Abstract
Transgenic broccoli (Brassica oleracea L. var. italica) cv. ‘Solan Green Head’ has been developed by using Agrobacterium tumefaciens strain harboring cryIAa gene for resistance against diamondback moth (Plutella xylostella). By combining the best treatments of 72 h pre-culturing and 48 h co-cultivation time period, a transformation frequency of 4.92 and 13.83% was obtained from cotyledon and hypocotyl explants, respectively. Supplementing the co-cultivation medium with acetosyringone in a concentration of 100 µM enhanced the transformation frequency to 17.92 and 32.11% in cotyledon and hypocotyl explants, respectively. The transgene (cryIAa) integration was confirmed by polymerase chain reaction using gene-specific primers and Southern blot analysis using digoxigenin nonradiolabelled DNA probe. Gene expression in the PCR-positive transgenic events had been confirmed by reverse transcriptase-PCR and quantitative real time-PCR. Insect bioassay proved the effectiveness of the transgene against infestation by diamondback moth (Plutella xylostella) larvae. To the best of our knowledge, this is the first report of optimization of a highly efficient transformation system and transgenic development in broccoli using cry1Aa gene for insect resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broccoli is a plant of the cabbage family Brassicaceae (formerly Cruciferae) and is classified as the Italica cultivar group of the species Brassica oleracea. Broccoli possesses abundant fleshy green flower heads arranged in a tree-like fashion on branches sprouting from a thick, edible stalk. Broccoli most closely resembles its close relative cauliflower, but is green rather than white. It is relatively new crop in North, South and Central America, Northern Europe and Asia. In broccoli, purple and green variants occur, though in USA, it is only green types that are grown. The green sprouting broccolis are classified in accordance to their maturity such as early, medium and late cultivars. In India, sprouting broccoli is hardly a commercial crop. A large number of F1 hybrid cultivars are being marketed by different seed companies in Japan, USA and Europe. Broccoli has a genetic characteristic of self-incompatibility, which encourages cross-pollination resulting in higher levels of variability.
Broccoli (B. oleracea L. var. italica) is an important nutritionally rich vegetable crop especially in calcium, antioxidants, vitamin A, vitamin K, β-carotene, riboflavin and iron content (Kumar et al. 2015; Kumar and Srivastava 2016a) and high selenium content. This cole crop is not only economically important, but also has anticancerous properties which are contributed by sulforaphane glucosinolate (Verma et al. 2014) and quinone reductase glutathione S-transferase (Kumar and Srivastava 2016b). Despite its significant nutritional value, the quality and quantity of produce is severely affected by environmental stress, pests and diseases because of a limited gene pool. Broccoli cv. “Solan Green Head” was developed by Department of Vegetable Science, Dr. Y. S. Parmar University of Horticulture and Forestry, Solan, India, and is used in crop breeding program because of its good yield potential and early maturity. However, this cultivar is largely affected by insect pests such as diamondback moth (Plutella xylostella), cabbage looper (Trichoplusia sp.), beetles (Phyllotreta cruciferae) and aphids (Brevicoryne brassicae). The diamondback moth, a serious havoc to broccoli, is considered to be the major pest of the crucifers worldwide and has become resistant to all major categories of insecticides (Gaur 2015; Parmar et al. 2017). The larvae attack flower buds and feed on tender leaves, causing severe damage. Development of insect-resistant broccoli cultivars through conventional plant breeding is not practicable due to non-availability of resistance sources in germplasm including their wild relatives. Therefore, genetically engineering broccoli genome with insect resistance genes such as Bt. (cry) gene against the diamondback moth is an attractive proposition. When Bt. crystal protein is ingested by the insect, it undergoes activation in the insect gut under the influence of an alkaline pH and gut proteases, and become active toxins that bind to the brush border membrane vesicles of the midgut epithelium cells and exert their toxicity by forming lytic pores, which ultimately kills the insect (Keshavareddy and Kumar 2016). Several attempts had been made earlier by scientists across the globe to enhance the yield and quality of broccoli using genetic transformation technique (Chen et al. 2007; Deng-Xia et al. 2011; Verma et al. 2014; Dhiman et al. 2015). However, because of the very low transformation frequency reported so far, genetic improvement in this crop remains hampered. So, for such genome-level interventions, the optimization and standardization of genetic transformation protocol is of utmost importance.
