Abstract
Intravascular thrombosis is one of the major causes of variety of cardiovascular disorders leading to high mortality worldwide. Fibrinolytic enzymes from microbial sources possess ability to dissolve these clots and help to circumvent these problems in more efficient and safer way. In the present study, fibrinolytic protease with higher fibrinolytic activity than plasmin was obtained from Serratia sp. KG-2-1 isolated from garbage dump soil. Response surface methodology was used to study the interactive effect of concentration of maltose, yeast extract + peptone (1:1), incubation time, and pH on enzyme production and biomass. Maximum enzyme production was achieved at 33 °C after 24 h at neutral pH in media containing 1.5% Maltose, 4.0% yeast extract + peptone and other trace elements resulting in 1.82 folds increased production. The enzyme was purified from crude extract using ammonium sulfate precipitation and DEAE-Sephadex chromatography resulting in 12.9 fold purification with 14.9% yield. The purified enzyme belongs to metalloprotease class and had optimal activity in conditions similar to physiological environment with temperature optima of 40 °C and pH optima of 8. The enzyme was found to be stable in various solvents and its activity was enhanced in presence of Na+, K+, Ba2+, Cu2+, Mn2+, Hg2+ but inhibited by Ca2+ and Fe3+. Hence, the obtained enzyme may be used as potential therapeutic agent in combating various thrombolytic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases such as myocardial infarction, arrhythmias and stroke are the leading cause of morbidity and mortality worldwide with 17 million deaths every year. Intravascular thrombosis is one of the major etiological conditions for such disorders. Traditionally, the treatment of thrombosis was relied, either on the use of antiplatelet and anticoagulating agents such as heparin and warfarin or on surgical treatments. But with the advent of our knowledge about the etiology of diseases or their mechanism at molecular level, the roles of enzymes in body became more clear. Therefore, enzymes have turned from an object of fundamental studies to an important medicinal/industrial tool. Fibrinolytic enzymes are potential candidates in the treatment of many cardiovascular disorders as thrombolytic agent. A variety of fibrinolytic enzymes are obtained from various sources viz. plants (Chung et al. 2010); animals (Huang et al. 2012); and microorganisms (Ju et al. 2012).
Among the clinically used fibrinolytic enzymes tissue plasminogen activator (t-PA), urokinase and bacterial streptokinase are widely used but suffers from various limitations such as short half-life, low specificity, re-occlusion, immunogenicity and high cost. Hence many investigations are being trailed to enhance the efficiency, economy and specificity of fibrinolytic therapy. Among various sources, the microbial fibrinolytic enzymes have attracted a great deal of medical interest due to their wide diversity, rapid growth, susceptibility to genetic manipulation as well as ease and economy of production (Peng et al. 2005; Bajaj and Sharma 2011). The enormous microbial diversity augments the probability of obtaining the enzyme with better therapeutic potential and desirable characteristics. Fibrinolytic microbial strains mainly belong to genus Bacillus (Sumi et al. 1987), while other strains belonging to genera like Staphylococcus, Pseudomonas, Fusarium, Rhizomucor and Streptomyces have also been reported (Choi et al. 2009; Simkhada et al. 2010).
Serralysin or Serrapeptidase is a metalloprotease isolated originally from the intestine of silkworm Bombyx mori (Bhargavi and Prakasham 2012). Later homologue of this enzyme was also reported from some Gram negative bacteria such as Pseudomonas aeruginosa, Serratia marcescens, Proteus mirabilis, Erwinia chrysanthemi (Bhargavi and Prakasham 2012; Wu et al. 2016) and a Gram Positive bacterium Deinococcus radiodurans (Basu and Apte 2008). This peptidase has fibrinolytic as well as analgesic and anti-inflammatory property with low antigenicity hence is an excellent candidate for treating cardiovascular diseases (Bhargavi and Prakasham 2013).
As cost is one of the important factors determining the use of enzymes in therapeutics, optimization of media for maximum production is considered important to reduce cost. Components of media such as carbon, nitrogen sources and fermentation conditions such as temperature, pH and incubation time significantly affect the physiology, production pattern and yield of the metabolites and thus needs to be optimized. Despite the availability of several media, production cost is still a major hindrance for the wide range application of enzyme at industrial scale. Consequently search for new optimized media to enhance yield which in turn may add economy to the process is an ongoing vogue. The use of statistical models helps in overcoming the limitations of traditionally used method of one-factor-at-a-time being more economical, time saving, less laborious and most importantly more reliable as they provide view of interactive effect of different parameters (Singh and Bajaj 2015).
The present study was focused on the search of potential fibrinolytic enzyme producing bacteria and in this voyage a potent producer identified as Serratia sp. was isolated from garbage dump soil sample. Optimization of media components using response surface methodology was studied and purification was carried out which is an ongoing process to improve their process applications (Bizuye et al. 2013). The purified enzyme was further characterized to study the effect of temperature, pH, metal ions, inhibitors, surfactants and solvents on enzyme activity and stability.
Methods
Media and chemicals
Fibrinogen (bovine) was procured from Alfa Aesar, India. Plasmin was obtained from Calbiochem, Germany. Agarose was procured from Sigma-Aldrich, India. Thrombin, DEAE-Sephadex, phenyl methyl sulfonyl fluoride, Iodoacetic acid, 1,10-phenanthroline, nutrient broth and agar powder were purchased from Hi-Media, India. All other chemicals were of analytical grade and were purchased from SD Fine chemicals Ltd., India and SRL, India.
