Abstract
The sessile nature of plants has enabled them to develop a higher level of plasticity in adaptation against the hostile environmental conditions such as biotic and abiotic stresses. The intricate molecular machineries in plants, consisting of signal sensors, signal transducers, and generation of response, are highly similar with animal’s signal transduction components; however, a large number of pathways also show a high level of divergence. G proteins are a part of signal transduction components, which play pivotal role in regulating a large number of physiological and developmental processes in both plants and animals. G proteins or guanine nucleotide-binding proteins serve as molecular switches, existing in two forms: an active conformation, when bound to GTP, and an inactive conformation, when bound to GDP. In active conformation, G proteins interact with downstream effector molecules to initiate various cellular responses. Based on their structure, they have been divided into heterotrimeric and monomeric G proteins, also called large and small G proteins, respectively, where the former is composed of three subunits (α, β, and γ) and latter is composed of single subunit (α). Plant growth and development is partially regulated by the combined activity of large and small G proteins. Besides, identification of profuse number of stress-related genes interacting with monomeric and heterotrimeric G-protein complex in both Arabidopsis and rice suggests their involvement in modulation of abiotic stress responses. Further, based on the functional analysis of several G proteins (large and small) regulating cell growth, cell proliferation, seed germination, sugar sensing, and hormonal responses indicate these proteins are equally essential for plant development. Due to increased demand of food crops to sustain growing population in adverse environmental conditions, the role of G proteins and modulation of GTPase signaling has been studied in plants to produce genetically modified crop plants with increased stress tolerance and productivity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plants being sessile have evolved an intricate network of molecular pathways to adapt to changes in their environment, including perception of signals by receptors, signal transduction via transducing molecules and proteins, and gene expression. The natural adaptation is a continuous process occurring by outbreeding over a span of several decades; however, to accelerate this process, plant biologists have adopted various approaches such as breeding and genetic manipulation of plants to generate resistant and tolerant varieties. With the global effort to sequence various plant genomes and availability of full genome sequence of model dicot like Arabidopsis (Arabidopsis Genome Initiative 2000) and model monocot crop like rice (Goff et al. 2002), a new field of functional genomics has emerged allowing analysis of the plant transcriptome, proteome, and metabolome employing techniques like epistasis analysis, microarrays, SAGE (serial analysis of gene expression), Y2H (yeast-two-hybrid), AP/MS (affinity purification and mass spectrometry), mutagenesis, and RNAi (RNA interference). Employing high-throughput technology, research in the past decades has identified key molecules acting in abiotic and biotic stress tolerance, including G proteins, also known as guanosine nucleotide-binding proteins. G family proteins are cytosolic proteins involved in transmitting a variety of extracellular signals into the cell through cell surface receptors to modulate cellular activities (Hepler and Gilman 1992). Their activity is regulated by certain cellular factors that control their ability to bind to GTP and hydrolyze it to GDP. G proteins when bound to GTP acquire an on or activated form, whereas GDP-bound form designates an inactivated or off state. Based on their structure, G proteins are mainly divided into two categories: monomeric small GTPases and heterotrimeric large G-protein complexes.
1.1 Heterotrimeric G Proteins
Heterotrimeric G protein (hereafter called G protein), also called large G protein, is a cytosolic protein and a complex containing three subunits including G-alpha (Gα), G-beta (Gβ), and G-gamma (Gγ) (Jones and Assmann 2004; Ma 2001). In animals, G proteins are involved in transmitting extracellular signals, such as, hormones, neurotransmitters, chemokines, and light to intracellular signaling components to modulate gene expression, culminating into a response (Gilman 1987; Wettschureck and Offermanns 2005). Animal G protein is a multigene family encoded by 23α, 6β, and 12γ genes (Hamm 1998; McCudden et al. 2005). Additionally, the G-protein signaling module is composed of G-protein-coupled receptors (GPCRs), containing seven-transmembrane α-helical proteins, acting upstream of G proteins and activating G proteins upon binding to a ligand. The human genome encodes for approximately 1,000 GPCRs, falling into six subgroups, comprising the largest receptor superfamily in metazoans (Kawasawa et al. 2003).
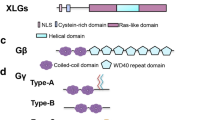
In comparison to mammals, plant G-protein family is relatively small; in Arabidopsis, Gα is encoded by a single-copy gene, designated as GPA1 (Ma et al. 1990), and single copy of Gβ, AGB1 (Weiss et al. 1994), and three genes encoding the Gγ subunits have been isolated: AGG1, AGG2, and AGG3 (Mason and Botella 2000, 2001; Chakravorty et al. 2011). The genome of crop plant rice contains one gene each encoding Gα (RGA1) and Gβ (RGB1) subunits and three genes encoding Gγ subunit (RGG1, RGG2, and RGG3) (Ishikawa et al. 1996; Kato et al. 2004). G-protein signaling components have also been identified in other plants: pea and soybean having two and four Gα-subunits, respectively (Ashikari et al. 1999; Misra et al. 2007; Choudhury et al. 2011). The α-, β-, and γ-subunits of G proteins link extracellular signals to intracellular effector through GPCRs. Interestingly, direct homologs of metazoan GPCR (G-protein-coupled receptors) proteins are altogether absent in plants. However, recently in Arabidopsis, GCR1 has been identified as a likely representative of GPCR-encoding gene because of its significant sequence similarity with them. The Arabidopsis GCR1 is predicted heptahelical protein, a hallmark of bona fide GPCRs (Josefsson and Rask 1997; Pandey and Assmann 2004; Chen et al. 2004a, 2004b). Using bioinformatics tools, the Arabidopsis genome is predicted to encode for 394 7TMpRs (seven-transmembrane putative receptors) (Moriyama et al. 2006). An additional regulator of G-protein signaling (RGS) reminiscent to GPCRs has been identified in Arabidopsis; AtRGS1 affects the intrinsic GTPase activity of Gα-subunit, keeping it in inactivated conformation. It appears that the Arabidopsis genome contains only one member of the RGS family, RGS1 (Chen et al. 2003). Additionally, 7 noncanonical, extra-large G (XLG) proteins were identified in plants, including 3 in Arabidopsis and 4 in rice (Lee and Assmann 1999). The physiological role of G proteins in plants was determined by the characterization of loss-of-function (lof) mutant and transgenic lines, in stomata opening/closure (Wang et al. 2001; Coursol et al. 2003; Chen et al. 2004a, 2004b), fungal defense (Llorente et al. 2005; Trusov et al. 2006; Trusov et al. 2009), oxidative stress (Joo et al. 2005; Booker et al. 2004), phytohormone signaling (gibberellic acid (GA)), and plant development (Ueguchi-Tanaka et al. 2000; Ullah et al. 2001; Ullah et al. 2002; Lease et al. 2001; Chen et al. 2006; Pandey et al. 2006). In mature leaves, G proteins transmit signals to molecules, including small GTPases, ion channels, and phospholipases, that are effectors in response to various biotic and abiotic stress conditions, including pathogen elicitation, ozone treatment, and water deficit.
1.1.1 Mechanism of Regulation of Heterotrimeric G Protein
Mechanism of mammalian G-protein activation involves binding of an activating ligand to its specific GPCR causing a change in its conformation, leading to the conversion of an inactive G protein to its active conformation. The GPCR acts as a guanine nucleotide exchange factor (GEF), causing Gα to exchange GDP for GTP (Gilman 1987). As a result, Gα–GTP separates from the Gβγ dimer, and both Gα–GTP and the Gβγ dimer separate from the receptor and can individually activate downstream effector molecules. Subsequent to signal propagation, the GTP that is bound to Gα is hydrolyzed to GDP, thereby inactivating Gα and allowing its reassociation with the Gβγ dimer to reform the inactive heterotrimeric G-protein complex.
In Arabidopsis, the mechanism of G-protein activation is different than in animals involving spontaneous disassociation of heterotrimer into Gαs and Gβγ dimer, which allows Gα to bind to GTP, and both the dissociated subunits separately activate downstream effectors (Urano et al. 2012). A 7TM-RGS protein, AtRGS1, promotes GTP hydrolysis of the Gαs, and this GDP-bound form then interacts with Gβγ dimer and returns to the resting state (Fig. 9.1a) (Johnston et al. 2007; Jones et al. 2011). Here RGS proteins act as GTPase-activating proteins (GAPs) for Gα, attenuating signaling by promoting the return of the G protein to the resting state. Genetic evidence is consistent with d-glucose being the ligand that stops the AtRGS1 GAP activity and allows AtGPA1 to self-activate (Fig. 9.1a) (Chen et al. 2003; Johnston et al. 2007; Chen et al. 2006; Grigston et al. 2008).
Graphic depicting regulatory mechanism of heterotrimeric and monomeric G protein: (a) G proteins or large G proteins act as a molecular switch and regulate many cellular processes. The G protein is made up of three subunits: α, β, and γ. The Gαs binds to the guanosine nucleotide, GDP, in the inactive state and forms a complex with Gβγ dimer. Spontaneous disassociation of heterotrimer into Gαs and Gβγ allows Gαs to bind to GTP, and both the dissociated subunits separately activate downstream effector molecules. A 7TM-RGS (transmembrane-regulator of G-protein signaling) protein, AtRGS1, promotes GTP hydrolysis of the Gαs, and this GDP-bound form then interacts with Gβγ dimer and comes back to the resting state. Here RGS proteins act as GTPase-activating proteins (GAPs) for Gαs, attenuating signaling by hastening the return of the G protein to the resting state. d-glucose acts as the ligand that halts AtRGS1 GAP activity and allows AtGPA1 to self-activate. (b) Monomeric GTP-binding proteins or small GTPases are molecular switches that are “activated” by GTP and “inactivated” by the hydrolysis of GTP to GDP. Monomeric GTPase activity is determined by the ratio of GTP/GDP-bound form, regulated by three classes of proteins, including guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). Upon stimulation by an upstream signal, a GEF protein converts the GDP-bound inactive form into a GTP-bound active conformation, through GDP/GTP replacement. In the activated state through the effector-binding domain, it interacts with downstream effector proteins to transduce the signal. The small GTPases also have a weak intrinsic GTPase activity for GTP hydrolysis and require a GTPase-activating protein (GAP) for efficient deactivation. Additionally, most small GTPases cycle between membrane-associated and cytosolic states. And only membrane-bound GTPases can be activated by GEF; their removal by cytosolic GDI proteins negatively regulates GTPases
1.1.2 Domain Structure of Heterotrimeric G Protein
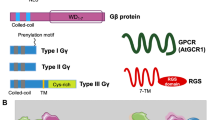
G proteins contain highly conserved G domain/fold, shared by Ras superfamily proteins and translation elongation factors. G domain is composed of 6 β-sheets, 5 α-helices, and 5 regions/loops designated G1–G5, which play a pivotal role in GTP binding and hydrolysis. The organization of G domain from N-terminus is N-β1-G1-α1-G2/SW1-β2-β3/G3-SW2-α2-β4-α3-β5/G4-α4-β6-G5-α5-C, where the G1 region or P-loop has a conserved GxxxxGK(S/T) motif (aka Walker A motif), required to contact α- and β-phosphate of GTP; the G2 region contains a conserved threonine residue for Mg2+ coordination important for GTP hydrolysis; G3 region (aka Walker B motif) is identified by sequence DxxG and the conserved aspartate (D) and glycine (G) bind Mg2+ and γ-GTP, respectively; G4 region has NKxD motif and the conserved aspartate contacts the guanine ring of GTP; and the G5 region is identified by E(A/C/S/T)(C/S)A(K/L) motif, which helps in guanine base recognition (Wittinghofer and Vetter 2011). Plant Gα-subunit is around 35–46 kDa protein, characterized by functional motifs including myristoylation and S-acetylation sites (for membrane targeting), N- and C-terminal receptor binding sites, and three switch regions (SW) that sense nucleotide binding (Fig. 9.2). The N-terminal helical region, a small interface at switch I (SWI), and a large interface at switch II (SWII) bind Gβγ dimer (Li et al. 2009; Temple and Jones 2007), whereas the helical domain shelters the guanine nucleotide-binding pocket. The P-loop, G45AGESGKS for NTP binding, the DxxGQ motif for GTP hydrolysis, and the NKxD motif for guanine recognition are conserved in plants (Temple and Jones 2007). The Gβs is 35–36 kDa protein, composed of an amino-terminal coiled-coil motif and a carboxyl-terminal WD40 (β-transducin) repeat domain (Fig. 9.2). The amino-terminus of Gβs forms the stable coiled-coil/hydrophobic interaction with the Gγs (Li et al. 2009), and the WD40 repeat domain contains the effector- and Gαs-binding surface. The effector-binding region on Gβs is normally masked by the GDP-bound form of Gαs; therefore, Gβγ dimer is active only after dissociating from the α-subunit (Wettschureck and Offermanns 2005). The Gγs is a small (less than 100 residue, 6–10 kDa) protein containing a coiled-coil region and a prenylation site at its carboxyl-terminus (Fig. 9.2) (Gilman 1987), required for its membrane targeting (Wei et al. 2008).
Graphic representation of domain structure α, β, and γ subunits of plant heterotrimeric G protein: plant Gαs is around 35–46 kDa protein, characterized by functional motifs including myristoylation and S-acetylation sites (for membrane targeting), 5 G domains, N- and C-terminal receptor binding sites, and 3 switch regions (SW) that sense nucleotide binding. The N-terminal helical region, a small interface at switch I, and a large interface at switch II bind Gβγ dimer, whereas the helical domain (HD) shelters the guanine nucleotide-binding pocket. The P-loop, G45AGESGKS for NTP binding, the DxxGQ motif for GTP hydrolysis, and the NKxD motif for guanine recognition are conserved in plants. The Gβs is 35–36 kDa protein, composed of an amino-terminal coiled-coil motif and a carboxyl-terminal WD40 repeat domain. The amino-terminus of Gβs forms the stable coiled-coil/hydrophobic interaction with the Gβs, and the WD40 repeat domain contains the effector- and Gαs-binding surface. The effector-binding surface on Gβs is normally masked by the GDP-bound form of Gαs; therefore, Gβγ dimer is active only after dissociating from the Gα-subunit. The Gγs is a small (less than 100 residue, 6–10 kDa) protein containing a coiled-coil region and a prenylation site at its carboxyl-terminus
1.2 Monomeric G Protein
Monomeric G proteins (21–30 kDa) are the small guanine nucleotide-binding proteins related to α-subunit of heterotrimeric G proteins, belonging to Ras (rat sarcoma oncoprotein) superfamily, named after human Ras genes, discovered as cellular homologs of the viral Ras oncogene (Parada et al. 1982).