In the present study, we have standardized a highly efficient transformation system in broccoli cv. ‘Solan Green Head’ using hypocotyl and cotyledon explants and developed transgenic plants harboring cryIAa gene expressing resistance against diamondback moth (Plutella xylostella).
Materials and methods
Genetic transformation
Plant material
The certified seeds of broccoli cv. Solan Green Head were procured from Department of Vegetable Science, Dr Y.S. Parmar University of Horticulture and Forestry, Solan (H.P.), and used for aseptic germination on half-strength MS basal medium (Murashige and Skoog 1962) containing 0.5% sucrose. Aseptically grown, 10- to 12-day-old seedlings were used as source of explants, i.e., cotyledon and hypocotyl for genetic transformation studies.
Agrobacterium tumefaciens strain
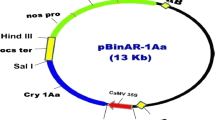
Agrobacterium tumefaciens strain LBA 4404 containing binary vector pBinAR-1Aa harboring cryIAa gene along with kanamycin resistance gene (npt-II) was used for co-cultivation experiments. This strain was obtained from ICAR-National Research Centre for Plant Biotechnology, New Delhi (Fig. 1). A single colony of A. tumefaciens strain was suspended into 10 ml liquid YMB medium containing 50 mg/l kanamycin at 28 °C temperature overnight with an agitation of 200 rpm. After 24 h, the cells were collected by centrifugation at 4000 rpm for 10 min, re-suspended into liquid MS medium. Agrobacterial suspension having 108 cells /ml having an OD of 0.521 at 540 nm wavelength was used for co-cultivation experiments.
Line diagram of gene construct: T-DNA of pBinAR-1Aa containing transcriptional fusion of CaMV 35S promoter with the coding region of cryIAa gene and NOS promoter with the coding region of npt-II gene (LB left border of T-DNA, RB right border of T-DNA, 35S CaMV35S promoter, NOS ter nopaline synthase terminator, NOS pro Nopaline synthase promoter, npt-II neomycin phosphor transferase-II, cryIAa-insect resistance gene, OCS ter octopine synthase terminator)
Pre-culturing, co-cultivation and development of independent putative transgenic events
Hypocotyl and cotyledon explants were cut into small pieces of 0.5–1.0 cm size and cultured on shoot regeneration medium (MS medium supplemented with 2.0 µM TDZ, 0.5 µM IAA for hypocotyl explants and MS medium containing 2.5 mg/l BAP, 0.5 mg/l NAA for cotyledon explants; already standardized in our laboratory by Kumar and Srivastava 2015a, b) for 24, 48 and 72 h to study the effect of pre-incubation on transformation frequency (Tables 1, 2). The pre-cultured explants were immersed in overnight grown fresh cultures of bacterial suspension for 15–20 s and then were blotted dry on pre-sterilized filter paper and cultured on shoot regeneration medium for different intervals of time, i.e., 48, 72 and 96 h to study the effect of co-cultivation time on transformation frequency. After co-cultivation, the explants were transferred to fresh selective shoot regeneration medium containing kanamycin (20 mg/l) and cefotaxime (400 mg/l) for selection of transformed cells and to inhibit further agrobacterial growth, respectively. Explants were periodically transferred to fresh selective shoot regeneration medium in order to check excessive bacterial growth. Selected clones (transformed cells/calli), which were growing on the selective shoot regeneration medium were further sub-cultured on the same medium to obtain independent putative transgenic shoots/events.
