Abstract
The heterotrimeric guanine-nucleotide-binding proteins (G-proteins) play a crucial role in signal transduction and regulate plant responses against biotic and abiotic stresses. Necrotrophic pathogens trigger Gα subunit and, in contrast, sometimes Gβγ dimers. Beneficial microbes play a vital role in the activation of heterotrimeric G-proteins in plants against biotrophic and necrotrophic pathogens. The subunits of G-protein (α, β, and γ) are activated differentially against different kinds of pathogens which in turn regulates the entry of the pathogen in a plant cell. Defense mediated by G-proteins in plants imparts resistance against several pathogens. Activation of different G-protein subunits depends on the mode of nutrition of the pathogen. The current review discussed the role of the three subunits against various pathogens. It appeared to be specific in the individual host–pathogen system as well as the role of effectors in the induction of G-proteins. We also discussed the G-protein-mediated production of reactive oxygen species (ROS), including H2O2, activation of NADPH oxidases, hypersensitive response (HR), phospholipases, and ion channels in response to microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
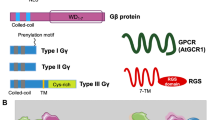
Heteromeric G-proteins (GPCRs, Gα, Gβ, and Gγ) are crucial for signal transduction in the plant immune system in response to phytopathogens. It triggers the expression of several downstream genes (Escudero et al. 2019). G-proteins are composed of three different subunits which includes, Gα, Gβ, and Gγ. They are activated by seven transmembrane G-protein coupled receptors (GPCRs). The Gα subunit has an inherent GTPase activity, and upon binding to GTP, the activated Gα dissociates from the Gβγ complex. The activated GTP-bound Gα subunit functions autonomously, while Gβ and Gγ subunits form a heterodimer. The separated Gα subunit and Gβγ complex then regulate the downstream signaling cascades (Oldham and Hamm 2008). Knock-down RGB1, which is a Gβ gene, affects several developmental aberrations, such as browning of the lamina, dwarf plant, sterile seeds, and narrow leaves (Utsunomiya et al. 2011).
The missing links of the complex network of plant immunity are being revealed layer by layer. However, several missing links between the pathways are yet to be studied. G-protein-coupled receptors (GPCR) encompass a large family of intrinsic transmembrane receptors. Upon binding to a ligand, the GPCR activates a cognate G-protein, which in turn triggers a myriad of secondary messengers that regulate many physiological responses within the plant cell. Recent studies in plants confirmed the direct role of G-proteins in plant biotic and abiotic stress responses, which includes the production of reactive oxygen species (ROS), activation of NADPH oxidases, ion channels, and phospholipases (Escudero et al. 2019). A heterotrimeric G-protein in Arabidopsis thaliana activated the jasmonate-mediated signaling response against necrotrophic pathogen Alternaria brassicicola. The barley homolog to the Mlo gene, which modulates plant defense responses, was known to be structurally similar to seven transmembrane GPCR protein family members. Plant NADPH oxidases produce ROS in response to pathogens that have diverse functions in different cellular contexts. The production of ROS by NADPH oxidase activates several signaling molecules resulting in the suppression of necrotrophic pathogens like A. brassicicola (Table 1). In contrast, a recent study confirmed an increased accumulation of Gα transcripts against the biotrophic pathogen Erysiphe pisi in pea (Patel et al. 2016).
The key objectives of the present review include (1) role of G-protein signaling in plant–microbe interaction has been unraveled. (2) The specific mode of nutrition of the phytopathogens in activation of specific heterotrimeric G-protein. (3) Beneficial microbes activate specific heterotrimeric G-protein in favor of the plant. (4) Role of G-protein-mediated signaling in plant disease resistance against specific pathogens. We have also discussed the role of G-proteins in the induction of plant cell death during interaction with biotic and abiotic stressors. Even though a lot of recent studies focused on the G protein signaling, this review throws light on the cascade of events in the signaling mechanism that activates the defense responsive genes.