The small GTPase superfamily in animals is divided into six distinct families, including Ras, Rho, Rab, Arf, Ran, and Miro (Kahn et al. 1992; Bischoff et al. 1999; Takai et al. 2001; Logan 2010). Members of each family are associated with specific biological function as Ras GTPases regulate cell proliferation; Arf (ADP-ribosylation factor) and Rab (Ras-like protein in brain) primarily regulate various steps in trafficking, where former is required for vesicle budding in the secretory system and latter controls transport and docking of specific vesicles to the plasma membrane. Ran (Ras-like nuclear protein) regulates trafficking of RNA and proteins across the nuclear membrane, and Miro (mitochondria Rho GTPase) GTPases regulate mitochondrial transport and morphology (Ridley et al. 1992; Nobes et al. 1998; Wennerberg et al. 2005; Reis et al. 2009).
In silico analysis identified 93 small GTPase superfamily members in Arabidopsis, classified into five small GTPase families: with 57 Rab GTPases, 21 Arf GTPases, 11 Rho GTPases, and 4 Ran GTPases. Interestingly, plant genomes do not encode homologs of Ras GTPases (Regad et al. 1993; Haizel et al. 1997; Vernoud et al. 2003). Furthermore, functional analysis of several members showed that they might regulate similar cellular processes in the plant cells as they do in animal cells. For example, Arf GTPases regulate vesicle trafficking between plasma membrane (PM) and cytoplasm (Geldner et al. 2001); the role of Ran GTPases is not known, but N-terminus of RanGAPs is homologous to nuclear matrix attachment proteins, providing new insight into their function in plant cells (Meier 2000). Rab GTPases comprise the largest family in Arabidopsis, functioning in vesicle trafficking (Batoko et al. 2000; Lu et al. 2001). Plants do not have true orthologs of Rho family GTPases, but have evolved a novel family of Rho-like proteins also known as Rho of plants (ROPs) (Zheng and Yang 2000; Shanmugam et al. 2013). Signaling proteins in plants play an important role in plant stress responses and pollen tube outgrowth (Kost et al. 1999; Li et al. 1999). In mammals 14 Rho family GTPases have been identified and divided into 7 subgroups (Nobes and Hall 1994; Aspenstrom 1999). In plants, two groups have been identified of which only one protein is Rho-like homolog while the rest are closely related to Rac (Newman et al. 1994; Yang and Watson 1993; Delmer et al. 1995; Winge et al. 1997).
1.2.1 Mechanism of Regulation of Monomeric G Protein
Small GTP-binding proteins are master molecular switches that are “activated” by GTP and “inactivated” by the hydrolysis of GTP to GDP, acting as global regulatory proteins in eukaryotic cells. In the cell, GTPase activity is determined by the ratio of GTP-/GDP-bound form, regulated by three classes of proteins, including guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). Upon stimulation by an upstream signal, a GEF protein converts the GDP-bound inactive GTPases to a GTP-bound active conformation, through GDP/GTP exchange. Once in the activated state, small GTPases through their effector-binding domain interacts with downstream effector proteins to transduce the signaling response. The activated conformation possesses weak intrinsic GTPase activity for GTP hydrolysis, requiring a GTPase-activating protein (GAP) for efficient deactivation. Additionally, most of the small GTPases frequently cycle between plasma membrane and cytosolic compartments; only membrane-bound GTPases can be activated by GEF, and removal by cytosolic GDI proteins negatively regulates GTPases. This complex mode of regulation and their ability to interact with variety of upstream and downstream targets create functional diversity in GTPase activity (Fig. 9.1b).
In animals, most RhoGEFs possess a Dbl (diffuse B-cell lymphoma) homology (DH) domain and a pleckstrin homology (PH) domain. However, no proteins with DH/PH domains have been identified in plants so far. Instead, plant-specific Rop nucleotide exchanger (PRONE) domain constitutes a large family in plants (Berken et al. 2005; Gu et al. 2006).
1.2.2 Domain Structure of Small GTPases
Members of Ras superfamily share common structural features including guanine nucleotide-binding domains and an effector-binding domain (Zheng and Yang 2000; Takai et al. 2001). Plant small GTPases contain five G domains (G1-G5) of which the G5 domain shows high sequence divergence among the GTPases. The G domains are essential for binding guanosine nucleotide and the associated Mg2+ ion and for GTP hydrolysis. The G1 motif associates tightly with the α- and β-phosphate, G2 motif contacts the γ-phosphate, and the G3 motif helps in positioning the water molecule for GTP hydrolysis through highly conserved glutamine residue. Switch 1 and 2 regions bind to the effector or regulatory molecules. Rac/Rop GTPases have a Rho insert region, and all the GTPases have hypervariable region at the C-terminus facilitating localization (Fig 9.3).
Graphic representation of domain structure of monomeric G protein: plant small GTPases contain five G domains (G1-G5) of which the G5 domain has highly divergent sequence. The G domains are essential for binding guanosine nucleotide and associated Mg2+ ion and for GTP hydrolysis. The G1 motif associates tightly with the α- and β-phosphate, the G2 motif contacts the γ-phosphate, and the G3 motif helps in positioning the water molecule for GTP hydrolysis through highly conserved glutamine residue. Switch 1 and 2 (SW1 and SW2) regions bind to effector of regulators. Rac/Rop GTPases have a Rho insert region, and all the GTPases have hypervariable (HVR) region at the C-terminus for localization
2 Small GTPases in Biotic and Abiotic Plant Stress
2.1 Rac/Rop Small GTPases in Biotic Stress
In plants, Rac/Rop small GTPases participate in diverse signal transduction events including defense responses, root hair development, pollen tube growth, ROS generation, and hormone responses (Nibau et al. 2006; Yang and Fu 2007; Shanmugam et al. 2013). Plants respond to pathogen attack by various ways including the activation of defense genes, formation of reactive oxygen species (ROS), synthesis of pathogenesis-related (PR) proteins, localized cell-wall reinforcement, and production of stress-related hormones and antimicrobial compounds. Moreover, plant defense system exists in two layers. The first layer is the PAMP (pathogen-associated molecular patterns)-triggered immunity (PTI) mediated by pattern recognition receptors (PRRs) (Dodds and Rathjen 2010), characterized by ion influx, mitogen-activated protein kinase (MAPK) activation and enhanced gene expression (Zipfel 2008). The second layer of defense is the effector-triggered immunity (ETI); it is in response to effector molecules secreted by pathogens, characterized by hypersensitive response (HR) including reactive oxygen species (ROS) production and programmed cell death (PCD) of infected tissue/cell (Jones and Dangl 2006).
ROS production is associated with PCD during resistance reaction to pathogens, and a plasma membrane NADPH oxidase (Rboh for respiratory burst oxidase homolog) similar to the neutrophil enzyme is suggested to be responsible for ROS production (Morel et al. 2004; Lamb and Dixon 1997; Hammond-Kosack and Jones 1996). In animals the oxidative burst produced by the NADPH oxidase in neutrophils is regulated by Rac2 (Babior 1999). Based on the assumption that the plant NADPH oxidase enzyme complex is similar to its mammalian counterpart, composed of six subunits including Rac (Bokoch 1994), the role of small GTPases was explored in plant defense response in ROS generation.
In rice, among seven Rac/Rop genes, OsRac1 and OsRacB have been associated with defense response (Miki et al. 2005; Ono et al. 2001; Jung et al. 2006). The constitutively active (CA), containing a G19V mutation, and dominant-negative (DN), with T24N mutation, forms of OsRac1, when introduced into seed-originated calli of the wild-type cv. Kinmaze and a lesion-mimic mutant of rice plant, sl (Sekiguchi lesion), induced and blocked ROS production, respectively. This observation suggests that OsRac1 mediates ROS production most likely by positive regulation of NADPH oxidase (Kawasaki et al. 1999). The role of NADPH oxidase in Rac-mediated ROS generation was supported by studies in tobacco, where Rac acts as a negative regulator of NADPH oxidase during ETI-triggered immune response. RT-PCR of tobacco cells upon elicitation with cryptogein, a fungal elicitor, exhibited repressed NtRac5 mRNA levels, and tobacco cells with CA-NtRac5 transgene showed decreased ROS production, by affecting the regulation of NtrbohD, a component of NADPH oxidase enzyme complex (Morel et al. 2004). OsRac1 regulation of NADPH oxidase is further supported by biochemical studies where OsRac1 interacts with the N-terminus of NADPH oxidase to regulate ROS production (Wong et al. 2007). Recent studies have also implicated PI3K as upstream regulator of OsRac1 in recruiting OsRac1 to PM to regulate NADPH oxidase activity and accelerate ROS production (Liu et al. 2012b). The role of Rac/Rop proteins in ROS production is conserved within plants and between plants and animals, pointing to existence of conserved signal transduction pathways in both the kingdoms, like maize Rac/Rop induces ROS production in mammalian cells (Hassanain et al. 2000). Similarly, human Rac was found to promote oxidative burst in soybean cell cultures (Park et al. 2000), and CA-GhRac13 (Gossypium hirsutum) transgene increases ROS production in soybean and Arabidopsis cell cultures, probably serving as a signal for xylem differentiation (Potikha et al. 1999).
In search for molecules acting in the same signaling pathway as OsRac1, Pit, an NLR protein, was identified. R gene encodes proteins with a nucleotide-binding site and leucine-rich repeat region (NLR family), which act as intracellular receptors for recognition of pathogen effectors (also called avirulence [Avr] proteins). R-mediated disease resistance results in a host response culminating in a HR and ROS production. Pit, an NLR family protein interaction with OsRac1 at the membrane, is supported by bimolecular fluorescence complementation (BiFC) assays using Vn- (N-terminus region of Venus) and Vc- (C-terminus region of Venus)tagged OsRac1 (Vn-CA-OsRac1) and Pit (PitWT-Vc) in rice protoplasts. Moreover, Förster resonance energy transfer (FRET) analysis using Raichu (Ras and interacting protein chimeric unit) confirmed activation of OsRac1 by Pit. Their role in HR and ROS production was confirmed by transient expression of autoactivated Pit, PitD485V, and DN-OsRac1 in N. benthamiana, where PitD485V in the absence of pathogen induce HR and ROS production, whereas, upon co-expression with DN-OsRac1, ROS production was visibly reduced. Phenotypic analysis of japonica rice cultivar K59, carrying Pit resistance gene in which OsRac1 was silenced by RNAi, showed enhanced fungal growth in RNAi lines, supporting the role of Pit acting upstream of OsRac1 in conferring resistance to rice blast fungus (Kawano et al. 2010). Using FRET probe Raichu-OsRac1 as an intracellular biosensor for monitoring the spatiotemporal activation of OsRac1 confirmed the induction of OsRac1 in response to chitin elicitation. The chitin-elicited defense response involves chitin binding and forming a complex with OsCEBiP, a chitin-binding protein, and OsCERK1, a receptor-like kinase at the membrane. A PRONE GEF, OsRacGEF1, was shown to regulate OsRac1 activity in chitin-elicited defense response. Phenotypic analysis of plants confirmed that OsRacGEF1 activation is required for chitin-induced immune response and resistance to rice blast fungus infection. OsRac1, OsRacGEF1, and OsCERK1 were shown to interact, supported by Y2H and in vitro binding assays, and the complex of OsRac1 and OsRacGEF1 was shown to localize to the PM by co-localization studies using OsRacGEF1-Venus and CFP-OsRac1-WT in rice protoplasts. Biochemical studies support the model where OsCERK1 phosphorylates OsRacGEF1 at the S549 residue, where the latter after activation regulates OsRac1. Based on these results, it can be hypothesized that OsCEBiP, OsCERK1, OsRacGEF1, and OsRac1 function as a “defensome” in chitin-triggered immunity (Akamatsu et al. 2013). These study indicate that OsRac1 function in plant defense response is regulated by GEF proteins acting upstream of Rac/Rops. Another GEF implicated in regulating OsRac1 in chitin-triggered immune response and ROS production is the human SWAP70 homolog in rice, OsSWAP70A, with conventional DH domain, which interacts and exhibits GEF catalytic activity towards OsRac1. OsSWAP70A activity towards OsRac1 was analyzed in vitro, by measuring replacement of unlabeled GDP with fluorescently labeled N-methylanthraniloyl (mant)-GTP. Transient expression of OsSWAP70A and OsRac1 together in N. benthamiana enhanced ROS production, whereas expressing OsSWAP70A alone had no effect. RNAi-mediated reduction of OsSWAP70A mRNA levels results in the suppression of chitin-induced defense response gene and ROS production, thereby indicating that OsSWAP70A positively regulates OsRac1 (Yamaguchi et al. 2012). In another study, the Arabidopsis SWAP70 was shown to function in both PAMP- and ETI-triggered immune responses, supported by the phenotypic analysis of T-DNA insertion mutant of AtSWAP70, which were more susceptible to pathogen growth (Yamaguchi and Kawasaki 2012).
Several independent studies have identified signaling components acting downstream of OsRac1. Expression of CA-OsRac1 transgene induces phytoalexin synthesis, a secondary metabolite with antimicrobial activity, and defense gene activation leading to resistance towards rice blast fungus (Ono et al. 2001; Fujiwara et al. 2006; Thao et al. 2007). Similarly, introduction of transgene, CA-gOsRac1, into rice, enhanced expression of PAL1 and PBZ1, defense-related genes, and induced HR-response like PCD. Moreover, it also activates genes previously known to be induced by Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae (Xoo) in cultured cells (Dang et al. 2013). OsRac1 has also been implicated in defense against the rice pathogen, the brown plant hopper (BPH), along with phytohormones, calcium, and MAPKs (Cheng et al. 2013).