Effect of acetosyringone on shoot regeneration and transformation frequency
To study the effect of acetosyringone on shoot regeneration and transformation frequency, the regeneration medium for cotyledon and hypocotyl explants was supplemented with different concentrations (50, 75, 100, 125 and 150 µM) of acetosyringone (Table 3). Acetosyringone, being heat labile in nature, wasadded to the molten MS medium through Millipore membrane filter sterilization. The explants were pre-incubated and co-cultivated for standardized time interval to study the effect of acetosyringone on transformation frequency.
Root regeneration and hardening
Selected kanamycin-resistant shoots of 3–4 cm length were then transferred to the selective root regeneration medium containing 0.10 mg/l NAA, 20 mg/l kanamycin and 400 mg/l cefotaxime. Regenerated putative transformed plantlets with well-developed roots and shoot system were transferred to plastic cups containing autoclaved peat moss for hardening. The acclimatized plantlets were then transferred to the earthen pots in the green house for further growth and development.
Molecular analysis of putative transgenic events
PCR and RT-PCR analysis
Genomic DNA was extracted from young leaves of putative transformed and control (non-transformed) plants by using the CTAB method described by Doyle and Doyle (1990). PCR analysis was performed to detect the coding region of 1kb (cryIAa) gene sequences using the designed forward and reverse primers (Table 4). PCR was carried out with a total of 35 cycles. Initial denaturation was carried out for 94 °C for 4 min. Further each cycle consisted of denaturation of 94 °C for 1 min, annealing at 50 °C for 1 min and extension at 72 °C for 2 min and a final extension at 72 °C for 4 min. PCR amplicons were visualized after electrophoresis on 1.2% agarose-ethidium bromide gel.
For RT-PCR analysis, total RNA was isolated from PCR-positive transgenic plantlets of broccoli by using Real Genomics Total RNA extraction kit as per the manufacturer’s instructions. RT-PCR amplification was carried out by using RealScript™ One Step RT-PCR kit. The RT-PCR products were analyzed by electrophoresis on a 1.2% agarose gel. The amplified DNA products in the gel were photographed by Alpha Imager® EC gel documentation system (USA).
Southern blot analysis
For Southern blot analysis, DIG nonradiolabeled detection Kit (PCR DIG Probe Synthesis Kit, Roche) was used for the detection of gene copy number in transgenic lines, as per the manufacturer’s instructions. DNA for Southern hybridization analysis (20 µg) was digested with HindIII. The labeling of probe, pre-hybridization, hybridization and detection were performed as per the protocol of digoxigenin (DIG) nonradiolabeled detection Kit, Roche.
qRT-PCR analysis
Quantitative real-time PCR (qRT-PCR)-based analysis was performed to study the expression of transgene (cryIAa). PCR reaction was performed in 12.5 µl mixture containing diluted cDNA samples (100 ng) as a template from RT-PCR-positive transgenic shoots, SYBR Green PCR master mix (Bio-Rad Laboratories), and 10 µM each of forward and reverse gene-specific primers. The reactions were performed using CFX96 system (Bio-Rad Laboratories; Hercules CA, USA) with the following program: 94 °C (3 min), 94 °C (30 s), corresponding annealing temperature (30 s) and 72 °C (20 s) at 40 cycles. All reactions were done in three technical replicates with rubisco gene as an internal control for normalization. The relative expression of each gene was calculated using comparative Ct value method.
Insect bioassay
The young leaves of transformed and non-transformed broccoli were excised and placed in small Petri dishes containing moistened filter paper. On each leaf, five third-instar larvae of diamondback moth were released and reared at 26–28 °C with 60% relative humidity. Each assay consisted of three replicates using larvae originating from the same egg cluster. For each replicate of either transformed or control plants, the percentage of larval mortality was calculated up to 72 h after the start of bioassay.
Statistical analysis
The data recorded for different parameters were subjected to completely randomized design (Gomez and Gomez 1984). Explants were evaluated for mean number of shoots per explant and percent shoot regeneration. The statistical analysis based on mean values per treatment was made using analysis of variance for completely randomized design (CRD).
