Abstract
This work investigated the properties of Paracoccus yeei VKM B-3302 bacteria isolated from activated sludge and immobilized in an N-vinylpyrrolidone-modified poly(vinyl alcohol) matrix. The developed hydrogel formed a network structure to enable the entrapment of microbial cells with their viability and biocatalytic properties preserved, which ensured the technological possibility of replicating expendable biosensor receptor elements. A new ratio of the components for the synthesis selected in this work enabled producing a copolymer of an earlier undescribed chemical structure, which can be efficiently used for immobilization of highly sensitive P. yeei bacteria. A biological oxygen demand (BOD) biosensor with these bacteria and matrix was shown to possess a long-time stability exceeding that described earlier, to have a broad substrate specificity and to exceed approximately tenfold the nearest analogues by its sensitivity and the lower boundary value of 0.05 mg/dm3. The biosensor enabled assays of water samples initially attributed to pure samples (the BOD range, 0.05–5.0 mg/dm3). BOD assays of water samples from various sources showed the use of the receptor element of this composition to enable the data that closely correlated with the standard method (R2 = 0.9990).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of immediate interest at present is the development of methods and instrumentation for rapid assays of natural-water and wastewater purity. One of the parameters that determine the integral pollution of water is the biochemical oxygen demand (BOD) index. The standard BOD technique requires incubation of an oxygen-saturated sample for 5 or 20 days (BOD5 or BOD20). In accordance with world practice, an efficient approach for the rapid assessment of BOD is to develop biosensors based on microorganisms oxidizing a broad range of organic substances (Jouanneau et al. 2014).
Bioreceptor elements of BOD sensors are developed using microbial associations (activated sludge, artificial consortia) as well as individual microbial cultures with certain consumer properties. An advantage of using microbial associations is their ability to oxidize a broad range of organic substrates; however, BOD biosensors on their basis are insufficiently stable because consortium’s composition changes in time (Ponamoreva et al. 2011). Individual cultures are more stable in the measurement mode, are capable of oxidizing various classes of organic substances, are stable under immobilization and resistant to negative factors of the environment (Abrevaya et al. 2014).
A key point in forming the stable biosensitive element of a BOD biosensor is the immobilization of microorganisms. Efficient methods of producing immobilized cells are associated with processes of their entrapment into natural and synthetic hydrogels; as a rule, these processes provide for mild conditions of cell entrapment into the supporting matrix. Produced matrices should ensure a high diffusion of substrates and products, as well as high cell retention (Burlage and Tillmann 2017). Application of various hydrogels has been shown to satisfy most of these requirements for BOD biosensors (Tag et al. 2000; Martínez et al. 2007; Fine et al. 2006; Commault and Weld 2016; Buenger et al. 2012; Fang et al. 2016). For instance, poly(carbamoyl)sulfonate was used to immobilize the yeasts Arxula adeninivorans LS3 and Saccharomyces cerevisiae (Tag et al. 2000; Martínez et al. 2007); calcium alginate and poly(vinyl alcohol) (PVA) were chosen for immobilization of luminescent yeasts (Fine et al. 2006); a polyurethane-based matrix was created for immobilization of aerobic microorganisms isolated from seawater (Commault and Weld 2016). Besides these hydrogels, widely applied are matrices based on photocrosslinkable resins, polyacrylonitrile, poly(ethylene glycol), carrageenan, agarose, chitosan and various protein structures (Buenger et al. 2012; Fang et al. 2016). The major problems of using these matrices in BOD biosensors are the complexity of production, decrease of microbial activity due to temperature treatment or use of toxic substances during the synthesis of the matrix, and low mechanical strength and stability of produced gel (Guisan and editor. 2006).
