Abstract
Conventional lead halide Perovskite Solar Cells (PSC) have toxicity and stability issues. Therefore it is crucial to look for lead-free inorganic perovskite material, such as La2NiMnO6, RbGeI3, CsGeI3, Cs2AgBiBr6 and others. There has been much work in the area of PSC using Cs2AgBiBr6 as an absorber, However, due to some critical issues of Cs2AgBiBr6, such as the film's broad bandgap which limits its capacity to absorb light, leading to corresponding PSC being typically restricted to around 4% efficiency. In this work, a lead-free PSC with Cs2AgBiBr6 as the absorber has been engineered to resolve the issues by considering the experimental works in the literature to increase efficiency to 6.3% from a maximum 4.48% as reported in the literature. The output response of both design approaches, as well as the potentiality of future designs, are investigated in terms of output parameters, i.e., Open-Circuit Voltage (VOC), Short-circuit Current density (JSC), Fill Factor (FF), and Power Conversion Efficiency (PCE). Besides, this work also focuses on eliminating Sulphur from ETLs by working on several sulphides and oxide-based Electron Transport Layers (ETLs). Several solar cell device structures have been analyzed for their numerical simulation with sulphide ETLs such as ZnS, WS2, CdS, CdZnS and oxide ETLs such as TiO2, ZnO, WO3, IGZO. To progress towards eco-friendly PSCs, alternatives to sulphide, several transparent oxide alternatives (TiO2, ZnO, WO3, IGZO) have been considered as ETLs. These ETLs have been further doped with Mg to enhance the performance parameters. Mg-doped based ETLs additionally behaving as Hole-blocking layers (HBLs) in corresponding PSCs leads to comparatively significantly better performance in a number of aspects, including VOC (1.21 V), PCE (5.74%), JSC, and FF. The solar cell design has been optimized for high performance through various techniques such as varying ETL and absorber thickness, interface defects variation, series/shunt resistance, band to band recombination, and bandgap grading through doping. Also an effective electric field (Eeff) that depicts the conduction's impact has been calculated. Best performing device among different designs among PSCs with sulphide and oxide-based ETLs has been ZnS/Cs2AgBiBr6/Cu2O (PCE-6.3%) and Mg-doped (20%) ZnO/Cs2AgBiBr6/Cu2O (PCE-5.74%). These results may help researchers in their efforts to find the best-suited materials for the design of high-performance PSCs in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Organic–inorganic halide perovskites (OIHP), being one of the main advancements of third-generation solar cells, have gained much attention since the highest PCE for these devices, is already reaching 25.7% (“Best Research-Cell Efficiencies” 2020), equivalent to that of silicon-based solar cells from 3.8% in 2009 in less than a decade (NREL 2022). Lead-based Perovskite solar cells (PSCs) have been facing hurdles to commercialization due to stability issues, Pb toxicity, and poor self-life (Zhang et al. 2020; Schade et al. 2021; Rafieirad and Ganji 2021). To address these issues, extensive research has been done to examine Pb-free inorganic perovskite materials as the core absorber layer in a solar cell (Shi et al. 2017; Zhang et al. 2017). The stoichiometric ratio of the OIHP materials is typically ABX3, where Metal cation Pb2+ and organic cations (Methylamine cation (MA+) or Formamidine cation (FA+)) make up the A, B, and X sites as well as halide anions (Cl−, I− and Br− respectively)(Jeon et al. 2015)(Yang et al. 2017). For instance, it has been suggested that changing the MA+ cations at the A site to Cs+ cations could boost the stability of the perovskite by increasing the breakdown energy from − 0.111 eV (MAPbI3) to − 0.069 eV (CsPbI3). Given the toxicity of the Pb element in the perovskite's B site, potential substitutes to reduce the toxicity of the optical absorption layer include tin (Sn2+) or silver (Ag+) mixed with bismuth (Bi3+). Even then, the photovoltage performance of CsSnI3-based solar cells is significantly reduced as the sensitive Sn2+ is easily oxidized to Sn4+ in air (Chen et al. 2019b, a). Creating an inorganic lead-free Perovskite absorption layer can address these issues (Shukla et al. 2021; Stranks et al. 2013). Henceforth, Cs2AgBiBr6-based perovskite solar cell (PSC) is being considered one of the most promising candidates in inorganic lead-free perovskite photovoltaic devices. While, the double substitutions of Pb2+ utilizing B site cations such as Ag+ and Bi3+ in perovskite could effectively improve the stability due to its enhanced Coulomb interaction energy, which leads to a high positive decomposition energy in Cs2AgBiBr6 (0.38 eV). A solar cell device with Cs2AgBiBr6 based absorbing layer can be a potential candidate for the greatest photovoltaic performance under optimum conditions. The Bi-based Cs2AgBiBr6 halide double perovskite is a suitable option for solar cells because it is lead-free and non-toxic. Cs2AgBiBr6 perovskite's applicability as an absorber layer is based on its excellent thermal and environmental stability, extended carrier recombination lifetime, high absorption coefficient, and correct bandgap (Gao et al. 2018). Numerous attempts have been made to improve the optoelectronic characteristics of Cs2AgBiBr6 PSCs, but the champion PCE of Cs2AgBiBr6 PSCs has been only 4.23% (Wang et al. 2018, 2012; Greul et al. 2017; Sirtl et al. 2022; Wang et al. 2021a, b), which is much lower than that of organic–inorganic hybrid lead-based PSCs. In this work, absorber layer has been engineered by various methods to optimize device perfformance.
Besides, absober ETL is another important layer in a PSC. When a hole-dominated device is furnished with a hole-blocking layer (ETL), the device's efficiency can be increased due to the electric field enhancement at the interface to the electron-injection contact. When choosing an ETL for PSCs, the significant elements to consider are electrical and optical characteristics, stability, and cost-effectiveness (Shukla et al. 2021). The ETL, which impacts the PV performance of PSCs, dominates the charge carrier extraction, transportation, and recombination processes. As a result, selecting an ETL with good band alignment, sufficient electron mobility, adequate light transmittance, and moisture resistance is critical (Wang et al. 2020). PSCs made of inorganic n-type metal oxides like TiO2 and ZnO are very efficient and stable (Li et al. 2019). Due to its good conduction band location, broad bandgap, and long carrier lifespan, TiO2 has been widely employed as the ETL for PSCs since 2009 (Kojima et al. 2009). Meanwhile, because of its good visual transparency, high electron mobility, large bandgap (3.4 eV), high thermal and electrochemical stability, low manufacturing cost, and great optoelectronic features, ZnO is a viable substitute for TiO2 as the ETL (Abdulrahman et al. 2020; Cao et al. 2020). This work considers several ETLs to optimize device performance.
Furher to enhance the performance of these ETLs, doping is one of the widely practised techniques. Mg-doped oxide-based ETL may show improved stability. Mg dopant addition may also improve conductivity and electron mobility rate. The films' crystallinity can be raised by doping Mg, and the optical absorbance can be further improved. Due to the creation of intermediate fermi states, magnesium significantly decreased band gap values from 3.37 to 2.9 eV (Arshad et al. 2021). This work analyses the effect of ETL thickness, Interface defect, and doping of Mg into TiO2 and ZnO on the PSC performance.
