Abstract
Standardization of metagenomic DNA extraction protocol is a pre-requisite for a successful metagenomic study aiming to screen and exploit the variety of microorganisms inhabiting a particular soil environment. Six methods reported earlier were used for isolation of metagenomic DNA in the present study. These methods suffered with regard to either poor yield or quality of DNA. Therefore, we developed an improved method for isolation of high-molecular weight and good quality metagenomic DNA from different soil samples. Our protocol combines the enzymatic (lysozyme and proteinase K) and chemical (CTAB and CaCl2) strategies to ensure efficient cell lysis and use of PEG and isopropanol for precipitation of humic impurities-free DNA. Our improved method gave high yield of good quality metagenomic DNA from diverse soils collected from garden, domestic waste dumping site, cellulose waste dumping site, sewage site, and tannery waste site. The good quality of the metagenomic DNA was evident by spectrophotometry data, PCR amplification of 16S rRNA gene and restriction digestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms are the most ubiquitous and abundant in all natural habitats of the earth including soil ecosystems, which are inhabited by a large number of microbial communities (Singh et al. 2014). The conventional culture-dependent techniques of screening microbial diversity do not allow characterization of the whole microbial community of a given habitat as specific nutrients and environmental conditions are required to culture different microbial species (Fatima et al. 2014). Normally, around 1% of the total soil microbial species are culturable using conventional plate culture technique (Verma and Satyanarayana 2011).
Metagenomics, a culture-independent approach, enables the study of unculturable microbial species by analysis of the genomic elements without culturing the host organisms. Therefore, this approach offers an opportunity to assess the microbial diversity of an ecosystem at a larger scale (Bashir et al. 2014) and isolation of industrially important enzymes and other useful biochemicals from microbial sources (Wilson 2009). Availability of good quality high-molecular weight DNA is a pre-requisite for a meaningful metagenomic study aiming to characterize and harness the benefits of microbial community (Siddhapura et al. 2010; Verma and Satyanarayana 2011).
Soil being a heterogeneous mixture of many chemicals possesses high concentration of some DNA contaminants and PCR inhibitors that make metagenomic DNA unsuitable for downstream molecular biology experiments (Nair et al. 2014). Humic acids and other phenolic compounds that co-precipitate with metagenomic DNA adversely affect the quality of DNA (Amorim et al. 2008) and interfere indirectly with the activity of Taq DNA polymerase during PCR (Verma and Satyanarayana 2011). Even minute quantity of humic acids restricts restriction digestion, PCR amplification and transformation processes (Gabor et al. 2003; Verma and Satyanarayana 2011).
The present study was undertaken with the aim of standardizing a protocol for the extraction of good quality high-molecular weight metagenomic DNA from different soil environments. We initially evaluated six different methods reported earlier for isolation of metagenomic DNA from garden soil and subsequently developed an improved method. This improved method was also evaluated for isolation of metagenomic DNA from soils collected from various waste dumping sites. Our method gives higher yield of good quality high-molecular weight metagenomic DNA from different types of soil and is suitable for downstream processing for metagenomic study.
Materials and methods
Sample collection and storage
The soil samples were collected from 5 to 10 cm depth using a soil sampler from different sites, which included (i) garden of Guru Gobind Singh Indraprastha University, New Delhi, (ii) domestic waste dumping site, Dwarka, New Delhi, (iii) cellulose waste dumping site, Dwarka, New Delhi, (iv) sewage-contaminated site, Dwarka, New Delhi, and (v) tannery waste dumping site, Jajmau, Kanpur. All the soil samples were transferred to the laboratory at 4 °C. Soil samples were filtered through 0.2 mm sieve and stored at −20 °C till DNA isolation.
Extraction of metagenomic DNA
Six methods published earlier for isolation of metagenomic DNA were used for the isolation and purification of metagenomic DNA initially from garden soil sample. These methods were reported by Zhou et al. (1996), Volossiouk et al. (1995), Tsai and Olson (1991), Siddhapura et al. (2010), Verma and Satyanarayana (2011), and Singh et al. (2014).