PVA is often used as a synthetic gel former as it is chemically and microbiologically stable, nontoxic and biocompatible (Szczesna-Antczak et al. 2004). However, its use as the base of the receptor element in a biosensor analyzer is made difficult as it forms water-soluble films of low mechanical strength. A way of creating network PVA structures is to introduce crosslinking agents (Aleshina et al. 2001). A new reagent used for modification of PVA with the view of immobilizing microorganisms to develop biosensor recognition elements is N-vinylpyrrolidone (NVP). It is known that a polymer on its basis, polyvinylpyrrolidone (PVP), readily forms complexes with many compounds (toxins, drug substances, dies), is biocompatible, nontoxic, is the basis of blood substitutes (Zhanybekova 2013), forms films when mixed with PVA (Bazilyuk and Melnik 2003). Also, it increases the activity of some microorganisms: for instance, the presence of PVP contributes to a significantly accelerated biodegradation of drug substances by Rhodococcus erythropolis (Demakov 2008).
An earlier work has shown a possibility of developing a stable biosensor receptor element by entrapping microorganisms into N-vinylpyrrolidone-modified PVA gel (Arlyapov et al. 2013). Investigation of the physical and chemical structure of this hydrogel and optimization of its composition enabled improving the metrological characteristics of the developed BOD biosensor. Formation of mechanically strong hydrogel films with immobilized microorganisms makes it possible to ensure the technological possibility of replicating biosensitive elements for modification of oxygen electrodes.
The aim of this work was to develop a matrix based on N-vinylpyrrolidone-modified poly(vinyl alcohol) for immobilization of Paracoccus yeei bacteria isolated from activated sludge and to study the characteristics of an amperometric BOD biosensor based on the produced biocatalyst.
Experimental
Production of N-vinylpyrrolidone-modified poly(vinyl alcohol)
N-Vinylpyrrolidone-modified poly(vinyl alcohol) was produced using a 5% aqueous solution of PVA, grade 16/1 (Russia); an aqueous solution of cerium (IV) ammonium nitrate (NH4)2Ce(NO3)6 (analytical grade, technical specs TU 6-09-4762-84, Russia) as an initiator; and N-vinylpyrrolidone (99%, Acros Organics, USA) as a crosslinking agent. The modification was performed at constant stirring in a nitrogen atmosphere at a temperature of 40 °C.
Recording of IR spectra
Dried films of NVP-modified poly(vinyl alcohol) were used. The IR spectra were recorded on an FMS 1201 infrared Fourier spectrometer (OOO Monitoring, Russia).
Recording of NMR spectra of modified poly(vinyl alcohol) and initial substances
The NMR spectra were recorded at the Centre of Magnetic Spectroscopy, N.M. Emanuel Institute of Biochemical Physics, Russian Academy of Sciences. The measurements were carried out at a Bruker Avance III 500 spectrometer with the working frequencies of 500.2 MHz and 125.8 MHz for proton and carbon, respectively. The structure of the produced copolymer with the component ratio of 160: 7: 1 (PVA: initiator: NVP) was established by a joint analysis of 1D 1H, 13C-DEPT and 2D COSY, HSQC, HMBC NMR spectra. The paper shows the data for only 1D NMR spectra.
We recorded the spectra of an aqueous solution of the reaction mixture at room temperature with the addition of 10% D2O (to stabilize the resonance conditions) and with water signal suppression. The chemical shifts of 1H and 13C were calibrated relative to the tetramethylsilane signal. All two-dimensional experiments were performed according to the standard Bruker techniques. The spectra were processed using Bruker’s TopSpin 3.0 software.
Scanning electron microscopy of gel structure
NVP-modified poly(vinyl alcohol) samples containing and not containing microbial cells were analyzed using a JSM-6510 LV scanning electron microscope (Jeol, Japan).
Determination of the fraction of crosslinked N-vinylpyrrolidone-modified poly(vinyl alcohol)
The fraction of the crosslinked polymer was determined by the extraction method. A dried sample of 0.01–0.02 g was weighed, poured with water and mixed for 2 h at 60 °C. After the extraction, water was separated by centrifugation, and the samples were dried to a constant weight at 60 °C. Extraction was repeated several times to a constant weight of the sample after drying. The experiment was conducted for 15 specimens of the same film with the statistical treatment of the obtained data. The percentage of the weight loss was determined by the formula:
where m1 is the weight before extraction and m2 is the weight of dried hydrogel after extraction.
