Abstract
Adaptable exploitation of the catalytic potential of membrane-bound d-sorbitol dehydrogenase (mSLDH) from Gluconobacter oxydans is desperately needed in the industrial-scale production of miglitol. In the present study, a carbonyl group-dependent colorimetric quantification method was developed for the assay of miglitol key intermediate 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose (6NSL), and a high-throughput screening process of positive mutants was processed. Combined with several rounds of ultraviolet irradiation mutagenesis and screening procedure, a positive mutant strain G. oxydans ZJB16009 was obtained with significant increase in mSLDH catalytic activity by 1.5-fold, which exhibited an extremely accelerated uptake rate of d-sorbitol, and the fermentation time was significantly shortened from 22 to 11 h. In a 5-L biotransformation system, 60 g/L substrate N-2-hydroxyethyl glucamine (NHEG) was catalyzed by the resting cells of the mutant strain within 36 h and accumulated 53.6 g/L 6NSL, showing a 33.6% increase in the product yield. Therefore, it was indicated that the established high-throughput screening method could provide a highly efficient platform for the breading of G. oxydans strain for the industrial biosynthesis of miglitol intermediate 6NSL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Miglitol (N-hydroxyethyl-1-deoxynojirimycin) is a pseudomonosaccharide α-glucosidase inhibitor in the treatment of non-insulin-dependent mellitus, which plays a role in preventing the digestion of carbohydrates and in establishing the control of blood glucose (Yang et al. 2008). The industrial production route of miglitol combines a biotechnological–chemical synthesis which integrates an enzymatic bioconversion towards N-2-hydroxyethyl glucamine (NHEG) catalyzed by the resting cells of Gluconobacter oxydans into a sequence of chemical steps, achieving an elegant, short and economical process (Schedel 2008). The substrate NHEG can be simply obtained from the reductive amination of D-glucose with up to 90% purity (Scaros 1999). The crucial step of regio- and stereo-selective dehydrogenation towards NHEG is usually achieved by microbial oxidation with resting cell of G. oxydans to produce 6-(N-hydroxyethyl) amino-6-deoxy-l-sorbose (6NSL), a key intermediate for the final synthesis of miglitol combined with a stereo-selective reductive ring closure (Zhang et al. 2011).
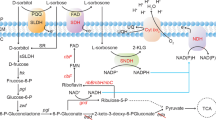
Gluconobacter oxydans strains are strict aerobes and use a variety of sugars, alcohols and polyols as substrates that are oxidized incompletely due to the absence of phosphofructokinase, succinyl-CoA synthetase, and succinate dehydrogenase (Prust et al. 2005; Kranz et al. 2017), which is advantageous for a broader range of industrial applications, such as 1,3-dihydroxyacetone (Hu and Zheng 2011; Hu et al. 2011, 2017), l-sorbose (vitamin C synthesis) (Deppenmeier et al. 2002) and miglitol (Schedel 2008). The majority of the oxidative reactions are catalyzed by membrane-bound dehydrogenases linked to the respiratory chain and their active sites are oriented towards the periplasm, so that substances that are used as energy sources can be oxidized in the periplasmic space without the need to enter the cytoplasm (Prust et al. 2005), facilitating a rapid and efficient accumulation of incompletely oxidized products (Deppenmeier et al. 2002). Among the varied kinds of membrane-bound dehydrogenases of G. oxydans, the prosthetic group pyrroloquinoline quinone (pQQ) dependent d-sorbitol dehydrogenase (mSLDH, EC 1.1.99.22) is verified to be responsible for the production of 6NSL for its important characteristic in the asymmetric oxidation of aminosorbitol derivatives in addition to its natural substrate of polyhydric alcohols (Yang et al. 2008; Gao and Wei 2006).
Nowadays, due to the limitations both in the activity and the stability of G. oxydans mSLDH, the development of mSLDH with desired functions has been boosted with advances in genomic data (Prust et al. 2005; Kranz et al. 2017; Gao et al. 2012), DNA recombination and protein expression technologies (Yuan et al. 2016; Gatgens et al. 2007; Saito et al. 1997). On the other hand, the traditional mutation strategy is still frequently implemented in the breeding of industrial strain and the availability of sensitive, facile and efficient high-throughput methods is generally needed to screening enzymes with high activity and specific substrate profile (Zheng et al. 2017; Liu et al. 2017a, b). Studies on the rapid selection model of DHA-over producing G. oxydans strain have been extensively reported (Lin et al. 2016). However, current literatures do not reveal any use of high-throughput screening method for the improved miglitol production. The miglitol intermediate 6NSL is traditionally analyzed by HPLC, GC/MS or NMR, providing an evaluation of conversion and stereoselectivity simultaneously; however, they are low throughput and time-consuming (Takahashi et al. 1995; Liu et al. 2014). Other methods such as silica gel thin-layer chromatography (Yang et al. 2008), conventionally used in the industrial analysis of miglitol intermediate 6NSL, suffers from specificity and accuracy, all of which largely hampered the adaptable exploitation of the catalytic potential of mSLDH. The reconstitution of an easily operated and high-throughput method for the quantitative analysis of 6NSL is therefore desperately needed (Liu et al. 2017b).