Role of effectors in triggering the G-protein signaling
The molecules, such as virulence factors or toxins, which are responsible for the alteration of structure and function of the host cell, are effectors and accountable for the activation of plant defense response. The effectors can be apoplastic (interact with extracellular targets) or cytoplasmic (translocated to cytoplasm) (Park et al. 2012).
In addition to receptors and modulator molecules, four effector molecules interact with G-protein which includes, thylakoid formation 1 (THF1) (Huang et al. 2006), the Arabidopsis cupin domain protein (AtPirin1/PRN1) (Lapik and Kaufman 2003), prephenate dehydrogenase 1 (PD1) (Holland and Jez 2018), and phospholipase (Zhao and Wang 2004; Mishra et al. 2006). It is reported that one Arabidopsis G-protein receives the external signals through more than three different kinds of GPCR-like molecules and transfers the information through at least four different types of effector molecules (Urano and Jones 2014).
A recent report demonstrated the role of Gα in the induction of resistance in Arabidopsis against Pseudomonas syringae (Lee et al. 2013). Overexpression of Gα and Gβ reduced the multiplication of nonhost bacterial pathogens and overexpression of Gβ, but not Gα reduced growth of host pathogens (Lee et al. 2013). Another report showed that heteromeric G-protein, primarily Gα, regulates the genes of JA signaling pathways in Arabidopsis (Okamoto et al. 2009; Li et al. 2019). Several defense responses mediated by AGB1 is (Escudero et al. 2019) independent of pathogen-dependent SA signaling. Accumulation of SA in the agb1 sid2 (isochorismate synthase 2), a double mutant showed enhanced disease susceptibility to P. syringae and Plectosphaerella cucumerina compared to single mutants.
Activation of G-protein signaling by beneficial microbes
Plants recognize external and internal signals, which regulate diverse responses in development and assimilation. Biochemical studies have confirmed the role of heterotrimeric G-proteins in different signaling pathways, which are regulated by several hormones produced by the plant in response to abiotic and biotic factors such as temperature, pathogen, etc. (Assmann 2005). Previous studies confirmed the essence of G-proteins in phytohormone signaling. Mutants lacking AGB1 and AtGPA1 affect the activity of phytohormones such as ABA (Wu and Urano 2018; Xu et al. 2015), brassinolides (Chen et al. 2004), gibberellin (Xu et al. 2015), and auxin (Zhang et al. 2018). Rice dwarf1 mutant (d1) lacking a functional Gα gene reduced HR and thus is susceptible to the infection of an avirulent rice blast fungal strain of Magnaporthe grisea (Kawano et al. 2010). The closure of the stomata by pathogen-associated molecular patterns (PAMPs) needs inhibition of inward K+ channels by the Gα subunit in Arabidopsis (Melotto et al. 2017). Gβ subunit is required to impart resistance against necrotrophic fungus P. cucumerina in Arabidopsis (Jorda et al. 2016; Pandey and Vijayakumar 2018). Patel et al. (2016) described the role of G-protein signaling mainly through Gα subunit during the tripartite interaction of the pea powdery mildew pathogen E. pisi in the presence of Trichoderma and Pseudomonas either in single or in combination. The study also revealed that activation of the Gα subunit during the interaction with bioagents which activates JA-mediated signaling (COI1 and LOX1 activation) upon challenging with E. pisi. The inhibition of pathogen development via ROS generation was observed following the activation of the Gα subunit. Activation of ABA (abscisic acid) signaling following ROS generation (H2O2 production), therefore, linked to the closure of stomata in both combined as well as single treatment of the beneficial microorganisms.
Role of G-protein in ROS production
Reactive oxygen species are superoxide anions and their dismutase product hydrogen peroxide (H2O2). Upon biotic and abiotic stresses, these ROS molecules activate a series of signal cascades (Mittler 2017). In Nicotiana benthamiana, both Gα and Gβ2 heterotrimer subunits account for H2O2 production. However, the hypersensitive response (HR) by Nep1 and boehmerin does not require a functional G-protein. Silencing of Gα and Gβ2 in plants compromise harpin-triggered cell death, suggesting the role of Gα and Gβ2 in plant harpin-induced cell death. G-protein mutants exhibited reduced expression of PR2b, EDS1, NbrbohA, and NbrbohB, giving an insight into the role of G-proteins in plant defense and signaling. Induced illustration of Arabidopsis ADH1 by ethylene shows the intricate signal network involving ethylene, G-protein, and H2O2 (Zhang et al. 2012).
