Abstract
Perovskite solar cells (PSCs) with high efficiency and low cost are being actively developed. In recent years, the role of lead-based PSCs has become very important in the solar cell industry. But its toxicity and instability present a significant challenge to the development of commercially viable products. Under such circumstances, Ti-based all-inorganic (PSC) materials are of paramount importance in the development of high-performance PSCs. This work examines the theoretical feasibility of a lead-free, environmentally friendly, and reliable Cs2TiBr6-based PSC. The analysis of various hole transport layers (HTLs) and electron transport layers (ETLs) is performed in order to select materials that would result in stronger perovskite solar cells with higher stability. The goal of this paper is to design a lead-free PSC using Cs2TiBr6 as an absorber layer in the form of Au/CuSbS2/ Cs2TiBr6/WO3/FTO. Each composite layer is optimized and analyzed through simulations utilizing SCAPS-1D software to achieve elevated performance. A maximum power-conversion efficiency of 20.40% is achieved for the proposed solar cell through optimization of the ETL, HTL, metal contact materials, defect density of the absorber and the thickness of the absorber, HTL, and FTO. These results will pave the way for developing eco-friendly and highly efficient perovskite photovoltaic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photovoltaic technology has been hailed as a promising technology for replacing depleted fossil fuels within the next few decades (Kabir et al. 2018). While solar cell technology has made tremendous advancements, the necessity for efficient, green, low-cost, and abundantly available materials remains a challenge (Jianbo Wang and Zhenming Xu 2017). Recent developments in materials which include their low cost, high stability, and simplicity of fabrication for new-generation solar cells have given rise to the most interesting topic (Mehrabian et al. 2021). The most widely available photovoltaic device is silicon solar cells, but their cost is high compared to conventional energy sources. It is obvious that cleanroom technology is required for Si to be used in photovoltaic applications, which greatly increases their inherent cost (Green 2009; Pizzini 2010). For solar cells, however, perovskite materials have shown high performance at very low costs, with their power-conversion efficiency (PCE) soaring from a few to more than 25% within a very short time frame, and they can be processed at room temperature (Kim and Kim 2021). Lead-based metal halide perovskites are attractive among these materials because of their high PCE. Halide perovskites (HPs) possess a triangular structure consisting of A, B, and X, where A represents a monovalent cation, B signifies a divalent metal cation, and X represents a halide anion (Md. Tohidul Islam et al. 2021). Essentially, these semiconductor materials display novel optoelectronic properties such as higher absorption coefficients, longer carrier diffusion distances, weakly bound exciton, and a wide bandgap tuning range (Alam and Ashraf 2020). Despite the improvement in PCE in PSCs, there remains a problem: the noxiousness of lead and the reality that the PSCs with the highest PCE consist of organic cations, like methylammonium and formamidinium ions (Alam et al. 2021). The ions in these materials are exceedingly volatile and hygroscopic, making the device heat and moisture intolerant. (Chen et al. 2018).The presence of lead in perovskites poses a threat to human health and the environment, which is a major concern for their commercial applications (Babayigit et al. 2016). Scientists are, therefore, taking a great deal of interest in proposing and investigating lead-free perovskites for high-efficiency solar cells (Giustino and Snaith 2016).
Many of these materials exhibit promising photovoltaic responses. One of these is the cesium titanium (IV) bromide (Cs2TiBr6) compound (Chen et al. 2018). Recent research has identified Cs2TiBr6 as a promising compound in Pb-free perovskites. Also, photoluminescence suggests that the non-radiative recombination rate is low, and diffusion lengths above 100 nm have been observed. Thermal stress and exposure to light and humidity have also been reported to have a high level of stability. Cs2TiBr6 is a very excellent option for Pb-free perovskite solar cells due to its combination of numerous desirable features for solar energy applications (Wang et al. 2020). Lead-free and non-toxic Ti-based A2 +1 Ti+4 X6 -1 double perovskites have innovative optoelectronic applications (Slami et al. 2019). Cs2TiBr6, a direct-energy bandgap perovskite compound with excellent optical, electrical, and photovoltaic properties, has the potential to replace methylammonium lead halide perovskite in next-generation solar applications (Ju et al. 2018; Pecunia et al. 2020). Cs2TiBr6 is a tolerable and stable perovskite semiconductor because of its strong titanium (Ti) (Grandhi et al. 2021).