The Cs2AgBiBr6 double perovskite has been proposed as a lead-free perovskite for use as an active layer in solar cells in this study. The device model has been validated by comparing it with experimental devices (Zhao et al. 2021; Wang et al. 2021b). The effect of variation in thickness of ETL, Interface defect variation in the device structures have been studied and analyzed. Further, the effect of the doping concentration of Mg into ZnO layer and TiO2 layer in PSCs have been analyzed. The output performances of these layers have been compared by altering input parameters and results have been compared with experimental works. Further for the validation of the work and authenticity of the device structure, more input parameters have been changed. The device structure have been analyzed for absorber study by changing the thickness of absorber layer, effect of variation in series/shunt resistance and temperature. Further, the absorber's recombination mechanism and band diagram have been considered.
2 Device structure and simulation methodology
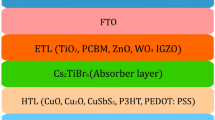
An n-i-p planar PSC structure as shown in Fig. 1, with FTO coated glass as the front contact, TiO2 (or as the ETL, Cs2AgBiBr6 as the light-absorbing material, Spiro-OMeTAD or Cu2O as the HTL, and Ag as the rear contact is shown in Fig. 1. Device has been initially calibrated using experimental parameters from literature Table 1 shows various experimental works as reported from literature with Cs2AgBiBr6 as absorber. Before proceeding towards, desigining various devices, device strtutures from experimental work has been simulated and further engineerimg has been done in the device. Device DS-a from Table 1 has been numerically simulated using SCAPS with TiO2 as the ETL, Cs2AgBiBr6 as absorber, and Spiro-OMeTAD as the HTL with corresponding parameters as reported in experimental works. The original structure's input parameters, which are listed in Table 1, the input parameter of FTO front contact and back contact had been obtained from theories and earlier works of literature (Pindolia et al. 2022).The device structure and performance parameters have been taken from the experimentally fabricated PSCs-based solar cell (Zhao et al. 2021; Wang et al. 2021b). Further, other HTL such as Cu2O has been tried as a possible replacement to obtain device DS-b.
Further several modifications in ETL and absorber with trying several materials have been done to optimize device performance. These materials must meet several characteristics to achieve improved device efficiency, including an appropriate bandgap, increased charge collection capacity, and correct thickness. Figure 2a shows the Current density vs Voltage (J–V) curve of devices from Table 1. J–V characteristics of Device DS-a agree to the experimentally reported work (Zhao et al. 2021). This helped us to calibrate the device for further study in this work. Figure 2b shows energy level diagram of different HTL, ETL, absorber and contacts for this study. The inorganic HTLs show higher current density as compared to Spiro-OMeTAD which is organic HTL. Also organic HTL has less hole mobilities. Higher the charge mobility, higher will be the current density. This is the reason of high JSC of inorganic HTLs.
a The Current density versus Voltage (J–V) curve of devices from Table 1. b Energy bands for different HTL, ETL, Cs2AgBiBr6, front contact and back contact
The Solar Cell capacitance Simulator (SCAPS) is implemented, in this research. SCAPS version 3.3.10 ("SCAPS3310") of April 2021, and subsequent versions of SCAPS by University of Gent has been taken in device modelling (Burgelman et al. 2000). It is utilised for modelling and simulating different solar structures. The SCAPS programme uses the continuity equations for electrons, holes, and Poisson's equation, which are three essential semiconductor equations (Niemegeers and Burgelman 1996).
where \(G_{n}\), \(G_{p}\) indicates the generation rate of electrons and holes, \(\mu_{n} \) and \(\mu_{p}\) are electron and hole mobilities, ξ is the electric field, D is diffusion coefficient, τ is the life time of electrons and holes, ε is permittivity,ψ is electrostatic potential, q is the electron charge, \(p_{t} \left( x \right) and n_{t\left( x \right)}\) are the concentrations of holes and electrons, \(N_{D} and N_{A}\) are shallow donor and acceptor concentrations.
SCAPS-1D software (Solar Cell Capacitance Simulator One Dimension) numerically solves one-dimensional equations that govern the semiconductor materials under steady-state conditions. It can help in simplifying and understanding solar cell basics. This also aids in identifying the significant parameters that affect their performance. The SCAPS-1D simulator has been used to analyze the PSC. The different types of device structures have been simulated at Standard Test Conditions (STC) of AM1.5G, 1000 W/m2, and T = 300 K.
3 Results and discussion
The primary goal of the current research project is to simulate and optimize the solar cell parameters such as PCE, JSC, VOC, and FF and well-matched with experimental reported efficient device (Zhao et al. 2021)(Wang et al. 2021b). In order to do this, the various device parameters (thickness, Interface defect, and doping of Mg into TiO2 and ZnO) have been analyzed for the best PCE using the SCAPS-1D simulator. After initially calibrating the device, several other device structures as per Table 2 have been analyzed. Table 3 (Azri et al. 2019; Mohandes et al. 2021) summarises the structure's input parameters (HTL, contacts and absorber) and Table 4 shows the paramters of the various ETL materials. In Sect. 3.1, the effect of variation in thickness of ETL and in Sect. 3.2, Interface defect variation in the Device Structures DS-1, DS-2, and DS-3 has been studied and analyzed. In Sect. 4.1, the effect of doping concentration of Mg into TiO2 layer in Device Structure, DS-8 to DS-10, further, in Sect. 4.2, the effect of doping concentration of Mg into ZnO layer in Device Structure DS-4 to DS-7 have been analyzed. In Sect. 4.4. effect of electrical properties with doping of Mg into ETL have been discussed. Section 5 discusses the electrical and thermal properties of devices DS-1, DS-2 and DS-3. Sections 5.1, 5.2 and 5.3 discusses the effect of variation in series resistance, effect of variation in shunt resistance respectively and the effect of variation in temperature have been respectively studied. In Sect. 6 study of the absorber layer has been performed for various analysis. In Sect. 6.1, the effect of variation in thickness of the absorber layer, in the 6.2 recombination mechanism, and in 6.3 electric properties at device level for absorber have been done. Further comparative study of all the devices designed in this work along with those reported in experimental works from literature, has been discussed.
The device structures DS-2-based perovskite active material have been suggested for the investigation. The commonly utilised substance Spiro-OMeTAD was initially selected as HTL in this study, which utilised TiO2, ZnS and ZnO as ETL. After that, Cs2AgBiBr6 PSCs were simulated to look into the photovoltaic (PV).
performance based on high-quality thin films. Cu2O was used as the material for the hole transport layer for greater efficiency. Both the TiO2/Cs2AgBiBr6/Spiro-OMeTAD and the TiO2/Cs2AgBiBr6/Cu2O solar cells were simulated for comparison.