Screening of fibrinolytic enzyme producing isolates
The isolate KG-2-1 showing high fibrinolytic activity was obtained from garbage dump soil sample collected from Hisar, Haryana (India). The soil sample was enriched in nutrient broth, serially diluted and plated on screening medium. The screening was primarily done on skim milk agar medium and the positive isolates forming clear zones around their colonies were further screened on fibrin plate formed by method as described by Astrup and Mullertz (1952), with some modifications. The fibrin plate was prepared by adding 10 mL of fibrinogen (0.6% w/v in 0.05 M Tris–HCl buffer pH 7.4) to 2% agarose solution containing 700 μL of thrombin (10 U in 0.05 M Tris–HCl buffer pH 7.4). The plate was allowed to stand for 30 min at room temperature to form fibrin clot and 6 mm wells were formed with the help of sterile core borer. The wells were then filled with crude enzyme extract obtained as supernatant by centrifuging 48 h culture in nutrient broth at 10,000 rpm for 15 min (Remi C-30 plus Centrifuge). The plates were studied after 16–18 h incubation. The isolate producing fibrinolytic enzyme degrade fibrin and form clear zone around wells. The isolate KG-2-1 showed high fibrinolytic activity and was selected for further studies.
Identification
The identification of strain KG-2-1 was carried out on the basis of phenotypic characterization and 16S rDNA sequencing as described by Mahajan et al. (2012). Analysis of the 16S rRNA gene sequence was determined by BLAST using the 16S rRNA Bacteria and Archaea dataset and phylogenetic tree was constructed using maximum likelihood method as implemented in Mega 6 software (Tamura et al. 2013).
Protease activity assay
The protease activity was assayed in accordance with the method as described by Kim et al. (2006), with slight modifications. Two mL of 1% w/v Casein solution was added to 0.5 mL of crude enzyme extract and was allowed to incubate at 37 °C for 20 min in waterbath. The reaction was terminated by adding 2.5 mL of Trichloro acetic acid (0.2 M) and incubating it at 70 °C for another 20 min. The mixture was then centrifuged at 10,000 rpm for 18 min and supernatant was analyzed for tyrosine content by observing its absorbance at 280 nm in UV–Vis spectrophotometer (BMG Labtech Spectro Star Nano, Germany) using Tris–HCl Buffer as blank and the suitable control. The standard curve was plotted using known concentrations of tyrosine solutions. The 1U of protease activity is equal to the amount of enzyme required to liberate 1 μmol of tyrosine from casein per min per mL under given assay conditions.
Preliminary screening of fermentation conditions and media components
Preliminary screening of fermentation conditions and media composition was carried out by one-factor-at-a-time approach. The Fibrinolytic protease enzyme production was carried out in protease production medium containing (% w/v): Dextrose 1.0, Peptone 0.5, Yeast Extract 0.5, K2HPO4 0.1, KH2PO4 0.1, MgSO4·7H2O 0.02, pH 7.2. Two % of freshly grown culture (16–18 h) with Absorbance 600 0.932 was used to seed the production media. The impact of fermentation conditions on enzyme production were studied by subjecting the culture to different incubation time (i.e. 24, 48, 72, and 96 h), temperature (i.e. 25, 30, 33, 37, and 40 °C), pH (i.e. 4, 7, 8, and 10), and agitation (i.e. 50, 100, 150, 200 rpm). Also the medium components were optimized by studying the effect of different carbon sources (1% w/w i.e. dextrose, starch, sucrose, fructose, and maltose), nitrogen source (1% w/w i.e. peptone, beef extract, yeast extract, urea and 1:1 yeast extract + peptone) and Inorganic salts (0.1% w/w K2HPO4, KH2PO4, Na2HPO4, NaH2PO4, MgSO4) on fibrinolytic protease production.
Optimization by response surface methodology
The optimum level and multiple regression analysis of different variables affecting the fibrinolytic protease production was determined by systemic design of different experiments using central composite design (Design Expert Software Version 7.0.0, Stat-Ease Inc., Minneapolis, MN, USA). Based on the preliminary studies minimum and maximum level of independent variables i.e., maltose (%), yeast extract + peptone (1:1) (%), incubation time (h) and pH were selected while all other variables were kept constant. Each factor was studied at 3 levels (−1, 0, +1). The total number of experimental runs are 2k + 2k + n 0 where, k = number of independent variables and n 0 = number of repetitions of experiments at center point. Thus, a total of 30 runs with 8 axial, 16 factorial and 6 central point are carried out (Table 1). Protease activity (R 1) and biomass (R 2) were taken as response variables. Protease activity was determined by protease activity assay and biomass was determined by centrifuging the 2 mL culture at 10,000 rpm for 15 min. The pellet thus obtained was washed with water and again centrifuged. Finally the pellet was separated and dissolved in 3 mL of distilled water and absorbance was measured at 600 nm in UV–Visible spectrophotometer. The statistical analysis of data was done and 3D plots of suggested models signifying the relationship between response variable and independent variables were plotted. The validation of model for maximum protease activity was performed on the basis of optimized levels of variable as given by point prediction method in CCD.