The formation of chromogenic products in the reaction between ketone/aldehyde groups and 2,4-dinitrophenylhydrazine (DNPHzine) under basic conditions is well-known (Oscar et al. 1926), and it has been developed for the assay of carbonyl reductases towards various ketones (Zhou et al. 2016). Our laboratory also employed this method in the screening of dehydrogenase with high enantioselectivity in the oxidative resolution of (R)- and (S)- α hydroxyacid into α-ketoacid (Xue et al. 2012). In present study, according to the typical α-hydroxyacid structure in 6NSL, the formation of chromogenic products in the reaction between 6NSL and DNPHzine under basic conditions was confirmed to be with high sensitivity and low background disturbance. A high-throughput screening approach was established that offers an easily operated and whole-cell system adaptable for the screening and characterization of the random mutagenesis in the breeding process of G. oxydans strain especially for the biosynthesis of 6NSL. A positive mutant strain ZJB16009 was obtained with a significant increase in the catalytic activity of mSLDH, leading to both efficient bioconversion rate and high product yield. To the best of our knowledge, this is the first report on the high-throughput assay for the quantification of 6NSL production with potential applications in the breeding of G. oxydans in biosynthesis of miglitol intermediate 6NSL.
Methods
Chemicals
The standard sample of DNPHzine, d-sorbitol, l-sorbose of analytical grade were purchased from J&K Chemical Co., Ltd. (Shanghai, China). The substrate NHEG (91.6% purity) was provided by Xinchang Pharmaceutical Factory of Zhejiang Medicine Co., Ltd., and was further purified by recrystallization from ethanol for two times to reach a 99.6% purity as identified by HPLC. All other chemicals were of analytical grade and were also purchased from commercial sources.
Establishment of DNPH method
The solution of DNPHzine (100 mL) was prepared with DNPHzine (198 mg) and hydrochloric acid (38%, 83.3 mL) in ultra-pure water. The general reductive reaction system was consisted of 150 µL 6NSL with different concentrations (0–50 g/L), 25 µL of DNPH solution (20 mM) and the mixture was incubated at 37 °C for 15 min (Zhou et al. 2016). General chromogenic reaction was performed by the addition of 25 µL of NaOH (8 mM) and then the absorbance of the bright-yellow solution in the micro-plates was determined at 445 nm by a microplate reader (Molecular Devices, Spectra Max, Sunnyvale, USA). The effect of the concentration of DNPH (1–100 mM) /alkali (1–12 mM)/incubation time (1–100 min) was optimized according to the general methods, respectively, to achieve high sensitivity. Standard curves of 6NSL with various concentrations (0–50 g/L) were determined by the DNPH method.
Microorganism and culture conditions
The G. oxydans ZJB-605 strain was originally screened from soil sample and conserved in China Center for Type Culture Collection (CCTCC No. M208069), and was stored at 4 °C on a agar slope consisted (g/L) yeast extract 10, CaCO3 20, glucose 40, agar 24 (pH 6.0). After inoculated into a 50-mL fermentation medium consisted (g/L) of D-sorbitol 80, yeast extract 24, K2HPO4 5, KH2PO4 5 (pH 6.0), the preculture was allowed to grow in an incubator shaker at 150 rpm at 28 °C for 24 h. The broth was then used for the subsequent UV mutagenesis.
UV mutagenesis and high-throughput screening procedure for 6NSL-overproducing mutants
The UV mutagenesis of the original G. oxydans was carried out according to a previous report (Hu and Zheng 2011), and the program of UV irradiation was conducted for several rounds combined with DNPH screening method until positive mutants could be sparsely obtained. In the screening procedure demonstrated in Fig. 1, single colony was picked up in 96-deep-well culture plates with 1.5 mL fermentation medium and was cultivated at 30 °C for 24 h. Cells were harvested by centrifugation at 1000×g for 30 min at 4 °C, washed thoroughly with distilled water, and then the resting cells were stored at 4 °C for biotransformation. The standard reaction mixture consisted of 60 g/L NHEG and 0.5 g/L MgSO4·7H2O, adjusted pH to 5.0 by titration of 38% hydrochloric acid, was added into micro-plates for 1 mL per well. And the bioconversion reaction was conducted in a shaker under 150 rpm at 15 °C for 24 h, the product concentration of 6NSL was then quantified by DNPH method. The re-screening procedure was subsequently implemented at shake-flask scale for the positive mutants with more than 40% higher in the relative activity of mSLDH, and the yield of 6NSL was further confirmed by HPLC analysis.