Role G-proteins in the pathogenesis
The rice dwarf mutant d1 with a non-functional Gα gene reduced HR, which makes plants susceptible to avirulent strain of the rice blast fungus Magnaporthe grisea (Ashikari et al. 1999; Fujisawa et al. 1999; Kawano et al. 2010). The Gα subunit also helps in the closure of stomata during the pathogen attack by inhibiting K+ channels through PAMPs (Melotto et al. 2017). Further, Trusov et al. (2006) described the role of Gβ in imparting resistance in Arabidopsis against necrotrophic pathogens. Gα protein XLG2 interacts directly with BIK1 (central cytoplasmic kinase) and FLS2 (Arabidopsis immune receptor). Gα, together with Gγ proteins (AGG1/2) and Gβ protein (AGB1), attenuates the degradation of BIK1 through proteasomes, which results in maximum plant immune activation (Liang et al. 2018). Upon activation of G-protein, the receptors flg22 and XLG2 dissociates from AGB1 and BIK1 phosphorylates its N terminus. Phosphorylation of XLG2 enhanced the production of ROS, and the most probable reason for the activity is the modulation of NADPH oxidase RbohD (Qi et al. 2017).
The role of extra-large G-proteins (XLGs) in defense regulation
A. thaliana deploys Gβγ dimer instead of Gα for multiple defense responses. Dimer of Gβγ partners directly with extra-large G-proteins (XLGs) for the activation of plant immunity. The phenomenon was confirmed by the use of mutants deficient in XLGs, Gβ, and Gγ, where resistance is compromised against several pathogens and production of ROS. Analysis of some other mutants such as double, triple, and quadruple XLGs and Gβγ functionally has been confirmed by the interaction of these in the same defense cascade (Maruta et al. 2015). The well-known fact is that heterotrimeric G-proteins have a role in the activation of plant immunity. The activation demonstrates the onset of innate immunity in the Arabidopsis by two different Gβγ dimers (Gβγ1 and Gβγ2), which majorly contributes in the resistance against several fungal pathogens (Jorda et al. 2016; Trusov et al. 2010; Delgado-Cerezo et al. 2012). However, these studies have not shown the role of Gα, due to mutant plant for the Gα unit have a truly insignificant effect on the plant immunity (Jorda et al. 2016; Escudero et al. 2019). The presence of three genes encoding Gα-like proteins in the Arabidopsis genome and are classified as extra-large G-proteins (XLGs) (Lee and Assmann 1999; Ding et al. 2008). These XLGs have two distinct regions, C-terminal and N-terminal. The C-terminal area is having conserved helical and GTPase domains like the established Gα. However, the N-terminal region has 400 amino acids for the nuclear localization in the plant cell (Ding et al. 2008).
G-protein regulates phytohormonal signaling
Heterotrimeric G-proteins are involved in plant stress signal network, which is tightly regulated by various phytohormones to cope-up with biotic and abiotic stress conditions (Assmann 2005). Transcriptomics studies in Arabidopsis Gβ mutant, agb1-2 suppressed many auxin-inducible genes such as GH3, Aux/IAA, and GST (Xu et al. 2015). At present, a very little transcriptomics data are available on the Gα mutant of Arabidopsis plants. Studies on G-protein regulated gene expression were performed in a Gα mutant gpa1-1 using an Affymetrix (ATH22K) microarray system (Okamoto et al. 2001). Analysis of the microarray results suggested the role of Gα subunit in regulating the expression of Jasmonic acid (JA)-induced genes. The study confirmed the response of plants to methyl jasmonate (MeJA) in chlorophyll loss, gene regulation, and root growth. Transcriptome analysis further revealed that there was a reduction in expression of 29% of JA-regulated genes, and mutation of the Gα subunit altered their expression. These results suggest the probable role of G-proteins in JA-mediated stress signaling in plants. Further, GPA1 mutants affect many other physiological responses in plants that are induced by ABA (Mishra et al. 2006) and GA (Grigston et al. 2008). During M. grisea infection, it was also observed that the Gα subunit activates the downstream GTPase OsRac1 signaling (Kawano et al. 2010).