3.1 Effect of variation in thickness of ETL
Electron transport layers and Hole transport layers are crucial for achieving increased PCE. With fall in charge carriers in ETL and HTL, there is a reduction in short circuit; however it also provides a sustainable approach for charge carriers to reach the output signal. The material must meet a number of characteristics to achieve improved device efficiency, including an appropriate charge collection capacity and correct thickness. By keeping the thickness of both the HTL and the absorber unchanged at 350 nm and 150 nm respectively, for TiO2/Cu2O/Cs2AgBiBr6 (DS-1), ZnS/Cu2O/Cs2AgBiBr6 (DS-2), ZnO/Cu2O/Cs2AgBiBr6 (DS-3) the thickness of the ETL has been varied from 10 to 60 nm. Figure 3a–d shows the variation of JSC, VOC, FF and PCE with ETL thickness change. For DS-1, DS-2, and DS-3 the fluctuation of current density is shown in Fig. 3a. The device structure shows that whenever the ETL changes from 10 to 60 nm, a little decrement of JSC value occurs for all device structures. For DS-1, VOC initially increases and then saturates because an extremely thick ETL layer will significantly add and then provide opportunities for recombination for the charges, whereas a very thin ETL will be ineffective at blocking holes. For DS-2, VOC reduces from 1.2167 to 1.2 V; for DS-3, VOC reduces from 1.22 to 1.2161 V as shown in Fig. 3b. As shown in Fig. 3c, there is minimal effect on the field factor for DS-1, DS-2, and DS-3. For DS-1, PCE increases from 5.64 to 5.72% as ETL thickness lowers. For DS-2, no significant change is observed in PCE, as seen in Fig. 3d. The equipment efficiency is 5.78% at all thickness values. For DS-3, PCE drops from 5.73 to 5.69% as ETL thickness increases. The decrement in JSC is due to the formation of extensive pinholes and uneven surfaces. On increasing the thickness, electron–hole pair recombination and series resistance increased, resulting in a decrement of the output performance parameters. These properties can be calculated by experimental techniques using a modified solvent engineering technique (You et al. 2016).
3.2 Interface defects variation
For a device to operate effectively, the junction's quality must be excellent since imperfections at the interface will increase the recombination rate, resulting in subpar performance. The best method for analyzing the interface defects is to put a thin layer at the interface because modelling recombination at the interface is quite challenging. Two thin interface layers are inserted between the absorber/electron transport layer (IL1) and the absorber/hole transport layer (IL2). One interface has been inserted using the SCAPS tools at the IL1 and IL2 junctions. The higher recombination rate causes instability and is responsible for the reduction in overall efficiency (Cao et al. 2020).
The defect densities at the interface in this simulation research range from 1 × 1014 to 1 × 1018 cm−2 and from 1 × 1010 to 1 × 1016 cm−2 for IL1 and IL2 respectively to improve the device efficiency of PSCs. The output performance efficiency, FF, JSC, and VOC are depicted in Figs. 4 and 5. As the density of interfacial defects rises, all values fall. This demonstrates the significance of interface in developing highly effective perovskite-based photovoltaic systems. The above statistics show that the fault density at the junction of IL1 significantly affects the device's performance. However, the defect density at the IL2 interface has little impact on the cell's functionality. This is because the IL1 generates more electron–hole pairs than the IL2 junction. The higher carrier density causes a higher recombination rate at the IL1 junctions. Because of this, IL1 perovskite junction has a more significant impact on device performance than IL2.
3.3 Other potential oxide and sulphide materials as ETLs
Our work's primary focus has been looking for prominent transparent conducting oxides as potential candidates for our work. In this section, other device structures' performances have been discussed with change in ETL materials. Few other potential oxides such as WO3 and IGZO and few sulphides such as WS2. CdS, CdZnS have been tried as ETLs. Keeping other layers the same as for other devices, like HTL being Cu2O with Cs2AgBiBr6 as the absorber, ETL material has been modified. By keeping the thickness of both the HTL and the absorber unchanged at 350 nm and 150 nm, respectively, device DS-11, DS-12, DS-13, DS-14 and DS-15 from Table 2 have been designed with WO3. IGZO, WS2. CdS, CdZnS as ETLs respectively. Figure 6a and b shows the study of variation of JSC, VOC, FF and PCE with change in the thickness of the ETL.
a Change in JSC, VOC, FF, PCE with variation in thickness for DS-11 with WO2 as ETL. b Change in JSC, VOC, FF, PCE with variation in thickness for DS-12 with IGZO as ETL, c Change in JSC, VOC, FF, PCE with variation in thickness for DS-13 with WS2 as ETL. d Change in JSC, VOC, FF, PCE with variation in thickness for DS-14 with CdZnS as ETL. e Change in JSC, VOC, FF, PCE with variation in thickness for DS-15 with CdS as ETL
4 Impact of Mg doping %age on TiO2 and ZnO layer
Further to analyse the effect of doping of Mg into oxide layer, in this study, transfer of Mg atoms into the ETL layer using different doping levels have been considered. Doping Mg into TiO2 and ZnO create Mg oxide (MgO) clusters and improve device efficiency. MgO has a broad band gap (7.7 eV) (Yousefi et al. 2013; Yousefi & Kamaluddin 2009) Because Mg has a lower electronegativity than other ions (χ = 1.31) and MgO has a large band gap (7.7 eV), it can combine with oxygen more readily to form a solid crystal structure (Kwak et al. 2016). Additionally, to reduce flaws in Mg-doped oxides, Mg atoms can mend dangling bonds and replace vacancies (Wang et al. 2016). Since the ionic radius of a Mg2+ ion is 0.66, being similar to Zn2+ (0.74), it can easily replace the Zn position in a ZnO lattice without appreciably altering the lattice size. On the other hand, the MgO energy gap causes the ZnO energy gap to grow since it is 7.7 eV more than the ZnO energy gap (Kharatzadeh et al. 2016). Additionally, Mg-doped ETL will alter oxygen vacancies and produce more electron–hole pairs, considerably enhancing ETL nanostructures' photocatalytic activity and perhaps raising the sensing film's adsorption capacity. To analyse the doping effect, device performance has been studied by variation Mg doping levels in the following Sects. 4.1 (For TiO2) and 4.2 (For ZnO).
4.1 Impact of Mg doping at significant amounts in TiO2 layers
This section examines the impact of an Mg-doped TiO2 compact layer on a PSC's performance. Experimental data for the bandgap of the layer with various doping levels have been taken to analyze the effect of varying Mg doping concentrations in the TiO2 compact layer, (Wang et al. 2015). Table 5 shows the information for adjusting the bandgap. As can be observed from Table 4, adding Mg to the TiO2 structure widens the bandgap.