Batch production of resting cells with mSLDH activity using sorbitol as substrate
The batch fermentation process of the origin strain and mutant strain was carried out in a 5-L multi-bioreactor system (Sartorius, Biostate B, Germany) using a sorbitol culture medium consisted of (g/L) yeast extract 24, d-sorbitol 80, K2HPO4 5, KH2PO4 5. All medium were sterilized by autoclaving at 115 °C for 20 min and the pH was adjusted to 6.0. The preculture was inoculated from a sorbitol broth agar plate and incubated in an orbital shaker (150 rpm, 30 °C) until the late exponential growth phase (about 22–24 h). A 10% (v/v) inoculum of these cells was added to the liquid fermentation medium and incubated at 30 °C for approximately 16 to 22 h, and the agitation speed was 400 rpm with a 1.5 vvm aeration rate (volumes of air/effective volumes of the bioreactor per minute). The samples (20 mL) were withdrawn from the bioreactor and centrifuged at 9000×g for 10 min. The supernatant was collected and used for the quantification of d-sorbitol and l-sorbose and the precipitate of cell was used to assay the mSLDH activity after washing with deionized water and dried to constant weight at 80 °C for the assay of biomass. Dissolved oxygen (DO) was detected by the dissolved oxygen electrode method as previously described (Bhave and Chattoo 2003).
Biotransformation of NHEG to 6NSL using resting cells
Cells reached early stationary phase were harvested by centrifugation at 9000×g at 4 °C for 10 min, washed twice with deionized water and resuspended in reaction solution to a final concentration of 40 g/L wet cell weight (WCW) [corresponding to 4.02 g/L dry cell weight (DCW)]. The substrate reaction solution consisted (g/L) NHEG 60, MgSO4·7H2O 5, resting cells WCW 40, was adjusted to pH 5.0 with 38% hydrochloric acid and was prepared just before the assay. The biotransformation of NHEG to 6NSL was firstly performed in shake flasks and then scaled-up in a 5 L stirred-bioreactor at 15 °C for 48 h, the pH was adjusted to 5.0 by titration of 2 M NaOH and the agitation speed in the bioreactor was 400 rpm with a 2.0 vvm aeration rate. The reaction was terminated by centrifugation (9000×g, 10 min) and supernatant was collected to determination the concentrations of NHEG and 6NSL.
Analytical methods
One unit of the catalytic activity of mSLDH towards NHEG (U) was defined as the amount of mSLDH enzyme that catalyzed the formation of 1 µmoL 6NSL per min at 15 °C. The concentrations of NHEG and 6NSL were analyzed by HPLC (Waters Co., Milford, MA, USA) equipped with a C-18 reverse phase column (250 mm × 4.6 mm) with a differential refractive index detector at a flow rate of 0.5 mL/min under 30 °C column temperature. The mobile phase consisted of ultra-pure water and methanol (98:2, v/v) with 4 mM sodium heptanesulfonate and 10 mM K2HPO4 was performed, and the pH was adjusted to 3.5 with phosphoric acid. Since there is no standard sample of 6NSL can be purchased from the commercial company, the product obtained from the bioconversion of NHEG by resting cells of G. oxydans at 15 °C for 48 h was performed by liquid chromatography–mass spectrometry (LC/MS) with a DECAX-60000 LCQ Deca XP (Thermo, America) and 2H nuclear magnetic resonance to confirm the quality and purity of 6NSL. In addition, one unit of mSLDH activity towards d-sorbitol (U) was defined as the amount of mSLDH enzyme that catalyzed the formation of 1 µmoL l-sorbose per min at 30 °C and it was determined by measuring the initial formation rate of l-sorbose within the first 20 min performed in a basal reaction mixture consisted of 20 mM phosphate buffer (pH 6.0), 20 g/L sorbitol, 5 g/L MgSO4·7H2O and an appropriate amount of resting cells collected from 20 mL fermentation broth. The concentrations of d-sorbitol and l-sorbose were assayed by HPLC (Waters Co., Milford, MA, USA) equipped with an Aminex HPX-87H chromatographic column and a differential refractive index detector at a flow rate of 0.6 mL/min under 60 °C column temperature using a mobile phase consisting of 5 mM H2SO4 aqueous solution.