Design of novel perovskite solar cell
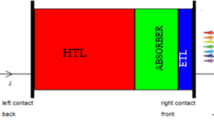
The structure of the multilayer Cs2TiBr6 solar cell employed in this investigation is shown in Fig. 1. The basic component of photovoltaic devices is an absorber layer, which captures photons and converts them into electron–hole pairs. The sandwich of two electrodes and an absorber layer may be sufficient for photovoltaic response, but the best solar cell performance is achieved when electrons and holes are spread uniformly inside the photovoltaic system (Chen et al. 2016).
A PSC can be produced when the electron transport layer (ETL) and the hole transport layer (HTL) are sandwiched together for balanced charge distribution (Said et al. 2019). Cs2TiBr6 is chosen as an absorber layer. Both HTL and ETL must perform three well-defined tasks in terms of layer selection: (i) extract holes/electrons from the absorb layer, (ii) block opposite charges from entering the absorb layer to prevent recombination, and (iii) collect holes/electrons from the absorb layer and guide them to their appropriate electrodes (Pitchaiya et al. 2020; Gu et al. 2016).
It is well known that ETL and HTL materials are important in the extraction of electrons and holes from perovskite absorber layers. Some examples of such materials are CuO, CuSbS2, Cu2O, P3HT, PEDOT: PSS. According to recent research, copper antimony sulphide (CuSbS2) is a potentially earth-abundant photoelectric absorber and appropriate hole transport material for developing solar cells, because it promotes the absorption of solar radiation in cells, which in turn leads to energy production (Divya Sharma 2022; Atowar Rahman 2954; Shivesh et al. 2021; Yang et al. 2014). From the studies carried out here, CuSbS2 has been identified as the most acceptable HTL among the other HTLs, for perovskite solar cells in terms of efficiency. Therefore, we opt CuSbS2 as HTL for this study. In the literature, TiO2 (Giordano et al. 2016), ZnO (Najafi et al. 2018), PCPM (Rai et al. 2020), WO3 (Rai et al. 2020), IGZO (Mozhgan Kazemzadeh Otoufi 2020), are utilized as electron transport layer (ETM) in the photovoltaic cell. Because of its high efficiency compared to all other ETLs, WO3 is regarded an ETL for perovskite performance. WO3 offers several unique benefits of solution processing, outstanding electrical characteristics, and high mobility among these electron transport materials. The performance of perovskite is improved by employing WO3 as an ETL (Rai et al. 2020). Here, WO3 is made as an ETL in this study. The Au/CuSbS2/ Cs2TiBr6/WO3/FTO perovskite solar cell is the optimum device.
Design and simulation
SCAPS software version 3.3.07, a one-dimensional (1-D) photovoltaic simulator created by ELIS, University of Gent, Belgium, was used in this investigation (Burgelman et al. 2000; Wang et al. 2020). To determine the density of electrons and holes individually, SCAPS 1D uses four different sets of equations (Verschraegen and Burgelman 2007). The following are the equations:
1. Differential form of the Poisson equation in terms of electrostatic potential is displayed below
where ф is the is the electrostatics potential, e is electronic charge, the permittivity of vacuum is ε0, εr is relative permittivity, NA stands for shallow acceptor impurity density while ND stands for shallow donor impurity density. Similarly, ρp denotes hole density distribution and ρn denotes electron density distribution, with n (x) and p(x) denoting electron and hole density as a function of x.
2. The Continuity equation is given as follows
where Jn and Jp represent the electron and hole current densities, G represents the generation rate, and R represents the recombination rate.
3. The electron and hole current densities are given by the Charge transport equation as follows
where µn is the electron mobility and µp is the hole mobility.
4. The absorption coefficient with respect to wavelength α (λ) can be calculated by the equation given below.
We use the following equation to get
where A, B are constant, h is plank constant, ν is frequency of photons and Eg is the band gap of the absorber layer. The band discontinuity between the interfaces of the different layers is small and therefore can be neglected for simplicity. The ambient temperature 300 K and the solar spectrum AM.1.5 (1000 W/m 2) is used for this study. All of the physical and electrical parameters required by SCAPS 1D for each layer have been gathered from scientific literature and are listed in Tables 1 and 2 below.