Cultivation of microbial cells
The yeast strains Ogataea angusta VKM Y-1397, Debaryomyces hansenii VKM Y-2482, Debaryomyces hansenii VKM Y-111 and Arxula adeninivorans VGI 78-6 were received from the All-Russian Collection of Microorganisms, Institute of Biochemistry and Physiology, Russian Academy of Sciences. Cells of the bacteria Paracoccus yeei BAA-599 T and Pseudomonas veronii DSM 11331 T were isolated from activated sludge of wastewater treatment facilities, the city of Tula (Kharkova et al. 2019). The microorganisms Paracoccus yeei BAA-599 T were deposited in the All-Russian Collection of Microorganisms under number B-3302.
The yeasts D. hansenii VKM Y-2482, D. hansenii VKM Y-111, A. adeninivorans VGI 78–6 and the bacteria P. yeei VKM B-3302 and Ps. veronii DSM 11331 T were cultivated on a rich mineral medium (liquid glucose–peptone nutrient medium). The composition of the liquid medium: glucose, 10 g/dm3 (OOO Diaem); peptone, 5 g/dm3 (OOO Diaem); yeast extract, 0.5 g/dm3 (OOO Diaem). The yeast O. angusta VKM Y-1397 was cultivated on a rich mineral medium (liquid yeast nutrient medium). The composition of the liquid medium: yeast extract, 0.1 g/dm3; leucine, 0.034 g/dm3; glycerol, 1.66 cm3; trace elements (H3BO3, ZnSO4, Na2MoO4, MnCl2, FeCl3) 0.2 cm3 (Sigma, USA).
The cultivation medium was sterilized by autoclaving at a temperature of 120 °C and pressure of 1.1 atm for 40 min. Cells were cultivated aerobically for 18–20 h in 750-cm3 shaken flasks at a temperature of 29 °C. Produced biomass was centrifuged at room temperature for 10 min at 10,000 rpm (a TG16WS centrifuge, OOO Polycom, Russia). The centrifugate was washed with a 20-mM phosphate buffer solution, pH 6.8 (or pH 7.6 for the yeast O. angusta VKM Y-1397). Sedimented cells were resuspended by the buffer solution, were distributed in Eppendorf microtubes and centrifuged on an Eppendorf centrifuge for 5 min at 10,000 rpm. Washed biomass was stored at a temperature of – 25 °C in microtubes without using a protective agent. Biosensors can be formed using both freshly grown and frozen bacterial cultures. The loss of activity when using thawed bacterial cells was no more than 10–15% as compared with freshly grown cells.
Growth curves
Microbial growth curves were obtained by overlapping the time intervals within which the optical density was measured. Optical densities of the suspension were measured spectrophotometrically on an SF-103 spectrophotometer (Akvilon, Russia) every two hours for 48 h at a wavelength of 540 nm and cuvette thickness of 1 cm relative to a cuvette with distilled water. The obtained dependences of optical density on time were used to plot the growth curves for the bacterial cells. The oxidative activity of cells was measured by the biosensor method; a solution of glucose and glutamic acid (GGA) at a concentration of 500 mg/dm3 was used as substrate. The experiment to study the activity of bacteria with respect to growth time was conducted on a freshly grown culture.
Immobilization of microorganisms using a dialysis membrane
For immobilization using a dialysis membrane, cell biomass was prediluted with a phosphate buffer solution at a ratio of 1:1. An amount of 5 μl of the produced suspension was applied onto a D977 dialysis membrane (Sigma, USA) and fixed on the surface of an oxygen electrode by a rubber ring.
Immobilization of microorganisms in modified PVA hydrogel
To produce a biocatalyst immobilized in NVP-modified PVA hydrogel, 20 mg of microbial cells was added to 100 μl of hydrogel. An even distribution of cells in hydrogel was achieved by shaking on a CM70M Sky Line Elmi Centrifuge & Vortex (ELMI, Latvia) for 5 min. The produced suspension was transferred to a plate (d = 5 mm) and left to dry at a temperature of 18–22 °C. After drying for 24 h, the bioreceptor was fixed on the surface of a Clark electrode by means of a nylon net.