The bandgap variations in TiO2 (3.2 eV) (Yadav et al. 2007) and MgO (7.8 eV) (Dette et al. 2014) lead to bandgap widening. Figure 6 shows variation in JSC, VOC, FF, PCE for devices DS-8, DS-9 and DS-10 with pure and Mg-doped TiO2 compact layers. As shown in Fig. 6, adding Mg to the Hole blocking layer (HBL) as an additive (up to 10%) enhances the efficiency of the PSC. The Mg-doped TiO2 layer was employed to boost PSC with an organic–inorganic Ch3NH3PbI3 absorber layer(Wang et al. 2015). In this work, Mg-doped ETL with a Cs2AgBiBr6 absorber has been employed to boost the PCE. The PCE of devices based on pure TiO2, 0.05 and 0.10 Mg-doped TiO2 layers are 5.71%, 5.72%, and 5.72%, respectively. The Hole Blocking Layer (HBL) in the structure is Mg-doped TiO2, which decreases the rate of electron and hole recombination. As shown in Fig. 7, enhancing the value of Mg in the HBL enhances the cell's JSC and VOC, implying that the recombination rate is reduced and thus more electrons are injected from the absorber layer into the HBL. By doping Mg the PSC films' crystallinity has been raised, and the optical absorbance has also been improved. Due to the creation of intermediate fermi states, magnesium significantly decreased band gap values from 3.37 to 2.9 eV (Arshad et al. 2021). Additionally, Mg-doped TiO2-based ETL showed improved stability. Mg dopant addition also improved conductivity and electron mobility rate. Mg as a dopant in TiO2 may increase the bandgap by causing the Conduction Band minimum (CBM) to move to better energy levels, hence increasing the bandgap. As a result, increasing CBM toward the absorber's level promotes electron injection from the absorber into the compact layer, resulting in improved cell performance.
4.2 Effect of doping concentration of Mg into ZnO layer
This section examines the impact of an Mg-doped ZnO compact layer on a PSC's performance. Experimental studies have been used to determine the bandgap energies of compact layers with various dopant concentrations (Etacheri et al. 2012) and used in our simulation. Table 6 shows the bandgap energies for ZnO layers with various Mg doping levels. Figure 7 shows variation in JSC, VOC, FF, PCE for devices DS-4, DS-5, DS-6 and DS-7 with pure and Mg-doped ZnO compact layers The efficiency of the cell with mildly Mg-doped (up to 10% Mg) is improved, as illustrated in Fig. 7. The cell's efficiency with a pure ZnO compact layer is 5.7%, while the cell's efficiency with 2, 5, 10, and 20% Mg-doped ZnO is 5.7%, 5.71%, 5.72%, and 5.74% respectively. The FF of the structure with a pure and 0.2 Mg-doped ZnO layer enhanced from 54.57 to 54.59%. The device structure having 2, 5, and 10% Mg-doped ZnO compact layers have FFs of 54.57%, 54.58%, and 54.58%, respectively. Adding magnesium to the ZnO structure pushes the CBM toward the absorber's level, making electron injection from the absorber to the compact layer easier.
Previously, the influence of a higher Mg concentration in the TiO2 structure for use in perovskite solar cells had been explored, and it had been discovered that a higher Mg density in the TiO2 structure would improve the perovskite solar cell's performance. According to our simulation results, 20% Mg in the ZnO structure increases the cell's efficiency, and the ideal level of Mg dopant, according to our estimates, is The increase in efficiency of the cell with a doping level of 20% Mg is because this doping level raises the conduction band minimum (CBM) of the HBL by 0.41 eV. A closer CBM of the HBL of the absorber may facilitate the electron injection from the absorber to the HBL. When CBM goes upward, on the other hand, it goes away from the CBM layer. As a result, it decreases the recombination rate inside the HBL and higher electron injection from the HBL's CBM. The fluctuation of the cell's VOC and JSC with pure and Mg-doped ZnO HBLs is also shown in Fig. 8. When the amount of Mg in the compact layer is raised, the 20%. JSC of the cell goes toward higher JSC. In fact, the presence of Mg impurity in the ZnO structure causes the bandgap energy to broaden, bringing the CBM closer. As a result, electrons would flow faster from the absorber's level to the compact layer's conduction band. However, the cell's VOC remains constant when the Mg level increases. The VOC for pure ZnO and Mg-doped ZnO models are both 1.216 V.
4.3 Comparing pure and Mg-doped TiO2 and ZnO compact layers
Researchers previously demonstrated the best device performance by utilizing a TiO2 blocking layer (Zhou et al. 2014; Yella et al. 2014). However, because of its abundance in nature, ease of manufacture, low cost, and high electron mobility, the ZnO blocking layer has got much attention (Paquin et al. 2015). As a result, higher cell efficiency and higher VOC for the ZnO HBL cell are expected. Using the ZnO blocking layer resulted in superior cell performance 5.74% versus 5.70%.
Figure 9. depicts the improvement in the efficiency of perovskite solar cells with various blocking layers. The improvement for the cell with 10% Mg-doped TiO2 compact layer is higher than that for the cell with up to 10% Mg-doped ZnO layer. At 20% Mg-doped ZnO the power conversion efficiency is 5.74%. The results suggest that utilizing a particular quantity of Mg impurity to boost the efficiency of the ZnO HBL cell is more successful than using TiO2 HBL.
4.4 Effect of doping of Mg into ETLs upon electrical properties
The device structure with various Mg doping levels (2%, 5%, 10% and 20%) having the bandgap (3.32 eV, 3.37 eV, 3.45 eV, 3.71 eV) respectively has been designed for different series and shunt resistance. The series resistance has been varied from 1 to 5 Ω cm2 in order to analyze the impact of doping on PSC performance Fig. 10 shows variation in JSC, VOC, FF, PCE for devices DS-4, DS-5, DS-6 and DS-7 Mg-doped ZnO compact layers. The efficiency of the cell decreases mildly with increase in Mg-doping as illustrated in Fig. 9. The cell's efficiency at 1 Ω cm2 is 5.69% and at 5 Ω cm2 is 5.52%. The FF of the structure with 20% doping is 52.66%. Adding magnesium to the ZnO structure electron injection from the absorber to the compact layer becomes easier. Due to the various charge recombination shunt resistance develops. To analyse the effect of change in shunt resistance, on ETL doping shunt resistance has been changed from 1000 to 8000 Ω cm2. The fluctuations in device parameters with series and shunt resistance are shown in Fig. 10(a) and 10(b) respectively. It has been found that as shunt resistance rises, PSC efficiency also rises. PCE = 5.64% and FF = 53.72% at 7000 Ω cm2, yet at 8000 Ω cm2, PCE stays at 5.64% and FF rises to 53.82%. Therefore, 7000 Ω cm2 can be considered as the optimum shunt resistance.
5 Study of electrical and thermal properties of the device
This section discusses the electrical and thermal properties of DS-1, DS-2 and DS-3. For the device's stability for variations in electrical and thermal conditions, the device needs to be tested with variation in temperature and electrical properties.
5.1 Effect of variation in series resistance
The front contact (FTO), HTL, perovskite layer, ETL, and back contact (Ag) provides the device's electrical resistance. The device's performance has been analyzed by changing the series resistance from 1 to 5 Ω cm2 in order to analyze the impact of series resistance on PSC performance. The fluctuation in device parameters with series resistance is shown in Fig. 11. With an increase in series resistance, VOC remains constant, JSC changes slightly, however, FF and PCE fall off. Therefore, a series resistance with a lower value performs better.