The role of ROS in plant defense is modulated by the interaction between NADPH oxidases and plant hormones. Exogenous application of ROS synergistically increased SA signaling towards the onset of HR (Le Thanh et al. 2017). ROS production by the activity of AtRbohD developed antagonism against the SA-mediated signaling pathway and resulted in cell death in the site of infection (Torres and Dangl 2005). The role of AtRbohD thus associated with the negative feedback regulation of SA and the ethylene pathway and thereby decreased cell death during infection by necrotrophic pathogens (Kadota et al. 2014). Simultaneous production of ROS and nitric oxide (NO) resulted in hypersensitive response (Torres et al. 2006) and led to regulation of ABA-mediated stomatal closure (Eisenach et al. 2017). However, the production of ROS has antagonistic activity towards the breakdown of basal host defense against necrotrophic pathogens such as Botrytis cinerea (Asai et al. 2008). Signaling through ROS is associated with the plant growth regulators such as ABA and gibberellin, which can modulate ROS production after infection (Achard and Genschik 2009; Sivakumaran et al. 2016). This interaction regulates the production of ROS signals and contributes to the multidisciplinary function of ROS in distinct types of plant–pathogen interactions. There is evidence associated with the regulatory role of G-protein-mediated signaling during plant–pathogen interaction, along with several developmental pathways and abiotic stresses (Temple and Jones 2007; Trusov et al. 2010).
G-protein-mediated defense regulation
Higher plants have a complex system to induce innate immunity against phytopathogens, including immune responses to conserved pathogen molecules or PAMPs (Chisholm et al. 2006; Jones and Dangl 2006). Pathogens should enter in the plant to establish the disease. Phytopathogens utilize the natural openings in the plant to enter inside (Melotto et al. 2006, 2008; Underwood et al. 2007). Recent research has shown the regulation of stomatal closure by guard cells can serve as the first line of defense against phytopathogen entrance (Melotto et al. 2006).
Heterotrimeric G-proteins participate in the responses of guard cells to PAMPs. PAMPs affect stomatal opening, K+ ion channel regulation is targeted by PAMPs. PAMP derived from bacterial flagellin (Flg2), also inhibits light-induced stomatal opening. PAMP-triggered stomatal response involves K + channel regulation, and this regulation is dependent on signaling through cognate PAMP receptors and a heterotrimeric G-protein (Zhang et al. 2008). Regulation of stomatal aperture by bacterial pathogens was altered in Gα and Gβ (Lee et al. 2013).
A very complex system of innate immunity is present in the plant system and woks against several plant pathogens, having specific conserved molecules such as PAMPs (Chisholm et al. 2006; Jones and Dangl 2006). Most of the plant pathogens enter through plant’s natural openings such as stomata (Underwood et al. 2007; Melotto et al. 2008). Heterotrimeric G-proteins are reported to control the guard cells for opening and closing the stomata, especially in response to PAMPs (Zhang et al. 2008). Lee et al. (2013) suggested the role of Gα and Gβ subunit of the heterotrimeric G-proteins in the regulation of stomatal aperture against the bacterial pathogen (P. syringae) in Arabidopsis plants.