Biosensor measurements
Electrochemical measurements were carried out using an Ekspert-009 analyzer (OOO Ekoniks-Ekspert, Russia) coupled with a computer operated by specialized software EXP2PR (OOO Ekoniks-Ekspert, Russia). Clark oxygen electrodes containing immobilized microbial cells were transducers. A biosensor response was a change of oxygen concentration (mg/dm3·s) in the near-electrode space owing to the biochemical reaction. As namely bacterial cells transform substrate when it is added into the cuvette, no biosensor response to the addition of an analyte was registered in their absence. Measurements were carried out in a 5-ml cuvette. For measurements, we used a sodium–potassium phosphate buffer solution (pH 6.8 or 7.6) the total concentration of the salts in which was 20 mM. The solution was mixed by a magnetic mixer (200 rpm). A mixture of glucose and glutamic acid at a ratio of 1:1 (w/w) used as the BOD5 detection standard in the Russian Federation and in international practice (Federal Environmental Regulatory Documents (PND F) 2003; Szczesna-Antczak 2004) was used as a model mixture. In accordance with regulatory documentation, BOD5 equal to 205 mg/dm3 was taken to correspond to a solution containing 150 mg/dm3 of glucose and 150 mg/dm3 of glutamic acid (BOD5 = 0.68 × CGGA). Between assays, the biosensor was stored at room temperature.
Determination of BOD5 by the standard dilution method
The dilution method was used as the reference method of BOD5 determination. Analysis was done in accordance with the technique indicated in Federal Environmental Regulatory Documents (PND F) 1997. The content of dissolved oxygen in analyzed samples was determined amperometrically in accordance with the standard technique.
Discussion
Development of the BOD-biosensor receptor element
Four yeast strains capable of oxidizing a broad range of organic substances and efficiently used earlier to develop BOD biosensors (Yudina et al. 2015) were used to form the biosensor receptor element. Besides, use was made of two bacterial strains isolated from activated sludge of wastewater facilities. These microorganisms were adapted to investigated samples, which ensured a high convergence of biosensor-assay results with those of the standard method (Kharkova et al. 2019).
Biological material, which made the basis of the BOD-biosensor receptor element, was chosen based on the ability of microorganisms to oxidize a broad range of organic substrates, by the stability during the immobilization on the Clark electrode and the stability during measurements. Encapsulation of the microbial suspension using a dialysis membrane was used as the simplest immobilization technique to perform.
From the data of Table 1, it is seen that the microorganisms D. hansenii VKM Y-2482, D. hansenii VKM Y-111 and P. yeei VKM B-3302 have the broadest spectrum of oxidized substrates. Herewith, the receptor element based on P. yeei has high long-time stability and the highest sensitivity. For this reason, the microorganisms P. yeei isolated from activated sludge were used in further work.
To develop a biosensor receptor element possessing a high sensitivity, it is necessary to use microorganisms with the maximum oxidative activity. An important factor affecting microbial activity is cultivation time. To choose an optimal cultivation time for P. yeei, we analyzed the dependence of the oxidative activity of cells, that comprise the receptor element, on growth time (Fig. 1).
From the data presented in Fig. 1, it is seen that the maximal activity of microorganisms is observed during the cultivation for 26 h, which corresponds to the onset of the growth declining phase.
An important stage in the formation of a stable receptor element for a BOD biosensor is the immobilization of microorganisms. The main requirements imposed on microbial immobilization matrices are a high diffusion of substrates and products, insolubility in aqueous media (availability of a network structure), high capacity, mechanical strength.
To produce a PVA-based network polymer, the linear polymer was modified by N-vinylpyrrolidone in the presence of cerium (IV) ammonium nitrate as an initiator of radical crosslinking. In the course of the investigation, the synthesis time and the reagents/initiator ratio were varied. The quality of the produced materials was assessed by their IR spectra and the crosslinked polymer fraction, which was determined by the swelling of films in water at a temperature of 60 °C. The results are given in Fig. 2 and Table 2.