Mutant strain stability
Hereditary stability of the positive mutant strains was further investigated by continuous subcultures. The slant of a mutant isolate was cultivated at 28 °C for 3–5 days. Totally, 10 generations of subsequent subcultures were performed. Bacteria of each passage were transferred into a shake flask and the corresponding resting cells were processed to determine the productivity of 6NSL by DNPH method. Only mutants with both high productivity and hereditary stability were selected to the next round of mutagenesis.
Results and discussion
Rapid quantitative determination of 6NSL
A simple colorimetric assay for rapid identification of 6NSL was established based on the chromogenic reaction of ketoacids with DNPH. As shown in Fig. 2, the bright-yellow colored hydrazone was homogeneously formed under alkaline condition, and the color was deepened with the increased concentration of 6NSL. According to the absorbance spectrum of the colorimetric assay, it was suitable to choose the 445 nm as the detection wavelength since 6NSL displayed a characteristic absorbance peak at around 455 nm, which was low detected from the samples of NHEG, glucose, d-sorbitol and l-sorbose when co-incubated with DNPHzine, as well as that of DNPHzine alone in the negative control (Fig. 3a), all above results indicated that the specificity of DNPH method in the assay of 6NSL. The calibration curve and the detection limitation of the DNPH method were further determined, respectively. Qualitative analysis and quantification of the product obtained from the bioconversion system catalyzed by the resting cell of G. oxydans was performed by LC/ESI-MS and the profile of liquid chromatogram demonstrated that 6NSL was detected at 7.0836 min with a purity of 92.1%, and the results from mass spectrometry displayed a peak at 224.1 [M + H+] which is equal to the molecular value of 6NSL (Fig. S1). According to the Beer–Lambert equation, the relationship between concentration of 6NSL and absorbance value at 445 nm is shown in Fig. 3b. A linear relationship was observed between NHEG concentration and OD445 nm from 5 to 50 g/L, when the concentration of NHEG was lower than 5 g/L, the color of the complex was so shallow that the color change was hardly observed; and the higher bright-yellow concentration (up to 50 g/L) led to higher color which was too dark to observe its variation. Afterwards, the reliability of the DNPH method was further evaluated against HPLC analysis, the concentration determined by DNPH is highly consistent with that obtained by HPLC and the Pearson correlation coefficient between DNPH and HPLC methods was 0.99 (Fig. 3c). All above results confirmed that the DNPH method is exceptionally accurate and specific towards 6NSL that can be simply performed on a 96-well plate with a universal microplate reader.
Bright-yellow colored hydrazone formation between 6NSL and DNPHzine. a 6NSL can react with DNPHzine to form a bright-yellow colored hydrazone under alkaline condition. b The color of different concentrations (0, 10, 20, 30, 40 and 50 g/L) of 6NSL with DNPHzine in 96-microplate, which was visible to the naked eye compared with the negative control
Rapid quantification of 6NSL concentration by a microplate reader under 445 nm. a Absorption spectra in the full-wavelength scan (300–700 nm) of NHEG, 6NSL, glucose, d-sorbitol and l-sorbose with DNPH and the negative control of DNPH alone. b The relationship between the concentration of 6NSL and the absorption value at 445 nm. The insert shows the linear range of the measurement. The directly proportional relationship 6NSL was y = 0.0566x + 0.0385 (R2 = 0.9969). c The concentration of 6NSL determined by HPLC and DNPH assay (R2 = 0.9921)
High-throughput screening of 6NSL-overproducing mutants
Ultraviolet irradiation is an easily accessible approach for screening high-yield strains for industrial application and was verified to be an effective approach in the mutagenesis of positive mutants in the breeding of G. oxydans (Hu and Zheng 2011; Ma et al. 2010). To obtain variants with improved activity of mSLDH, a rapid screening model for UV mutagenesis was established based on DNPH method as demonstrated in Fig. 1. The UV exposure time was optimized between 0 and 150 s and the highest rate of positive mutation rate of 51.5% was obtained after exposure for 60 s with a survival rate of 5–10% (Fig. S2). Several rounds of random mutations were performed and approximately 1400 mutants of G. oxydans were screened as demonstrated in Fig. 4. Among them, 2% of the variants displayed up to 40% increase in mSLDH activity. A positive mutant strain ZJB16009 was obtained and the catalytic activity towards NHEG was further confirmed in shake-flask system. As demonstrated in Table 1, compared with the original strain ZJB-605, the mutant strain ZJB16009 demonstrated a significant increase in mSLDH catalytic activity towards NHEG. The conversion rate of NHEG was increased from 55.8 to 79.7% and accumulated 44.2 g/L 6NSL within 24 h, showing a 42.6% increase compared with that of the original strain. Therefore, the intrinsic capability for high throughput in DNPH method was verified, contributing to speeding up the breeding process of industrial strain of G. oxydans mutants in the biosynthesis of miglitol intermediate 6NSL.