5.2 Effect of variation in shunt resistance
Shunt resistance develops in the PSC through a variety of charge recombination pathways. In order to analyze the effect of shunt resistance on PSC performance, the device's operation has been analyzed by varying the shunt resistance from 1000 to 8000 Ω cm2. The fluctuation in device parameters with shunt resistance is shown in Fig. 12. It has been found that as shunt resistance rises, PSC efficiency also rises. PCE = 5.66% and FF = 54.12% at 7000 Ω cm2, yet at 8000 Ω cm2, PCE stays at 5.66% and FF rises to 54.12%. Therefore, 7000 Ω cm2 can be considered as the optimum shunt resistance.
5.3 Effect of variation in temperature
Further, the performance of the PSC with variation in the temperature from 280 to 400 K has been analyzed in order to comprehend how the PSC behaves with changes in environmental temperature. Figure 13 illustrates the variation of device performance with temperature. JSC and PCE all decrease as temperature rises. The reason for this drop in performance is that as temperature rises, the saturation current and recombination rate rise (Ahmed et al. 2021).
6 Study of absorber layer of device
Till now, the output performance of a lead-free perovskite solar cells in this study has been analysed by varying the thickness of ETL, Interface defect in the Device Structures DS-1, DS-2, and DS-3. Further, the effect of the doping concentration of Mg into the ZnO layer (DS-4, DS-5, DS-6 and DS-7) and the effect of the doping concentration of Mg into the TiO2 layer (DS-8, DS-9 and DS-10) in device structure has been analyzed. These layers' output performances and results have been compared with experimental works as shown in Table 7.
In this section, the study has been done for the absorber layer. The absorber being the most significant layer of PSC has to be thoroughly analyzed. For the Device Structures DS-1, DS-2, and DS-3, absorber study have been performed by considering the effect of variation in thickness of the absorber layer. Further, the effect of variation in series/shunt resistance and temperature has been analyzed for these devices. Thereafter, the absorber's recombination mechanism and band diagram have been studied to understand the electrical properties.
6.1 Effect of variation in thickness of absorber layer
The absorber thickness is one of the important, influential elements in device efficiency. The influence of absorbent layer thickness on system performance has been explored by varying absorbent layer thicknesses in the range of 150 nm to 250 nm as shown in Fig. 14. The findings reveal that the perovskite material absorbs a larger number of photons at the optimal width of ETL. JSC increases as the absorber layer's thickness increases, giving better conductivity at longer wavelengths. As a result, the number of electron–hole pairs in the device increases, which improves its performance. It has been observed that the thickness of perovskite absorbing material must be less than the electron and hole diffusion lengths (Ke et al. 2017). It enables electrons and holes to reach their respective electrodes, resulting in power generation. As a result of increasing the layer thickness, more light is absorbed, resulting in increased JSC, VOC, FF, and PCE. Increasing the thickness of the absorbent layer, on the other hand, reduces the back contact recombination current density (Liu et al. 2017). As a result, some of the photo-generated electron–hole pairs recombine at the back contact, causing the back contact recombination current density to decrease with each increment of absorbent layer thickness. For DS-1, DS-2 and DS-3, the ETL and HTL layers' thickness remains unchanged (i.e. at 350 nm for HTL and 50 nm for ETL), whereas absorber layer thickness varies from 150 to 250 nm. In DS-1, DS-2 and DS-3, thickness variation is most significant as the PCE decreases when absorber thickness increases due to the adequate energy band alignment. For DS-1, DS-2 and DS-3, the peak value for PCE are 6.16%, 6.19% and 6.30%, respectively at 50 nm absorber thickness. Figure 14c shows a very significant effect of absorber layer thickness variation on the FF in DS-1, DS-2 and DS-3 due to high series resistance. With the increase in absorber layer thickness in DS-1, DS-2 and DS-3, JSC increases significantly due to more absorbed energy in this layer, which creates more electron–hole pairs, as illustrated in Fig. 14b. For all device structures DS-1, DS-2 and DS-3, the VOC changes from 1.24 to 1.19 V at 50 nm and 250 nm, respectively, showing the minimal effect of absorber layer thickness variation.
6.2 Recombination mechanism for absorber
Three types of recombination, including radiative recombination, Auger electron, and hole capture coefficient, are mostly considered. The maximum spectral brightness of semiconductors with low defect density is often slightly greater than the bandgap; nonetheless, the radiative recombination in the absorber occurs at energies lower than the bandgap values. As a result, at this energy level, radiative recombination has a large density of states, which in fact restricts the open circuit voltage in absorbers. The radiative recombination coefficient (Br) varies depending on whether a semiconductor has a direct or indirect bandgap. Br values for direct and indirect bandgap semiconductors commonly fall between 10–11 to 10–9 cm3 s−1 and 10–15 to 10–13 cm3 s−1, respectively (Niemegeers et al. 2014). Radiative recombination is one of the main concerns limiting the efficiency in polycrystalline solar cells. As a result, here radiative recombination values have been tuned and optimised for the optimized Device DS-2. Radiative recombination for the absorber layer is plotted against PCE, JSC, FF values ranging from 10–5 to 10–12 in Fig. 15a. In Fig. 15b and c, Auger electron/hole recombination variations from 10–20 to 10–28 are shown, and in Fig. 15d the open circuit voltage for all band-to-band recombination is shown. According to estimates, Br has stabilised to around 10–8, and for Auger electron/hole capture recombination, it has stabilised between 10–24 and 10–26 respectively.
6.3 Electrical study of absorber Cs2AgBiBr6 at semiconductor level
In this article, device structure cell's photovoltaic performance (efficiency) is examined in relation to its electrical characteristics. Figure 16. shows the band diagram of Cs2AgBiBr6. One of the key factors affecting the performance of solar cells and the current transport across the heterojunction is band alignment. By changing the elemental composition of absorber, which further takes a positive or negative band offset value depending on the absorber layer bandgap, the energy difference of the conduction band can be changed. Further, the band diagram of absorber has been analyzed further to understand tuning the efficiency with change in electric field for the absorber of device.
The internal electric field produced by the p–n junction, which ought to be similar to the depletion-based calculation with Ec the conduction, an effective electric field (Eeff) from Eq. 4 that depicts the conduction's impact Eeff is shown in Fig. 17 alongside the internal electric field that SCAPS-1D estimated for an absorber depth of 0–0.6 um. Figure 17. shows the absorber internal electric field calculated by SCAPS-1D and notch-created effective electric field Eeff as a function of the depth. A red line represents the electric field as computed by SCAPS. At the stated depth position, the electric field linear approximation crosses the absolute value of the depth generated electric field.This leads us to the conclusion that the generation rate rises sharply with depth position as anticipated. The recombination rate in the device structure greatly rises when the depth is in it, even though in an ideal scenario we should observe a total collection of carriers generated in the depletion region, as demonstrated by our analysis of carrier collection and recombination rate.