The IR spectra of initial components’ mixtures (Fig. 2) are observed to have an absorption band at ν = 1625 cm−1 corresponding to the C=C double bonds of N-vinylpyrrolidone. The band vanishes in an hour in the process of the synthesis, and, as the result of the running reaction, a new bond > CH–O–CH < forms. This bond yields three characteristic absorption bands at 1136, 935 and 917 cm–1 in its IR spectrum (Fig. 2). The fraction of the crosslinked polymer also increases with synthesis time from 50 up to 55% (Table 2).
The properties of modified polymer are also affected by the ratio of NPV. With the NPV lacking, the sample of produced copolymer dissolves at a temperature of 60 °C (Table 2). The maximal fraction of cross-linked polymer (70%) was obtained in the polymer at a ratio of PVA: NPV: initiator = 160: 70: 10 and synthesis time of 3 h. However, this polymer, as the polymer with a component ratio of 160: 35: 5, significantly hampered the diffusion of oxygen as well as the products of reaction, which could lead to a decrease in the biosensor response. Further in our research we used a copolymer with the ratio of components being 160: 7: 1 and synthesis time lasting for 3 h.
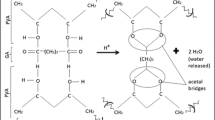
To establish the structure of the produced cross-linked polymer, we studied the reaction products by one- and two-dimensional NMR spectroscopy. The proton spectra are shown in Fig. 3a.
In the proton NMR spectrum of a copolymer solution, there is a signal of the NH4+ group of cerium (IV) ammonium nitrate (6.90–7.22 ppm) and there are no proton signals of the N-vinylpyrrolidone group CH2=CH−, which is indicative of the complete running of the reaction. In the proton spectrum (Fig. 3), well seen are the broadened signals of CH2(8) (1.50–1.70 ppm) and CH (6,7) (3.86–4.06 ppm) groups of the poly(vinyl alcohol) chain. Apart from proton signals of three CH2 groups—1.96–2.14 CH2 (2), 2.29–2.45 CH2 (3); 3.35–3.59 CH2 (1)—there is one more signal of the CH2 (4) group, 1.27 ppm. Its upfield shift indicates that this CH2 group belongs to the crosslinking fragment. Figure 3b presents a 13C (DEPT) spectrum of NVP-modified poly(vinyl alcohol). Three groups of signals—42.0–42.7 ppm (CH2), as well as 63.2–66.6 ppm and 72.3–74.7 ppm (CH)—correspond to the CH2 and CH groups in the carbon spectrum. The occurrence of two groups of signals for the CH groups is explained by the fact that one of them (at 63.2–66.6 ppm) pertains to the unsubstituted hydroxyl (CH–OH), and the other (at 72.3–74.7 ppm) belongs to the hydroxyl group, to which N-vinylpyrrolidone is attached. Besides the proton signals of three CH2 groups – 1.96–2.14 CH2 (2), 2.29–2.45 CH2 (3), 3.35–3.59 CH2 (1) there is also one more signal of a CH2 group (4): 1.27 ppm (19.7 ppm in the carbon spectrum). Its upfield shift implies unequivocally that this CH2 group pertains to the crosslinking fragment. The CH proton of group (5) is not seen in the 1H spectrum, because it gets under a suppressed water signal. Apart from the crosslinking signals, signals of two methyl CH3 (10) groups, 1.32 and 1.40 ppm (14.3 and 18.4 ppm in the carbon spectrum), and two CH (9) groups, 5.57 and 5.85 ppm (56.2 and 71.3 ppm in the carbon spectrum) are also seen in the proton and carbon spectra, which suggests the occurrence of an ethylpyrrolidone fragment in the polymer. Thus, proceeding from the analysis of two NMR spectra, the structure of the produced polymer corresponds to that presented in the insert of Fig. 3. It is important to emphasize that this particular structure is not similar to the one synthesized earlier for a different ratio of components in the same copolymer (Arlyapov et al. 2013).
The structure of a copolymer specimen with a molar component ratio of PVA: NPV: initiator = 160: 7: 1 was studied by scanning electron microscopy (Fig. 4).