Results and discussion
Genetic transformation
During genetic transformation experiments, a pre-culturing period of 72 h followed by 48 h co-cultivation period with agrobacterial cells was found the best to obtain an efficient transformation frequency. Similar results were reported by Ravanfar et al. (2014) in cotyledon and shoot tip explants of broccoli. After co-cultivation, the explants were transferred to fresh selective shoot regeneration media containing antibiotics (cefotaxime 400 mg/l and kanamycin 20 mg/l). Callus formation was seen after 3 weeks from cut edges of cultured explants and shoot regeneration was observed after 7–8 weeks in cultured hypocotyl and cotyledon tissues. The cells/tissues which were not transformed turned brown or black on the selective media after 5 weeks in culture. The highest transformation frequency (4.92%) was obtained on MS medium containing 2.5 mg/l BAP, 0.5 mg/l NAA, 20 mg/l kanamycin & 400 mg/l cefotaxime in case of cotyledon explants, whereas in hypocotyl explants, the highest transformation frequency (13.83%) was obtained on MS medium supplemented with 2.0 µM TDZ, 0.5 µM IAA, 20 mg/l kanamycin and 400 mg/l cefotaxime, when pre-culturing and co-cultivation were carried out for 72 and 48 h, respectively (Tables 1, 2; Fig. 2a, b). A co-cultivation period of 2 days (48 h) was found optimal for cotyledon and hypocotyl transformation in broccoli as reported in other studies (Chen et al. 2007; Bhalla and Singh 2008; Awasthi and Srivastava 2013). The transformation rate varies from genotype to genotype and explant types in the same genotype. In our studies, hypocotyl explants responded better than cotyledon explants in terms of transformation rate. Min et al. (2007) have also reported that in Brassica species, hypocotyl explants showed high frequency of shoot regeneration than cotyledon explants. In another study, Song et al. (2012) also reported higher transformation frequency in hypocotyl explants (4%) as compared to cotyledon explants (0.7%).
Genetic transformation in broccoli (Brassica oleracea L. var. italica cv. Solan Green Head). a Co-cultivation of hypocotyl explants with Agrobacterium strain on shoot regeneration medium for 48 h. b Initiation of adventitious shoots from hypocotyl explants on selective shoot regeneration medium after 20–25 days in culture. c In vitro regenerated transgenic shoots obtained from hypocotyl explants after 45–50 days of culturing. d Shoot multiplication and elongation. e Root regeneration from in vitro developed putative transgenic shoots of broccoli after 20 days of culturing. f Acclimatization of in vitro regenerated putative transgenic plantlets of broccoli cv. Solan Green Head
Effect of acetosyringone on shoot regeneration and transformation frequency
In cotyledon and hypocotyl explants, different concentrations of acetosyringone (50, 75, 100, 125, 150 µM) were tried at standardized 72 h of pre-culturing and 48 h of co-cultivation time interval to investigate the effect of acetosyringone on transformation frequency. At a concentration of 100 µM acetosyringone, the transformation frequency was enhanced to 17.92% in cotyledon and 32.11% in hypocotyl explants (Table 3). The positive effect of acetosyringone in enhancing transformation frequency had been reported earlier in Brassica species (Song et al. 2012; Gambhir 2014; Gaur 2015). Actually, addition of acetosyringone to the regeneration medium during pre-culturing and co-cultivation increases the number of transformed cells by increasing the virulence of Agrobacterium cells and stimulates T-DNA transfer (Costa et al. 2006; Husaini 2010).