Table 1 Photovoltaic device parameters used for these simulations are reported here, where doping concentration are just given for initial estimation which are optimized in later stages.
Results and discussion
This section includes an analysis of various HTLs and ETLs for an innovative device, as well as an optimizations of thickness of HTL, absorber, FTO and the optimization of absorber defect density for Cs2TiBr6 absorber.
ETL, HTL, Optimization for Cs2TiBr6 perovskite absorber
Figure 2 illustrates the performance of all device configurations with each HTL and ETL variation with standard thickness of 30 nm as given above in the literature (Tables 1 and 2), while keeping Au as back metal contact. The inorganic and organic materials such as CuO, CuSbS2, Cu2O, P3HT, and PEDOT:PSS as Hole Transport Materials (HTMs) are analyzed on the design in the present work. The parameters associated with each HTMs are listed in Table 2. The J-V characteristics on various HTLs due to the effect of ZnO ETL are studied and given in Table.3. Similarly the effect of HTLs on various ETLs are also studied and are given in Tables 4, 5 and 6. From these analysis, it came to know that CuSbS2 is a good HTL compared with other HTL combination on Cs2TiBr6 absorber PSC. Based on the J-V characteristics of the HTMs, which shows the Tables 3, 4, 5, 6 and 7, the product performance for each of the ETLs employed in the device. Performance is found to be very high for the configuration: Glass/FTO/WO3/Cs2TiBr6/CuSbS2/ Au. The attained PCE is 20.90%, FF is 82.57%, JSC is 24.9535 mA/cm2, and VOC is 0.9050 V.
The obtained results show that inorganic HTL improves the PCE of the device. Inorganic HTL has several advantages, including increased stability, excellent transparency, and band alignment. Multiple simulations were run to improve the ETL and HTL layers for PSC enhancement. The PCE variability of the Cs2TiBr6 PSCs using WO3 as ETM and CuSbS2 as HTM is found to be maximum.
Absorber layer thickness optimization
The thickness of the perovskite absorbing film plays a crucial role on the performance of solar cells. It has been discovered that if the film thickness is too thin, photons are poorly absorbed in the film, resulting in low PCE. If the film thickness is too thick, carriers recombine, resulting in reduced solar cell PCE. For the purpose of finding the optimal thickness of perovskite, multiple thicknesses of absorber have been simulated. Figure 3 depicts a graph of the PCE, and Voc, of PSCs, whereas Fig. 4 depicts a graph of the Jsc and FF properties of PSCs as a function of absorber thickness.
It is seen that all the solar cell output parameters improved greatly with the increase in absorber layer thickness. The value of Voc is obtained to be 0.9030 V at 400 nm thickness and increases up to 0.905 V at 900 nm. It is also observed that Jsc increased linearly up to an absorber thickness of 900 nm and then almost saturated with increasing absorber thickness. Jsc of 23.9535 mA/cm2 is estimated for a thickness of 900 nm. This is due to the increase in photon absorption with increasing thickness at a higher wavelength. Similarly, FF and PCE also increased with the increase in thickness. A FF of 82.57% is obtained at a thickness of 900 nm. The efficiency of the PSC is calculated 17.90% at a thickness of 900 nm. It can also be perceived from Figs. 3 and 4 that when the thickness exceeds 900 nm, all the photovoltaic parameters almost undergo saturation. This is due to the recombination of electrons and holes in the CS2TiBr6 layer before reaching the contacts. Thus, in the present work, the thickness of the absorber layer is optimized to be 900 nm, which is crucially important to reduce the charge recombination and hence improve the PV performance. At a perovskite optimized thickness of 900 nm, these graphs reveal a maximum PCE of 17.90%, Voc of 0.9050 V, Jsc of 23.9535 mA/cm2, and FF of 82.57%.