Comparative studies on the characteristics of sorbitol fermentation
It has been shown that due to the incomplete respiratory chain, the coupling between respiratory chain and ATP production is very poor in G. oxydans, which is mainly mediated by the pQQ-dependent quinoprotein mSLDH, using of oxygen as final electron acceptor (Prust et al. 2005). According to the broad substrate spectrum of mSLDH in G. oxydans, various low-cost polyols and sugar alcohols were used to investigate the effect of different carbon resources on the mSLDH activity produced by the mutant strain ZJB16009. Glycerol/sorbitol was confirmed to be the optimum carbon resource in shake flasks compared with glucose, fructose and maltose (data not shown), probably due to their specific structure according to the Bertrand and Hudson rule (Kulhánek 1989). The fermentation peculiarity of the mutant strain ZJB1009 was further investigated in a multi-bioreactor system compared with the original strain ZJB-605. As can be seen in Fig. 5, the mutant strain ZJB16009 showed similar growth profile compared with that of the original strain though the biomass value was a bit decreased at the late stage of fermentation (4.46 vs. 5.06 g DCW/L). At 12 h, sorbitol in culture medium was totally taken up by ZJB16009 and resulted in the accumulation of 77.9 g/L sorbose; while there was still 42.34 g/L sorbitol remained in the medium of the original strain, which takes another 10 h to consume up. Over 90% of sorbitol was determined be dehydrogenated into sorbose, which was consistent to a previous report (Xu et al. 2014). Subsequently, profiles of the OD value was gradually dropped from 100% air saturation to less than 5%, and then it sharply increased to 100% after the sorbitol was exhausted at the late stage of fermentation, referring that the OD was mainly utilized in the biotransformation of d-sorbitol to l-sorbose through the incomplete oxidation respiratory chain of G. oxydans (Prust et al. 2005). Thus, the culture duration of G. oxydans ZJB16009 under sorbitol fermentation has been shortened by 11 h. In addition, as shown in Fig. 6, at 9 h, the maximal activity of mSLDH per unit volume was achieved by the mutant strain G. oxydans ZJB16009, which was 1.5-fold higher than that of the original strain. Subsequently, the mSLDH activity was gradually reduced. The obvious reduction of mSLDH activity per unit volume was also observed in the original strain ZJB-605 though it occurred 1 h later, probably due to the later deprivation of the d-sorbitol. Dissolved oxygen in the fermentation process of G. oxydans may be another vital factor which is confirmed to regulates a genome-wide transcriptional response, and the mRNA level of mSLDH encoded by sldAB gene was only 10% compared with that under the O2-saturated condition (Hanke et al. 2012). Thus, DO control strategy during the fermentation process needs further exploitation to improve the production of high level of mSLDH activity, which has been confirmed in the improved production of DHA in our previous study (Hu and Zheng 2011).
Enhanced biotransformation of NHEG to 6NSL in mutant strain ZJB16009
The substrate concentration of NHEG used in mSLDH biotransformation are usually low because of their toxicity due to the structural similarities with D-glucose (Merfort et al. 2006). Key parameters involved in biotransformation system including catalyst loading, Mg2+ concentration and the pH value was preliminary optimized in shake-flask bioconversion system (data not shown). For the high yield of 6NSL to avoid its degradation (Landis et al. 2002), the temperature in the bioconversion system was maintained at 15 °C. To further assess the potential use of ZJB16009 in the industrial biosynthesis of 6NSL, scaled-up biotransformation system was performed in a pH-controlled conversion process in combination with a continuous high oxygen supply. As can be calculated from the time course of the overall biocatalytic process from NHEG to 6NSL (Fig. 7), the concentration of 6NSL was dramatically increased at the initial stage of the reaction and after 48 h of biotransformation, only 1.7 g/L NHEG was remained in the reaction system catalyzed by ZJB16009 compared with 18.5 g/L by the original strain ZJB-605. Correspondently, the concentration of the product 6NSL was approximately increased to 53.6 g/L after 36 h bioconversion, while there was only 40.1 g/L 6NSL accumulated catalyzed by the original strain, showing a 33.6% increase in the product yield. All above results indicated that the mutant strain ZJB16009 exhibited a significant increase in the catalytic activity towards substrate NHEG and resulted in an outstanding productivity that approximately up to 60 kg substrate/m3 can be oxidized quantitatively in less than 36 h with a biomass concentration of about 4 kg cell dry weight/m3, and the specific oxidation activity was around 2 mM of substrate NHEG oxidized per h per g DCW cell, leading to a obvious increase in ratio of substrate to catalyst (S/C-ratio, 15) and in the product yield (89.3%), compared with that of the original strain ZJB-605.