7 Comparative study of designed devices with different experimentally fabricated PSCs
Comparative analysis of efficiency and device parameters for experimental, simulated, and proposed works reported in the literature with this work has been shown in Table 6. Different experimental works (Zhao et al. 2021; Wang et al. 2021b) and simulated work (Alam et al. 2021) on double perovskite-based devices report various device structures having different ETLs with corresponding device parameters. Considering experimentally reported works, device TiO2/Cs2AgBiBr6/Spiro-OMeTAD shows the highest PCE of 4.23%; (Wang et al. 2021b). However, with our optimization and device engineering, PCE of device with same device structure (DS-1) using Cu2O as HTL, PCE increased to 6.16%. Among several TCOs as tried in this work to discard sulphur from ETLs, such as TiO2, ZnO, WO3, IGZO, TiO2 performs best in terms of PCE. With the doped verions of ZnO and TiO2 with Mg (Device DS-3 to DS 10), although there is not much difference in PCE, but Mg-doped ETL will alter oxygen vacancies and produce more electron–hole pairs, considerably enhancing ETL nanostructures' photocatalytic activity and perhaps raising the sensing film's adsorption capacity, which may help achieve more enhanced PCE experimentally.
Among Suplhor based ETLs as well as among our work on different PSCs (DS-1 to DS-15), device DS-2 with ZnS as ETL shows the highest PCE of 6.30%. However, considering sulphur a hazardous material, TCO alternatives can be considered as discussed in this work. ZnS as ETL has been considered for proposed device structures in this work, as ETL has been reported to be better than ZnO, TiO2 in literature with improved stability and no appreciable hysteresis in PSC (Chen et al. 2019b, a). Further, simulated work reported lead-free double PSCs (Alam et al. 2021) device ZnO/Cs2AgBiBr6/P3HT shows PCE of 4.48%, which is significantly low. Hole-blocking layers (HBLs) on perovskite solar cells made of Mg-doped ETL outperform in a number of aspects, including greater open-circuit voltage (VOC) (1.21 V), power conversion efficiency (5.74%), short-circuit current, and fill factor. Table 8 shows the best performing devices in our work.
8 Conclusion
In this work, the PCE of PSCs with Cs2AgBiBr6 as absorber has been enhanced using various device engineering methods. For absorber study, thickness modulation and other electrical studies including the effect of recombination mechanism and electric field has been performed. Besides absorber, work also focuses on sulphide Electron Transport Layers (ETLs) such as ZnS, WS2, CdS, CdZnS and oxide ETLs such as TiO2, ZnO, WO3, IGZO and other related device analysis methods, significantly impacting cell performance. By tuning the thickness, interface defects, and doping of ETL material (with Mg), the solar cell design has been analyzed for optimum performance. In our work on different PSCs, sulphide ETL-based PSC (ZnS/Cs2AgBiBr6/Cu2O) shows the highest PCE of 6.3%. Further, as sulhide ETL is replaced by oxide ETLs, Mg-doped ZnO based PSC still reaches more than 6% of PCE. These results are quite motivating as experimentally reported literature on Cs2AgBiBr6-based PSC, is still reaching the PCE of around 4%. Optimum doping of Mg into oxide ETLs at 10% and at 20% of for device structures TiO2/Cs2AgBiBr6/Cu2O and ZnO/Cs2AgBiBr6/Cu2O have significantly improved the performance of the PSC device to 5.72% and 5.74% respectively. Besides, using TCOs as ETLs can be important to realizing transparent PSCs. In the future, to create eco-friendly PSC, there should be a continuous effort to remove lead and sulphur elements from PSCs without compromising with PCE. This work may prove to be a significant contribution in this direction.
Data availability
Not applicable.
References
Abdulrahman, A.F., Ahmed, S.M., Ahmed, N.M., Almessiere, M.A.: Enhancement of ZnO nanorods properties using modified chemical bath deposition method: effect of precursor concentration. Crystals 10, 386 (2020). https://doi.org/10.3390/cryst10050386
Ahmed, S., Jannat, F., Khan, M.A.K., Alim, M.A.: Numerical development of eco-friendly Cs2TiBr6 based perovskite solar cell with all-inorganic charge transport materials via SCAPS-1D. Optik (Stuttg) 225, (2021). https://doi.org/10.1016/j.ijleo.2020.165765
Alam, I., Mollick, R., Ashraf, M.A.: Numerical simulation of Cs2AgBiBr6-based perovskite solar cell with ZnO nanorod and P3HT as the charge transport layers. Phys. B Condens. Matter. (2021). https://doi.org/10.1016/j.physb.2021.413187
Arshad, Z., Khoja, A.H., Shakir, S., Afzal, A., Mujtaba, M.A., Soudagar, M.E.M., Fayaz, H., Saleel, C.A., Farukh, S., Saeed, M.: Magnesium doped TiO2 as an efficient electron transport layer in perovskite solar cells. Case Stud. Therm. Eng. 26, 101101 (2021). https://doi.org/10.1016/j.csite.2021.101101
Azri, F., Meftah, A., Sengouga, N., Meftah, A.: Electron and hole transport layers optimization by numerical simulation of a perovskite solar cell. Sol. Energy 181, 372–378 (2019). https://doi.org/10.1016/j.solener.2019.02.017
Bansal, S., Aryal, P.: Evaluation of new materials for electron and hole transport layers in perovskite-based solar cells through SCAPS-1D simulations. 2017 IEEE 44th Photovolt. Spec. Conf. PVSC 2017, 3220–3223 (2017). https://doi.org/10.1109/PVSC.2017.8366107
Burgelman, M., Nollet, P., Degrave, S.: Modelling polycrystalline semiconductor solar cells. Thin Solid Films 361, 527–532 (2000). https://doi.org/10.1016/S0040-6090(99)00825-1
Cao, Z., Li, C., Deng, X., Wang, S., Yuan, Y., Chen, Y., Wang, Z., Liu, Y., Ding, L., Hao, F.: Metal oxide alternatives for efficient electron transport in perovskite solar cells: Beyond TiO2 and SnO2. J. Mater. Chem. A 8, 19768–19787 (2020). https://doi.org/10.1039/d0ta07282f
Chen, J., Luo, Z., Fu, Y., Wang, X., Czech, K.J., Shen, S., Guo, L., Wright, J.C., Pan, A., Jin, S.: Tin(IV)-tolerant vapor-phase growth and photophysical properties of aligned cesium tin halide perovskite (CsSnX3; X = Br, I) nanowires. ACS Energy Lett. 4, 1045–1052 (2019a). https://doi.org/10.1021/acsenergylett.9b00543