The role of subunits of G-proteins in plant defense has been confirmed with mutant studies. Rice dwarf mutant d1 is one homolog of Gα subunit induce HR response and resistance against avirulent races of the rice blast pathogen M. grisea (Kawano et al. 2010). However, in Arabidopsis, mutation of Gα subunit GPA1 exhibits minor or no effect on the response. In contrast, GPCR subunit AGB1 induces against some fungal pathogens (Jorda et al. 2016; Trusov et al. 2010; Delgado-Cerezo et al. 2012). Therefore, the study confirmed that the mutant of Arabidopsis agb1 is more susceptible to disease compared to the wild-type plants against the necrotrophic pathogens such as P. cucumerina, Botrytis cinerea, and Alternaria brassicicola, as well as against the hemibiotrophic vascular fungus Fusarium oxysporum. However, agb1 mutant showed no response against biotrophic pathogens such as oomycete Hyaloperonospora arabidopsidis and P. syringae compared to the wild-type plants (Jorda et al. 2016; Pandey and Vijayakumar 2018). It was proved that Arabidopsis Gα has a decisive role in the activation of defense responses against P. syringae through stomatal closure, which ultimately restricts the pathogen entry into the plant leaves (Melotto et al. 2017; Hind et al. 2016; Lee et al. 2013). Two different pathways are involved, which includes, the action of Gβγ dimer independently from Gα, and another is the formation of heterotrimer by the replacement of Gα.
Three subunits of heterotrimeric G-proteins can function in diverse ways depending upon the host plant and mode of nutrition of the pathogens used in the experiment. The Gβ protein AGB1 is necessary for defense response in plants. However, Gγ subunits (AGG1 and AGG2) and AGB1 work together for generating defense responses, because mutant agb1 and double mutants agg1 agg2 showed similar responses against the necrotrophic fungal pathogen P. cucumerina (Delgado-Cerezo et al. 2012).
A report by Brenya et al. (2016) suggested the activity of G-proteins in viral diseases such as cucumber mosaic virus (CMV) and turnip mosaic virus (TuMV) in A. thaliana, they reported the negative regulation of salicylic acid, jasmonic acid, and abscisic by Gβ during infection of CMV and TuMV. Results from Brenya et al. (2016) suggested that G-proteins play a positive role in the suppression of viral disease by promoting necrosis of the cells.
G-protein as an activator of receptor kinases
One of the largest family of proteins, receptor-like kinases (RLKs), has various roles related to the development and stress signaling in plants (Liang et al. 2018). Over 600 RLKs have been reported (Shiu and Bleecker 2001) in the model plant A. thaliana. RLKs have an extracellular domain, a cytoplasmic kinase domain, and a single transmembrane domain to be shared in most of the RLKs. The extracellular domain is believed to be involved in recognition of ligands, which later is responsible for activation of the cytoplasmic kinase domain. Flagellin-sensitive2 (FLS2), a receptor for PAMPs and chitin elicitor receptor kinase1 (CERK1), is the member of the RLK family. EFR and FLS2 are the receptor kinases exposed on the membrane surface involved in the activation of EF-Tu and bacterial flagellin, respectively (Gomez-Gómez and Boller 2000; Liang et al. 2018). However, CERK1 is reported (Miya et al. 2007; Wan et al. 2008) for the chitin and other components of the fungal cell wall. Other RLKs such as Brassinosteroid Insensitive1-associated receptor kinase1 (BAK1) said (Cao et al. 2013; Chaparro-Garcia et al. 2011) to be the co-receptor of EFR and FLS2. Xa21 in rice is another example of RLKs functions as a receptor for a peptide obtained from the AvrXa21 (Park et al. 2010).
G-protein subunits including AGB1 and AGG1/AGG2 functions downstream of RLKs such as SOBIR1, FLS2, EFR, and CERK1 towards the activation of resistance responses against pathogens. Liu et al. (2013) described that RLKs function upstream of the G-protein subunits-like activity of GPCR in plant heterotrimeric G-proteins. Some of the results still question the role of AGB1 and AGG1/AGG2 downstream to RLKs for the activation of plant immunity (Stateczny et al. 2016). A recent report by Liu et al. (2018) confirmed that RACK1 (receptor for activated C kinase 1) is involved in the regulation of G-protein signaling pathway for defense in woody plants.