Conversion process of the substrate NHEG to product 6NSL using G. oxydans resting cell. The reactions were performed in reaction mixtures consisted of 40 g/L wet cell weight, 100 mM Tris–HCl (pH 5.0) and 60 g/L NHEG at 15 °C; concentrations of NHEG remained in the bioconversion system catalyzed by the original strain ZJB-605 (black filled square) and the mutant strain ZJB16009 (red filled square); concentrations of 6NSL accumulated in the original strain ZJB-605 (black filled circle) and the mutant strain ZJB 16009 (red filled circle)
Stability of the mutant strain ZJB16009
In the process of mutagenic breeding, back mutations often occurred in the positive mutants and led to a decline in the production performance. Therefore, the genetic stability of ZJB16009 was further investigated. As shown in Fig. 8, after serial passages of the strain ZJB16009 from the slant culture to shake-flask cultivation for 10 generations, the catalytic activity of mSLDH towards NHEG to 6NSL in ZJB16009 remained almost the same and the concentration of 6NSL accumulated in the reaction system reached to approximately to 55 g/L, all of which confirmed the stability of ZJB16009 in the catalytic activity of mSLDH. In addition, the genetic background of mSLDH in the mutant strain ZJB16009 was further investigated and the open reading frames of sldBA gene was cloned and sequenced, only a synonymous mutation within SldA region (G297A) was identified for the degenerate codon of glutamine. Other factors such as the promoter activation of the gene cluster of SldAB and its prosthetic group pqqABCDE (Prust et al. 2005), the amino acid mutation in the subunit III AdhS (Zhang et al. 2016) and the efficiency of electron transfer in the oxidative reaction (Gao et al. 2014), related to the mSLDH activity of G. oxydans, need further exploration to elucidate the genetic mechanism underlying the improved catalytic activity of mSLDH in the mutant strain ZJB16009. Therefore, sequencing and wide range of genetic mining of ZJB16009 are further considered.
Conclusion
In the present work, a high-throughput screening approach was established for the quick quantification of 6NSL. A positive mutant strain ZJB16009 was obtained with improved mSLDH activity by 1.5-fold and its outstanding performance in the biosynthesis of miglitol intermediate 6NSL was further confirmed in a 5-L biotransformation system that 60 g/L NHEG was catalyzed into 53.6 g/L 6NSL within 36 h, showing a 33.6% increase in the product yield. This research thus provides an easily operated and whole-cell system adaptable for the breeding process of G. oxydans strain in the biosynthesis of miglitol intermediate 6NSL.
Abbreviations
- mSLDH:
-
Membrane-bound d-sorbitol dehydrogenase
- 6NSL:
-
6-(N-Hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose
- NHEG:
-
N-2-Hydroxyethyl-glucamine
- pQQ:
-
Pyrroloquinoline quinone
- DNPHzine:
-
2,4-Dinitrophenylhydrazine
- DO:
-
Dissolved oxygen
- WCW:
-
Wet cell weight
- DCW:
-
Dry cell weight
- LC/MS:
-
Liquid chromatography–mass spectrometry
References
Bhave SL, Chattoo BB (2003) Expression of vitreoscilla hemoglobin improves growth and levels of extracellular enzyme in Yarrowia lipolytica. Biotechnol Bioeng 84(6):658–666. https://doi.org/10.1002/bit.10817
Deppenmeier U, Hoffmeister M, Prust C (2002) Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol 60(3):233–242. https://doi.org/10.1007/s00253-002-1114-5
Gao KL, Wei DZ (2006) Asymmetric oxidation by Gluconobacter oxydans. Appl Microbiol Biotechnol 70(2):135–139. https://doi.org/10.1007/s00253-005-0307-0
Gao L, Zhou J, Liu J, Du G, Chen J (2012) Draft genome sequence of Gluconobacter oxydans WSH-003, a strain that is extremely tolerant of saccharides and alditols. J Bacteriol 194(16):4455–4456. https://doi.org/10.1128/JB.00837-12