Chen, R., Cao, J., Duan, Y., Hui, Y., Chuong, T.T., Ou, D., Han, F., Cheng, F., Huang, X., Wu, B., Zheng, N.: High-efficiency, hysteresis-less, UV-stable perovskite solar cells with cascade ZnO–ZnS electron transport layer. J. Am. Chem. Soc. 141, 541–547 (2019b). https://doi.org/10.1021/jacs.8b11001
Das, S., Chakraborty, K., Choudhury, M.G., Paul, S.: Studies on thickness and internal quantum efficiency of Cs2AgBiBr6 based double perovskite material for photovoltaic application. J. Nano Electron. Phys. 13, 3018 (2021). https://doi.org/10.21272/jnep.13(3).03018
Dette, C., Pérez-Osorio, M.A., Kley, C.S., Punke, P., Patrick, C.E., Jacobson, P., Giustino, F., Jung, S.J., Kern, K.: TiO2 anatase with a bandgap in the visible region. Nano Lett. 14, 6533–6538 (2014). https://doi.org/10.1021/nl503131s
Etacheri, V., Roshan, R., Kumar, V.: Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis. ACS Appl. Mater. Interfaces 4(5), 2717–2725 (2012)
Gao, W., Ran, C., Xi, J., Jiao, B., Zhang, W., Wu, M., Hou, X., Wu, Z.: High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2 % efficiency. ChemPhysChem 19, 1696–1700 (2018). https://doi.org/10.1002/cphc.201800346
Greul, E., Petrus, M.L., Binek, A., Docampo, P., Bein, T.: Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 5, 19972–19981 (2017). https://doi.org/10.1039/c7ta06816f
Jeon, N.J., Noh, J.H., Yang, W.S., Kim, Y.C., Ryu, S., Seo, J., Seok, S.I.: Compositional engineering of perovskite materials for high-performance solar cells. Nature 517, 476–480 (2015). https://doi.org/10.1038/nature14133
Ke, W., Stoumpos, C.C., Zhu, M., Mao, L., Spanopoulos, I., Liu, J., Kontsevoi, O.Y., Chen, M., Sarma, D., Zhang, Y., Wasielewski, M.R., Kanatzidis, M.G.: Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en}FASnI3. Sci. Adv. 3, 1–10 (2017). https://doi.org/10.1126/sciadv.1701293
Kharatzadeh, A., Jamali-Sheini, F., Yousefi, R.: Excellent photocatalytic performance of Zn(1 - x)MgxO/rGO nanocomposites under natural sunlight irradiation and their photovoltaic and UV detector applications. Mater. Des. 107, 47–55 (2016). https://doi.org/10.1016/j.matdes.2016.06.007
Kojima, A., Teshima, K., Shirai, Y., Miyasaka, T.: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). https://doi.org/10.1021/ja809598r
Kwak, C.H., Woo, H.S., Abdel-Hady. F., et al.: Vapor-phase growth of urchin-like Mg-doped ZnO nanowire networks and their application to highly sensitive and selective detection of ethanol. Sensors Actuators, B Chem 223, 527–534 (2016). https://doi.org/10.1016/j.snb.2015.09.120
Li, Z., Wang, R., Xue, J., Xing, X., Yu, C., Huang, T., Chu, J., Wang, K.L., Dong, C., Wei, Z., Zhao, Y., Wang, Z.K., Yang, Y.: Core-shell ZnO@SnO2 nanoparticles for efficient inorganic perovskite solar cells. J. Am. Chem. Soc. 141, 17610–17616 (2019). https://doi.org/10.1021/jacs.9b06796
Liu, Y., Li, X., Wang, J., Xu, L., Hu, B.: An extremely high power factor in Seebeck effects based on a new n-type copper-based organic/inorganic hybrid C6H4NH2CuBr 2I film with metal-like conductivity. J. Mater. Chem. A 5, 13834–13841 (2017). https://doi.org/10.1039/c7ta03015k
Mohandes, A., Moradi, M., Nadgaran, H.: Numerical simulation of inorganic Cs2AgBiBr6 as a lead-free perovskite using device simulation SCAPS-1D. Opt. Quantum Electron. 53, 1–22 (2021). https://doi.org/10.1007/s11082-021-02959-z
Ngoupo, A.T., Ouédraogo, S., Zougmoré, F., Ndjaka, J.M.B.: Numerical analysis of ultrathin Sb2Se3-based solar cells by SCAPS-1D numerical simulator device. Chin. J. Phys. 70, 1–13 (2021). https://doi.org/10.1016/j.cjph.2020.12.010
Niemegeers, A., Burgelman, M.: Numerical modelling of ac-characteristics of CdTe and CIS solar cells. In: Conference Record of the Twenty Fifth IEEE Photovoltaic Specialists Conference-1996, pp. 901–904. (1996). https://doi.org/10.1109/pvsc.1996.564274
Niemegeers, A., Burgelman, M., Decock, K., Verschraegen, J., Degrave, S.: SCAPS manual. University of Gent, 13 (2014)
NREL, Best Research-Cell Efficiency Chart, in, 2022, Available: https://www.nrel.gov/pv/cell-efficiency.html
Paquin, F., Rivnay, J., Salleo, A., Stingelin, N., Silva, C.: Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 3, 10715–10722 (2015). https://doi.org/10.1039/b000000x
Pindolia, G., Shinde, S.M., Jha, P.K.: Optimization of an inorganic lead free RbGeI 3 based perovskite solar cell by SCAPS-1D simulation. Sol. Energy 236, 802–821 (2022)
Rafieirad, R., Ganji, B.A.: Efficiency improvement of perovskite solar cells by utilizing cuins thin layer: modeling and numerical study. IEEE Trans. Electron Devices. 68, 4997–5002 (2021). https://doi.org/10.1109/TED.2021.3102536
Rai, N., Rai, S., Singh, P.K., Lohia, P., Dwivedi, D.K.: Analysis of various ETL materials for an efficient perovskite solar cell by numerical simulation. J. Mater. Sci. Mater. Electron. 31, 16269–16280 (2020). https://doi.org/10.1007/s10854-020-04175-z
Salem, M.S., Shaker, A., Othman, M.S., Al-Bagawia, A.H., Fedawy, M., Aleid, G.M.: Numerical analysis and design of high performance HTL-free antimony sulfide solar cells by SCAPS-1D. Opt. Mater. (amst). (2022). https://doi.org/10.1016/j.optmat.2021.111880
Schade, L., Mahesh, S., Volonakis, G., Zacharias, M., Wenger, B., Schmidt, F., Kesava, S.V., Prabhakaran, D., Abdi-Jalebi, M., Lenz, M., Giustino, F., Longo, G., Radaelli, P.G., Snaith, H.J.: Crystallographic, optical, and electronic properties of the Cs2AgBi1−xInxBr6 double perovskite: understanding the fundamental photovoltaic efficiency challenges. ACS Energy Lett. 6, 1073–1081 (2021). https://doi.org/10.1021/acsenergylett.0c02524
Shi, Z., Guo, J., Chen, Y., Li, Q., Pan, Y., Zhang, H., Xia, Y., Huang, W.: Lead-free organic-inorganic hybrid perovskites for photovoltaic applications: recent advances and perspectives. Adv. Mater. (2017). https://doi.org/10.1002/adma.201605005
Shukla, R., Kumar, R.R., Pandey, S.K.: theoretical study of charge carrier lifetime and recombination on the performance of eco-friendly perovskite solar cell. IEEE Trans. Electron Devices 68, 3446–3452 (2021). https://doi.org/10.1109/TED.2021.3078063