Standardization of metagenomic DNA extraction protocol
5 g soil samples (triplicate) from each of the five soil types were mixed with 10 ml of extraction buffer [100 mM Tris/HCl (pH 8.0), 100 mM EDTA (pH 8.0), 100 mM sodium phosphate buffer (pH 8.0), 1.5 M NaCl, 1% (w/v) CTAB, 100 mM CaCl2, 10 mg proteinase K/ml and 10 mg lysozyme/ml] in oakridge tubes and incubated at 37 °C for 1 h in incubator shaker at 200 rpm. After adding 2 ml of 20% (w/v) SDS, the mixture was incubated in water bath at 65 °C for 2 h with invert mixing after every 10–15 min. The tubes were centrifuged at 7000g for 20 min at 4 °C to collect the supernatant. The soil pellets were further extracted twice by adding 4.5 ml of extraction buffer and 0.5 ml of 20% (w/v) SDS, followed by incubation at 65 °C for 15 min. The supernatants of three extractions were pooled and mixed with equal volume of chloroform/isoamylalcohol (24:1, v/v). The tubes were centrifuged again at 14,000g for 20 min at 4 °C to collect the upper aqueous phase. The crude DNA was precipitated by adding 0.1 volume 3 M sodium acetate along with 0.4 volume 30% (w/v) PEG-8000 (poly ethylene glycol) to the aqueous phase and incubated at −20 °C. After 2 h, the crude DNA was pelleted by centrifugation at 14,000g for 15 min at 4 °C, washed once with 70% (v/v) ethanol (room temperature) and air dried. The dried pellets were dissolved in 1 ml of 1× TE buffer and mixed with equal volume chloroform/isoamylalcohol (24:1, v/v). The aqueous phase was collected by centrifugation at 14,000g for 15 min at 4 °C and DNA was precipitated using 0.7 volume isopropanol followed by overnight incubation at −20 °C. The DNA was pelleted again by centrifugation at 14,000g for 15 min at 4 °C. The pellets were washed with 5 M NaCl, followed by 70% (v/v) ethanol and air dried. The dried pellet was dissolved in 200 μl 1× TE buffer.
Agarose gel electrophoresis
To assess and compare the quality of metagenomic DNA isolated using different methods, an equal volume (3 µl) of metagenomic DNA extracts was loaded on to 0.8% (w/v) agarose gel along with 2 µl of HindIII digested lambda DNA marker (Fermentas). After electrophoresis, gel was stained with ethidium bromide and visualized for the presence of DNA under UV using Alpha Innotech gel documentation system.
Comparison of yield and purity of metagenomic DNA
To assess the yield of metagenomic DNA isolated from garden soil using different methods and from various soil samples using our improved method, absorbance was measured at 230, 260, and 280 nm using eppendorf BioPhotometer plus. Quality of metagenomic DNA was estimated using absorbance ratio as A260 nm/230 nm (DNA/humic acid) and A260 nm/280 nm (DNA/protein).
Assessment of quality of metagenomic DNA isolated using improved method
PCR amplification of 16S rRNA gene
To validate the quality of metagenomic DNA isolated from different soil types following earlier methods and our improved method, PCR amplification of 16S rRNA gene was performed using universal forward primer B27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and reverse primer U1492R (5′-CGG TTA CCT TGT TAC GAC TT-3′). The PCR was carried out for all samples in a reaction volume of 30 µl containing 1× GoTaq buffer (Promega), 10 pmol each of forward and reverse primers, 0.05 U GoTaq DNA polymerase, 2.5 mM MgCl2 and 0.2 mM dNTPs mix along with 100 ng template metagenomic DNA. The PCR was carried out in Master cycler (Eppendorf) under optimum conditions: (1) initial denaturation at 95 °C for 5 min; (2) 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 30 s and extension at 72 °C for 1 min 30 s; (3) final extension at 72 °C for 10 min; and (4) hold at 4 °C.