Development of complete independent putative transgenic plantlets
In vitro developed putative transgenic shoots were further multiplied on the same selective shoot regeneration medium (Fig. 2C, D). The putative transgenic shoots (3–4 cm in length) were excised and transferred to the selective root regeneration medium containing 0.10 mg/l NAA, 20 mg/l kanamycin & 400 mg/l cefotaxime. Roots initiated after 8–10 days of inoculation and within 20–25 days well developed roots were observed (Fig. 2e). After the complete development of transgenic broccoli plantlets, they were taken out from the culture tubes and washed gently under running tap water to remove adhering medium. The plantlets were kept in 0.5% bavistin solution for 5–10 min and placed in sterilized cocopeat. The plantlets were watered and covered with polythene bags to maintain high humidity. Water was sprayed daily. The transgenic plantlets showed initial sign of establishment in plastic pots after 1 week of hardening (Fig. 2f). The polythene bags were punctured gradually after 1 week of hardening and 50–60% survival of plants were observed during acclimatization. Most of the putative transgenic plantlets regenerated from cotyledon and hypocotyl explants appeared phenotypically/morphologically normal.
Molecular analysis of putative transgenic plants
PCR and RT-PCR analysis
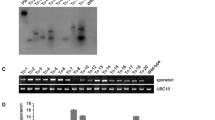
The presence/integration of transgenes (cryIAa) into the genome of broccoli was confirmed by PCR using gene-specific primers and total genomic DNA was isolated from 30 randomly selected putative transgenic plantlets along with control plantlets by the CTAB method. Gene-specific primers (Table 4) were used to amplify 1.0 kb fragment of cryIAa gene by polymerase chain reaction. Out of 30 randomly selected putative transgenic events, 21 had shown the amplification of cryIAa gene there by indicating the presence/integration of cryIAa gene into the genome of transgenic plantlets of broccoli (Fig. 3).
Transgene expression at transcriptional level was checked by RT-PCR using Real Genomics Total RNA extraction kit as per the manufacturer’s instructions. Eleven putative transgenic events of broccoli inferred positive for transcriptional expression of cryIAa gene out of 21 PCR positive events (Fig. 4). The most plausible explanation for this may be that while doing PCR analysis using gene-specific primers, spurious/false bands may appear due to the presence of agrobacterial cells inside the transgenic plants showing them PCR positive. The other reason may be the silencing of transgene resulting into null expression at transcriptional level. RT-PCR had been used by many workers to see the gene expression at mRNA level in transgenic broccoli plants (Rafat et al. 2010; Ravanfar and Aziz 2014). In another study, Deng-Xia et al. (2011) developed transgenic broccoli with cryIBa3 gene for incorporation of insect resistance and confirmed the gene expression by RT-PCR.
qRT-PCR and Southern blot analysis
All the RT-PCR positive plants were subjected to QRT-PCR to confirm the real time expression of transgene. In quantitative real time-PCR (qRT-PCR) assay, a positive reaction was detected by accumulation of a fluorescent signal. The ct value for all the transgenic plantlets used for real time-PCR analysis were below 26 and hence it indicated the greater amount of target nucleic acid (cry IAa gene) in the broccoli transgenic plantlets. Figure 5a depicts the comparison of expression of transgene with negative control and the transgenic event S9 showed maximum expression level of transgene, i.e., cryIAa followed by S22 and S23. The transgenic events showing positive signal in qRT-PCR were subjected to Southern blot analysis for confirmation of copy number of transgene.
QRT-PCR, Southern blot analysis and bioassay studies. a Real time-PCR comparison of cryIAa gene in regenerated transgenic plantlets of broccoli (Brassica oleracea L. var. italica cv. Solan Green Head). NC negative control (non-transformed), (S1, S2, S9, S10, S11, S12, S13, S14, S22, S23 and S30-transformed plantlets of broccoli cv. Solan Green Head. b Southern blot analysis of transgene in transformed plantlets of broccoli (Brassica oleracea L. var. italica cv. Solan Green Head). WT Wild type non-transformed (control); (S1, S2, S9, S13, S22 & S23—transgenic plantlets of broccoli). c–f Insect bioassay with diamondback moth (DBM); c infested non-transgenic (control) plants (leaves samples) of broccoli after 48 h. d Infested transgenic plants (leaves samples) of broccoli after 48 h. e Live larvae of diamondback moth (DBM) on damaged non-transgenic plants (leaves samples) after 72 h and f larval mortality of diamondback moth (DBM) larvae on transgenic plants (leaf samples) after 72 h
For Southern analysis, the isolated, purified and quantified DNA (plasmid DNA and genomic DNA of qRT-PCR positive transgenic plantlets and control plantlet) was used for blotting with DIG-nonradiolabeled DNA probe. Out of the six RT-PCR positive shoots, 4 shoots were confirmed positive for integration of transgene with single copy gene insertion (Fig. 5b). PCR and Southern hybridization-based gene integration was also reported in broccoli in earlier studies by Chen et al. (2001). Ravanfar and Aziz (2014) also confirmed integration of transgene (AtHSP101) in the genome of broccoli by PCR and Southern hybridization.