Using proteomics, OsCCR1 encoding Oryza sativa cinnamoyl-CoA reductase 1, an enzyme involved in lignin biosynthesis, was identified as a downstream effector of OsRac1. In the cell wall, lignin polymerization requires peroxidase activity using H2O2. The role of OsCCR1 in plant defense was supported by the fact that OsCCR1expression level is enhanced by sphingolipid elicitor. OsCCR1 and OsRac1 form a complex, evidenced by Y2H and in vitro binding assay, where OsRac1 activates OsCCR1. Transgenic cell cultures expressing CA-OsRac1 accumulate lignin through enhanced OsCCR1 activity and ROS production. Most likely, OsRac1 regulates lignin synthesis by activating NADPH oxidase and OsCCR1 in rice defense response (Kawasaki et al. 2006). OsMT2b, a rice metallothionein gene, was identified as a downstream effector of OsRac1. Metallothionein are involved in ROS scavenging and metal homeostasis. OsMT2b expression is downregulated synergically by rice blast-derived elicitor and OsRac1, supporting its function downstream of OsRac1. OsMT2b overexpressing cells showed reduced H2O2 production in contrast to homozygous OsMT2b:Tos17 inserted mutant and OsMT2b RNAi-silenced transgenic cells. Most likely OsRac1 functions by downregulating OsMT2b and activating NADPH oxidase in blast defense response (Wong et al. 2004)
Trancriptomics revealed the signaling components such as transcription factors acting downstream of OsRac1. Microarray analysis of CA-OsRac1 transgenic plants exhibited enhanced levels of RAI1 (Rac immunity 1), a transcription factor. In order to identify genes regulated by RAI1, microarray analysis of cells containing RAI1-GR (glucocorticoid receptor), a dexamethasone (DEX)-inducible construct was performed; induction with DEX-induced expression of OsWRKY19 and PAL1 (phenylalanine ammonia lyase) defense-related genes also upregulated in response to chitin and sphingolipids. Phenotypic analysis of RAI1 T-DNA activation-tagged and RNAi transgenic lines, showing increased resistance to rice blast fungus infection, further supported the role of RAI1 in plant defense response. Moreover, in vitro binding assay supports binding between RAI1 and MAPK3/6, and BiFC assay using RAI1-Vn and OsMAPK3/6-Vc constructs in rice protoplasts supports in vivo interaction. qPCR of OsMAPK3/6 overexpression (OE) transgenic lines showed enhanced expression of PAL1 and OsWRKY19, supporting the model that OsRac1 activates RAI1 through OsMAPK3/6 to regulate expression of defense-related genes (Kim et al. 2012). Another transcription factor regulated by OsRac1 in chitin-triggered immunity is OsRap2.6. OsRac1 has been shown to interact with RACK1 (receptor for activated kinase C1). BiFC assay in rice protoplast with Vn- and Vc-tagged OsRap2.6, RACK1, and OsMAPK3/6 showed these proteins interact and localize in nucleus and the cytoplasm. Moreover, OsRap2.6, PAL1, and PBZ1 are all induced in response to chitin. Phenotypic analysis of OsRap2.6 RNAi and OE lines showed that the former are highly susceptible, whereas the latter are more resistant to infection by rice blast fungus, Magnaporthe oryzae (Wamaitha et al. 2012), indicating OsRap2.6 might act downstream of OsRac1, RACK1, and MAPK3/6 in plant defense response.
In a study OsRacB, a close ortholog of barley HvRacB gene, transcript levels were downregulated in response to infection by rice blast fungus. Interestingly, transgenic plants overexpressing OsRacB showed increased symptom development. Moreover, OsRacB requires PM localization for initiating response evident from the GFP:OsRacB-transformed onion cells and Arabidopsis protoplasts (Jung et al. 2006). These results indicate that OsRacB functions as a negative regulator as opposed to OsRac1 acting as positive regulator during rice blast fungus defense response.
To find out the role of the remaining Rop genes in defense responses, a tissue-specific expression pattern and subcellular localization of rice Rac/Rop genes were done using semiquantitative RT-PCR and transient expression system using GFP fusion proteins. Moreover, their role in disease resistance by testing single Rac/Rop RNAi plants against the rice blast fungus showed OsRac2, OsRac6, and OsRac7 expressions at a very low level in leaf blades. Infection assays showed OsRac1 as positive regulator of blast resistance, corroborating earlier results (Ono et al. 2001), whereas OsRac4 and OsRac5 act as negative regulators of blast resistance (Chen et al. 2010).
Three ROP proteins HvRACB, HvRAC1, and HvRAC3 in barley (Hordeum vulgare) are linked to both development and pathogen responses (Schultheiss et al. 2005; Pathuri et al. 2008; Hoefle et al. 2011). HvRACB functions as a common signaling component for both biotic and abiotic stresses, supported by phenotypic analysis of CA-HvRACB transgenic plants, exhibiting enhanced susceptibility to powdery mildew fungus (Blumeria graminis f. sp. hordei [Bgh]) and increased water loss in detached leaves due to reduced responsiveness to ABA, leading to failure in stomata closing (Schultheiss et al. 2005). In barley defense response, HvRACB acts as a negative regulator of disease resistance to Bgh (Schultheiss et al. 2002; Schultheiss et al. 2003). Knockdown of HvRACB expression by RNAi reduced susceptibility to powdery mildew by increasing resistance to penetration by Bgh, whereas the CA-HvRACB transgene enhanced susceptibility to Bgh. The mechanism of HvRACB action depends on the actin cytoskeleton disorganization and the mildew resistance locus (MLO). MLO encodes a seven-transmembrane protein and acts as negative regulator of cell death (Piffanelli et al. 2002; Schultheiss et al. 2002, 2003; Hoefle et al. 2011; Huesmann et al. 2011). HvMAGAP1 (microtubule-associated ROP GTPase-activating protein 1) was identified as a susceptibility factor in barley (Huesmann et al. 2011). Transient-induced gene silencing or OE of HvMAGAP1 resulted in enhanced or reduced susceptibility to Bgh, respectively. Like HvRACB, HvMAGAP1 also influences the polarity of cortical microtubules in interaction with Bgh, initiating MT disorganization to accommodate fungus haustoria. Moreover, interaction between HvMAGAP1 and HvRACB is required for the defense response, established in vitro, by Y2H and using FRET, in planta (Hoefle et al. 2011). The Arabidopsis homologs of HvMAGAP1, AtROPGAP1, and AtROPGAP4 also function as plant defense. T-DNA insertion knockout enhanced susceptibility to the virulent powdery mildew fungus Erysiphe cruciferarum, indicating functional conservation of ROPGAPs in pathogen response in both monocots and dicots (Huesmann et al. 2011).
Adopting a proteomic approach, HvRBK1 (Hordeum vulgare Rop-binding protein kinase1) was identified as a downstream effector of HvRACB and HvRAC1 signaling pathways involved in MT reorganization in a Y2H assay. In in vitro assay, kinase activity of HvRBK1 was observed to be induced by CA-HvRACB or activated HvRAC1. Transient-induced gene silencing of barley HvRBK1 supported penetration by Bgh, and transient knockdown influenced MT stability in barley epidermal cells; all these results support a model where HvRACB activates HvRBK1 to bring about cytoskeleton reorganization (Huesmann et al. 2012). It is evident from research in both rice and barley that the mechanism of action of Rac/Rop protein function in plant defense is different, where in rice OsRac1 functions by activating enzymes involved in ROS generation and regulating gene expression; HvRACB primarily affects the MT organization in the infected cells by activating HvRBK1.
Phosphatidic acid (PA), a secondary messenger, is implicated in numerous biotic and abiotic stresses including pathogen elicitation, wounding, freezing, hyperosmotic stress, and water deficit (Canonne et al. 2011). Studies have shown that PA mediates PCD and ROS generation in whole leaf and at single-cell level by activating AtROP2, which is shown to enhance ROS production in an in vitro assay. Although, epistasis analysis where in absence of exogenous PA, no spontaneous ROS production was observed in CA-ROP2 transgenic plants; suggested activation of ROP2 alone is not sufficient to induce ROS generation (Park et al. 2004).
Mutations in plant signaling molecule salicylic acid (SA)-mediated pathways heighten disease susceptibility to pathogens, while induction of SA-dependent defense response reduces pathogen growth (Durrant and Dong 2004). SA-dependent signaling pathway known as systemic acquired resistance (SAR) induces expression of PR (pathogenesis-related) genes including phytoalexins and PR proteins. Microarray analysis of DN-AtROP6 plants revealed a role for AtROP6 in SA-mediated defense response by causing major changes in gene expression associated with the constitutive SA-mediated plant defense responses. The total level of SA in DN-AtROP6 transgenic plants resembled those of wild-type plants, inoculated with a virulent powdery mildew pathogen. The constitutive SA-associated response in DN-AtROP-6 was suppressed in mutants defective in SA signaling (norexpressor of PR genes1 (npr1)) or biosynthesis (salicylic acid induction deficient 2 (sid2)) (Poraty-Gavra et al. 2013), supporting Rac/Rops as negative regulators in SAR.
Similarly, in other plant species, the role of Rac/Rop GTPases in plant defense mechanism is conserved. Rac/Rop GTPases were shown to be involved in both PTI- and ETI-triggered immunity as their expression was found to be induced in pathogen-inoculated chickpea leaf and elicitor-treated cell culture (Ichinose et al. 1999); for example, in tobacco silencing of NtRop by expression of Medicago, Ms-Rac1 antisense cDNA causes defects in defense response (Schiene et al. 2000).
2.2 Rac/Rop GTPases in Abiotic Stress
Stomata closure in aerial tissue is an important approach adopted by plants to respond to rapid water loss during drought, orchestrated by phytohormone ABA. ABA is also the key mediator of plant responses to other abiotic stresses including salinity and cold, and therefore is known as the stress hormone. The ABA signaling components include ABA receptors (PYL/PYR/RCAR family), a group of type 2C phosphatases (PP2C) (Park et al. 2009), three members of the SNF1-related protein kinase2 family (Fujii et al. 2009), and molecules regulating ABA signaling including NADPH oxidase (Jiang and Zhang 2002; Zhang et al. 2009a, 2009b) and heterotrimeric G protein (Pandey and Assmann 2004).
Genetic and biochemical analysis in Arabidopsis points towards the involvement of ROP family GTPases in ABA signaling. Acting as negative regulator, expression of CA-AtROP6 reduced ABA-mediated stomata closing (Lemichez et al. 2001). Zheng et al. in 2002 demonstrated AtROP10 as negative regulator of ABA-mediated seed germination, stomata closing, and root elongation. A null rop10 mutant exhibits enhanced responses to ABA in seed germination, root elongation, and stomata closure, whereas CA-ROP10 reduces ABA inhibition of seed germination. Li and group in 2012 confirmed that ROP11 also acts as a negative regulator of ABA-mediated stomata closure, seed germination, and drought response. Expressing CA-ROP11 allele inhibiting stomata closure and DN-ROP11 allele exhibited opposite effect. ABA primarily modulates the localization of ROP11, causing it to accumulate in the nucleus for transcriptional regulation. Moreover, phenotypic analysis of rop10rop11 double mutant showed that they act in parallel pathways in ABA-mediated responses. Li et al. ( 2012a, 2012b), using luciferase complementation imaging (LCI), Y2H assay, and co-immunoprecipitation assay, showed that activated ROP11 binds ABI1 (ABA insensitive), a PP2C phosphatase, and allows the downstream SnRKs to be activated, which further activate TFs and upregulate stress-related genes like RAD29.
Insight into the mechanism of RacRop GTPase action was elucidated by studies providing a link between actin cytoskeleton remodeling in guard cells and ABA-induced stomata closure. Microscopic analysis of WT/GFP-mTn (GFP-mouse talin) and WT/MAP4-GFP (microtubule-associated protein 4-GFP), expressing GFP fusion proteins targeted to actin (Kost et al. 1998) and tubulin (Mathur and Chua 2000), respectively, supported ABA-mediated actin cytoskeleton reorganization during stomata closure. A role for ABA in actin cytoskeleton reorganization was further supported by studies done in abi1-1/GFP-mTn transgenic lines, where ABI1 is an upstream regulator of ABA signaling in stomata closure (Leung et al. 1994). abi1-1 mutants display a wilty phenotype associated with impaired stomata closure. In abi1-1/GFP-mTn transgenic lines, both actin cytoskeleton and stomata closure remain unaffected (Meyer et al. 1994; Gosti et al. 1999). AtRac1/AtROP3/ARAC3/Rop6AT was isolated in a reverse genetic approach to identify genes acting in ABA signaling to induce stomata closure in Arabidopsis (Winge et al. 1997; Li et al. 1998; Lemichez et al. 2001). A role for AtRac1/AtROP3 was confirmed by observing actin cytoskeleton organization in AtRac1-T20N/GFP-mTn, a DN Rac (Ridley et al. 1992), and AtRac1-G15V/GFP-mTn, a CA Rac (Diekmann et al. 1991), transgenic lines, where the former promoted actin cytoskeleton reorganization. Furthermore, WT/AtRac1-T20N transgenic lines blocked the ABA-induced stomata closure and restored stomata closure defects of abi1-1 /AtRac1-T20N transgenic lines. In conclusion, AtRac1 acts as a negative regulator linking the actin cytoskeleton remodeling and ABA signaling during drought response and water homeostasis in Arabidopsis (Lemichez et al. 2001).
Hypoxia/oxygen deficiency causes switch from mitochondrial respiration to anaerobic fermentation (Sauter 2000). Ethanolic fermentation relies on activity of pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH); both the enzymes are activated upon hypoxia (Fukao et al. 2006). Importance of ADH and PDC (pyruvate decarboxylase) has been shown by mutant analysis, where adh and pdc mutants of maize and Arabidopsis rapidly succumb to hypoxia, confirming the importance of ethanolic fermentation under oxygen stress (Ismond et al. 2003; Kürsteiner et al. 2003).