Restriction digestion
Restriction digestion of metagenomic DNA isolated from different soils using different isolation methods was performed using Sau3AI (Bsp143I) (10 U/µl). 2 µg metagenomic DNA of each of the five soil samples was treated with 1 U of enzyme in 50 µl reaction volume and incubated at 37 °C for 10 min. The digestion reaction was terminated by placing the mixture at 80 °C for 20 min and the digested DNA was visualized on 1.2% (w/v) agarose gel with uncut metagenomic DNA as reference.
Results and discussion
Standardization of protocol for isolation of metagenomic DNA
An ideal metagenomic DNA isolation protocol should ensure unbiased cell lysis and extraction of DNA (Rajendhran and Gunasekaran 2008). Several methods reported for soil metagenomic DNA extraction have mainly focused on cell lysis and DNA purification steps (Verma and Satyanarayana 2011; Devi et al. 2015). Microbial cell lysis can be successfully achieved using mechanical/physical (freezing/thawing, crushing in liquid N2, ultrasonication or bead-beating), chemical (CTAB, SDS, EDTA or PVPP treatment), and enzymatic (lysozyme, proteinase K or achromopeptidase) methods, either using a single or combination of these methods (Urban and Adamczak 2008).
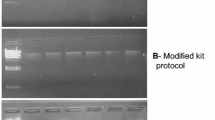
Initially, metagenomic DNA from garden soil was extracted using popular CTAB extraction buffer method developed by Zhou et al. (1996). They reported successful extraction of high-molecular weight metagenomic DNA from eight soil samples collected from different regions of Australia, Canada, Chile, Russia and USA. High-molecular weight metagenomic DNA was obtained from our garden soil samples using this method (Fig. 1a). However, the quality of DNA was not satisfactory for downstream processing and the DNA was also degraded. Some workers have modified this method to obtain pure high-molecular weight DNA (Sharma et al. 2007; Verma and Satyanarayana 2011; Singh et al. 2014). Therefore, we also evaluated two of these modified methods. Verma and Satyanarayana (2011) suggested the addition of PVPP (polyvinylpolypyrrolidone) and powdered activated charcoal (PAC) in CTAB extraction buffer followed by DNA precipitation using PEG-8000 and isopropanol. They extracted DNA from soil and sediment samples collected from Maharashtra, Rajasthan, Uttar Pradesh and Uttaranchal. Use of PAC and PVPP removes humic impurities significantly. PEG-8000 restricts co-precipitation of humic impurities with metagenomic DNA. High-molecular weight metagenomic DNA was obtained from our garden soil sample using this method (Fig. 1e); however, the quality of the DNA was not suitable for further processing. Another modified method was developed by Singh et al. (2014) for extraction of high-molecular weight, inhibitor-free metagenomic DNA from soil and sediment samples from Jammu and Kashmir. They reported removal of humic impurities using CTAB extraction buffer, DNA precipitation by PEG/NaCl followed by DNA purification using 2% (w/v) CaCl2 solution. CaCl2 prevents the oxidation of humic substances to quinones, which covalently bind with DNA and hinder the enzymatic processes like PCR amplification and restriction digestion. However, significantly poor amount of DNA was obtained at our hand after purification as no DNA band was observed on agarose gel (Fig. 1f).