Purification of fibrinolytic enzyme
The enzyme was purified by ammonium sulfate precipitation method followed by dialysis and column chromatography. Five hundred mL of optimally grown culture was centrifuged at 10,000 rpm for 15 min at 4 °C and supernatant was collected. The enzyme was precipitated by slowly adding increasing amount of solid (NH4)2SO4 till 75% saturation with gentle stirring to avoid frothing. Pellet was recovered by centrifugation at 10,000 rpm for 30 min at 4 °C, dissolved in minimum amount of buffer and dialyzed against 0.05 M Tris–HCl buffer (pH 7.4) overnight to remove excessive ammonium sulfate. The dialyzed enzyme was then subjected to DEAE- Sephadex column (25 × 2 cm) pre-equilibrated with 0.05 M Tris–HCl buffer (pH 7.4). The elution of the adsorbed protein was carried out with 0.2–0.8 M NaCl in the same buffer at a flow rate of 30 mL/h. The active fractions were pooled, concentrated and subjected to SDS-PAGE for analyzing its purity and Molecular weight. The protein content (by Bradford method) (Bradford 1976) and enzyme activity was determined at each step. SDS-PAGE was performed using 7% stacking gel and 10% resolving gel by the method as described by Laemmli (1970) with some modifications. The gel was then stained with Coomassie Brilliant Blue for 1 h and destained till all the background stain was removed. The broad range molecular weight marker (Promega) was run simultaneously with the sample for the determination of molecular weight of the purified enzyme.
Characterization of purified fibrinolytic enzyme
Effect of temperature and pH on enzyme activity and stability
The effect of temperature on activity of the purified enzyme was studied by carrying out the protease activity assay at different temperature ranging from 10 to 90 °C. Similarly the effect of pH was studied by carrying out the activity assay in buffers of different pH (3–12) i.e., Acetate buffer (pH 3–6), Tris HCl buffer (pH 7–8), Glycine buffer (pH 9–10) and Disodium phosphate buffer (pH 11–12). The % relative activity was calculated considering maximum activity as 100%. While to study the effect of temperature and pH on stability of enzyme, the purified enzyme was incubated at different temperatures (10–90 °C) and pH (buffer of pH 3–12) for 30 min. After incubation the enzyme % residual activity was calculated.
Effect of metal ions on enzyme activity
The effect of different metal ions viz. Na+, Ca2+, K+, Mg2+, Al2+, Ba2+, Fe3+, Cu2+, Zn2+, Co2+, Mn2+ and Hg2+ on enzyme activity was studied by adding either of the metal salts in enzyme assay mixture at final concentration of 2 mM while the mixture including neither of them was used as control. The % relative activity was calculated against control.
Effect of inhibitors and solvents on enzyme activity
The effect of different inhibitors such as phenyl methyl sulfonyl fluoride (PMSF), Ethylene diamine tetra acetic acid (EDTA), Iodoacetic acid and 1,10-phenanthroline was studied by incubating the enzyme with these inhibitors for 30 min at 40 °C. The final concentration of inhibitors used was 2 mM and activities at the end of incubation period were determined. Similarly the residual activities after incubation with different solvents such as ethanol, methanol, isopropanol, n-butanol, acetone, tween-20, β-mercaptoethanol, acetic acid, dimethyl sulfoxide (DMSO), glycerol, gluteraldehyde, acetaldehyde, sodium dodecyl sulphate (SDS) and cetrimonium bromide (CTAB) were measured. The % relative activities with respect to control which exclude all the additives were calculated.
Results and discussion
Isolation, screening and identification of fibrinolytic enzyme producing strain
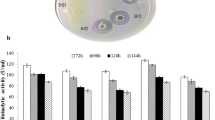
To isolate a potent fibrinolytic enzyme producer strain, different samples were collected and screened on skim milk agar and fibrin plate. Out of the total isolates obtained, 76 isolates showed proteolytic activity and 37 strains among these displayed fibrinolytic activity also. The strain KG-2-1 isolated from garbage dump soil sample showed significantly high fibrinolytic activity (Fig. 1a) and was selected as potent strain for further studies. Upon phenotypic characterization it was found to be Gram–ve thin short rods with smooth, round, red coloured, translucent, flat colonies with entire margin. The strain was positive for catalase, caseinase, DNAase production, citrate utilization and Methyl Red (MR)-Voges-Proskauer (VP) test, while it showed negative results for amylase, urease and indole production. In addition, it was able to ferment xylose, sucrose, sorbitol, cellobiose, raffinose, inositol and salicin, etc. 16S rRNA gene sequencing analysis using BLAST against the Bacteria and Archaea 16S rRNA dataset showed that strain KG-2-1 was closely related to members of Serratia species (similarity value of 98%) with the closest relatives being members of S. marcescens (similarity value of 99%). A phylogenetic tree representing strain KG-2-1 is shown in Fig. 1b. Strain KG-2-1 has been deposited in the Microbial Culture Collection, Pune, India, under accession number MCC 3079 and the 16S rRNA gene has been deposited in GenBank under accession number KX785163. Microbial strains isolated from natural sources have been explored for numerous biotechnological and pharmaceutically important compounds with better therapeutic potential and desirable properties. In the present work, highly active fibrinolytic enzyme producing bacterial strain Serratia sp. KG-2-1 was isolated from natural habitat viz. garbage dump soil sample. Various other fibrinolytic enzyme producing microorganisms including Bacillus subtilis A26 (Agrebi et al. 2009), Pseudomonas sp. TKU015 (Wang et al. 2009), Bacillus cereus NK1 (Venkataraman et al. 2010), Serratia sp. RSPB11 (Bhargavi and Prakasham 2013), B. subtilis ZA400 (Ahn et al. 2015), Bacillus sp. IND7 (Ponnuswamy et al. 2016) have also been isolated from wide diversity of natural habitat viz. soil and marine habitat.