HTL layer thickness optimization
Figure 5 displays the PSC performance analysis employing CuSbS2 layer as an HTL that ranges from 30 to 90 nm in thickness. In the optimized thickness of 90 nm, PCE reached 18.38%. The VOC and FF increase as the thickness increases, peaking of 0.9716 V and 78.67% at 90 nm, respectively, while the current density (Jsc) is 24.0448 mA/cm.2
Effect of front electrode on PSCs performance
The performance parameters of PSCs parameters with ITO (Indium Tin Oxide) and FTO (Fluorine doped Tin Oxide) as the front transparent electrode are compared in J-V characteristics curves which are depicted in Fig. 6a, b. When the PCE is considered, it is visible that the FTO electrode is a better choice for this PSC to be a use to a solar cell than the ITO electrode.
Optimization of FTO layer thickness
The PSC performs much better when FTO is employed as the front electrode. Figure 7 exhibits PSC properties vs. FTO thickness within range of 50–550 nm. The FF, Jsc, VOC, and PCE first increases rapidly with the FTO thickness. Therefore, the optimized FTO thickness is around 50 nm which gives VOC of 1.02 V, JSC of 20.13 mAcm-2, FF of 88.2% and PCE of 19.2%.
Optimization of absorber defect density
A key factor that has a substantial impact on device performance is the total defect density of the active layer. In addition to the increased recombination caused by the more defects in the absorber layer, the higher defect concentrations result in a faster rate of degradation of the film, reduced stability, and lower performance of the device (Ouslimane et al. 2021). Figure 8 shows the PCE, while Table 8 shows how the J-V characteristics of the design are affected by varying interface defect densities. The defect density of absorber interfaces is examined on a scale ranging from 1013 cm−3 to 1017 cm−3. It is evident that all cell performances degrade as the number of defects increases in the absorber layer. There is a significant impact of defect density on FF and JSC. A defect density of 1017 cm–3 results in a FF and JSC as low as 28.44% and 15.0321 mA/cm2 (Table 8). Additionally, as the defect density rises from 1013 cm−3 to 1017 cm −3, the efficiency is drastically lowered from 20.4 to 3.06%. In terms of defect density, PSCs based on Cs2TiBr6 have the maximum PCE of 20.40% when compared to other defect densities as illustrated in Fig. 8. This table displays the outcomes of Voc, JSC, and FF for various defect densities and it is found that the defect density corresponding to 1013 cm−3 has better solar cell parameters.
The impact of the rear metal work function on the PSC’s execution
When the work function of a metal is larger, it can be represented as the amount of energy or photons efficient. Studies have found that solar cells become more efficient when their work functions are larger. When the work function value rises while the carrier's barrier height falls, the ohmic contact rises. PSCs have used a variety of metals as back contact electrodes, as shown in Table 9 with Fig. 9
Simulations have been performed involving copper (Cu), iron (Fe), carbon (C), and gold (Au) as back contacts for PSCs. The ETL and electrode must be coupled in an Ohmic way for electrons to be collected to the load. It is appropriate for back contact materials to own a relatively high work function. In addition, as the work function of the back metal contact is reduced below 5.1 eV, the photovoltaic parameters gradually decline. Figure 9 shows that increasing the work function of the back contact materials improves the efficiency of PSCs. It reaches its maximum PCE of 20.4%, when its back contact is 5.1 eV. The best performing back electrode material is Au which proves the PCE curve of Fig. 9 that yields the VOC of 1.5628 V, JSC of 14.3404 mA/cm2, and FF of 90.01%
The impact of temperature on PSC performance
Different geographical regions use solar cells at different occasion of the year with varying weather conditions and climates, which affect the operation temperature of the photovoltaics. Simulations were run at various operating temperatures to see how temperature affects PSC’s execution. The effect of temperature change on the performance of solar cells is depicted in Fig. 10. From the plots, it is noticeable that the PCE, FF and Voc decrease with temperature. Hence, the PSC offers better performance at 300 k.
Optimized device
The novel optimized design is illustrated in Fig. 11. The quantum efficiency (Q.E) of a solar cell is a measure of the ratio of number of carriers it collects to the number of photons it receives. Sandwiched absorbing layers that can only absorb photons in the range of 790 nm can be more effective.