Concluding remarks
It is interesting to know the role of G-proteins in plant defense signaling against pathogens. G-proteins are involved in the regulation of different developmental as well as physiological processes in plants, but still, so many missing links need to be bridged for complete understanding. This review also suggested the role of G-proteins in the plant defense pathways against phytopathogens. We have also suggested the role of beneficial plant–microbe such as P. fluorescens in defense response running through the G-protein signaling cascade. Knowledge of the role of receptors, as well as beneficial microorganisms, could open newer eco-friendly strategies for the plant disease control. G-protein-induced programmed cell death (PCD), closure of stomata, and production of disease-resistance-related proteins could be the strategies for the protection of plants against pathogens. However, in-depth study is needed to enhance our understanding of the role of G-proteins for activation of induced immunity in the plants for plant disease management.
References
Achard P, Genschik P (2009) Releasing the brakes of plant growth: how GAs shutdown DELLA proteins. J Exp Bot 60(4):1085–1092
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20(5):1390–1406
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Natl Acad Sci 96(18):10284–10289
Assmann SM (2005) G proteins go green: a plant G protein signaling FAQ sheet. Science 310:71–73
Brenya E, Trusov Y, Dietzgen RG, Botella JR (2016) Heterotrimeric G-proteins facilitate resistance to plant pathogenic viruses in Arabidopsis thaliana (L.) Heynh. Plant Signal Behav 11(8):e1212798
Cao Y, Aceti DJ, Sabat G, Song J, Makino SI, Fox BG, Bent AF (2013) Mutations in FLS2 Ser-938 dissect signaling activation in FLS2-mediated Arabidopsis immunity. PLoS Pathog 9(4):e1003313
Chaparro-Garcia A, Wilkinson RC, Gimenez-Ibanez S, Findlay K, Coffey MD, Zipfel C, Schornack S (2011) The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana. PLoS One 6(1):e16608
Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM (2004) GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol 135(2):907–915
Cheng Z, Li JF, Niu Y, Zhang XC, Woody OZ, Xiong Y, Sheen J (2015) Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521(7551):213
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124(4):803–814
Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jorda L, Somerville S (2012) Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant 5(1):98–114
Ding L, Pandey S, Assmann SM (2008) Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J 53(2):248–263
Eisenach C, Baetz U, Huck NV, Zhang J, De Angeli A, Beckers GJ, Martinoia E (2017) ABA-induced stomatal closure involves ALMT4, a phosphorylation-dependent vacuolar anion channel of arabidopsis. Plant Cell 29(10):2552–2569
Escudero V, Jorda L, Sopena-Torres S, Melida H, Miedes E, Munoz-Barrios A, Bulone V (2017) Alteration of cell wall xylan acetylation triggers defense responses that counterbalance the immune deficiencies of plants impaired in the β-subunit of the heterotrimeric G-protein. Plant J 92(3):386–399
Escudero V, Torres MA, Delgado M, Sopena-Torres S, Swami S, Morales J, Molina A (2019) Mitogen-activated protein kinase phosphatase 1 (MKP1) negatively regulates the production of reactive oxygen species during Arabidopsis immune responses. Mol Plant Microbe Interact 32(4):464–478
Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Iwasaki Y (1999) Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci 96(13):7575–7580
Gomez-Gómez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5(6):1003–1011
Grigston JC, Osuna D, Scheible WR, Liu C, Stitt M, Jones AM (2008) D-Glucose sensing by a plasma membrane regulator of G signaling protein, AtRGS1. FEBS Lett 582(25–26):3577–3584
Hind SR, Strickler SR, Boyle PC, Dunham DM, Bao Z, O’Doherty IM, Vinatzer BA (2016) Tomato receptor flagellin-sensing 3 binds flgII-28 and activates the plant immune system. Nat Plants 2(9):16128
Holland CK, Jez JM (2018) Reaction mechanism of prephenate dehydrogenase from the alternative tyrosine biosynthesis pathway in plants. ChemBioChem 19(11):1132–1136