Preliminary fermentation studies for Serratia sp. KG-2-1
Media components and fermentation conditions are the important factors affecting bioprocess and economy of the enzyme production. Preliminary optimization studies were carried out using one-factor-at-a-time approach. Different organisms have different physiological requirements that could influence the bioprocessing. To determine the optimum physiological conditions strain was subjected to different temperature, pH, and agitation conditions.
Effect of fermentation conditions on enzyme production
Enzymes are secreted during late exponential or stationary phase of bacterial growth cycle. Hence the monitoring of culture for maximum production with time is vital. The time profile analysis in this study showed that enzyme production started before 24 h, reached maximum at 48 h, continued till 72 h and decreased thereafter (Fig. 2a). Similar observations were reported in previous studies on Serratia sp. like optimum duration for significant enzyme production in Serratia sp. varied from 24 to 48 h (Bhargavi and Prakasham 2012). Temperature is an important factor influencing the secretion of enzyme extracellularly as it alters the physical state of cell membrane affecting substrate uptake and its utilization. Temperature analysis study showed significant enzyme production in the range of 25–37 °C with maximum production at 33 °C (237.5 U/mL) and very little activity of 19.8 U/mL at 40 °C (Fig. 2a). This is in accordance with the previous studies on Serratia sp. like the optimum temperature for Serratia sp. RSPB11 is 32 °C, while that of Serratia sp. DT3 is 28 °C, and of S. marcescens 7 is 40 °C (Bhargavi and Prakasham 2012). The optimum temperature ranged from 30 to 55 °C and even up to 85 °C have also been reported in other strains (Kim and Choi 2000; Choi et al. 2009; Lee et al. 2010; Kim et al. 2011; Vijayaraghavan et al. 2016). In addition, the effect of pH indicated that maximum enzyme production occurred at neutral to slightly alkaline pH in the range of pH 7–8 and is sharply decreased on either side (Fig. 2a). pH dependence production was observed in various strains with optimum pH varying from 5 to 10 (Kim and Choi 2000; Kim et al. 2011). In addition to this, agitation rate which indirectly indicates the dissolved oxygen level, extent of content mixing and thus nutrient availability in the fermentation broth also affects the cell growth and enzyme production. The study on effect of agitation rate on the enzyme production showed that the maximum enzyme production occurred at 150 rpm (243.2 U/mL) (Fig. 2a).
Effect of media components on enzyme production
Among the different carbon sources used in the study maltose resulted in maximum enzyme production i.e., 201.2 U/mL followed by sucrose, starch, fructose and dextrose (Fig. 2b). Nitrogen source besides being served as nitrogen precursors for cellular components also alters the pH and is therefore among the most significant factor influencing the production and stability of enzymes (Bajaj et al. 2014). Amongst all nitrogen sources used, yeast extract, peptone, and beef extract supported more or less equal enzyme production although it was less than initially used production media. In addition, urea did not support high enzyme production. Therefore, 1:1 ratio of yeast extract and peptone was used for further studies (Fig. 2c). Fibrinolytic protease producing bacterial strains successfully utilized different low-cost complex nitrogen sources, such as soybean meal, peptone, gelatin, beef extract and malt extract producing significant titers of fibrinolytic protease (Venkataraman et al. 2010; Bajaj et al. 2014). The inorganic salts also influence the enzyme production at low concentration. The salts used in the study influenced the enzyme production in the order KH2PO4 > K2HPO4 > NaH2PO4 > MgSO4 > Na2HPO4 with activity 202.7, 189.8, 182.8, 169.2 and 163.9 U/mL, respectively (Fig. 2d). The effect of changing the concentration of KH2PO4 from 0.1 to 0.4% did not significantly affect the production, therefore 0.2% is selected for further optimization studies. This might be due to the fact the fibrinolytic protease under study belongs to metalloprotease category which although requires metal ions for their activity but not their production. However, the production of serine protease is dependent upon the concentration of metal ions due to feedback inhibition during translocation across the plasma membrane (Mahajan et al. 2012).