Insect bioassay studies
Finally, the expression and efficacy of transgene (cryIAa) against diamondback moth was evaluated by bioassay using third-instar larvae of diamondback moth. A high degree of insect mortality was reported during insect bioassays in transgenic plants. The larvae fed on transgenic leaves were severely stunted in growth when compared to larvae fed on control (non-transformed) leaves after 1 day from the start of bioassay (Fig. 5c, d). Transgenic plants demonstrated 100% mortality of larvae at the day 3 after the bioassay was started; whereas, the larvae on control plants (non-transformed) were growing healthy (Fig. 5e, f).
In conclusion, by optimizing the different parameters in Agrobacterium-mediated insect resistance (cryIAa) gene transformation technique in broccoli, relatively high transformation efficiency was demonstrated. Bt–broccoli cv. Solan Green Head expressing cryIAa gene in the present investigation showed promise of practical utility in insect-pest management. The standardized genetic transformation protocol of broccoli can be further exploited to develop transgenic plantlets with biotic and abiotic stress resistant/tolerant gene(s) in future.
References
Awasthi M, Srivastava DK (2013) Pyramiding of Bt-genes (cry1Ab and cry1Aa) in cauliflower (Brassica oleracea L. var. botrytis) for insect resistance using an Agrobacterium-mediated gene transfer technique. In: Proceedings of National symposium on plant tissue culture and biotechnology for food and nutritional security, Abstract p. 72
Bhalla PL, Singh MB (2008) Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat Protoc 3:181–189
Chen LFO, Hwang JY, Charng YY, Sun CW, Yang SF (2001) Transformation of broccoli (Brassica oleracea var. italica) with isopentenyltransferase gene via Agrobacterium tumefaciens for post-harvest yellowing retardation. Mol Breed 7:243–257
Chen LO, Chin HL, Kelkarc SM, Chang YM, Shawa JF (2007) Transgenic broccoli (Brassica oleracea L. var. italica) with antisense chlorophyllase (BOCLH1) delays post harvest yellowing. Plant Sci 174(1):25–31
Costa MS, Miguel CL, Oliveira MM (2006) An improved selection strategy and the use of acetosyringone in shoot induction medium increase almond transformation efficiency by 100-fold. Plant Cell Tiss Org Cult 85:205–209
Deng-Xia Y, Lei C, Yu-Mei L, Mu Z, Yang-Yong Z, Zhi-Yuan F, Li-Mei Y (2011) Transformation of cabbage (Brassica oleracea L. var. capitata) with Btcry1Ba3 gene for control of diamondback moth. Agric Sci China 10(11):1693–1700
Dhiman K, Verma S, Srivastava DK (2015) Plant regeneration, genetic transformation and expression of gus gene in broccoli. Veg Sci 41(8):129–134
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissues. Focus 12:13–15
Gambhir G (2014) Studies on Agrobacterium-mediated insect resistance gene transfer in cabbage (Brassica oleracea L. var. capitata) and molecular analysis of regenerated plantlets. Ph. D. Thesis, Dr YS Parmar University of Horticulture and Forestry, Nauni. Solan (H.P.), India
Gaur A (2015) Studies on Agrobacterium-mediated insect resistance gene [cry1A(a)] transfer in cauliflower (Brassica oleracea L. var. botrytis). Thesis PhD, Dr. Y.S. Parmar University of Horticulture and Forestry, Nauni, Solan (H.P.), India
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Husaini AM (2010) Pre- and post-agroinfection strategies for efficient leaf disk transformation and regeneration of transgenic strawberry plants. Plant Cell Rep 29:97–110