As a result of the modification of poly(vinyl alcohol) by N-vinylpyrrolidone, a hydrogel of a network structure is produced (Fig. 4). For a chosen ratio of PVA: NPV: initiator = 160: 7: 1, a copolymer forms, which possesses a homogeneous structure and whose pores vary from 1 up to 10 μm in size (Fig. 4a). At the entrapment of P. yeei cells into the matrix (the diameter of bacteria, 0.5–0.9 μm) they are evenly distributed along the bulk of the carrier. In Fig. 4b, it is seen that after the immobilization the bacteria are retained in the matrix, and the matrix itself has a sufficiently porous structure, which contributes to the passage of oxygen and organic substances to microorganisms and the release of enzyme reaction products from them. Thus, the produced matrix in combination with P. yeei microorganisms can be efficiently used for the development of a biosensor.
Determination of the characteristics of a BOD biosensor based on the developed receptor element
An important characteristic of the assay is its selectivity, i.e., the possibility of detecting each component of the assayed object irrespective of others. In the case of a biosensor assay, its selectivity is determined by the substrate specificity of biomaterial used to form the sensor’s receptor element. When developing the receptor element of a biosensor for BOD detection it is preferable to use microbial cells that possess a broad substrate specificity (low selectivity). The broad substrate specificity is, in this case, an advantage, because it leads to an increase in the accuracy of BOD assay results. This work assessed the substrate specificity of P. yeei bacteria, immobilized in the N-vinylpyrrolidone-modified PVA matrix, with respect to 33 substrates of various classes of organic compounds. As substrates, we chose mainly readily oxidized organic substances, whose occurrence in water reservoirs leads to a significant decrease of the level of dissolved oxygen and the further eutrophication of aquatic ecosystems. Figure 5 presents data on the substrate specificity of the biosensor based on P. yeei bacteria.
Bacteria P. yeei immobilized in PVA-modified gel possess the highest sensitivity to glucose; the response to it was taken for 100%. Bacteria oxidize substances of all presented classes of organic compounds: alcohols, carbohydrates, carboxylic acids, amino acids, nitrophenols and surfactants that can be found in wastewaters. Valuable from the point of view of practice are responses to sodium dodecylsulfate, sodium dodecyl benzenesulfonate (detergents’ components) and nitrophenols (widespread industrial toxicants), as well as the absence of a toxic effect of these substrates at their brief impact on immobilized P. yeei bacteria. The obtained results suggest that, when determining BOD5 values in water samples of various origins (industrial, household, synthetic wastewaters), a high correlation can be obtained between the readings of the developed biosensor and the standard method.
A calibration dependence of biosensor response on BOD5 in a measuring cuvette for the developed biosensor is shown in Fig. 6.
Bioreceptors based on microbial whole cells are of catalytic type, i.e., the biological response in these systems is provided for by microbial enzyme reactions. The dependences shown in Fig. 6 are satisfactorily approximated by a Michaelis–Menten-type equation
where Rmax is the maximal rate of oxygen uptake by immobilized microorganisms achieved at [S] → ∞; KM is the effective Michaelis constant, i.e., the substrate concentration at which R = Rmax/2.
To reduce the error of the assay, use is made, as a rule, of the linear segment of the calibration curve bounded from above by the Michaelis constant КM (5.0 mg/dm3). The lower boundary of the linear segment corresponds to the lower boundary of the determined contents and was calculated by the statistical method, proceeding from the criterion of the value of the relative standard deviation of the measurement results (Sr(C)) < 0.33. The lower boundary of the determined BOD5 values was 0.05 mg/dm3. Thus, the linear range of the dependence of biosensor response on BOD5 is within the limits of 0.05–5.0 mg/dm3.
Table 3 presents the major analytical and metrological characteristics of a biosensor based on P. yeei bacteria. The characteristics of the developed biosensor were determined using a model GGA mixture.
Thus, it can be noted that the use of N-vinylpyrrolidone-modified PVA (the component ratio, PVA: NPV: initiator = 160: 7: 1) for immobilization of P. yeei bacteria made it possible to improve the main characteristics of the biosensor as compared with its analog based on the yeast D. hansenii described in (Arlyapov et al. 2013). A new ratio of the components for the synthesis selected in this work enabled producing a copolymer of an earlier undescribed chemical structure, which can be efficiently used for immobilization of highly sensitive P. yeei bacteria. The lower boundary of the assayed BOD5 values decreased more than tenfold, and the sensitivity of the biosensor increased ~ 50 times; herewith, the operational life of the BOD biosensor without replacement of the bioreceptor element remained on the previous level (about 45 days).