Optimization by response surface methodology
Response surface methodology is a mathematical and statistical technique for testing multiple process variables by empirical model building and is advantageous being less laborious, efficient, less time consuming and more informative about the interactive effects. Medium optimization by statistical analysis has been successfully and efficiently applied for the production of numerous compounds and fermentation process (Venkataraman et al. 2010). As the production of enzymes significantly depends upon the media components and their interactive effect, therefore central composite design was employed to determine their optimum level aiming for maximum enzyme production. 30 experimental runs were carried out to study protease activity as well as biomass and results were subjected to various polynomial models (Table 1). It was found that both the responses i.e., Protease activity (R 1) and biomass (R 2) were fitted well in quadratic model without any transformation. The regression equation for quadratic models showing the relationship between the independent variables and the responses R 1 and R 2 can be expressed as
where A, B, C, D represents maltose, yeast extract + peptone (1:1), incubation time and pH, respectively. The data confirms significant factors affecting the protease activity (R 1) were the synergistic effect of linear contribution of A and B, interactive contribution of AB, BC and CD, and quadratic contribution of C 2 and D 2 as indicated by positive sign. And antagonistic effect of linear contribution of C and D, interactive contribution of AC, AD and BD, and quadratic contribution of A 2 and B 2 as indicated by the negative signs. While that for biomass (R 2) it is synergistic effect of linear contribution of A, B and C, interactive contribution of AD, BD and CD, quadratic contribution of D 2 and antagonistic effect of linear contribution of D and interactive contribution of AB, AC and BC, and quadratic contribution of A 2, B 2 and C 2.
Table 2 summarizes the various factor effects and their associated p values. It can be observed that maltose, YE + peptone, incubation time and pH significantly affected the enzyme production.
The polynomial models were further subjected to ANOVA analysis to check the adequacy of suggested models (Table 3). The F value and p value for both responses indicated that quadratic model was the best fitted model with adequate significance and non significant lack of fit. The coefficient of determination (R 2) was 0.92 and 0.74 for protease activity and biomass, respectively, indicating a better correlation between experimental and predicted responses. The value of Adequate Precision (measure of signal to noise ratio) was 14.02 and 5.56 for R 1 and R 2, respectively, indicating the adequate signal (>4) and therefore model can be used to navigate the design space.
On the basis of model equations 3-D response surface graphs were plotted (Fig. 3) to study the interactive effect of different parameters. Figure 3a shows that as the concentration of maltose increased protease activity also increased while at higher concentration it becomes constant. Similar effect was seen with yeast extract + peptone (1:1), but the increase was more prominent at lower concentration of maltose. In addition to this, the maximum enzyme production was observed around neutral to slightly alkaline pH, therefore, activity decreased with increase in pH (Fig. 3b). Similar observation was made by Kim et al. (2006) in B. subtilis TP-6 and Bhargavi and Prakasham (2012) for Serratia sp. RSPB11 where maximum enzyme production was achieved at pH 7. The results obtained in Fig. 3c shows that maximum enzyme production occurred up to 24 h and decreases thereafter. This decrease in activity was due to exhaustion of nutrients with time, production of inhibitory products and inadequate mixing. So, by increasing the concentration of yeast extract + peptone (1:1) during later phases i.e., after 60 h, a comparative increase in protease activity was observed. The perturbation graph showed that the variable A, B, C, D has significant effect on protease activity (Fig. 4a).
Figure 3d shows the interactive effect of yeast extract + peptone (1:1) and maltose on biomass, maintaining other factors at zero level. It can be inferred that at lower concentration of maltose, biomass increased rapidly as the concentration of B increased and become almost constant at its higher concentration. However, the change in biomass with maltose concentration is not much prominent. Similarly observations of increasing effect of B concentration were made in case of interactive effect studies with pH. Biomass increases with increase in concentration of B with comparatively more effect at neutral pH then alkaline pH (Fig. 3e). The interactive effect of time and concentration of yeast extract + peptone (1:1) on biomass gave a high curvature graph showing initial increase in biomass at lower concentration of B with time reached maximum in middle, become constant and then decreased. At higher concentration of B, biomass increased up to 60 h and then decreased rapidly (Fig. 3f). In addition, the perturbation graph showed that all the variable A, B, C, D had significant effect on biomass production but the effect of C was most prominent among all (Fig. 4b). Therefore, optimization using statistical techniques resulted in 1.82 folds increase in protease production as compared to unoptimized medium.
Validation of statistical model showed that experimental values were very close to the predicted values. Validation studies were carried out by using 1.5% maltose, 4.0% yeast extract + peptone (1:1), 24 h incubation time and neutral pH. The experimental response of 250.41 U/mL and 6.58 (Absorbance600) was close to the predicted response of 243.75 U/mL and 6.51 (Absorbance600) of protease activity and biomass, respectively, verifying the validity of the model.
Purification of fibrinolytic protease
The enzyme was precipitated from crude extract using ammonium sulfate (75% saturation) and dialyzed, resulting in 9.1 and 10.9 fold purification and 20.0 and 16.8% yield, respectively. The sample was then subjected to DEAE-Sephadex column and eluted with NaCl. The fractions with 0.5 M NaCl elution were found to be active which were then pooled and concentrated resulting in 12.9 fold purification with 14.9% product recovery. The results of the each step of purification are summarized in Table 4. The purified enzyme when subjected to SDS-PAGE, gave a single band with molecular weight of approximately 52 kDa (Fig. 5) which was similar to the other reported literature on Serratia sp. In the previous studies also fibrinolytic enzymes from different microorganisms were purified by protein precipitation by ammonium sulfate/ethanol followed by dialysis and chromatography resulting in purified enzymes with different yield of 6.5–95.0% and 4.9–56.0 fold purification (Mahajan et al. 2012). The purification of protease enzyme from S. marcescens TW1 by ammonium sulfate and acetone precipitation followed by dialysis and DEAE cellulose chromatography resulted in purified enzyme with molecular weight 50 kDa and 8.5 fold purification with 50% recovery yield (Tariq and Reyaz 2012). Similarly, fibrinolytic protease from Bacillus amyloliquefaciens CH86-1 purified by ammonium sulfate precipitation and CM-Sephadex and Phenyl Sepharose column chromatography yielded enzyme with 100.2 U/mg specific activity with 1.22% recovery and purification folds of 25.96 times (Lee et al. 2010). A metalloprotease (50 kDa) from S. marcescens RSPB11 was purified by 80% ammonium sulfate salting out, gel filtration and Ion exchange chromatography yields enzyme with 15 fold purification and 17% yield (Bhargavi and Prakasham 2013).