Gao LL, Hu YD, Liu J, Du GC, Zhou JW, Chen J (2014) Stepwise metabolic engineering of Gluconobacter oxydans WSH-003 for the direct production of 2-keto-L-gulonic acid from D-sorbitol. Metab Eng 24:30–37. https://doi.org/10.1016/j.ymben.2014.04.003
Gatgens C, Degner U, Bringer-Meyer S, Herrmann U (2007) Biotransformation of glycerol to dihydroxyacetone by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 76(3):553–559. https://doi.org/10.1007/s00253-007-1003-z
Hanke T, Richhardt J, Polen T, Sahm H, Bringer S, Bott M (2012) Influence of oxygen limitation, absence of the cytochrome bc(1) complex and low pH on global gene expression in Gluconobacter oxydans 621H using DNA microarray technology. J Biotechnol 157(3):359–372. https://doi.org/10.1016/j.jbiotec.2011.12.020
Hu ZC, Zheng YG (2011) Enhancement of 1,3-dihydroxyacetone production by a UV-induced mutant of Gluconobacter oxydans with DO control strategy. Appl Biochem Biotechnol 165(5–6):1152–1160. https://doi.org/10.1007/s12010-011-9332-x
Hu ZC, Zheng YG, Shen YC (2011) Use of glycerol for producing 1,3-dihydroxyacetone by Gluconobacter oxydans in an airlift bioreactor. Bioresour Technol 102(14):7177–7182. https://doi.org/10.1016/j.biortech.2011.04.078
Hu ZC, Tian SY, Ruan LJ, Zheng YG (2017) Repeated biotransformation of glycerol to 1,3-dihydroxyacetone by immobilized cells of Gluconobacter oxydans with glycerol- and urea-feeding strategy in a bubble column bioreactor. Bioresour Technol 233:144–149. https://doi.org/10.1016/j.biortech.2017.02.096
Kranz A, Vogel A, Degner U, Kiefler I, Bott M, Usadel B, Polen T (2017) High precision genome sequencing of engineered Gluconobacter oxydans 621H by combining long nanopore and short accurate Illumina reads. J Biotechnol 258:197–205. https://doi.org/10.1016/j.jbiotec.2017.04.016
Kulhánek M (1989) Microbial dehydrogenations of monosaccharides. Adv Appl Microbiol 34:141–182
Landis BHM, Heeren JK, And R, Wang PT (2002) Bioconversion of N-Butylglucamine to 6-deoxy-6-butylamino Sorbose by Gluconobacter oxydans. Org Process Res Dev 6(4):547–552
Lin X, Liu S, Xie G, Chen J, Li P, Chen J (2016) Enhancement of 1,3-dihydroxyacetone production from Gluconobacter oxydans by combined mutagenesis. J Microbiol Biotechnol 26(11):1908–1917. https://doi.org/10.4014/jmb.1604.04019
Liu ZQ, Zhang XH, Xue YP, Xu M, Zheng YG (2014) Improvement of Alcaligenes faecalis nitrilase by gene site saturation mutagenesis and its application in stereospecific biosynthesis of (R)-(−)-mandelic acid. J Agric Food Chem 62:4685–4694. https://doi.org/10.1021/jf405683f
Liu ZQ, Dong SC, Yin HH, Xue YP, Tang XL, Zhang XJ, He JY, Zheng YG (2017a) Enzymatic synthesis of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase in an aqueous-organic solvent system. Bioresour Technol 229:26–32. https://doi.org/10.1016/j.biortech.2016.12.098
Liu ZQ, Wu L, Zhang XJ, Xue YP, Zheng YG (2017b) Directed evolution of carbonyl reductase from Rhodosporidium toruloides and its application in stereoselective synthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate. J Agric Food Chem 65(18):3721–3729. https://doi.org/10.1021/acs.jafc.7b00866
Ma L, Lu W, Xia Z, Wen J (2010) Enhancement of dihydroxyacetone production by a mutant of Gluconobacter oxydans. Biochem Eng J 49(1):61–67. https://doi.org/10.1016/j.bej.2009.11.011
Merfort M, Herrmann U, Ha SW, Elfari M, Bringer-Meyer S, Gorisch H, Sahm H (2006) Modification of the membrane-bound glucose oxidation system in Gluconobacter oxydans significantly increases gluconate and 5-keto-D-gluconic acid accumulation. Biotechnol J 1(5):556–563. https://doi.org/10.1002/biot.200600032
Oscar LB, Sc. D, Gladys FIC VE (1926) The use of 2,4-dinitrophenylhydrazine as a reagent for aldehydes and ketones. Analyst 51(599):77–78
Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF, Ehrenreich A, Gottschalk G, Deppenmeier U (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23(2):195–200. https://doi.org/10.1038/nbt1062
Saito YIY, Hayashi H, Imao Y, Akashi T, Yoshikawa K, Noguchi Y, Soeda S, Yoshida M, Niwa M, Hosoda J, Shimomura K (1997) Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-Keto-L-Gulonate, a precursor of L-ascorbic acid, in a recombinant G-oxydans strain. Appl Environ Microbiol 63:454–460
Scaros RGBLPWMPM (1999) Process for microbially oxidizing N-substituted glucamines. US, US5916784
Schedel M (2008) Regioselective oxidation of aminosorbitol with Gluconobacter oxydans, key reaction in the industrial 1-deoxynojirimycin synthesis. Biotechno Biotransform II 8b:295–311
Takahashi ENK, Furui M, Mori T (1995) R-(–)-mandelic acid production from racemic mandelic acids by Pseudomonas polycolor with asymmetric degrading activity. J Ferment Bioeng 79(5):439–442
Xu S, Wang X, Du G, Zhou J, Chen J (2014) Enhanced production of L-sorbose from D-sorbitol by improving the mRNA abundance of sorbitol dehydrogenase in Gluconobacter oxydans WSH-003. Microb Cell Fact 13:146. https://doi.org/10.1186/s12934-014-0146-8
Xue YP, Wang W, Wang YJ, Liu ZQ, Zheng YG, Shen YC (2012) Isolation of enantioselective alpha-hydroxyacid dehydrogenases based on a high-throughput screening method. Bioprocess Biosyst Eng 35(9):1515–1522. https://doi.org/10.1007/s00449-012-0741-1
Yang XP, Wei LJ, Lin JP, Yin B, Wei DZ (2008) Membrane-bound pyrroloquinoline quinone-dependent dehydrogenase in Gluconobacter oxydans M5, responsible for production of 6-(2-hydroxyethyl) amino-6-deoxy-L-sorbose. Appl Environ Microbiol 74(16):5250–5253. https://doi.org/10.1128/AEM.00272-08
Yuan J, Wu M, Lin J, Yang L (2016) Combinatorial metabolic engineering of industrial Gluconobacter oxydans DSM2343 for boosting 5-keto-D-gluconic acid accumulation. BMC Biotechnol 16(1):42. https://doi.org/10.1186/s12896-016-0272-y
Zhang JB, Zhang XL, Wang DH, Zhao BX, He G (2011) Biocatalytic regioselective oxidation of N-hydroxyethyl glucamine for synthesis of miglitol. Adv Mater Res 197–198 (21):51–55
Zhang H, Shi LL, Lin JP, Sun M, Wei DZ (2016) Effective improvement of the activity of membrane-bound alcohol dehydrogenase by overexpression of adhS in Gluconobacter oxydans. Biotechnol Lett 38(7):1131–1138. https://doi.org/10.1007/s10529-016-2084-5
Zheng YG, Yin HH, Yu DF, Chen X, Tang XL, Zhang XJ, Xue YP, Wang YJ, Liu ZQ (2017) Recent advances in biotechnological applications of alcohol dehydrogenases. Appl Biochem Biotechnol 101:987–1001. https://doi.org/10.1007/s00253-016-8083-6
Zhou JY, Xu GC, Han RZ, Dong JJ, Zhang WG, Zhang RZ, Ni Y (2016) Carbonyl group-dependent high-throughput screening and enzymatic characterization of diaromatic ketone reductase. Catal Sci Technol 6(16):6320–6327. https://doi.org/10.1039/c6cy00922k
Acknowledgements
We would like to thank Xinchang Pharmaceutical Factory of Zhejiang Medicine Co., Ltd., for offering the substrate NHEG.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LY18B020019) and Zhejiang Provincial Public Technology Application Research Project (No. 2015C31049).
Author information
Authors and Affiliations
Contributions
XK designed the experiment and wrote the manuscript; NNW established the high-throughput screening method and obtained the mutant strain ZJB16009; PHY and YHL performed the fermentation and bioconversion experiment; ZCH revised the manuscript; YGZ was the group superior.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ke, X., Wang, NN., Yu, PH. et al. Biosynthesis of miglitol intermediate 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose by an improved d-sorbitol dehydrogenase from Gluconobacter oxydans. 3 Biotech 8, 231 (2018). https://doi.org/10.1007/s13205-018-1251-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1251-x