Huang J, Taylor JP, Chen JG, Uhrig JF, Schnell DJ, Nakagawa T, Jones AM (2006) The plastid protein thylakoid formation1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell 18(5):1226–1238
Ishikawa A (2009) The Arabidopsis G-protein β-subunit is required for defense response against Agrobacterium tumefaciens. Biosci Biotechnol Biochem 73(1):47–52
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jorda L, Sopena-Torres S, Escudero V, Nunez-Corcuera B, Delgado-Cerezo M, Torii KU, Molina A (2016) ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Front Plant Sci 7:897
Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Zipfel C (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54(1):43–55
Kawano Y, Chen L, Shimamoto K (2010) The function of Rac small GTPase and associated proteins in rice innate immunity. Rice 3(2):112
Lapik YR, Kaufman LS (2003) The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 15(7):1578–1590
Le Thanh T, Thumanu K, Wongkaew S, Boonkerd N, Teaumroong N, Phansak P, Buensanteai N (2017) Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J Plant Interact 12(1):108–120
Lee YRJ, Assmann SM (1999) Arabidopsis thaliana ‘extra-large GTP-binding protein’ (AtXLG1): a new class of G-protein. Plant Mol Biol 40(1):55–64
Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC (2009) A type I–secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326(5954):850–853
Lee S, Ishiga Y, Clermont K, Mysore KS (2013) Coronatine inhibits stomatal closure and delays hypersensitive response cell death induced by nonhost bacterial pathogens. Peer J 1:34
Li L, Su B, Qi X, Zhang X, Song S, Shan X (2019) JA-induced endocytosis of AtRGS1 is involved in G-protein mediated JA responses. Int J Mol Sci 20(15):3779
Liang X, Ma M, Zhou Z, Wang J, Yang X, Rao S, Chen S (2018) Ligand-triggered de-repression of Arabidopsis heterotrimeric G proteins coupled to immune receptor kinases. Cell Res 28(5):529
Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Zhang Y (2013) Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol 161(4):2146–2158
Liu CY, Xu YZ, Fan W, Long DP, Cao BN, Xiang ZH, Zhao AC (2018) Identification of the genes involved in heterotrimeric G-protein signaling in mulberry and their regulation by abiotic stresses and signal molecules. Biol Plant 62(2):277–286
Maruta N, Trusov Y, Brenya E, Parekh U, Botella JR (2015) Membrane-localized extra-large G proteins and Gβγ of the heterotrimeric G proteins form functional complexes engaged in plant immunity in Arabidopsis. Plant Physiol 167(3):1004–1016
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126(5):969–980
Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Howe GA (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine-and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55(6):979–988
Melotto M, Zhang L, Oblessuc PR, He SY (2017) Stomatal defense a decade later. Plant Physiol 174(2):561–571
Mishra NS, Tuteja R, Tuteja N (2006) Signaling through MAP kinase networks in plants. Arch Biochem Biophys 452(1):55–68
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19
Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. PNAS 104(49):19613–19618
Okamoto H, Matsui M, Deng XW (2001) Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13(7):1639–1652
Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E (2009) High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant physiol 149(2):825–834
Oldham WM, Hamm HE (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9(1):60
Pandey S, Vijayakumar A (2018) Emerging themes in heterotrimeric G-protein signaling in plants. Plant Sci 270:292–300
Park CJ, Han SW, Chen X, Ronald PC (2010) Elucidation of XA21-mediated innate immunity. Cell Microbiol 12(8):1017–1025
Park CH, Chen S, Shirsekar G, Zhou B, Khang CH, Songkumarn P, Valent B (2012) The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 24(11):4748–4762
Patel JS, Sarma BK, Singh HB, Upadhyay RS, Kharwar RN, Ahmed M (2016) Pseudomonas fluorescens and Trichoderma asperellum enhance expression of Gα subunits of the pea heterotrimeric G-protein during Erysiphe pisi infection. Front Plant Sci 6:1206
Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38:92–100
Shiu SH, Bleecker AB (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. PNAS 98(19):10763–10768
Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJ, Mur LA (2016) ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci 7:709
Stateczny D, Oppenheimer J, Bommert P (2016) G protein signaling in plants: minus times minus equals plus. Curr Opin Plant Biol 34:127–135
Swain DM, Sahoo RK, Chandan RK, Ghosh S, Kumar R, Jha G, Tuteja N (2019) Concurrent overexpression of rice G-protein β and γ subunits provide enhanced tolerance to sheath blight disease and abiotic stress in rice. Planta 250(5):1505–1520
Takahashi T, Murano T, Ishikawa A (2018) SOBIR1 and AGB1 independently contribute to nonhost resistance to Pyricularia oryzae (syn. Magnaporthe oryzae) in Arabidopsis thaliana. Biosci Biotechnol Biochem 82(11):1922–1930
Temple BR, Jones AM (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58:249–266
Thung L, Chakravorty D, Trusov Y, Jones AM, Botella JR (2013) Signaling specificity provided by the Arabidopsis thaliana heterotrimeric G-protein γ subunits AGG1 and AGG2 is partially but not exclusively provided through transcriptional regulation. PloS one 8(3)
Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress, and development. Curr Opinion Plant Biol 8(4):397–403
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141(2):373–378
Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR (2006) Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140(1):210–220
Trusov Y, Jorda L, Molina A, Botella JR (2010) Signaling and communication in plants series, G-proteins and plant innate immunity. In: Yalovsky FB, Jones A (eds) Integrated G-proteins signaling in plants. Springer, Berlin, pp 221–250
Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cellular Microbiol 9(7):1621–1629
Urano D, Jones AM (2014) Heterotrimeric G protein-coupled signaling in plants. Annu Rev Plant Biol 65:365–384
Urano D, Miura K, Wu Q, Iwasaki Y, Jackson D, Jones AM (2016) Plant morphology of heterotrimeric G protein mutants. Plant Cell Physiol 57(3):437–445
Utsunomiya Y, Samejima C, Takayanagi Y, Izawa Y, Yoshida T, Sawada Y, Iwasaki Y (2011) Suppression of the rice heterotrimeric G protein β-subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J 67(5):907–916
Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey G (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20(2):471–481
Wu TY, Urano D (2018) Genetic and systematic approaches toward G protein-coupled abiotic stress signaling in plants. Front Plant Sci 9:1378
Xu DB, Chen M, Ma YN, Xu ZS, Li LC, Chen YF, Ma YZ (2015) A G-protein β subunit, AGB1, negatively regulates the ABA response and drought tolerance by down-regulating AtMPK6-related pathway in Arabidopsis. PLoS One 10(1):e0116385
Xue J, Gong BQ, Yao X, Huang X, Li JF (2019) BAK1-mediated phosphorylation of canonical G protein alpha during flagellin signaling in Arabidopsis. J Integr Plant Biol. https://doi.org/10.1111/jipb.12824
Zhang W, He SY, Assmann SM (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56(6):984–996
Zhang H, Wang M, Wang WEI, Li D, Huang Q, Wang Y, Zhang Z (2012) Silencing of G proteins uncovers diversified plant responses when challenged by three elicitors in Nicotiana benthamiana. Plant Cell Environ 35(1):72–85
Zhang T, Xu P, Wang W, Wang S, Caruana JC, Yang HQ, Lian H (2018) Arabidopsis G-protein β subunit AGB1 interacts with BES1 to regulate brassinosteroid signaling and cell elongation. Front Plant Sci 8:2225
Zhao J, Wang X (2004) Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 279(3):1794–1800
Zheng XY, Zhou M, Yoo H, Pruneda-Paz JL, Spivey NW, Kay SA, Dong X (2015) Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. PNAS 112(30):9166–9173
Acknowledgements
There is no acknowledgement for the current work.
Funding
No funding is available for the current work.
Author information
Authors and Affiliations
Contributions
All the authors equally contributed for the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest related to this study to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Patel, J.S., Selvaraj, V., Gunupuru, L.R. et al. Plant G-protein signaling cascade and host defense. 3 Biotech 10, 219 (2020). https://doi.org/10.1007/s13205-020-02201-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02201-9