Characterization of purified fibrinolytic enzyme
Effect of temperature and pH on enzyme activity and stability
The effect of temperature on activity of purified fibrinolytic protease from Serratia sp. KG-2-1 showed that the activity increased with the increase in temperature. Maximum enzyme activity was observed at 40 °C (100%) then decreased gradually up to 60 °C with relative activity of 69.28 and 67.75% at 50 and 60 °C, respectively. While it significantly decreased thereafter with approximately 33% relative activity. Similar observations were made in other Serratia sp. like S. marcescens RSPB11, S. marcescens NRRLB-23112 and S. marcescens ATCC 25419 have optimal temperature of 37, 42 and 45 °C, respectively (Bhargavi and Prakasham 2013). The pH studies showed significantly high activity between pH 7–10 with maximum activity at pH 8 hence is stable near physiological pH. Other microorganisms also produced enzyme with similar optimal temperature and pH like fibrinolytic enzyme from Rhizopus chinensis 12 (Liu et al. 2005), Fusarium sp. CPCC 480097 (Wu et al. 2009), Streptomyces sp. CS684 (Simkhada et al. 2010) had optimum temperature of 45 °C and optimum pH 10.5, 8.5 and 7.5, respectively.
Also the studies on stability of the purified enzyme at different temperature and pH ranges showed that the enzyme is stable at various temperature and pH. The enzyme showed relatively high stability between 10–30 °C retaining 94.87, 94.83 and 99.79% of activity at respective temperatures while at higher temperature activity decreased (Fig. 6a). The purified enzyme showed higher stability between pH 6–8 retaining more than 90% of its activity while it retained 87.22 and 74.23% of its activity at pH 5 and 9, respectively. While with further increase in pH only 18% residual activity was observed at pH 12. Also the stability was very low at acidic pH (Fig. 6b).
Effect of metal ions on enzyme activity
To study whether metal ions could participate in the regulation of enzyme activity, different metal salts NaCl, CaCl2, KCl, MgCl2, Al(NO3)3, BaCl2, FeCl3, CuSO4, ZnCl2, CoCl2, MnSO4 and Hg2SO4 were added to the reaction mixture. The purified enzyme was stable and even had stimulatory catalytic effect in the presence of many metal ions. Na+, K+, Ba2+, Cu2+, Mn2+ and Hg2+ enhanced the enzyme activity showing maximum activity with Ba2+ (165.81% relative activity). Ca2+ and Fe3+ showed strong inhibition while Mg2+, Al2+ and Zn2+ also decreased the activity (Fig. 7a). Cations such as Na+ and K+ commonly occur in the living system so their stimulatory effect on enzyme activity favors its therapeutic potential being used as thrombolytic agent. On the contrary fibrinolytic enzyme from Streptomyces sp. CS684 was inhibited by Co2+, Zn2+, Cu2+ and Fe2+, but slightly enhanced by Ca2+ and Mg2+ (Simkhada et al. 2010) while that from Fusarium sp. CPCC 480097 was stimulated by 1 mM/L Ca2+, Zn2+ and inhibited by 3 mM/L of Co2+, Cu2+ and Zn2+ (Wu et al. 2009).