Keshavareddy G, Kumar ARV (2016) Bacillus thuringiensis. In: Omkar (ed) Ecofriendly pest management for food security. Academic Press, London, pp 443–477
Kumar P, Srivastava DK (2015a) Effect of potent cytokinin thidiazuron (TDZ) on in vitro morphogenic potential of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Indian J Plant Physiol 20(4):317–323
Kumar P, Srivastava DK (2015b) High frequency organogenesis in hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Physiol Mol Biol Plants 21(2):279–285
Kumar P, Srivastava DK (2016a) Biotechnological advancement in genetic improvement of broccoli (Brassica oleracea L. var. italica), an important vegetable crop: a review. Biotech Lett 38(7):1049–1063
Kumar P, Srivastava DK (2016b) Biotechnological application in in vitro plant regeneration studies of Broccoli (Brassica oleracea l. var. italica), an important vegetable crop: a review. Biotech Lett 38(4):561–571
Kumar P, Gambhir G, Gaur A, Srivastava DK (2015) Molecular analysis of genetic stability in in vitro regenerated plants of broccoli (Brassica oleracea L. var. italica). Curr Sci 109(8):1470–1475
Min BW, Cho YN, Song MJ, Noh TK, Kim BK, Chae WK, Park YS, Choi YD, Harn CH (2007) Successful genetic transformation of Chinese cabbage using phosphomannose isomerase as a selection marker. Plant Cell Rep 26:337–344
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Parmar N, Singh KH, Sharma D, Singh L, Kumar P, Nanjundan J, Khan YJ, Chauhan DK, Thakur AK (2017) Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3 Biotech 7:239. https://doi.org/10.1007/s13205-017-0870-y
Rafat A, Aziz MA, Rhashid AA, Abdullah SNA, Kamaladini H, Sirchi MHT, Javadi MB (2010) Optimization of Agrobacterium tumefaciens-mediated transformation and shoot regeneration after co-cultivation of cabbage (Brassica oleracea subsp. capitata) cv. KY cross with At hsp101 gene. Sci Hortic 124:1–8
Ravanfar SA, Aziz MA (2014) Shoot tip regeneration and optimization of Agrobacterium tumefaciens-mediated transformation of Broccoli (Brassica oleracea var. italica) cv. Green Marvel. Plant Biotech Rep. https://doi.org/10.1007/s11816-014-0340-5
Ravanfar SA, Aziz MA, Rashid AA, Shahida S (2014) In vitro adventitious shoot regeneration from cotyledon explant of Brassica oleracea subsp. italica and Brassica oleracea subsp. capitata using TDZ and NAA. Pakistan J Bot 46(1):329–335
Song GQ, Walworth AE, Hancock JF (2012) Agrobacterium tumefaciens-mediated transformation of rutabaga (Brassica napus var. napobrassica) cultivar “American Purple Top Yellow”. In Vitro Cell Dev Biol Plant 48:383–389
Verma H, Kumar P, Gambhir G, Srivastava DK (2014) Agrobacterium-mediated transformation of broccoli. Crop improv 41(2):139–147
Acknowledgements
Authors are highly thankful to the Project Director, National Research Centre on Plant Biotechnology, New Delhi, for providing Agrobacterium strain and for allowing to do molecular analysis work. The senior author (PK) thankfully acknowledges Department of Science and Technology, New Delhi, for providing INSPIRE fellowship during doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, P., Gambhir, G., Gaur, A. et al. Development of transgenic broccoli with cryIAa gene for resistance against diamondback moth (Plutella xylostella). 3 Biotech 8, 299 (2018). https://doi.org/10.1007/s13205-018-1316-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1316-x