G (2017) Propionic acid production from corn stover hydrolysate by Propionibacterium acidipropionici. Biotechnol Biofuels 10:1–13
Wang P, Shen C, Li L, Guo J, Cong Q, Lu J (2020) Simultaneous production of propionic acid and vitamin B12 from corn stalk hydrolysates by Propionibacterium freudenreichii in an expanded bed adsorption bioreactor. Prep Biochem Biotech 50(8):763–767. https://doi.org/10.1080/10826068.2020.1734942
Wemmenhove E, van Valenberg HJF, Zwietering MH, van Hooijdonk TCM, Wells-Bennik MHJ (2016) Minimal inhibitory concentrations of undissociated lactic, acetic, citric and propionic acid for Listeria monocytogenes under conditions relevant to cheese. Food Microbiol 58:63–67. https://doi.org/10.1016/j.fm.2016.03.012
Xie C, Coda S, Chamlagain B, Varmanen P, Piironen P, Katina K (2019) Co-fermentation of Propionibacterium freudenreichii and Lactobacillus brevis in Wheat Bran for in situ Production of Vitamin B12. Front Microbiol 10:1541. https://doi.org/10.3389/fmicb.2019.01541
Yang H, Wang Z, Lin M, Yang ST (2018) Propionic acid production from soy molasses by Propionibacterium acidipropionici: Fermentation kinetics and economic analysis. Bioresour Technol 250:1–9. https://doi.org/10.1016/j.biortech.2017.11.016
Zhang A, Yang ST (2009a) Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol Bioeng 104:766–777. https://doi.org/10.1002/bit.22437
Zhang A, Yang ST (2009b) Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem 44:1346–1351. https://doi.org/10.1016/j.procbio.2009.07.013
Zhang A, Sun J, Wang Z, Yang ST, Zhou H (2015a) Effects of carbon dioxide on cell growth and propionic acid production from glycerol and glucose by Propionibacterium acidipropionici. Bioresour Technol 175:374–381
Zhang K, Yu C, Yang ST (2015b) Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process Biochem 50:173–179
Zhang W, Wang JJ, Gao Y, Zhang LL (2020) Bacterial cellulose synthesized with apple pomace enhanced by ionic liquid pretreatment. Prep Biochem Biotech 50(4):330–340. https://doi.org/10.1080/10826068.2019.1692222
Zhu L, Wei P, Cai J, Zhu X, Wang Z, Huang L, Xu Z (2012) Improving the productivity of propionic acid with FBB-immobilized cells of an adapted acid-tolerant Propionibacterium acidipropionici. Bioresour Technol 112:248–253. https://doi.org/10.1016/j.biortech.2012.01.055
Zhuge X, Liu L, Shin HD, Li J, Du G, Chen J (2014) Improved propionic acid production from glycerol with metabolically engineered Propionibacterium jenseni by itntegrating fed-batch culture with a pH shift control strategy. Bioresour Technol 152:519–525
Funding
N/A.
Author information
Authors and Affiliations
Contributions
KP—designed study, performed research, analyzed data, wrote the paper (60%). EL—designed study (20%). EH-S: performed research (10%). KP—performed research (10%). My co-authors confirm the contribution mentioned above.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human and animals participants performed by any of the authors.
Informed consent
N/A.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Piwowarek, K., Lipińska, E., Hać-Szymańczuk, E. et al. Propionic acid production from apple pomace in bioreactor using Propionibacterium freudenreichii: an economic analysis of the process. 3 Biotech 11, 60 (2021). https://doi.org/10.1007/s13205-020-02582-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02582-x