According to Fig. 12, more than 98% photons are alive having wavelength between 300 and 800 nm, and less than 2% has been recombined via other processes (Auger and SRH). Hence, the sandwiched layer at 300–800 nm absorbs virtually all incident photons to create electron–hole pairs and carriers with minimal recombination by the built-in field.
Figures 13 and 14 provide the details of the spectral response and J-V characteristic curves of the optimized device. Furthermore, the device also has an excellent spectral response, which is in the visible range, with the PCE of 20.40%, Jsc of 24.3404 mA/cm2, Voc of 0.9717 V, and FF of 86.24%.
Comparative analysis
Table 10 presents the comparison of existing Cs2TiBr6-based PSCs. This material is relatively new in the realm of PSCs, and it has received little research. From Table 10, we find that our optimized device performs better than existing works on Cs2TiBr6-based PSCs. This device appears to have comparatively favorable outcomes based on our test results, and it is ecologically friendly because it is a pure inorganic Ti-based perovskite, and it uses totally inorganic charge transport layers, the environment span end is projected to be extremely stable.
Conclusion
In this research, a lead-free, environmentally friendly, and stable Cs2TiBr6-based perovskite solar cell is presented, which integrates organic and inorganic charge transportation materials, in addition to different electron transport materials and metal contacts. By carefully selecting the HTL, ETL, back metal contacts, as well as the parameters of the absorb layer, which includes thickness and defect density, the device attains maximum performance and appears stable at 300 K. Despite using several back contact metals, the device has the maximum effectiveness with Au with a work function of 5.1 eV. Optimized solar cells retain their QE of 98% nearly at 800 nm wavelength. Analysis of the consequences of diverse HTL and ETL materials on invention enactment and optimization of layer thickness, back metal contacts and temperature caused in the evolution of an innovative high-performance combination of FTO/WO3/Cs2TiBr6/Cu2SbS2/Au. This proposed system displays a peak PCE of 20.40%, a JSC of 24.3042 mA/cm2, VOC of 0.9717 V, and a FF of 86.24%, also a QE of 98% at the visible spectrum. Designing lead-free, eco-friendly Ti-based PCS for future technologies may benefit from the proposed work.
References
Ahmed S, Jannat F, Abdul Kaium Khan MD, Alim MA (2021) Numerical development of eco-friendly Cs2TiBr6 based perovskite solar cell with all-inorganic charge transport materials via SCAPS-1D. Int J Light Electron Optics 225:165765
Alam I, Mollick R, Ashraf MA (2021) Numerical simulation of Cs2AgBiBr6-based perovskite solar cell with ZnO nanorod and P3HT as the charge transport layers. Phys B Condens Matter 618:413187
Alam I, Ashraf MA (2020) Effect of different device parameters on tin-based perovskite solar cell coupled with In2S3 electron transport layer and CuSCN and Spiro-OMeTAD alternative hole transport layers for high-efficiency performance. Energy Sources Part A Recover Util Environ Effects
Ameri M, Mohajerani E, Ghafarkani M, Safari N, Alavi SA (2019) The investigation of the unseen interrelationship of grain size, ionic defects, device physics and performance of perovskite solar cells. J Phys D Appl Phys 52:125501
Atowar Rahman M (2022) Numerical modeling of ultra-thin CuSbS2 heterojunction solar cell with TiO2 electron transport and CuAlO2: Mg BSF layers. Opt Mater Express 12:2954
Babayigit A, Ethirajan A, Muller M, Conings B (2016) Toxicity of organometal halide perovskite solar cells. Nat Mater 15:247–251