Effect of inhibitors and solvents on enzyme activity
The purified enzyme from Serratia sp. KG-2-1 was stable in many additives under study. Enzyme inhibitor like PMSF had little effect on enzyme activity suggesting that it does not belong to serine protease category. While cystein protease inhibitor iodoacetic acid, 1,10-phenanthroline and metal chelator EDTA strongly inhibited the activity with 17.08, 17.48 and 16.51% residual activity. The observation signifies the presence of metal ions for enzymatic activity alongwith the presence of cysteine moiety in its active site. Bhargavi et al. also isolated a metalloprotease from Serratia sp. RSPB11 but its activity was retained in presence of PMSF as well as idoacetic acid (Bhargavi and Prakasham 2013). Fibrinolytic protease belonging to metalloprotease class had also been obtained from Rhizopus chinensis 12 (Liu et al. 2005), Fusarium sp. CPCC 480097 (Wu et al. 2009), Streptomyces sp. CS684 (Simkhada et al. 2010), B. subtilis K42 (Hassanein et al. 2011) and Candida guilliermondii NRRL Y-2075 (Rashad et al. 2012). Also, the enzyme had 85% of activity as compared to control in the presence of ethanol, methanol, n-butanol and CTAB. While some solvents such as isopropanol, acetone, tween-20, acetic acid, DMSO, glycerol and SDS showed stimulatory effect on enzyme activity. However, the activity of enzyme was strongly inhibited in presence of β-mercaptoethanol, gluteraldehyde and acetaldehyde (Fig. 7b). The organic solvents lead to aggregation of enzyme particles which are more stable than free enzyme. Also the solvent molecule disrupts/removes the hydration layer around enzyme resulting in compaction of enzyme molecules. Such compact molecules have high surface energy which stabilizes the enzyme (Sellek and Chaudhuri 1999). Besides being stable, the stimulatory effect might be due to decreased flexibility along with favourable structural modification which is necessary for enzyme activity. Similar results were observed in lipase obtained from Penicillium corylophilum with residual activity of 123 and 135% in the presence of ethanol and acetone, respectively (Romero et al. 2014). Such solvent stable enzymes have also been obtained from other bacterial strains like P. aeruginosa, Bacillus pumilus, B. cereus BG1 (Gupta and Khare 2009). Similarly SDS and other surfactants lead to favorably folded and compact barrel motif structure resulting in maximum possible enzyme activity. In some previous studies, various surfactants including SDS was observed to had stabilizing as well as stimulating effect on activity of different enzymes such as alkaline protease from Aspergillus terreus (Niyonzima and More 2015) and α-amylase from saliva and porcine pancreas (Valls et al. 2012). Therefore, the enzyme isolated from Serratia sp. KG-2-1 was a cysteine type metalloprotease with stability in the presence of various metal ions, solvents and detergents.
Thus, the present study resulted in isolation, purification and characterization of highly potent fibrinolytic enzyme from Serratia sp. KG-2-1 with activity (Specific activity 493.8 U/mg) higher than that of plasmin. The attempt to improve the economy of the production process was carried out. The use of simple, less laborious, less time consuming, efficient, more reliable and more informative statistical method of response surface methodology for media optimization resulted in 1.82 folds enhancement in the activity. The purified belongs to metalloprotease category with optimum activity at conditions similar to physiological environment. In addition, it was found to be stable not only in various organic solvents but also in both ionic and non-ionic surfactants hence it has wide industrial potential besides its pharmaceutical importance.
References
Agrebi R, Haddar A, Hajji M, Frikha F, Manni L, Jellouli K, Nasri M (2009) Fibrinolytic enzymes from a newly isolated marine bacterium Bacillus subtilis A26 characterization and statistical media optimization. Can J Microbiol 55:1049–1061
Ahn MJ, Ku HJ, Lee SH, Lee JH (2015) Characterization of novel fibrinolytic enzyme, BsfA, from Bacillus subtilis ZA400 in Kimchi reveals its pertinence to thrombosis treatment. J Microbiol Biotechnol 25:2090–2099
Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem 40:346–351
Bajaj BK, Sharma P (2011) An alkali-thermotolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotechnol 28:725–732
Bajaj BK, Singh S, Khullar M, Singh K, Bhardwaj S (2014) Optimization of fibrinolytic protease production from Bacillus subtilis I-2 using agro-residues. Braz Archives Bio Technol 57:653–662
Basu B, Apte SK (2008) A novel serralysin metalloprotease from Deinococcus radiodurans. Biochem Biophys Acta 1784:1256–1264
Bhargavi PL, Prakasham RS (2012) Proteolytic enzyme production by isolated Serratia sp RSPB11: role of environmental parameters. Curr Trends Biotechnol Pharm 6:55–65
Bhargavi PL, Prakasham RS (2013) A fibrinolytic, alkaline and thermostable metalloprotease from the newly isolated Serratia sp RSPB11. Int J Biol Macromol 61:479–486
Bizuye A, Sago A, Admasu G, Getachew H, Kassa P, Amsaya M (2013) Isolation, optimization and characterization of protease producing bacteria from soil and water in Gondar town, North West Ethiopia. Int J Bacteriol Virol Immunol 3:20–24
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Choi NS, Song JJ, Chung DM, Kim YJ, Maeng PJ, Kim SH (2009) Purification and characterization of a novel thermoacid-stable fibrinolytic enzyme from Staphylococcus sp. strain AJ isolated from Korean salt-fermented Anchovy-joet. J Ind Microbiol Biotechnol 36:417–426