The developed BOD biosensor based on this bioreceptor element exceeds the known analogs by orders of magnitude with respect to its sensitivity and is not inferior by its rapidity. In particular, it has a smaller lower limit of the determined BOD5 values as compared with the predominant majority of the described prototypes (Jouanneau et al. 2014; Li et al. 2016; Wang et al. 2015). Besides, a single BOD assay using this analyzer is less time-consuming (Ponamoreva et al. 2011; Li et al. 2016; Wang et al. 2015). Herewith, the developed biosensor is distinguished with a narrower range of determined BOD values, which is easily eliminated by diluting the assayed sample.
Analysis of water samples
We analyzed samples of water using the developed biosensor and by the standard dilution method. Samples were wastewaters of the municipal sewage treatment works taken at various stages of purification, effluents of a food processing plant, natural waters including pond water polluted with fuel and lubricants. Sample taking and BOD5 determination by the standard dilution method were carried out according to the regulatory documents acting in the Russian Federation Federal Environmental Regulatory Documents (PND F) 1997. When determining the BOD5 of wastewater samples using the developed biosensor, the sample was prediluted. The value of dilution was adjusted such that the sensor response is within the linear segment of the calibration dependence (Fig. 6). Figure 7 shows a correlation between the BOD values determined using the biosensor and those by the standard dilution method.
Thus, the values of BOD5 determined using a P. yeei-based biosensor coincide with those obtained by the standard method with account for the confidence interval. Correlation with the standard method for a biosensor based on P. yeei microorganisms immobilized in modified PVA hydrogel was R2 = 0.9990, which is higher than for earlier described analogs (Jouanneau et al. 2014; Arlyapov et al. 2013; Raud et al. 2012).
Conclusions
We developed a BOD biosensor based on P. yeei VKM B-3302 bacteria immobilized in N-vinylpyrrolidone-modified poly(vinyl alcohol) hydrogel. P. yeei bacteria were shown to possess a broad substrate specificity and to be capable of oxidizing substrates from various classes of organic compounds. The developed hydrogel formed a network structure to enable the entrapment of microbial cells with their viability and biocatalytic properties preserved, which ensured the technological possibility of replicating expendable biosensor receptor elements. A new ratio of the components for the synthesis selected in this work enabled producing a copolymer of an earlier undescribed chemical structure, which can be efficiently used for immobilization of highly sensitive P. yeei bacteria. The lower boundary of BOD concentrations detected by the developed biosensor was 0.05 mg/dm3, which enables assays of high-purity water samples.
We determined the BOD indices of water samples from various sources. The use of activated sludge cells immobilized in N-vinylpyrrolidone-modified PVA was shown to enable registration of data that highly correlated with those of the standard method. The obtained results indicate the possibility of using the developed biosensor analyzer as a prototype of pilot devices for serial application.
References
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E (2014) Analytical applications of microbial fuel cells Part I: Biochemical oxygen demand. Biosensors Bioelectron 63:580–590
Aleshina EY, Yudanova TN, Skokova IF (2001) Production and properties of polyvinyl alcohol spinning solutions containing protease C and polyhexamethylene guanidine. Fibre Chem 33(6):421–423
Arlyapov VA, Yudina NYu, Asulyan LD, Alferov SV, Alferov VA, Reshetilov AN (2013) BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzyme Microb Technol 53:257–262
Bazilyuk TN, Melnik NP, Menzheres GYa (2003) Modification of poly(vinyl alcohol) by poly-N-vinylpyrrolidone. Voprosy Khimii I Khimicheskoi Tekhnologii 1:57–60 (in Russian)
Buenger D, Topuz F, Groll J (2012) Hydrogels in sensing applications. Prog Polym Sci 37(12):1678–1719
Burlage RS, Tillmann J (2017) Biosensors of bacterial cells. J Microbiol Methods 138:2–11
Commault A, Weld RJ (2016) Whole-cell biosensors for monitoring bioremediation. In: Lear G (ed) Biofilms in bioremediation: current research and emerging technologies, chap 5. Caister Academic Press, pp 75–92
Demakov VA, Maksimova YuG, Maksimov AYu (2008) Immobilization of microbial cells: biotechnological aspects. Biotekhnologiia 2:30–45 (in Russian)
Fang D et al (2016) A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochimica Acta 222:303–311
Federal Environmental Regulatory Documents (PND F) 14. 1:2:3:4. 123–97 (1997) Quantitative chemical analysis of waters. Methods of measuring the biochemical oxygen demand after n days of incubation (BODtotal) in surface fresh, ground, drinking, effluent and purified effluent waters. Moscow, 25 p. (in Russian).