Metagenomic DNA isolated from garden soil using different methods. M HindIII digested λ DNA marker, a lanes 1–3 Zhou et al. (1996) method, b lanes 4–6 Volossiouk et al. (1995) method, c lanes 7–9 Tsai and Olson (1991) method, d lanes 10–12 Siddhapura et al. (2010) method, e lanes 13–15 Verma and Satyanarayana (2011) method, f lanes 16–18 Singh et al. (2014) method, g lanes 19–21 improved method
In other attempts, physical cell lysis protocols involving crushing in liquid N2 (Volossiouk et al. 1995) and freeze–thaw process (Tsai and Olson 1991) were used to extract garden soil metagenomic DNA. Volossiouk et al. (1995) isolated metagenomic DNA from six farm soil types representing diverse regions of Canada. They facilitated microbial cell lysis by crushing soil sample in liquid N2 followed by SDS buffer-phenol extraction and ethanol precipitation. Highly degraded metagenomic DNA was obtained in our sample as shown in Fig. 1b possibly resulting due to the grinding of soil in liquid N2. Freeze–thaw method exploited by Tsai and Olson (1991) for the isolation of metagenomic DNA from soil sample of South California and pond sediment sample of Oak Ridge, Tennessee, USA, was also not found suitable considering the relatively poor DNA yield as evident by very faint band of metagenomic DNA (Fig. 1c).
Lastly, we evaluated indirect metagenomic DNA isolation (microbial cell separation from soil sample prior to cell lysis) protocol reported by Siddhapura et al. (2010) for saline soil of coastal Gujarat and Sambhar Lake, Rajasthan. In this method also, the yield of the garden soil DNA was poor (Fig. 1d). This method is comparatively more time consuming as microbial cells are first separated from soil samples after overnight incubation.
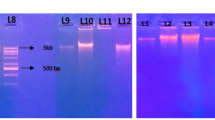
Although, metagenomic DNA was obtained from the garden soil samples using all the six methods used in the present study with varying yield, the quality of the DNA was not suitable for further processing. Therefore, there was a need for standardization of the protocol for isolation of metagenomic DNA from diverse types of soil keeping in mind advantages and limitations of the above-mentioned methods. Our improved method is based on the use of enzymatic (lysozyme and proteinase K) and chemical (CTAB and CaCl2) strategies for cell lysis to ensure the efficient cell disruption and recovery of metagenome representing diverse soil communities, followed by precipitation of humic impurities free metagenomic DNA using PEG and isopropanol. A secondary precipitation step using isopropanol is necessary to remove PEG completely as it is believed that PEG acts as interfering agent in PCRs (Verma and Satyanarayana 2011; Devi et al. 2015). We used calcium chloride as purifying agent in the extraction buffer before cell lysis, considering the possibility that calcium ions bind to functional groups (carboxylic and phenolic groups) of humic impurities, thereby inhibiting them to form quinones, which form covalent bonds with DNA. We also used 30% (w/v) PEG-8000 along with 3 M sodium acetate for precipitation of pure DNA as PEG has been used for precipitating soil metagenomic DNA without co-precipitating humic impurities. PEG works as crowding agent and increases the speed of DNA precipitation using some law of physics. Sodium acetate along with PEG improves the purity of DNA by providing monovalent cations. This improved method yielded high-molecular weight DNA in all the soil samples collected from different waste dumping sites as evident by sharp high intensity bands of DNA visualized on 0.8% (w/v) agarose gel as shown in Figs. 1g and 2.
Comparison of yield and quality of metagenomic DNA
The yield of the metagenomic DNA isolated from garden soil using different methods was further quantified by spectrophotometry and the quality was compared considering absorbance ratio A260/280 and A260/230 (Table 1). The yield of the garden soil metagenomic DNA isolated using Siddhapura et al. (2010) method was relatively poor and the quality was also not suitable for further processing. It is also reported that metagenomic DNA of only 25–30% of the bacteria can be extracted by indirect isolation because of strong adherence of bacteria with soil particles (Steffan et al. 1988; Verma and Satyanarayana 2011). In the present study, good yield of the garden soil metagenomic DNA was obtained using Zhou et al. (1996) and Verma and Satyanarayana (2011) methods, while very poor amount of DNA was obtained using Singh et al. (2014) method.