In animals, cellular hypoxia triggers H2O2 (ROS) production, leading to increased transcription of hypoxia-inducible factor 1α (HIF1α) via a Rho-GTP-dependent mechanism (Turcotte et al. 2003). In plants, hypoxia leads to H2O2 production by triggering the activity of ADH (Baxter-Burrell et al. 2002). In Arabidopsis, ROPGAP4 was identified in an attempt to identify genes induced under hypoxia stress, using a Ds-GUS transposon gene trap (Baxter-Burrell et al. 2003). Loss-of-function ropgap4 mutants exhibited increased ADH mRNA, indicating ROPGAP4 negatively regulates ADH. Moreover, the DN form of ROP2 (35S:DN-Rop2) did not show any increase in ADH mRNA, whereas the CA-ROP2 (35S:CA-Rop2) lines were hypersensitive to oxygen deprivation, supporting ROP2 as positive regulator in signaling during hypoxia. Even though H2O2 levels were found to increase significantly in ropgap4 mutants under hypoxic conditions, no rise in H2O2 was detected in 35S:DN-Rop2 seedlings. These experimental results support the hypothesis, where ROPGAP4 acts as a negative feedback regulator of Rop GTPases, which are involved in defense responses and H2O2 production required for ADH induction. A feedback loop is formed where H2O2 activates RopGAP4, which in turn inhibits Rop2. Rop2 inactivation subsequently leads to reduction in hydrogen peroxide release (Baxter-Burrell et al. 2003).
Further, studies in tobacco revealed the role of Rac/Rops in salinity stress. NtRop1 of tobacco when expressed in Arabidopsis conferred salt sensitivity. It was speculated that NtRop1-induced salt sensitivity is caused by increased H2O2 production, which acts as a second messenger molecule during salt stress (Cao et al. 2008), inducing PM H+-ATPase activity resulting in increased K+ to Na+ ratio (Zhang et al. 2007).
2.3 Ran GTPases
Plants respond to various biotic and abiotic stresses by modulating transcriptional activity of various stress-related genes or alternatively by regulating posttranscriptional nucleocytoplasmic trafficking of proteins and noncoding RNA molecules through Ran (Ras-related nuclear protein)-dependent karyopherin (importin β) protein (Mosammaparast and Pemberton 2004; Mazzucoteli et al. 2008; Chinnusamy et al. 2008). Ran GTPase activity is regulated by several proteins including GAPs, GEFs, and RanBP1. Ran in GDP-bound form, generated by action of RanGAP1, exists in the cytoplasm, whereas the GTP-bound form, generated by RCC1 (regulator of chromosome condensation 1), a GEF, exists in the nucleus. This asymmetric distribution of Ran GTPases is pivotal for their functional activities (Bischoff and Ponstingl 1991; Bischoff et al. 1994). Ran-binding protein 1 (RanBP1) acts as an accessory factor to increase RanGAP1-mediated nucleotide hydrolysis by inhibiting the GEF activity of RCC1, thereby promoting inactivated Ran (Bischoff et al. 1995). In plants, the identification of RanBP1 and RanGAP (Ach and Gruissem 1994; Haizel et al. 1997; Rose and Meier 2001; Pay et al. 2002) revealed their high sequence similarity and subcellular localization with animal Ran proteins, pointing to functional conservation in nucleocytoplasmic trafficking and mitotic process (Ach and Gruissem 1994; Haizel et al. 1997).
Ran GTPases are encoded by a family of 4 genes in Arabidopsis and 3 genes in rice (Haizel et al. 1997; Vernoud et al. 2003). Several independent studies have implicated Ran GTPases in plant development, for example, overexpression (OE) of wheat Ran, TaRAN1, in Arabidopsis and rice resulted in an elevated mitotic index and prolonged life cycle (Wang et al. 2006). Besides, Ran GTPases are also affected by oxidative stress, causing reduction in ATP levels, leading to decrease in Ran GTP levels, and their disordered distribution (Yasuda et al. 2006).
Virus-induced gene silencing (VIGS) of NbRanBP1 in N. benthamiana exhibited stress responses, such as reduced mitochondrial membrane potential and excessive production of ROS (Cho et al. 2008). A number of studies in both Arabidopsis and rice using transgenic and RT-PCR approaches have shown involvement of Ran in phytohormone signaling and salt stress response (Miche et al. 2006). OsRAN2 overexpression results in increased sensitivity to salt, osmotic stress, and ABA treatment, and RT-PCR analysis of transgenic lines showed reduced OsRAN2 trancript levels in salt and osmotic stresses and ABA treatment, supporting their role as negative regulators in abiotic stress response (Zang et al. 2010). Furthermore, three ABA- or stress-responsive genes, AtNCED3, PLC1, and AtMYB2, encoding a key enzyme in ABA synthesis, a phospholipase C homolog, and a putative transcription factor, respectively, showed differentially induced expression in OsRAN2 OE plants under salt and ABA treatment conditions (Liu and Zhu 1997; Xiong et al. 2002; Verslues et al. 2006). In order to find out the mechanism of OsRAN2 action, OsRAN2 and Lc (leaf color transcription factor) were co-expressed in tobacco epidermal cells, where LC acts as a maize reporter gene for nuclear transport (Shieh et al. 1993), and it was observed that OsRAN2 disturbed the nuclear transport of Lc, suggesting that the OsRAN2 hypersensitivity phenotype could be due to its effect on nucleocytoplasmic trafficking of regulatory proteins such as transcription factors (Zang et al. 2010).
In Oryza sativa, OsRAN2 was identified to confer resistance towards cold stress. The transcript level of OsRAN2 increased several fold under cold stress condition. Insight into the mechanism of OsRAN2 action came from the analysis of the mean mitotic index for OsRAN2 OE and OsRAN2 RNAi lines, which showed an enhanced and reduced mean mitotic index, suggesting its role in cell cycle to confer cold stress tolerance. Subcellular localization of OsRAN2:GFP revealed GFP fluorescence in nucleus at the spindle and tubulin during metaphase and anaphase pointing towards a function in maintaining cell division during cold stress. These results were further corroborated by OsRAN2 RNAi plants, exhibiting aberrant organization of spindles during mitosis and that OsRAN2 helps in nucleocytoplasmic transport of tubulin and formation of intact nuclear envelop during cold conditions. In conclusion, OsRAN2 regulates cold resistance in rice by maintaining cell division and by promoting accumulation of intranuclear tubulin at the end of mitosis and formation of intact nuclear envelope (Chen et al. 2011).
A recent report has shown that OsRAN1 is ubiquitously expressed in rice and exhibit enhanced transcript levels upon cold and IAA treatment, where auxin is known for its effects on cell division. OsRAN1 OE results in improved cold tolerance. It was found that at molecular level, it helps to maintain cell division and cell cycle progression and promote formation of intact nuclear envelope (Xu and Cai 2014). In conclusion, Ran GTPases confer cold stress tolerance by regulating the plant cell cycle; interestingly, cell division has been previously closely related to stress tolerance in plants (Kitsios and Doonan 2011).
2.4 RAB GTPases
RABs and SNAREs (soluble N-ethylmaleimide-sensitive (NSF) attachment protein receptors) are conserved regulatory molecules involved in membrane trafficking. RABs promote the tethering of transport vesicles/organelles to target membranes, followed by membrane fusion, which is executed by specific combinations of three glutamine-contributing SNAREs (Qa-, Qb-, and Qc-SNARE) and one arginine-contributing (R-)SNARE in a stable complex (Saito and Ueda 2009; Wickner and Schekman 2008).
Arabidopsis genome encodes 57 Rab GTPases (Pereira-Leal and Seabra 2001), grouped into 8 clades (RabA-RabH) (Segev 2001; Rutherford and Moore 2002; Vernoud et al. 2003). Among them, Rab5 (RabF) forms the largest clade (Stenmark et al. 1994), with three homologs encoded in Arabidopsis genome including AtRabF1 (Ara6), AtRabF2a (Rha1), and AtRabF2b (Ara7) (Rutherford and Moore 2002; Vernoud et al. 2003). Co-expression of ARA6-Venus and ARA7-GFP under their native regulatory elements showed overlapping expression pattern in various endosomal population in Arabidopsis, confirming their role in endocytosis (Ebine et al. 2012).
Endocytosis, traditionally a constitutive housekeeping process, has recently been implicated in trafficking in stress responses in tobacco (Leyman et al. 1999). It has been shown that in yeast, oxidative stress inhibits vesicle trafficking between cytosol and PM, a phenotype partially restored by OE of Arabidopsis synaptobrevin homolog, providing tolerance to lethal concentrations of H2O2 (Levine et al. 2001).
Various Rab GTPases have been identified and implicated in biotic and abiotic stresses using SSH, differential display (DD), and northern blot techniques in different plant species. Phenotypic analysis of mutants and various proteomics approach helped to understand the mechanism of Rab GTPase action in conferring stress tolerance.
AtRabG3e (AtRab7) was isolated by DD approach for genes induced under abiotic and biotic stresses including salt, superoxide, SA, and necrogenic pathogens (virulent P. syringae bacteria or B. cinerea fungus) (Mazel et al. 2004). Overexpression of AtRabG3e in Arabidopsis thaliana leads to less sensitivity to salt and osmotic stress than WT plants. Analysis of general membrane endocytosis in AtRabG3e transgenic plants revealed accelerated uptake of membrane dye FM1-43, which could be due to faster vesicle trafficking at the end point, i.e., endosome-vacuole fusion, supporting the hypothesis that in the transgenic plants Na+ is transported into the vacuoles, reducing the overall cytosolic Na+ content. Moreover, transgenic plants exhibited reduced ROS content, due to decrease in the cytosolic Na+ content (Mazel et al. 2004). Another Rab5 member, ara6, lof confers hypersensitivity to salt stress, whereas OE transgenic plants showed elevated resistance to salinity stress and high-sorbitol stress (Ebine et al. 2012). Similarly, in rice root cells, ARA6 is secreted in the apoplast in response to salinity stress (Zhang et al. 2009a, 2009b).
PgRab7 of Pennisetum glaucum, isolated by subtractive hybridization (SSH) of treated plants, showed enhanced transcript level upon cold, dehydration, and NaCl and IAA treatment. The role of PgRab7 in abiotic stress was confirmed by overexpression of PgRab7 in heterologous system, enhancing NaCl and mannitol tolerance in transgenic tobacco plants (Agarwal et al. 2008). In a similar study, Prosopis juliflora, an abiotic stress-tolerant tree species of Fabaceae, was used for isolating genes functioning in abiotic stress tolerance. Northern analysis of leaf tissue revealed upregulation of PjRAB7 during salt stress conditions. Moreover, expressing PjRAB7 in heterologous system conferred salt tolerance to tobacco, probably by sequestering sodium in the vacuoles, suggesting functional conservation of Rab GTPase in different plant species (George and Parida 2011). Adopting differential display (DD) approach Rab2 was identified in the desiccation-tolerant grass Sporobolus stapfianus in response to drought and rehydration in leaves. RT-PCR analysis showed RAB2 transcript accumulation in response to decreased relative water content (RWC), whereas rehydration resulted in decreased levels. Furthermore, ABA was also found to function in this pathway as exogenous ABA slightly increased the RAB2 transcript accumulation (O’Mahony and Oliver 1999). SmGTP related to Rab2 gene family was identified in a salt stress SSH expression library of model grass species Lolium temulentum, exhibiting strong expression under salt and drought stress, a pattern similar to dehydration-specific gene such as delta 1-pyrroline-5-carboxylate synthetase (P5CS) and cold response gene COR413 (Dombrowski et al. 2008).
The mechanism of Rab GTPase tolerance to biotic stress was shown in wheat, TaRab7, a Rab GTPase gene. It was isolated by reverse transcription technique from wheat cDNA library obtained from leaves infected with Puccinia striiformis f. sp. tritici (Pst), wheat stripe rust pathogen. It showed highest homology to BdRab7-like small GTPase from Brachypodium distachyon, OsRab7 (Oryza sativa), AtRabG3b (Arabidopsis), and HvRab7 (Hordeum vulgare). qRT-PCR analysis of TaRab7 showed enhanced expression levels under both abiotic (salinity, drought, and low temperature) and biotic (Pst infection) stresses. Furthermore, knocking down TaRab7 expression by virus-induced gene silencing (VIGS) enhanced the susceptibility of wheat cv. Suwon 11 towards an avirulent Pst race CYR23 (Liu et al. 2012a, 2012b). Previous studies have shown that Rab7 interacts with various downstream effectors (Zerial and McBride 2001) and plays a critical role in vesicular transport from early to late endosomes in the cytoplasm (Feng et al. 1995; Mukhopadhyay et al. 1997). In HeLa cells, Rab7 is critical for lysosomal aggregation and fusion in perinuclear region (Bucci et al. 2000). Transient subcellular localization assays in onion peel cells using pCaMV35S:TaRab7-GFP fusion construct revealed the cytoplasmic, perinuclear, and nuclear localization pattern, consistent with its role in regulating lysosome and phagosome to detoxify the toxic material secreted by pathogen into the host cells (Liu et al. 2012a, 2012b).
Studies in Arabidopsis have shown the involvement of auxins in stress response and tolerance. RABA (RAB11) group/clade contains 26 members, divided further into six subgroups (RABA1–RABA6). Cell biological and physiological analysis of RABA1 members implicated them in transport between trans-Golgi network and PM and their requirement during salt stress tolerance. Mutant and complementation analysis showed that the four RABs – RABA1a, RABA1b, RABA1c, and RABA1d – act redundantly in salt stress tolerance and are not involved in regulating intracellular distribution of sodium (Asaoka et al. 2012; Asaoka et al. 2013) and probably act by affecting the localization of PIN, auxin efflux transporters (Feraru et al. 2011), supported by the role of auxins in salt stress tolerance (Park et al. 2011). RABA1, subgroup of (RAB11) family members in Arabidopsis, shows a different mechanism for salt tolerance; it is involved in salinity stress by regulating the transport of sodium across the PM and accumulation in vacuoles as the quadruple mutants do not affect the sodium content or distribution (Asaoka et al. 2013).