Fine T, Leskinen P, Isobe T, Shiraishi H, Morita M, Marks RS, Virta M (2006) Luminescent yeast cells entrapped in hydrogels for estrogenic endocrine disrupting chemical biodetection. Biosens Bioelectron 21(12):2263–2269
Guisan JM editor (2006) Immobilization of enzymes and cells. 2nd ed. Totowa, New Jersey: Humana Press 450 p
ISO 5815–1:2003, 2003. Water Quality – Determination of Biochemical Oxygen Demand after n Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition
Jouanneau S, Recoules L, Durand MJ, Boukabache A, Picot V, Primault Y, Thouand G (2014) Methods for assessing biochemical oxygen demand (BOD): A review. Water Res 49:62–82
Kharkova AS, Arlyapov VA, Turovskaya AD, Avtukh AN, Starodumova IP, Reshetilov AN (2019) Mediator BOD biosensor based on cells of microorganisms isolated from activated sludge. Appl Biochem Microbiol 55(2):189–197
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, Xia S (2016) A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem Eng J 112:219–225
Martínez M, Hilding-Ohlsson A, Viale AA, Cortón E (2007) Membrane entrapped Saccharomyces cerevisiae in a biosensor-like device as a generic rapid method to study cellular metabolism. J Biochem Biophys Methods 70(3):455–464
Ponamoreva ON, Arlyapov VA, Alferov VA, Reshetilov AN (2011) Microbial biosensors for detection of biological oxygen demand (a review). Appl Biochem Microbiol 47(1):1–11
Raud M, Tenno T, Jogi E, Kikas T (2012) Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzyme Microb Technol 50(4–5):221–226
Szczesna-Antczak M, Antczak T, Bielecki S (2004) Stability of extracellular proteinase productivity by Bacillus subtilis cells immobilized in PVA-cryogel. Enzyme Microb Technol 34(2):168–176
Tag K, Lehmann M, Chan C, Renneberg R, Riedel K, Kunze G (2000) Measurement of biodegradable substances with a mycelia-sensor based on the salt tolerant yeast Arxula adeninivorans LS3. Sensors and Actuators B 67:142–148
Wang J, Bian C, Li Y, Tong J, Sun J, Hong W, Xia S (2015) A renewable BOD microsensor based on magnetically functionalized microorganism and ultramicroelectrode array. IEEE Sensors 2015:1–4
Yudina NYu, Arlyapov VA, Chepurnova MA, Alferov SV, Reshetilov AN (2015) A yeast co-culture-based biosensor for determination of waste water contamination levels. Enzyme Microb Technol 78:46–53
Zhanybekova AG et al (2013) N-Vinylpyrrolidone as a source of new excipients for pharmaceutical technology. Vestnik Kazakhskogo Natsionalnogo Meditsynskogo Universiteta 5–3:101–103 (in Russian)
Acknowledgements
The reported study was funded by the RFBR, project number 20–33-70078, and a grant of the President of the Russian Federation for the State Support of Young Russian PhD Scientists, agreement No. MK-1349.2020.3. The authors acknowledge Victor Selivanov for providing linguistic help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arlyapov, V.A., Yudina, N.Y., Asulyan, L.D. et al. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 10, 207 (2020). https://doi.org/10.1007/s13205-020-02199-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02199-0