The protocols of Volossiuok et al. (1995) and Tsai and Olson (1991) methods are based on physical cell lysis by crushing soil in liquid N2 and repeated freeze–thaw cycles, respectively. The yield and quality of the garden soil DNA were not satisfactory in both the methods (Table 1). The improved method developed in the present study allowed the isolation of garden soil metagenomic DNA with comparatively higher yield of 15.55 ± 0.8 µg/g and quality of the DNA was also found best in this method as estimated on the basis of spectrophotometric data given in Table 1. As this improved method was found suitable for the isolation of good quality metagenomic DNA from garden soil, it was later evaluated for its suitability for isolation of metagenomic DNA from different waste site soil samples. The spectrophotometric data presented in Table 2 indicate that the improved method is suitable for the isolation of good quality metagenomic DNA from samples representing different soil environments.
PCR amplification of 16S rRNA gene
Humic impurities indirectly affect the DNA polymerase enzyme activity by chelating Mg2+ ions during PCR. Thus, to assess the efficacy of the improved method for the elimination of humic impurities, PCR amplification of 16S rRNA gene was performed with metagenomic DNA isolated from garden soil using different methods along with metagenomic DNA isolated from different soil samples using our improved method. No faithful amplification of target locus was observed in the case of garden soil metagenomic DNA isolated using different methods except our improved method (Fig. 3a). Amplification products of expected nearly 1.5 kb were obtained from metagenomic DNA of all the five different soil samples as visualized on 1.2% (w/v) agarose gel (Fig. 3b).
PCR amplification of 16S rRNA gene. a M 1 kb DNA ladder; PCR amplification products from garden soil metagenomic DNA isolated using different methods. Lane 1 Zhou et al. (1996) method, lane 2 Volossiouk et al. (1995) method, lane 3 Tsai and Olson (1991) method, lane 4 Siddhapura et al. (2010) method, lane 5 Verma and Satyanarayana (2011) method, lane 6 Singh et al. (2014) method, lane 7 improved method. b M 1 kb DNA ladder, lanes 1–5 PCR amplification product from metagenomic DNA isolated using improved method from garden soil, domestic waste site soil, cellulose waste site soil, sewage-contaminated soil and tannery waste soil, respectively
Restriction digestion of metagenomic DNA
Proper restriction digestion of DNA is an essential step towards construction of a metagenomic library. The presence of humic impurities may hinder this restriction process. Thus, restriction digestion of the garden soil metagenomic DNA isolated using different methods along with the metagenomic DNA isolated from all different soil samples using our improved method was performed using Sau3AI (Bsp143I) enzyme to further assess the suitability of the improved method. Desired partial digestion was observed only for garden soil metagenomic DNA isolated using our improved method (Fig. 4a). Further, the desired partial digestion of metagenomic DNA isolated from different soils using our improved method was evident by the uniform smear seen on the agarose gels indicating elimination of humic impurities from the metagenomic DNA (Fig. 4b).
Restriction digestion of metagenomic DNA. a M 1 kb DNA ladder, undigested and restriction digested garden soil metagenomic DNA isolated using Zhou et al. (1996) [lanes 1, 1′], Volossiouk et al. (1995) [lanes 2, 2′], Tsai and Olson (1991) [lanes 3, 3′], Siddhapura et al. (2010) [lanes 4, 4′], Verma and Satyanarayana (2011) [lanes 5, 5′] and improved method [lanes 6, 6′], respectively. b M 1 kb DNA ladder, undigested and restriction digested metagenomic DNA isolated using improved method from garden soil (lanes 1, 1′), domestic waste site soil (lanes 2, 2′), cellulose waste site soil (lanes 3, 3′), sewage-contaminated soil (lanes 4, 4′) and tannery waste site soil (lanes 5, 5′), respectively
Conclusions
In conclusion, all the six methods used for their suitability for extraction of metagenomic DNA from garden soil yielded metagenomic DNA albeit with varying quantity; however, the quality of the DNA was not found suitable for the downstream processing like PCR amplification and restriction digestion required for the preparation of metagenomic library due to the presence of humic acid and protein impurities. Our improved method of metagenomic DNA isolation ensured higher yield of good quality contamination-free DNA in comparison to other methods evaluated in this study. Addition of CaCl2 in the extraction buffer in our improved method helped in the elimination of humic impurities prior to cell lysis by binding of calcium ions with humic impurities. Next, DNA precipitation using 30% (w/v) PEG-8000 helped in the elimination of remaining humic impurities by inhibiting their co-precipitation with metagenomic DNA. This improved method is rapid, cost-effective and applicable for samples collected from diverse soil environments.