In Mangifera indica, MiRab5 is upregulated in response to cold, salinity, and PEG stress in addition to its involvement in seed germination (Liu et al. 2014). Arabidopsis CPRabA5e (where CP represents chloroplast) is a chloroplast-localized protein found to regulate oxidative stress signaling. The role of CPRabA5e was further validated by Y2H screen where 13 proteins previously predicted to be involved in stress and localized in thylakoids and plastoglobules were identified (Karim et al. 2014). However, the exact mechanism of CPRab5e activity is not known yet.
Proteome analysis of rice seedlings during drought stress and recovery upon rewatering revealed that 9 small GTP-binding proteins are highly expressed specifically under severe drought stress, while 3 out of 9 were induced in all stress conditions with highest expression in drought. Among these 9 stress-induced small GTP-binding proteins, 4 belong to Rab GTPases family, indicating Rab GTPases role in plant stress tolerance is conserved throughout the plant kingdom (Mirzaei et al. 2012).
2.5 ARF GTPases
High concentrations of salt arrest plant development and lead to plant cell death by disrupting ion and water homeostasis, inhibition of metabolism, and damage to membranes (Huh et al. 2002). Photosynthetic activity is suppressed by salt stress, and the reduction in photosynthetic activity can be accounted for by the decline in chlorophyll content (Al-Aghabary et al. 2004). Plants suffering from salt stress often display symptoms of oxidative damage as indicated by marked accumulation of ROS such as H2O2 (Hasegawa et al. 2000). MDA (malondialdehyde) is recognized parameter for lipid peroxidation, leading to increased permeability of membranes to various ions (Mittler 2002). Plants overcome salt stress by either transporting excess Na+ into the vacuoles or by prohibiting its entry into plant cells, accomplished by various transporters (Apse et al. 1999; Zhang and Blumwald 2001; Rus et al. 2001) or by accumulating osmolytes.
ADP-ribosylation factor (Arf) GTPases are important regulators of membrane-trafficking pathways, initially identified due to their ability to stimulate the ADP-ribosyltransferase activity of cholera toxin A (Moss and Vaughan 1998). They are involved in generation of the three types of vesicle coat proteins (COPI, COPII, and clathrin) (Kirchhausen 2000). Plant genome encodes for Arf-like (Arl) GTPases with protein sequence highly similar to that of Arf GTPases in animals. Knockout of an Arl GTPase, TITAN, leads to dramatic alterations in mitosis and cell cycle control during seed development in Arabidopsis (McElver et al. 2000). Suppression subtractive hybridization (SSH) in Medicago falcate, tolerant to abiotic stresses, identified MfARL1, an Arl GTPase, which confers greater salt tolerance. Functional characterization of MfARL1 in Arabidopsis confirmed its role in salt tolerance. The OE transgenic plants showed reduced accumulation of Na+ and reduced expression levels of AtHKT1, a sodium transporter, which probably reduces the influx of Na+ into the plant thereby conferring resistance to salt (Wang et al. 2013). Function of AtHKT1 in sodium transport has been previously shown, where AtHKT1 mutation suppress sos3 mutant phenotypes. Analysis of ion contents in the sos3hkt1 mutant demonstrated that AtHKT1 is involved in mediating Na+ influx into the plant cells (Rus et al. 2001). Moreover, the photosynthetic activity and chlorophyll content of the MfARL1 OE transgenic lines were not affected by salt stress when compared to wild-type plants. The MDA and H2O2 content of transgenic plants was less, whereas the wild-type plants exhibited increased levels of H2O2 and MDA. Increased level of H2O2 in wild-type plants was due to decreased CAT (catalase) activity, whereas salt stress has no effect on CAT activity in the transgenic lines. In conclusion, AtHKT1 provides greater salt tolerance by regulating a sodium transporter and CAT activity.
Similarly, ARF1 of Jatropha curcas is induced in response to abiotic stresses, particularly upon treatment with PEG, which induces osmotic stress, and cold treatment (Qin et al. 2011).
2.6 MIRO GTPases
Miro GTPases function in mitochondrial trafficking and morphology in eukaryotes. The role for AtCBG (calcium-binding GTPase)/AtMIRO2, a Miro GTPase, has been explored in abiotic stress. Expression of AtMIRO2 was upregulated in salt stress and ABA treatment suggesting its involvement in abiotic stress. Phenotypic analysis of AtMIRO2 mutant confirmed their sensitivity towards both salt and ABA stress (Jayasekaran et al. 2006).
2.7 Seed Plant (Spermatophyte)-Specific Small GTPase
In plants, circadian clock regulates signaling pathways such as light, hormone, and stress (Covington et al. 2008). Plants respond to high salinity stress, by increasing cytosolic calcium, by activating the SOS1-mediated changes in ion homeostasis, and subsequently by generating secondary messengers such as phospholipids and ROS, which activate kinase cascade to regulate transcription of stress-inducible genes. These responses can be ABA dependent and independent and are linked to circadian clock (Sanchez et al. 2011). Kevei et al. in 2007 identified LIP1 (LIGHT INSENSITIVE PERIOD 1), a small GTPase, in a genetic screen for novel circadian clock mutant in Arabidopsis. lip1 mutant displayed increased sensitivity to salt stress, affecting morphogenesis and ploidy. qRT-PCR analysis confirmed that LIP1 does not function in ABA-dependent or ABA-independent salt stress signaling pathways leading to expression of osmoprotectant genes like RAD29A (RESPONSIVE TO DESICCATION 29A), RAD29B, or RAB18 (RESPONSIVE TO ABA) and ionic stress genes like SOS2, a Na+/H+ antiporter and activator of SOS1, respectively, though its function at the posttranslational level was not ruled out. Another possible explanation for salt stress sensitivity could be the role of LIP1 in endoreplication, affecting the ploidy level (Terecskei et al. 2013). Previous studies have implicated endoreplication in seedling development upon exposure to stress including high temperature and drought (Engelen-Eigles et al. 2001). Cookson et al. in 2006 showed that increase in extent of endoreduplication reduced the negative impact of drought on the final leaf size.
3 Large G Proteins in Biotic and Abiotic Stress
3.1 Biotic Stress
Several independent studies using inhibitors and agonists of G proteins in different plant species supported the role of G proteins in defense signaling (Legendre et al. 1992; Beffa et al. 1995; Gelli et al. 1997). Particularly, changes in cytosolic calcium concentrations, often observed in elicitor-treated plant cells, are regulated by G proteins (Aharon et al. 1998). Calcium then activates the downstream signaling pathway including calcium-activated kinases (Romeis et al. 2000), calmodulin (Heo et al. 1999), and NADPH oxidase (Sagi and Fluhr 2001).
Studies in rice have indicated the specificity of G-protein signaling in plant defense response. Rice d1 mutants, lacking functional RGA1 (Gαs), showed reduced defense response to avirulent strains of bacterial blight (Xanthomonas oryzae pv. Oryzae [Xoo]) and rice blast fungus (Magnaporthe grisea) (Komatsu et al. 2004). A role supported by elevated RGA1 mRNA levels upon rice blast infection and sphingolipid elicitor (SE) treatment in leafs and cultured cells. Further analysis supported G-protein function in ETI-triggered defense response, as there was suppression of ROS production and PBZ1 expression by SE in the d1 mutant cell cultures. Moreover, Rac signaling was placed genetically downstream of G proteins based on epistasis analysis using CA-OsRac1, where OsRac1 restored the defense signaling, by promoting the PR gene expression and ROS production in d1 mutant plants (Suharsono et al. 2002).
Similarly, Arabidopsis mutants of gpa1 gene, encoding GPA1, have slightly increased resistance to several pathogens though the link between G proteins and plant defense is conclusively established through the Arabidopsis Gβγ dimer. Mutants deficient in AGB1 (Gβs) are more susceptible to the fungal pathogens Alternaria brassicicola, Botrytis cinerea, Fusarium oxysporum, and Plectosphaerella cucumerina (Llorente et al. 2005; Trusov et al. 2009). Upon infection with A. brassicicola or treatment with methyl jasmonate (MeJA), where latter is produced during both biotic and abiotic stress response, agb1 mutants exhibit delay in the induction of the MeJA-induced PR genes, PDF1.2, OPR3, and PAD3 (Trusov et al. 2009), whereas expression of the SA-dependent PR1 was increased after infection with P. cucumerina (Llorente et al. 2005). It was concluded that the Gγs confer functional selectivity to Gβγ dimer. Among the three Arabidopsis Gγs, AGG1 has been implicated in plant defense response against F. oxysporum and A. brassicicola (Trusov et al. 2007), whereas AGG3 has no defense-related response and the role of AGG2 is not clear (Delgado-Cerezo et al. 2012). In conclusion, G protein plays an important role in both PTI- and ETI-triggered immune responses. Besides, the canonical G-protein subunits, one of three extra-large α-subunit, XLG2 (Lee and Assmann 1999), is linked to plant defense. xlg2 mutant shows enhanced susceptibility to P. syringae and reduced induction of the pathogenesis-related gene PR2 indicating positive regulatory role in plant defense response. Microarray analysis revealed that, aside from PR2, other pathogen-inducible genes are downregulated in xlg2 mutant in response to P. syringae infection, whereas overexpression of XLG2 resulted in accumulation of aberrant transcripts for several defense-related genes. Biochemical analysis revealed that XLG2 physically interacts with AGB1 and that the interaction is restricted to infected tissues (Zhu et al. 2009).
G protein have been shown to be involved in elicitor-induced HR in plants by VIGS-induced silencing of the Gα, Gβ1, and Gβ2 subunits of Nicotiana benthamiana G-protein complex. The silenced plants when treated with bacterial and fungal elicitors showed impaired HR including stomata closure, nitric oxide (NO) production, and ROS accumulation in guard cells. Besides, transcription of various plant defense-related genes was also impaired, including NbrbohA (NADPH oxidase), NOA1 (associated with NO), and NIA (responsible for NO production) (Zhang et al. 2012).
3.2 Abiotic Stress
Sessile nature of plants constantly exposes them to changing environmental conditions giving rise to oxidative stress. A mild oxidative stress induces antioxidant defenses, whereas severe stress leads to rapid necrosis or cell death. The induction and execution of PCD is regulated by various signaling molecules including ROS, jasmonic acid (JA), salicylic acid (SA), and ethylene (Lam et al. 1999). Ozone is a major oxidative stress and a major pollutant that damages crops, forests, and urban vegetation. The main symptoms of ozone damage are foliar lesions and reduction in stomata aperture mediated through ABA signaling (Mansfield 1998), and it shares signaling pathways and gene expression pattern with hypersensitive response (HR) (Tamaoki et al. 2003).
Arabidopsis plants with null mutations in the gpa1 (Gαs) and agp1 (Gβγ dimer) are less and more sensitive, respectively, to ozone damage than the wild-type plants. Analysis of gpa1 mutant plants exhibited ozone-resistant phenotype, indicated by lack of leaf curling in response to ozone (Booker et al. 2004). At molecular level, it was shown that Gαs activates membrane-bound NADPH oxidases, encoded by AtrbohD and AtrbohF genes, whereas the Gβγ dimer stimulates ROS production in chloroplast. The role of gpa1 in activating NADPH oxidases was further supported by analysis of atrbohD and atrbohF double-mutant plants, which are more resistant to ozone than wild-type plants. Moreover, a link between G-protein and Rac signaling has been provided, where Gαs acts through the Rop (Baxter-Burrell et al. 2002; Suharsono et al. 2002) and where Rop is activated by phosphatidic acid (PA), a product of phospholipase Dα (PLDα), which in turn is inhibited by Gαs. A direct interaction was shown between PLDαl and GPA1, where binding inhibited PLDα1 activity, relieved upon GTP addition, suggesting in vivo G-protein activation leads to PLDα1 activation. PA upon activation and dissociation of Gαs inhibits the activity of ABI-1 and ABI-2 (ABA insensitive), negatively regulating ABA signaling through stress-activated MAPK cascade (Zhang et al. 2004). gpa1 mutant also exhibit reduced ozone sensitivity at single-guard-cell level, indicated by reduced ABA inhibition of guard cell inward K+ channels and altered ABA promotion of slow anion currents (Wang et al. 2001). ABA is known to mediate its effects by acting through second messenger, S1P (sphingosine-1-phosphate) (Ng et al. 2001; Coursol et al. 2003) and through the effector molecule, PLCs (phosphatidylinositol-phospholipases) (Jacob et al. 1999; Zhang et al. 2004). Guard cells of gpa1 mutants exhibit insensitivity to inhibition of stomata opening by S1P. Moreover, studies supported existence of two parallel pathways: S1P dependent and independent (Wang et al. 2001; Coursol et al. 2003).
The role for G proteins in ABA-mediated response is further validated by studies in gcr1 mutants, exhibiting hypersensitivity to ABA and S1P in both stomata opening and promotion of stomata closure, indicating that GCR1 acts as negative regulator of GPA1-mediated ABA responses in guard cells. A hypothesis further supported by higher levels of expression of drought- and ABA-regulated genes after exogenous ABA treatment and inhibited improved recovery following drought stress in gcr1 mutant plants (Pandey and Assmann 2004). PLCs, the downstream effectors of ABA, have been shown to both genetically and physically interact with G-protein signaling component, indicated by studies done in BY2 tobacco cells overexpressing GCR1, where GCR1 activates DNA synthesis in PLC-dependent manner (Apone et al. 2003).
G proteins have been shown to function in closure of stomata aperture by production of H2O2 induced by ExtCaM (extracellular calmodulin) and NO (nitric oxide). G-protein involvement in H2O2 production is evident by analysis of gpa1 mutants; ExtCaM-induced H2O2 production is impaired in gpa1 mutants, indicated by impaired stomata closure. ExtCaM-mediated NO generation is regulated by GPA1, whereas GPA1 activation of NO production depends on H2O2 (Chen et al. 2004a, 2004b).