Behrouznejad F, Shahbazi S, Taghavinia N, Wu H-P, Wei-Guang Diau E (2016) A study on utilizing different metals as the back contact of CH3NH3PbI3 perovskite solar cells. J Mater Chem A 4:13488–13498
Burgelman M, Nollet P, Degrave S (2000) ‘Modelling polycrystalline semiconductor solar cells.’ Thin Solid Films 6:361–362
Chakraborty K, Choudhury MG, Paul S (2019) Numerical study of Cs2TiX6(X = Br−, I−, F− and Cl−) based perovskite solar cell using SCAPS-1D device simulation. Solar Energy 194:886–892
Chen K, Hu Q, Liu T, Zhao L, Luo D, Wu J, Zhang Y, Zhang W, Liu F, Russell TP, Zhu R, Gong Q (2016) ‘Charge-carrier balance for highly efficient inverted planar heterojunction perovskite solar cells.’ Adv Mater 28(48):10718–10724
Chen M, Ju M-G, Carl A, Zong Y, Grimm RL, Gu J, Zeng XC, Zhou Y, Padture NP (2018) Cesium Titanium (IV) bromide thin films based stable lead-free perovskite solar cells. Joule 2:558–570
Deepthi Jayan K, Sebastian V (2021) Comprehensive device modelling and performance analysis of MASnI3 based perovskite solar cells with diverse ETM, HTM and back metal contacts. Sol Energy 217:40–48
DivyaSharma RajeshMehra (2022) BalwinderRaj, Optimization of tin based perovskite solar cell employing CuSbS2 as HTL: A numerical simulation approach. Opt Mater 134:113060
Du HJ, Wei-Chao W, Jian-Zhuo Z (2016) Device simulation of lead-free CH3NH3SnI3 perovskite solar cells with high efficiency. Chin Phys B 25(10):108802
Giordano F, Abate A, Baena JPC, Saliba M, Matsui T, Im SH, Zakeeruddin SM, Nazeeruddin MK, Hagfeldt A, Graetzel M (2016) Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat Commun 7:10379
Giustino F, Snaith HJ (2016) Toward, lead-free perovskite solar cells. ACS Energy Lett 1(6):1233–1240
Grandhi G, Matuhina A, Liu M, Annurakshita S, Ali-Löytty H, Bautista G, Vivo P (2021) Lead-free cesium titanium bromide double perovskite nanocrystals. Nanomaterials 11:1458
Green MA (2009) The path to 25% silicon solar cell efficiency: History of silicon cell evolution. Prog Photovolt Res Appl 17:183–189
Gu PY, Wang N, Wu A, Wang Z, Tian M, Fu Z, Sun XW, Zhang Q (2016) An azaacene derivative as promising electron-transport layer for inverted perovskite solar cells. Chem Asian J 11:2135–2138
Huan L, Sun X, Li C, Xu R, Xu J, Du Y, Wu Y, Ni J, Cai H, Li J (2016) Electron transport layer-free planar perovskite solar cells: further performance enhancement perspective from device simulation. Sol Energy Mater Sol Cells 157:1038–1047
Ju M-G, Chen M, Zhou Y, Garces HF, Dai J, Ma L, Padture NP, Zeng XC (2018) Earth-abundant nontoxic titanium(IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett 3:297–304
Kabir E, Kumar P, Kumar S, Adelodun AA, Kim K-H (2018) ‘Solar energy: Potential and future prospects.’ Renew Sustain Energy Rev 82:894–900
Kim GH, Kim DS (2021) Development of perovskite solar cells with >25% conversion efficiency. Joule 5(5):1033–1035
Lakhdar N (2020) Hima, Abdelkader, electron transport material effect on performance of perovskite solar cells based on CH3NH3GeI3. Opt Mater 99:109517
Mehrabian M, Afsha EN, Yousefzadeh SA (2021) Simulating the thickness effect of the graphene oxide layer in CsPbBr 3- based solar cells Masood. Mater Res Express 8:035509
Moiz SA, Alahmadi ANM, Aliohani AJ (2021) Design of a novel lead-free perovskite solar cell for 17.83% efficiency. IEEE Access 9:54254–54263
Mottakin M et al (2021) Design and modelling of eco-friendly CH3NH3SnI3-based perovskite solar cells with suitable transport layers. Energies 14:7200
Najafi M, Di Giacomo F, Zhang D, Shanmugam S, Senes A, Verhees W, Hadipour A, Galagan Y, Aernouts T, Veenstra S (2018) Highly efficient and stable flexible perovskite solar cells with metal oxides nanoparticle charge extraction layers. Nano. Micro Small 14:1702775