References
Amorim JH, Macena TNS, Lacerda-Junior GV, Rezende RP, Dias JCT, Brendel M, Cascardo JCM (2008) An improved extraction protocol for metagenomic DNA from a soil of the Brazilian Atlantic rainforest. Genet Mol Res 7:1226–1232
Bashir Y, Singh SP, Konwar BK (2014) Metagenomics: an application based perspective. Chin J Biol 2014:1–7
Devi SG, Fatima AA, Radha S, Arunraj R, Curtis WR, Ramya M (2015) A rapid and economical method for efficient DNA extraction from diverse soils suitable for metagenomic applications. PLoS ONE 10:e0132442
Fatima F, Pathak N, Rastogi VS (2014) An improved method for soil DNA extraction to study the microbial assortment within rhizospheric region. Mol Biol Int 2014:1–6
Gabor EM, de Vries EJ, Janssen DB (2003) Efficient recovery of environmental DNA for expression cloning by indirect extraction methods. FEMS Microbiol Ecol 44:153–163
Nair HP, Vincent H, Bhat SG (2014) Evaluation of five in situ lysis protocols for PCR amenable metagenomic DNA from mangrove soils. Biotechnol Rep 4:134–138
Rajendhran J, Gunasekaran P (2008) Strategies for accessing soil metagenome for desired applications. Biotechnol Adv 26:576–590
Sharma PK, Caplash N, Kaur J (2007) An improved method for single step purification of metagenomic DNA. Mol Biotechnol 36:61–63
Siddhapura PK, Vanparia S, Purohit MK, Singh SP (2010) Comparative studies on the extraction of metagenomic DNA from the saline habitats of coastal Gujarat and Sambhar Lake, Rajasthan (India) in prospect of molecular diversity and search for novel biocatalysts. Int J Biol Macromol 47:375–379
Singh R, Devi T, Verma V, Rasool S (2014) Comparative studies on the extraction of metagenomic DNA from various soil and sediment samples of Jammu and Kashmir region in prospect for novel biocatalysts. IOSR J Environ Sci Toxicol Food Technol 8:46–56
Steffan RJ, Goksoyr J, Bej AK, Atlas R (1988) Recovery of DNA from soils and sediments. Appl Environ Microbiol 54:2908–2915
Tsai YL, Olson BH (1991) Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol 57:1070–1074
Urban M, Adamczak M (2008) Exploration of metagenomes for new enzymes useful in food biotechnology—a review. Polish J Food Nutr Sci 58:11–22
Verma D, Satyanarayana T (2011) An improved protocol for DNA extraction from alkaline soil and sediment samples for constructing metagenomic libraries. Appl Biochem Biotechnol 165:454–464
Volossiouk T, Robb EJ, Nazar RN (1995) Direct DNA extraction for PCR-mediated assays of soil organisms. Appl Environ Microbiol 61:3972–3976
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20:295–299
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322
Acknowledgements
SKV thanks the University Grants Commission, Government of India for the award of a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Verma, S.K., Singh, H. & Sharma, P.C. An improved method suitable for isolation of high-quality metagenomic DNA from diverse soils. 3 Biotech 7, 171 (2017). https://doi.org/10.1007/s13205-017-0847-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0847-x