Recently, eATP (extracellular ATP) has been reported as a signaling molecule, existing in the apoplast of plant cells. In gpa1 mutant plants, eATP-promoted stomata opening, cytoplasmic ROS generation, and Ca2+ and H+ efflux are all suppressed, indicating G-protein involvement during eATP signaling (Hao et al. 2012). Proteomics has identified MAPK (mitogen-activated protein kinase) as a downstream effector molecule for G-protein-mediated signaling in response to ABA and MeJA (Methyl Jasmonate), supported by yeast two-hybrid assays and upregulation of PsGβ and PsMAPK3 (Pisum sativum MAPK3), upon treatment with MeJA and ABA (Bhardwaj et al. 2011).
Besides, Arabidopsis and rice, the signaling pathways involving G protein during abiotic stress conditions are also conserved in other plants. Overexpression of AGG3 under the control of CaMV35S promoter in Camelina sativa resulted in transgenic seeds with less sensitivity to ABA, osmotic, and salt stress during germination and post germination. In addition, stomata in CaMV35:AGG3 plants are more sensitive to ABA, imparting better drought recovery (Roy Choudhary et al. 2004).
Some plants respond to hypoxia or oxygen deprivation like underwater loging by enhanced ethylene production (Cohen and Kende 1987; Wang and Arteca 1992; Zarembinski and Theologis 1993). Ethylene signaling regulates adaptation to hypoxic conditions by promoting morphological, anatomical, and metabolic adaptations (He et al. 1996a; He et al. 1996b; Steffens and Sauter 2009). RGA1, the rice homolog of Gαs, functions downstream of ethylene and H2O2 under oxygen deprivation condition to induce cell death, indicated by reduced cell death in rga1 (d1) mutant plants, with reduced D1 mRNA (Steffens and Sauter 2010). The cell death phenotype of rga1 mutant plants could not be restored by treatment with ethylene or H2O2, indicating that G proteins act downstream of these signaling molecules. Microarray analysis revealed that the D1 gene itself was not regulated in epidermal cells by ethylene or H2O2, pointing to posttranscriptional regulation of the D1-dependent G-protein activation (Suharsono et al. 2002; Steffens and Sauter 2009).
Studies in maize further support the role of G protein in hypoxia condition. Maize roots incubated with GTPγS, an activator of G proteins, at normoxic conditions, resulted in cell death and aerenchyma formation. This could probably occur due to constitutively activated G proteins, as GTPγS is nonhydrolyzed substrate for GTPases. Moreover, formation of aerenchyma was accompanied by an increase in cellulase activity, where cellulases contribute to the degradation of the cell wall and thus to the removal of the cell corpses. These results demonstrated a central role for G protein in hypoxia by activating cellulases (He et al. 1996a).
ABA, a plant stress hormone, is known to regulate adaptation of plants to environmental stresses including cold, drought, and salt by modulating the expression of stress-responsive genes and controlling the stomatal aperture to regulate water content (Spartz and Gray 2008). BnGA1, encoding Brassica napus Gαs, is shown to be induced in response to ABA. BnGA1 is upregulated by salinity and drought stress tolerance while downregulated by temperature stresses (Gao et al. 2010) supporting the role of BnGA1 in ABA-mediated responses. Similarly, Arabidopsis GPA1 acts as a regulator of transpiration efficiency (TE). gpa1 mutant plants, despite having guard cells that are hyposensitive to ABA-induced inhibition of stomata opening, exhibit increased TE under ample water and drought stress conditions and when treated with exogenous ABA, most likely due to increased stomatal density. GPA1 regulation of stomatal density was further supported by analysis of gpa1 mutant plants, which showed reduced stomatal density (Nilson and Assmann 2010a). This supports the role of GPA1 downstream of ABA signaling. AtRGS1 was identified as one of the components of G-protein signaling during salt stress in Arabidopsis. Lof AtRGS1 (rgs1-2 mutant) in presence of salt showed better survival and low senescence as opposed to lof Gβs (agb1-2 mutant), which showed low survival and accelerated senescence under salt stress. Most likely in the presence of Na+, AtRGS1 undergoes encosytosis due to increased glucose levels, thereby activating the Gαs and releaving the heterotrimer. In conclusion Gαs and Gβs act antagonistically during salt stress affecting the survival and senescence process (Colaneri et al. 2014).
Asakura and Kurosaki (2007) demonstrated that the expression of Gαs in carrot seedlings was transiently decreased by treatment with high concentration of NaCl and decreased by the exposure to high temperature, and no induction was seen at low temperature in carrot. In pea, the expression of Gαs increases upon treatment with high concentration of NaCl and high temperature (Misra et al. 2007).
Functional genomics approach has shown conservation of G-protein signaling across plant species during biotic and abiotic stresses. Transcription profiling of rice rga1 showed upregulation in the transcript level following NaCl, cold, and drought stresses. Where higher temperatures downregulate RGA1 transcript levels, heavy metal(loid) stress exhibits upregulation. Similar, transcription profiling of rice RGG1 and RGG2 showed that their transcript levels are upregulated following NaCl, cold, heat, and ABA treatments. However, in drought stress, only RGG1 is upregulated. Promoter analysis of RGG1 and RGG2 revealed presence of stress-related cis-regulatory signature motifs, supporting their role in abiotic stress response (Yadav et al. 2012; Yadav et al. 2013). Similar study involving in silico characterization of cis-regulatory elements of RGB1, encoding the Gβs of Indica rice, revealed the presence of stress-related cis-regulatory elements. Transcript profiling revealed enhanced RGB1 transcript levels under KCl, cold, drought (dehydration), and micronutrient (Mn2+ and Zn2+) stresses. Moreover, nuclear localization of RGB1 supports its possible interaction with transcription factors in regulating salt stress-responsive genes (Yadav et al. 2014).
4 Conclusions and Future Perspectives
Increased demand of high-yielding food crops and depleting natural resources has resulted in exponential growth in the number of genetically modified crops. To efficiently generate genetically engineered plants, use of high-throughput technologies has led to rapid identification and subsequent manipulation of target genes. Primarily, target genes are involved in signal transduction pathways, which either increase the yield or generate stress-tolerant crop plants. Using high-throughput techniques like proteomics, metabolomics, and phenomics along with functional genomics approach has identified several key signaling molecules in plant stress responses, including large and small G proteins. G proteins are excellent molecular switches in various cellular and biological processes and can serve as excellent targets for generating stress-tolerant crop plants. Monomeric GTPases have been shown to act as molecular switches in various abiotic and biotic stress conditions signaling downstream of heterotrimeric G proteins, elicitor molecules, phytohormones like ABA, ethylene, and others. In GTP-bound form, they undergo conformation change and activate downstream effector molecules like NADPH oxidase, MAPKs, SnRKs, metallothionein, and transcription factors, to generate response either by modulating enzyme activity or gene expression. Similarly, research has shown that heterotrimeric G proteins act in signaling pathways in response to pathogens, ROS, salt, and others. The ease of manipulation of pathways involving large and small G proteins by using constitutively active or dominant-negative forms has made them excellent targets to generate stress-tolerant high-yielding crop plants. Although research done in the past several decades has delineated many signaling pathways involved in both biotic and abiotic stresses as evident from Fig. 9.4, the picture is far from complete, and future efforts have to be focused on establishing the intricate network and cross talk between various networks to find a target pathway for creating genetically modified crop plants.
Schematic depiction of various signaling pathways involving small and large G proteins during biotic and abiotic stress responses in plants: the G proteins and small GTPases are activated in response to various abiotic and biotic stimuli and second messenger molecules to activate several signaling pathways. The G-protein subunits activate downstream effectors when activated, and one of the effector molecules is Rac GTPase thus providing a link between G proteins and small GTPases. The Rac GTPase acts in plant defense response by forming a defensome at the membrane consisting CEBiP (chitin-binding protein), CERK (chitin-activated receptor kinase), and Pit (NLR protein). The Rac complex then activates various effector molecules including Rboh (NADPH oxidase), MT2b (metallothionein), CCR1 (cinnamoyl-CoA reductase1), MAPK (mitogen-activated kinase), and RBK (receptor-like kinase). Downstream of effector molecules is transcription factor such as RAI (Rac immunity) and Rap2.6, which regulate transcription of defense-related gene such as PBZ1, PAL1, WRKY1, and MT2b, leading to response and stress tolerance
References
Ach RA, Gruissem W (1994) A small nuclear GTP-binding protein from tomato suppresses a schizosaccharomyces pombe cell-cycle mutant. Proc Natl Acad Sci U S A 91:5863–5867
Agarwal PK, Agarwal P, Jain P, Reddy HK, Sopory SK (2008) Constitutive overexpression of a stress-inducible small GTP-binding protein PgRab7 from Pennisetum glaucum enhances abiotic stress tolerance in transgenic tobacco. Plant Cell Rep 27:105–115
Aharon GS, Gelli A, Snedden WA, Blumwald E (1998) Activation of a plant plasma membrane Ca2+ channel by TGalpha1, a heterotrimeric G protein alpha-subunit homologue. FEBS Lett 424(1–2):17–21
Akamatsu A, Wong HL, Fujiwara M, Okuda J, Nishide K, Uno K, Imai K, Umemura K, Kawasaki T, Kawano Y, Shimamoto K (2013) An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 13(4):465–476
Al-Aghabary K, Zhu Z, Shi QH (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 27:2101–2115
Apone F, Alyeshmerni N, Wiens K, Chalmers D, Chrispeels MJ, Colucci G (2003) The G-protein-coupled receptor GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C. Plant Physiol 133:571–579
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 258:1256–1258
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Asakura Y, Kurosaki F (2007) Cloning and expression of dcga gene encoding a subunit of GTP-Binding protein in carrot seedlings. Biol Pharm Bull 30:1800–1804
Asaoka R, Uemura T, Ito J, Fujimoto M, Ito E, Ueda T, Nakano A (2012) Arabidopsis RABA1 GTPases are involved in transport between the trans-golgi network and the plasma membrane, and are required for salinity stress tolerance. Plant J 73:240–249
Asaoka R, Uemura T, Nishida S, Fujiwara T, Ueda T, Nakano A (2013) New insights into the role of Arabidopsis RABA1 GTPases in salinity stress tolerance. Plant Signal Behav 8(9):pii, e25377
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc Natl Acad Sci U S A 96:10284–10289
Aspenstrom P (1999) The Rho GTPases have multiple effects on the actin cytoskeleton. Exp Cell Res 246:20–25
Babior BM (1999) NADPH oxidase: an update. Blood 93:1464–1476
Batoko H, Zheng HQ, Hawes C, Moore I (2000) A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12:2201–2218
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296(5575):2026–2028
Baxter-Burrell A, Chang R, Springer P, Bailey-Serres J (2003) Gene and enhancer trap transposable elements reveal oxygen deprivation-regulated genes and their complex patterns of expression in Arabidopsis. Ann Bot 91 Spec:129–141
Beffa R, Szell M, Meuwly P, Pay A, Vögeli-Lange R, Métraux JP, Neuhaus G, Meins F Jr, Nagy F (1995) Cholera toxin elevates pathogen resistance and induces pathogenesis-related gene expression in tobacco. EMBO J 14(23):5753–5761
Berken A, Thomas C, Wittinghofer A (2005) A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436:1176–1180
Bhardwaj D, Sheikh AH, Sinha AK, Tuteja N (2011) Stress induced β subunit of heterotrimeric G-proteins from Pisum sativum interacts with mitogen activated protein kinase. Plant Signal Behav 6(2):287–292
Bischoff FR, Ponstingl H (1991) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 54:80–82
Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H (1994) RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci U S A 91:2587–2591
Bischoff FR, Krebber H, Smirnova E, Dong W, Ponstingl H (1995) Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO J 14:705–715
Bischoff F, Molendijk A, Rajendrakumar CS, Palme K (1999) GTP-binding proteins in plants. Cell Mol Life Sci 55:233–256
Bokoch GM (1994) Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr Opin Cell Biol 6(2):212–218
Booker FL, Burkey KO, Overmyer K, Jones AM (2004) Differential response of G-protein Arabidopsis thaliana mutants to ozone. New Phytol 162:633–641
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480
Canonne J, Froidure-Nicolas S, Rivas S (2011) Phospholipases in action during plant defense signaling. Plant Signal Behav 6(1):13–88
Cao Y, Li Z, Chen T, Zhang Z, Zhang J, Chen S (2008) Overexpression of a tobacco small G protein gene NtRop1 causes salt sensitivity and hydrogen peroxide production in transgenic plants. Sci China C Life Sci 51(5):383–390
Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR (2011) An atypical heterotrimeric G-protein c-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J 67:840–851
Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP (2003) A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 301:1728–1731
Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM (2004a) GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol 135:907–915
Chen YL, Huang R, Xiao YM, Lu P, Chen J, Wang XC (2004b) Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiol 136:4096–4103
Chen Y, Ji F, Xie H, Liang J, Zhang J (2006) The regulator of G-protein signaling proteins involved in sugar and abscisic acid signaling in Arabidopsis seed germination. Plant Physiol 140:302–310
Chen L, Shiotani K, Togashi T, Miki D, Aoyama M, Wong HL, Kawasaki T, Shimamoto K (2010) Analysis of the Rac/Rop small GTPase family in rice: expression, subcellular localization and role in disease resistance. Plant Cell Physiol 51(4):585–595
Chen N, Xu Y, Wang X, Du C, Du J, Yuan M, Xu Z, Chong K (2011) OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant Cell Environ 34:52–64
Cheng X, Zhu L, He G (2013) Towards understanding of molecular interactions between rice and the brown planthopper. Mol Plant 6(3):621–634
Chinnusamy V, Gong Z, Zhu JK (2008) Nuclear RNA export and its importance in abiotic stress response of plants. Curr Top Microbiol Immunol 326:235–255