Chung DM, Choi NS, Chun HK, Maeng PJ, Park SB, Kim SH (2010) A new fibrinolytic enzyme (55 kDa) from Allium tuberosum: purification, characterization, and comparison. J Med Food 13:1532–1536
Gupta A, Khare SK (2009) Enzymes from solvent-tolerant microbes: useful biocatalysts for non-aqueous enzymology. Crit Rev Biotechnol 29:44–54
Hassanein WA, Kotb E, Awny NM, El-Zawahry YA (2011) Fibrinolysis and anticoagulant potential of a metallo protease produced by Bacillus subtilis K42. J Biosci 36:773–779
Huang MX, Ye Y, Chen YX, Han YL (2012) Partial purification and characterization of fibrinolytic enzymes from yellow mealworm. Int J Pept Res Ther 18:153–161
Ju X, Cao X, Sun Y, Wang Z, Cao C, Liu J, Jiang J (2012) Purification and characterization of a fibrinolytic enzyme from Streptomyces sp. XZNUM 00004. World J Microbiol Biotechnol 28:2479–2486
Kim SH, Choi NS (2000) Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp strain DJ-4 screened from Doen-Jang. Biosc Biotechnol Biochem 64:1722–1725
Kim SB, Lee DW, Cheigh CI, Choe EA, Lee SJ, Hong YH, Choi HJ, Pyun YR (2006) Purification and characterization of a fibrinolytic subtilisin-like protease of Bacillus subtilis TP-6 from an Indonesian fermented soybean, Tempeh. J Ind Microbiol Biotechnol 33:436–444
Kim HC, Choi BS, Sapkota K, Kim S, Lee HJ, Yoo JC, Kim SJ (2011) Purification and characterization of a novel, highly potent fibrinolytic enzyme from Paecilomyces tenuipes. Process Biochem 46:1545–1553
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee AR, Kim GM, Park JY, Jo HD, Cha J, Song YS, Chun J, Kim JH (2010) Characterization of a 27 kDa fibrinolytic enzyme from Bacillus amyloliquefaciens CH86-1 isolated from Cheonggukjang. J Korean Soc Appl Biol Chem 53:56–61
Liu XL, Du LX, Lu FP, Zheng XQ, Xiao J (2005) Purification and characterization of a novel fibrinolytic enzyme from Rhizopus chinensis 12. Appl Microbiol Biotechnol 67:209–214
Mahajan PM, Nayak S, Lele SS (2012) Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: media optimization, purification and characterization. J Biosci Bioeng 113:307–314
Niyonzima FN, More SS (2015) Purification and characterization of detergent-compatible protease from Aspergillus terreus gr. 3. Biotech 5:61–70
Peng Y, Yang X, Zhang Y (2005) Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol 69:126–132
Ponnuswamy V, Arumugaperumal A, Samue GPV, Mariadhas VA, Naif AA (2016) Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in in vitro clot lysis. Front Microbiol 7:361. doi:10.3389/fmicb.2016.00361
Rashad MM, Mahmoud AE, Al-Kashef AS, Nooman MU (2012) Purification and characterization of a novel fibrinolytic enzyme by Candida guilliermondii grown on sunflower oil cake. J Appl Sci Res 8:635–645
Romero CM, Baigori MD, Baron AM, Krieger N, Pera LM (2014) Activity and stability of lipase preparations from Penicillium corylophilum: potential use in biocatalysis. Chem Eng Technol 37:1987–1992
Sellek GA, Chaudhuri JB (1999) Biocatalysis in organic media using enzymes from extremophiles. Enzyme Microb Technol 25:471–482
Simkhada JR, Mander P, Cho SS, Yoo JC (2010) A novel fibrinolytic protease from Streptomyces sp. CS684. Process Biochem 45:88–93
Singh S, Bajaj BK (2015) Medium optimization for enhanced production of protease with industrially desirable attributes from Bacillus subtilis K-1. Chem Eng Commun 202:1051–1060
Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 43:1110–1111
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tariq AL, Reyaz AL (2012) Characterization of UTI associated protease from Serratia marcescens strain TW1 and its immunogenic properties. Int Res J Biotechnol 3:88–95
Valls C, Rojas C, Pujadas G, Garcia-Vallve S, Mulero M (2012) Characterization of the activity and stability of amylase from saliva and detergent. Biochem Mol Bio Edu 40:254–265
Venkataraman D, Ilangovan S, Sampathkumar MV, Victoria MJ, Pasha SPBS, Pandian SBRK, Gurunathan S (2010) Medium optimization and immobilization of purified fibrinolytic URAK from Bacillus cereus NK1 on PHB nanoparticles. Enzy Microbial Technol 47:297–304
Vijayaraghavan P, Vincent SGP, Arasu MV, Al-Dhabi NA (2016) Bioconversion of agro-industrial wastes for the production of fibrinolytic enzyme from Bacillus halodurans IND18: purification and biochemical characterization. Electro J Biotechnol 20:1–8
Wang S, Chen H, Liang T, Lin Y (2009) A novel nattokinase produced by Pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochem 44:70–76
Wu B, Wu L, Chen D, Yang Z, Luo M (2009) Purification and characterization of a novel fibrinolytic protease from Fusarium sp. CPCC 480097. J Ind Microbiol Biotechnol 36:451–459
Wu D, Ran T, Wang W, Xu D (2016) Structure of a thermostable serralysin from Serratia sp. FS14 at 1.1A resolution. Acta Cryst 72:10–15
Acknowledgements
The author Kapila Taneja gratefully acknowledges Department of Science and Technology, Govt. of India for providing financial support in the form of DST-INSPIRE Fellowship. Authors also thank Microbial Culture Collection, NCCS, DBT, Govt. of India for phenotypic characterization of samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have seen the manuscript and approved the same in its present form. There is no declaration of interest for the present manuscript submitted in the journal.
Rights and permissions
About this article
Cite this article
Taneja, K., Bajaj, B.K., Kumar, S. et al. Production, purification and characterization of fibrinolytic enzyme from Serratia sp. KG-2-1 using optimized media. 3 Biotech 7, 184 (2017). https://doi.org/10.1007/s13205-017-0808-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0808-4