Otoufi MK, Ranjbar M, Kermanpur A, Taghavinia N (2020) Enhanced performance of planar perovskite solar cells using TiO2/SnO2 and TiO2/WO3 bilayer structures: Roles of the interfacial layers. Sol Energy 208:697–707
Ouslimane T, Et-taya L (2021) Lahoucine Elmaimouni, Abdellah Benami, Impact of absorber layer thickness, defect density, and operating temperature on the performance of MAPbI3 solar cells based on ZnO electron transporting material. Heliyon 7:e06379
Pecunia V, Occhipinti LG, Chakraborty A, Pan Y, Peng Y (2020) Lead-free halide perovskite photovoltaics: Challenges, open questions, and opportunities. APL Mater 8:100901
Pitchaiya S, Natarajan M, Santhanam A, Asokan V, Yuvapragasam A, Ramakrishnan VM, Palanisamy SE, Sundaram S, Velauthapillai D (2020) ‘A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application.’ Arabian J Chem 13(1):2526–2557
Pizzini S (2010) Towards solar grade silicon: Challenges and benefits for low cost photovoltaics. Sol Energy Mater Sol Cells 94:1528–1533
Rafsun Jani MD et al (2020) Exploring Solar Cell Performance of Inorganic Cs2TiBr 6 Halide Double Perovskite: A Numerical Study. Superlattices Microstruct 146:106652
Rai N, Rai S, Singh PK, Pooja Lohia DK, Dwivedi, (2020) Analysis of various ETL materials for an efficient perovskite solar cell by numerical simulation. J Mater Sci Mater Electron 31:16269–16280
Said AA, Xie J, Zhang Q (2019) Recent progress in organic electron transport materials in inverted perovskite solar cells. Small 15:1900854
Samanta M, Ahmed SI, Chattopadhyay KK, Bose C (2020) Role of various transport layer and electrode materials in enhancing performance of stable environment-friendly Cs2TiBr6 solar cell. Optik Int J Light Electron Optics 217:164805
Shivesh K, Alam I, Kushwaha AK, Kumar M, Singh SV (2021) Investigating the theoretical performance of Cs2TiBr6-based perovskite solar cell with La-doped BaSnO3 and CuSbS2 as the charge transport layers. Int J Energy Res 46:6045–6064
Slami A, Bouchaour M, Merad L (2019) Numerical Study of Based Perovskite Solar Cells by SCAPS-1D. Int J Energy Environ 13:17–21
Tohidul Islam Md, Rafsun Jani Md, Shorowordi K, Hoque Z, Gokcek AM, Vattipally V, Nishat SS, Ahmed S (2021) Numerical simulation studies of Cs3Bi2I9 perovskite solar device with optimal selection of electron and hole transport layers. Optik 231:166417
Verschraegen J, Burgelman M (2007) ‘Numerical modeling of intra-band tunneling for heterojunction solar cells in SCAPS.’ Thin Solid Films 515(15):6276–6279
Wang J, Zhenming X (2017) Environmental friendly technology for aluminum electrolytic capacitors recycling from waste printed circuit boards. J Hazard Mater 326:1–9
Wang T, Li YY, Mitzi DB (2020) Is Cs2TiBr6 a promising Pb-free perovskite for solar energy applications? J Mater Chem A 8:2–7
Wang K, Olthof S, Subhani WS, Jiang X, Cao Y, Duan L, Wang H, Du M, Liu SF (2020) Novel inorganic electron transport layers for planar perovskite solar cells: Progress and prospective. Nano Energy 68:104289
Yang Bo, Wang L, Han J, Zhou Y, Song H, Chen S, Jie Zhong Lu, Lv DN, Tang J (2014) CuSbS2 as a promising earth-abundant photovoltaic absorber material: a combined theoretical and experimental study. Chem Mater 26:3135–3143
Zuo C, Ding L (2015) Solution-processed Cu2O and CuO as whole transport materials for efficient perovskite solar cells. Epub 11(41):5528–5532
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mercy, P.A.M., Wilson, K.S.J. Design of an innovative high-performance lead-free and eco-friendly perovskite solar cell. Appl Nanosci 13, 3289–3300 (2023). https://doi.org/10.1007/s13204-022-02745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02745-7