Cho HY, Park JA, Pai HS (2008) Physiological function of NbRanBP1 in Nicotiana benthamiana. Mol Cells 26:270–277
Choudhury SR, Bisht NC, Thompson R, Todorov O, Pandey S (2011) Conventional and novel gamma protein families constitute the heterotrimeric G-protein signaling network in soybean. PLoS One 6:e23361
Cohen E, Kende H (1987) In vivo 1-aminocyclopropane-1- carboxylate synthase activity in internodes of deep-water rice: enhancement by submergence and low oxygen levels. Plant Physiol 84:282–286
Colaneri AC, Tunc-Ozdemir M, Huang JP, Jones AM (2014) Growth attenuation under saline stress is mediated by the heterotrimeric G protein complex. BMC Plant Biol 14:129
Cookson SJ, Radziejwoski A, Granier C (2006) Cell and leaf size plasticity in Arabidopsis: what is the role of endoreduplication? Plant Cell Environ 29:1273–1283
Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM (2003) Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423:651–654
Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL (2008) Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol 9:R130
Dang TT, Shimatani Z, Kawano Y, Terada R, Shimamoto K (2013) Gene editing a constitutively active OsRac1 by homologous recombination-based gene targeting induces immune responses in rice. Plant Cell Physiol 54(12):2058–2070
Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jordá L, Hernández-Blanco C, Sánchez-Vallet A, Bednarek P, Schulze-Lefert P, Somerville S, Estevez JM, Persson S, Molina A (2012) Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant 5(1):98–114
Delmer DP, Pear JR, Andrawis A, Stalker DM (1995) Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol Gen Genet 248:43–51
Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A (1991) Bcr encodes a GTPase-activating protein for p21rac. Nature 351:400–402
Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11(8):539–548
Dombrowski JE, Baldwin JC, Martin RC (2008) Cloning and characterization of a salt stress-inducible small GTPase gene from the model grass species Lolium temulentum. J Plant Physiol 165(6):651–661
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Ebine K, Miyakawa N, Fujimoto M, Uemura T, Nakano A, Ueda T (2012) Endosomal trafficking pathway regulated by ARA6, a RAB5 GTPase unique to plants. Small GTPases 3:23–27
Engelen-Eigles G, Jones RJ, Phillips RL (2001) DNA endoreduplication in maize endosperm cells is reduced by high temperature during the mitotic phase. Crop Sci 41:1114–1121
Feng Y, Press B, Wandinger-Ness A (1995) Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol 131:1435–1452
Feraru E, Feraru MI, Asaoka R, Paciorek T, De Rycke R, Tanaka H, Nakano A, Friml J (2011) BEX5/RabA1b regulates trans-Golgi network-to-plasma membrane protein trafficking in Arabidopsis. Plant Cell 24:3074–3086
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462:660–664
Fujiwara M, Umemura K, Kawasaki T, Shimamoto K (2006) Proteomics of Rac GTPase signaling reveals its predominant role in elicitor-induced defense response of cultured rice cells. Plant Physiol 140:734–745
Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18(8):2021–2034
Gao Y, Li T, Liu Y, Ren C, Zhao Y, Wang M (2010) Isolation and characterization of gene encoding G protein α subunit protein responsive to plant hormones and abiotic stresses in Brassica napus. Mol Biol Rep 37(8):3957–3965
Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Gelli A, Higgins VJ, Blumwald E (1997) Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol 113(1):269–279
George S, Parida A (2011) Over-expression of a Rab family GTPase from phreatophyte Prosopis juliflora confers tolerance to salt stress on transgenic tobacco. Mol Biol Rep 38(3):1669–1674
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Goff SA, Ricke D, Lan T-H et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. Japonica). Science 296:92–100
Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11:1897–1910
Grigston JC, Osuna D, Scheible WR, Liu C, Stitt M, Jones AM (2008) D-glucose sensing by a plasma membrane regulator of G signaling protein, AtRGS1. FEBS Lett 582:3577–3584
Gu Y, Li S, Lord EM, Yang Z (2006) Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase- dependent polar growth. Plant Cell 18:366–381
Haizel T, Merkle T, Pay A, Fejes E, Nagy F (1997) Characterization of proteins that interact with the GTP-bound form of the regulatory GTPase Ran in Arabidopsis. Plant J 11:93–103
Hamm HE (1998) The many faces of G protein signaling. J Biol Chem 273(2):669–672
Hammond-Kosack KE, Jones JD (1996) Resistance gene-dependent plant defense responses. Plant Cell 8(10):1773–1791
Hao LH, Wang WX, Chen C, Wang YF, Liu T, Li X, Shang ZL (2012) Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol Plant 5(4):852–864
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hassanain HH, Sharma YK, Moldovan L, Khramtsov V, Berliner LJ, Duvick JP, Goldschmidt-Clermont PJ (2000) Plant Rac proteins induce superoxide production in mammalian cells. Biochem Biophys Res Commun 272:783–788
He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW (1996a) Ethylene biosynthesis during aerenchyma formation in roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiol 112:1679–1685
He CJ, Morgan PW, Drew MC (1996b) Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol 112:463–472
Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ, Park CY, Park HC, Choi JY, Cho MJ (1999) Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc Natl Acad Sci U S A 96(2):766–771
Hepler JR, Gilman AG (1992) G proteins. Trends Biochem Sci 17(10):383–387
Hoefle C, Huesmann C, Schultheiss H, Börnke F, Hensel G, Kumlehn J, Hückelhoven R (2011) A barley ROP GTPase activating protein associates with microtubules and regulates entry of the barley powdery mildew fungus into leaf epidermal cells. Plant Cell 23(6):2422–2439
Huesmann C, Hoefle C, Hückelhoven R (2011) ROPGAPs of arabidopsis limit susceptibility to powdery mildew. Plant Signal Behav 6(11):1691–1694
Huesmann C, Reiner T, Hoefle C, Preuss J, Jurca ME, Domoki M, Fehér A, Hückelhoven R (2012) Barley ROP binding kinase1 is involved in microtubule organization and in basal penetration resistance to the barley powdery mildew fungus. Plant Physiol 159(1):311–320
Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29:649–659
Ichinose Y, Toyoda K, Barz W (1999) cDNA cloning and gene expression of three small GTP-binding proteins in defense response of chickpea. Biochim Biophys Acta 1489:462–466
Ishikawa A, Iwasaki Y, Asahi T (1996) Molecular cloning and characterization of a cDNA for the b-subunit of a G protein from rice. Plant Cell Physiol 37:223–228
Ismond KP, Dolferus R, de Pauw M, Dennis ES, Good AG (2003) Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol 132(3):1292–1302
Jacob T, Ritchie S, Assmann SM, Gilroy S (1999) Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci U S A 96:12192–12197
Jayasekaran K, Kim KN, Vivekanandan M, Shin JS, Ok SH (2006) Novel calcium binding GTPase (AtCBG) involved in ABA-mediated salt stress signaling in Arabidopsis. Plant Cell Rep 25:1255–1262
Jiang M, Zhang J (2002) Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen JG, Siderovski DP, Jones AM, Willard FS (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci U S A 104:17317–17322
Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5:572–578
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jones JC, Temple BR, Jones AM, Dohlman HG (2011) Functional reconstitution of an atypical G protein heterotrimer and regulator of G protein signaling protein (RGS1) from Arabidopsis thaliana. J Biol Chem 286:13143–13150
Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17:957–970
Josefsson LG, Rask L (1997) Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur J Biochem 249:415–420
Jung YH, Agrawal GK, Rakwal R, Kim JA, Lee MO, Choi PG, Kim YJ, Kim MJ, Shibato J, Kim SH, Iwahashi H, Jwa NS (2006) Functional characterization of OsRacB GTPase—a potentially negative regulator of basal disease resistance in rice. Plant Physiol Biochem 44:68–77
Kahn RA, Der CJ, Bokoch GM (1992) The ras superfamily of GTP-binding proteins: guidelines on nomenclature. FASEB J 6:2512–2513
Karim S, Alezzawi M, Garcia-Petit C, Solymosi K, Khan NZ, Lindquist E, Dahl P, Hohmann S, Aronsson H (2014) A novel chloroplast localized Rab GTPase protein CPRabA5e is involved in stress, development, thylakoid biogenesis and vesicle transport in Arabidopsis. Plant Mol Biol 84(6):675–692
Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y (2004) Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J 38:320–331
Kawano Y, Akamatsu A, Hayashi K, Housen Y, Okuda J, Yao A, Nakashima A, Takahashi H, Yoshida H, Wong HL, Kawasaki T, Shimamoto K (2010) Activation of a Rac GTPase by the NLR family disease resistance protein pit plays a critical role in rice innate immunity. Cell Host Microbe 7(5):362–375
Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimoto K (1999) The small GTP binding protein rac is a regulator of cell death in plants. Proc Natl Acad Sci U S A 96:10922–10926
Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci U S A 103(1):230–235
Kawasawa Y, McKenzie LM, Hill DP, Bono H, Yanagisawa M, RIKEN GER Group, GSL Members (2003) G protein-coupled receptor genes in the FANTOM2 database. Genome Res 13(6B):1466–1477
Kevei E, Gyula P, Fehér B, Tóth R, Viczián A, Kircher S, Rea D, Dorjgotov D, Schäfer E, Millar AJ, Kozma-Bognár L, Nagy F (2007) Arabidopsis thaliana circadian clock is regulated by the small GTPase LIP1. Curr Biol 17:1456–1464
Kim SH, Oikawa T, Kyozuka J, Wong HL, Umemura K, Kishi-Kaboshi M, Takahashi A, Kawano Y, Kawasaki T, Shimamoto K (2012) The bHLH Rac immunity1 (RAI1) is activated by OsRac1 via OsMAPK3 and OsMAPK6 in rice immunity. Plant Cell Physiol 53(4):740–754
Kirchhausen T (2000) Three ways to make a vesicle. Nat Rev Mol Cell Biol 1:187–198
Kitsios G, Doonan JH (2011) Cyclin dependent protein kinases and stress responses in plants. Plant Signal. Behav. 6(2):204–209
Komatsu S, Yang G, Hayashi N, Kaku H, Umemura K, Iwasaki Y (2004) Alterations by a defect in a rice G protein a subunit in probenazole and pathogen-induced responses. Plant Cell Environ 27:947–957
Kost B, Spielhofer P, Chua N-H (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16:393–401
Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH (1999) Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol 145:317–330
Kürsteiner O, Dupuis I, Kuhlemeier C (2003) The pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol 132(2):968–978
Lam E, Pontier D, del Pozo O (1999) Die and let live: programmed cell death in plants. Curr Opin Plant Biol 2:502–507
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC (2001) A mutant Arabidopsis heterotrimeric G-protein beta subunit affects leaf, flower, and fruit development. Plant Cell 13:2631–2641
Lee YR, Assmann SM (1999) Arabidopsis thaliana “extra-large GTP-binding protein” (AtXLG1): a new class of G-protein. Plant Mol Biol 40:55–64
Legendre L, Heinstein PF, Low PS (1992) Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem 267(28):20140–20147
Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15(14):1808–1816
Leung J, Bouvier-Durand M, Morris P-C, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264:1448–1452
Levine A, Belenghi B, Damari-Weisler H, Granot D (2001) Vesicle associated membrane protein of Arabidopsis suppresses bax-induced apoptosis in yeast downstream of oxidative burst. J Biol Chem 276:46284–46289
Leyman B, Geelen D, Blatt MR (1999) Localization and control of expression of Nt-Syr, a tobacco SNARE protein. Plant J 24:369–381
Li H, Wu G, Ware D, Davis KR, Yang Z (1998) Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol 118:407–417
Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11:1731–1742
Li JH, Liu YQ, Lu P, Lin HF, Bai Y, Wang XC, Chen YL (2009) A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol 150:114–124
Li Z, Kang J, Sui N, Liu D (2012a) ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J Integr Plant Biol 54(3):169–179
Li Z, Li Z, Gao X, Chinnusamy V, Bressan R, Wang ZX, Zhu JK, Wu JW, Liu D (2012b) ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J Integr Plant Biol 54(3):180–188
Liu J, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Liu F, Guo J, Bai P, Duan Y, Wang X, Cheng Y, Feng H, Huang L, Kang Z (2012a) Wheat TaRab7 GTPase is part of the signaling pathway in responses to stripe rust and abiotic stimuli. PLoS One 7(5):e37146
Liu J, Zhou J, Xing D (2012b) Phosphatidylinositol 3-kinase plays a vital role in regulation of rice seed vigor via altering NADPH oxidase activity. PLoS One 7(3):e33817
Liu ZL, Luo C, Dong L, Van Toan C, Wei PX, He XH (2014) Molecular characterization and expression analysis of a GTP-binding protein (MiRab5) in Mangifera indica. Gene 540(1):86–91
Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A (2005) ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 43:165–180
Logan DC (2010) Mitochondrial fusion, division and positioning in plants. Biochem Soc Trans 38:789–795
Lu C, Zainal Z, Tucker GA, Lycett GW (2001) Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell 13:1819–1833
Ma H (2001) Plant G-proteins: the different faces of GPA1. Currr Biol 11:R869–R871
Ma H, Yanofsky MF, Meyerowitz EM (1990) Molecular cloning and characterization of GPA1, a G protein a subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci U S A 87:3821–3825
Mansfield TA (1998) Stomata and plant water relations: does air pollution create problems? Environ Pollut 101:1–11
Mason MG, Botella JR (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein gamma-subunit cDNA. Proc Natl Acad Sci U S A 97(26):14784–14788
Mason MG, Botella JR (2001) Isolation of a novel G-protein gamma-subunit from Arabidopsis thaliana and its interaction with Gbeta. Biochim Biophys Acta 1520(2):147–153
Mathur J, Chua N-H (2000) Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12:465–477
Mazel A, Leshem Y, Tiwari BS, Levine A (2004) Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol 134:118–128
Mazzucoteli E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L (2008) Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci 174:420–431
McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005) G-protein signaling: back to the future. Cell Mol Life Sci 62:551–577
McElver J, Patton D, Rumbaugh M, Liu C, Yang LJ, Meinke D (2000) The TITAN5 gene of Arabidopsis encodes a protein related to the ADP ribosylation factor family of GTP binding proteins. Plant Cell 12:1379–1392
Meier I (2000) A novel link between ran signal transduction and nuclear envelope proteins in plants. Plant Physiol 124:1507–1510
Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264:1452–1455
Miche L, Battistoni F, Gernmer S, Belghazi M, Reinhold-Hurek B (2006) Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol Plant Microbe Interact 19:502–511
Miki D, Itoh R, Shimamoto K (2005) RNA silencing of single and multiple members in a gene family of rice. Plant Physiol 138:1903–1913