Sirtl, M.T., Hooijer, R., Armer, M., Ebadi, F.G., Mohammadi, M., Maheu, C., Weis, A., van Gorkom, B.T., Häringer, S., Janssen, R.A.J., Mayer, T., Dyakonov, V., Tress, W., Bein, T.: 2D/3D hybrid Cs2AgBiBr6 double perovskite solar cells: improved energy level alignment for higher contact-selectivity and large open circuit voltage. Adv. Energy Mater. (2022). https://doi.org/10.1002/aenm.202103215
Stranks, S.D., Eperon, G.E., Grancini, G., Menelaou, C., Alcocer, M.J.P., Leijtens, T., Herz, L.M., Petrozza, A., Snaith, H.J.: Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342, 341–344 (2013). https://doi.org/10.1126/science.1243982
Wang, Y., Fan, H., Zhao, G., Liu, D., Du, L., Wang, Z.: High quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency. Chem. Phys. Chem. 19(14), 1696–1700 (2012). https://doi.org/10.1111/febs.12037
Wang, J., Qin, M., Tao, H., Ke, W., Chen, Z., Wan, J., Qin, P., Xiong, L., Lei, H., Yu, H., Fang, G.: Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. DOI 10(1063/1), 121104 (2015)
Wang, D., Wright, M., Elumalai, N.K., Uddin, A.: Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 147, 255–275 (2016). https://doi.org/10.1016/j.solmat.2015.12.025
Wang, M., Zeng, P., Bai, S., Gu, J., Li, F., Yang, Z., Liu, M.: High-quality sequential-vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells. Sol. RRL 2, 1–6 (2018). https://doi.org/10.1002/solr.201800217
Wang, S., Wang, A., Deng, X., Xie, L., Xiao, A., Li, C., Xiang, Y., Li, T., Ding, L., Hao, F.: Lewis acid/base approach for efficacious defect passivation in perovskite solar cells. J. Mater. Chem. A 8, 12201–12225 (2020). https://doi.org/10.1039/d0ta03957h
Wang, B., Li, N., Yang, L., Agnese, C.D., Jena, A.K., Miyasaka, T., Wang, F.: Organic dye/Cs2 AgBiBr6 double perovskite heterojunction solar cells. J. Am. Chem. Soc. 143(36), 14877–14883 (2021a)
Wang, B., Li, N., Yang, L., Dall’agnese, C., Jena, A.K., Sasaki, S.I., Miyasaka, T., Tamiaki, H., Wang, X.F.: Chlorophyll derivative-sensitized TiO2 electron transport layer for record efficiency of Cs2AgBiBr6 double perovskite solar cells. J. Am. Chem. Soc. 143, 2207–2211 (2021b). https://doi.org/10.1021/jacs.0c12786
Yadav, M.K., Ghosh, M., Biswas, R., Raychaudhuri, A.K., Mookerjee, A., Datta, S.: Band-gap variation in Mg- and Cd-doped ZnO nanostructures and molecular clusters. Phys. Rev. B Condens. Matter Mater. Phys. 76, 1–9 (2007). https://doi.org/10.1103/PhysRevB.76.195450
Yang, W.S., Park, B.W., Jung, E.H., Jeon, N.J., Kim, Y.C., Lee, D.U., Shin, S.S., Seo, J., Kim, E.K., Noh, J.H., Seok, S.I.: Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017). https://doi.org/10.1126/science.aan2301
Yella, A., Heiniger, L.P., Gao, P., Nazeeruddin, M.K., Grätzel, M.: Nanocrystalline rutile electron extraction layer enables low-temperature solution processed perovskite photovoltaics with 13.7% efficiency. Nano Lett. 14, 2591–2596 (2014). https://doi.org/10.1021/nl500399m
You, J., Meng, L., Song, T.B., Guo, T.F., Chang, W.H., Hong, Z., Chen, H., Zhou, H., Chen, Q., Liu, Y., De Marco, N., Yang, Y.: Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 11, 75–81 (2016). https://doi.org/10.1038/nnano.2015.230
Yousefi, R., Kamaluddin, B.: Fabrication and characterization of ZnO and ZnMgO nanostructures grown using a ZnO/ZnMgO compound as the source material. Appl. Surf. Sci. 256, 329–334 (2009). https://doi.org/10.1016/j.apsusc.2009.08.025
Yousefi, R., Zak, A.K., Jamali-Sheini, F.: Growth, X-ray peak broadening studies, and optical properties of Mg-doped ZnO nanoparticles. Mater. Sci. Semicond. Process 16, 771–777 (2013). https://doi.org/10.1016/j.mssp.2012.12.025
Zhang, C., Gao, L., Hayase, S., Ma, T.: Current advancements in material research and techniques focusing on lead-free perovskite solar cells. Chem. Lett. 46, 1276–1284 (2017). https://doi.org/10.1246/cl.170345
Zhang, T., Li, H., Ban, H., Sun, Q., Shen, Y., Wang, M.: Efficient CsSnI3-based inorganic perovskite solar cells based on a mesoscopic metal oxide framework via incorporating a donor element. J. Mater. Chem. A 8, 4118–4124 (2020). https://doi.org/10.1039/c9ta11794f
Zhao, D., Liang, C., Wang, B., Liu, T., Wei, Q., Wang, K., Gu, H., Wang, S., Mei, S., Xing, G.: Overcoming the limitation of Cs2AgBiBr6 double perovskite solar cells through using mesoporous TiO2 electron extraction layer. Energy Environ Mater 1317–1322 (2021). https://doi.org/10.1002/eem2.12249
Zhou, H., Chen, Q., Li, G., Luo, S., Song, T.B., Duan, H. S., Yang, Y.: Interface engineering of highly efficient perovskite solar cells. Science 345(6196), 542–546 (2014)
Acknowledgements
The authors acknowledge the Department of Science and Technology (DST), Govt. of India for the financial assistance provided under DST SERB Project (File No. SRG/2021/002110) to carry out the present work. Dr. Amitesh Kumar would like to thank DST SERB for providing a Start-up Research Grant for this project to conduct research at NIT Patna. Mr. Parshuram Singh would like to thank the Ministry of Education and NIT Patna for the research fellowship. The authors are thankful to NIT Patna for providing research facilities.
Funding
The authors acknowledge the Department of Science and Technology (DST), Govt. of India for the financial assistance provided under DST SERB Project (File No. SRG/2021/002110) to carry out the present work.
Author information
Authors and Affiliations
Contributions
PS: Conceptualization, Methodology, Software, Data curation, Visualization, Investigation Writing—Original draft preparation. AK: Conceptualization, Methodology, Supervision, Reviewing and Editing. Visualization, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Not applicable.
Consent for publication
The authors have given Consent for Publication as per the journal policy.
Ethical approval
Not applicable. The work presented in this manuscript is modeling only for the proposed solarcell. No experiment was performed on the human body and living organism/animal. So, ethical approval from an ethical committee is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, P., Kumar, A. Device engineering of double perovskite based solar cells towards high-performance, eco-friendly solar cells. Opt Quant Electron 55, 304 (2023). https://doi.org/10.1007/s11082-023-04580-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-04580-8