Mirzaei M, Pascovici D, Atwell BJ, Haynes PA (2012) Differential regulation of aquaporins, small GTPases and V-ATPases proteins in rice leaves subjected to drought stress and recovery. Proteomics 12(6):864–877
Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N (2007) Heterotrimeric G-protein complex and 21 G-protein-coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross-talk with phospholipase C. Plant J 51:656–669
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Morel J, Fromentin J, Blein JP, Simon-Plas F, Elmayan T (2004) Rac regulation of NtrbohD, the oxidase responsible for the oxidative burst in elicited tobacco cell. Plant J 37(2):282–293
Moriyama EN, Strope PK, Opiya SO, Chen Z, Jones AM (2006) Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol 7:R96
Mosammaparast N, Pemberton LF (2004) Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol 14:547–556
Moss J, Vaughan M (1998) Molecules in the ARF orbit. J Biol Chem 273:21431–21434
Mukhopadhyay A, Funato K, Stahl PD (1997) Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem 272:13055–13059
Newman T, de Bruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M (1994) Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol 106:1241–1255
Ng CK, Carr K, McAinsh MR, Powell B, Hetherington AM (2001) Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410:596–599
Nibau C, Wu HM, Cheung AY (2006) RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci 11:309–315
Nilson SE, Assmann SM (2010a) The alpha-subunit of the Arabidopsis heterotrimeric G protein, GPA1, is a regulator of transpiration efficiency. Plant Physiol 152(4):2067–2077
Nobes C, Hall A (1994) Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev 4:77–81
Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P (1998) A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol 141:187–197
O’Mahony PJ, Oliver MJ (1999) Characterization of a desiccation-responsive small GTP-binding protein (Rab2) from the desiccation-tolerant grass Sporobolus stapfianus. Plant Mol Biol 39(4):809–821
Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K (2001) Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci U S A 98:759–764
Pandey S, Assmann SM (2004) The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein alpha subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16(6):1616–1632
Pandey S, Chen JG, Jones AM, Assmann SM (2006) G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 141:243–256
Parada LF, Tabin CJ, Shih C, Weinberg RA (1982) Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus Ras gene. Nature 291:474–478
Park J, Choi HJ, Lee S, Lee T, Yang Z, Lee Y (2000) Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol 124(2):725–732
Park J, Gu Y, Lee Y, Yang Z, Lee Y (2004) Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol 134(1):129–136
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Park J, Kim YS, Kim SG, Jung JH, Woo JC, Park CM (2011) Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol 156:537–549
Pathuri IP, Zellerhoff N, Schaffrath U, Hensel G, Kumlehn J, Kogel KH, Eichmann R, Hückelhoven R (2008) Constitutively activated barley ROPs modulate epidermal cell size, defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep 27(12):1877–1887
Pay A, Resch K, Frohnmeyer H, Fejes E, Nagy F, Nick P (2002) Plant Ran GAPs are localized at the nuclear envelope in interphase and associated with microtubules in mitotic cells. Plant J 30:699–709
Pereira-Leal JB, Seabra MC (2001) Evolution of the Rab family of small GTP binding proteins. J Mol Biol 313:889–901
Piffanelli P, Zhou F, Casais C, Orme J, Jarosch B, Schaffrath U, Collins NC, Panstruga R, Schulze-Lefert P (2002) The barley MLP modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiol 129(3):1076–1085
Poraty-Gavra L, Zimmermann P, Haigis S, Bednarek P, Hazak O, Stelmakh OR, Sadot E, Schulze-Lefert P, Gruissem W, Yalovsky S (2013) The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol 161(3):1172–1188
Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A (1999) The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol 119:849–858
Qin X, Lin F, Lii Y, Gou C, Chen F (2011) Molecular analysis of ARF1 expression profiles during development of physic nut (Jatropha curcas L.). Mol Biol Rep 38(3):1681–1686
Regad F, Bardet C, Tremousaygue D, Moisan A, Lescure B, Axelos M (1993) cDNA cloning and expression of an Arabidopsis GTP-binding protein of the ARF family. FEBS Lett 316:133–136
Reis K, Fransson A, Aspenstrom P (2009) The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS Lett 583:1391–1398
Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401–410
Romeis T, Piedras P, Jones JD (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12(5):803–816
Rose A, Meier I (2001) A domain unique to plant RanGAP is responsible for its targeting to the plant nuclear rim. Proc Natl Acad Sci U S A 98:15377–15382
Roy Choudhary S, Westfall CS, Laborde JP, Bisht NC, Jez JM, Pandey S (2004) Two chimeric regulators of G-protein signaling (RGS) proteins differentially modulate heterotrimeric G-protein cycle. J Biol Chem 287:17870–17881
Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci U S A 98:14150–14155
Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family-another enigma variation. Curr Opin Plant Biol 5:518–528
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126(3):1281–1290
Saito C, Ueda T (2009) Chapter 4: functions of RAB and SNARE proteins in plant life. Int Rev Cell Mol Biol 274:183–233
Sanchez A, Shin J, Davis SJ (2011) Abiotic stress and the plant circadian clock. Plant Signal Behav 6:223–231
Sauter M (2000) Rice in deep water: “how to take heed against a sea of troubles”. Naturwissenschaften 87:289–303
Schiene K, Puhler A, Niehaus K (2000) Transgenic tobacco plants that express an antisense construct derived from a Medicago sativa cDNA encoding a Rac-related small GTP-binding protein fail to develop necrotic lesions upon elicitor infiltration. Mol Gen Genet 263:761–770
Schultheiss H, Dechert C, Kogel KH, Huckelhoven R (2002) A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol 128:1447–1454
Schultheiss H, Dechert C, Kogel KH, Huckelhoven R (2003) Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J 36:589–601
Schultheiss H, Hensel G, Imani J, Broeders S, Sonnewald U, Kogel KH, Kumlehn J, Hückelhoven R (2005) Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol 139(1):353–362
Segev N (2001) Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol 13:500–511
Shanmugam T, Sharma M, Pandey A, Pandey GK (2013) Small GTPases: Rho of plant (ROP) in development and stress signaling. In: Pandey GK (ed) Stress-mediated signaling in plants II, Plant Stress 7(Special Issue 1)., Global science books pp 10–15
Shieh MW, Wessler SR, Raikhel NV (1993) Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol 101:353–361
Spartz AK, Gray WM (2008) Plant hormone receptors: new perceptions. Genes Dev 22:2139–2148
Steffens B, Sauter M (2009) Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an auto-amplified signal pathway. Plant Cell 21:184–196
Steffens B, Sauter M (2010) Heterotrimeric G protein signaling is required for epidermal cell death in rice. Plant Physiol 151:732–740
Stenmark H, Valencia A, Martinez O, Ullrich O, Goud B, Zerial M (1994) Distinct structural elements of rab5 define its functional specificity.EMBO J. 13(3):575–583
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci U S A 99(20):13307–13312
Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81:153–208
Tamaoki M, Nakajima N, Kubo A, Aono M, Matsuyama T, Saji H (2003) Transcriptome analysis of O3-exposed Arabidopsis reveals that multiple signal pathways act mutually antagonistically to induce gene expression. Plant Mol Biol 53:443–456
Temple BRS, Jones AM (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58:249–266
Terecskei K, Tóth R, Gyula P, Kevei E, Bindics J, Coupland G, Nagy F, Kozma-Bognár L (2013) The circadian clock-associated small GTPase LIGHT INSENSITIVE PERIOD1 suppresses light-controlled endoreplication and affects tolerance to salt stress in Arabidopsis. Plant Physiol 161(1):278–290
Thao NP, Chen L, Nakashima A, Hara S, Umemura K, Takahashi A, Shirasu K, Kawasaki T, Shimamoto K (2007) RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell 19:4035–4045
Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR (2006) Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140:210–220
Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR (2007) Heterotrimeric G protein β subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 19:1235–1250
Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR (2009) Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J 58:69–81
Turcotte S, Desrosiers RR, Béliveau R (2003) HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci 116(Pt 11):2247–2260
Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M (2000) Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci U S A 97(21):11638–11643
Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292:2066–2069
Ullah H, Chen JG, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129:897–907
Urano D, Jones JC, Wang H, Matthews M, Bradford W, Bennetzen JL, Jones AM (2012) G protein activation without a GEF in the plant kingdom. PLoS Genet 8:e1002756
Vernoud V, Horton AC, Yang ZB, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131:1191–1208
Verslues PE, Guo Y, Dong C-H, W-j M, Zhu J-K (2006) Mutation of SAD2, an importin b-domain protein in Arabidopsis, alters abscisic acid sensitivity. Plant J 47:776–787
Wamaitha MJ, Yamamoto R, Wong HL, Kawasaki T, Kawano Y, Shimamoto K (2012) OsRap2.6 transcription factor contributes to rice innate immunity through its interaction with receptor for activated kinase-C 1 (RACK1). Rice (N Y) 5(1):35
Wang TW, Arteca RN (1992) Effects of low O(2) root stress on ethylene biosynthesis in tomato plants (Lycopersicon esculentum Mill cv Heinz 1350). Plant Physiol 98:97–100
Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292:2070–2072
Wang X, Xu YY, Han Y, Bao SL, Du JZ, Yuan M, Xu ZH, Chong K (2006) Overexpression of RAN1 in rice and Arabidopsis alters primordial meristem, mitotic progress, and sensitivity to auxin. Plant Physiol 140:91–101
Wang TZ, Xia XZ, Zhao MG, Tian QY, Zhang WH (2013) Expression of a Medicago falcata small GTPase gene, MfARL1 enhanced tolerance to salt stress in Arabidopsis thaliana. Plant Physiol Biochem 63:227–235
Wei Q, Zhou WB, Hu GZ, Wei JM, Yang HQ, Huang JR (2008) Heterotrimeric G-protein is involved in phytochrome A-mediated cell death of Arabidopsis hypocotyls. Cell Res 18:949–960
Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H (1994) Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc Natl Acad Sci U S A 91(20):9554–9558
Wennerberg K, Rossman KL, Der CJ (2005) The Ras superfamily at a glance. J Cell Sci 118:843–846
Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159–1204
Wickner W, Schekman R (2008) Membrane fusion. Nat Struct Mol Biol 15:658–664
Winge P, Brembu T, Bones AM (1997) Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol 35(4):483–495
Wittinghofer A, Vetter IR (2011) Structure-function relationships of the 6 domain, a canonical switch motif. Annu Rev Biochem 80:943–971
Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K (2004) Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol 135(3):1447–1456
Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19:4022–4034
Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277:8588–8596
Xu P, Cai W (2014) RAN1 is involved in plant cold resistance and development in rice (Oryza sativa). J Exp Bot. 65(12):3277–3287
Yadav DK, Islam SM, Tuteja N (2012) Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal Behav 7(7):733–740
Yadav DK, Shukla D, Tuteja N (2013) Rice heterotrimeric G-protein alpha subunit (RGA1): in silico analysis of the gene and promoter and its upregulation under abiotic stress. Plant Physiol Biochem 63:262–271
Yadav DK, Shukla D, Tuteja N (2014) Isolation, in silico characterization, localization and expression analysis of abiotic stress-responsive rice G-protein β subunit (RGB1). Plant Signal Behav 9:e28890
Yamaguchi K, Kawasaki T (2012) Function of Arabidopsis SWAP70 GEF in immune response. Plant Signal Behav 7(4):465–468
Yamaguchi K, Imai K, Akamatsu A, Mihashi M, Hayashi N, Shimamoto K, Kawasaki T (2012) SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J 70(3):389–397
Yang Z, Fu Y (2007) ROP/RAC GTPase signaling. Curr Opin Plant Biol 10:490–494
Yang Z, Watson JC (1993) Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc Natl Acad Sci U S A 90:8732–8736
Yasuda Y, Miyamoto Y, Saiwaki T, Yoneda Y (2006) Mechanism of the stress-induced collapse of the Ran distribution. Exp Cell Res 312:512–520
Zang AP, Xu XJ, Neill S, Cai WM (2010) Overexpression of OsRAN2 in rice and Arabidopsis renders transgenic plants hypersensitive to salinity and osmotic stress. J Exp Bot 61:777–789
Zarembinski TI, Theologis A (1993) Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol Biol Cell 4:363–373
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Zhang H-X, Blumwald E (2001) Transgenic salt tolerant tomato plants accumulate salt in the foliage but not in the fruits. Nat Biotechnol 19:765–768
Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci U S A 101:9508–9513
Zhang F, Wang Y, Wang D (2007) Role of nitric oxide and hydrogen peroxide during the salt resistance response. Plant Signal Behav 2(6):473–474
Zhang L, Tian LH, Zhao JF, Song Y, Zhang CJ, Guo Y (2009a) Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis. Plant Physiol 149:916–928
Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009b) Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21:2357–2377
Zhang H, Wang M, Wang W, Li D, Huang Q, Wang Y, Zheng X, Zhang Z (2012) Silencing of G proteins uncovers diversified plant responses when challenged by three elicitors in Nicotiana benthamiana. Plant Cell Environ 35(1):72–85
Zheng Z-L, Yang Z (2000) The Rop GTPase: an emerging signaling switch in plants. Plant Mol Biol 44:1–9
Zheng ZL, Nafisi M, Tam A, Li H, Crowell DN, Chary SN, Schroeder JI, Shen J, Yang Z (2002) Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14:2787–2797
Zhu H, Li GJ, Ding L, Cui X, Berg H, Assmann SM, Xia Y (2009) Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gb subunit of heterotrimeric G protein and functions in disease resistance. Mol Plant 2:513–525
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Acknowledgment
We are thankful to Delhi University and Department of Biotechnology (DBT), India, for research support in our lab.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Pandey, A., Sharma, M., Pandey, G.K. (2015). Small and Large G Proteins in Biotic and Abiotic Stress Responses in Plants. In: Pandey, G. (eds) Elucidation of Abiotic Stress Signaling in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2211-6_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2211-6_9
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2210-9
Online ISBN: 978-1-4939-2211-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)