Abstract
Self-organized TiO2 nanotubes (TiNTs) have been synthesized by electrochemical anodization in an electrolyte containing ethylene glycol, ammonium fluoride, and de-ionized water at 40 V for 3 h. TiNTs were then annealed at different temperatures in air (that is 340 °C, 450 °C and 670 °C). All of the annealed TiNTs were then loaded with NiO using 0.2 M precursor concentration. Furthermore, TiNTs annealed at 450 °C were additionally loaded with NiO using 1.6 M precursor concentration. The XRD data revealed the formation of low-temperature anatase phase at 340 °C, high-temperature anatase at 450 °C and dominantly rutile phase of TiNTs at 670 °C. Addition of NiO to the TiNTs was also confirmed by XRD. SEM images revealed the morphological evolution of TiNTs at different annealing temperatures as well as loading of NiO. Raman spectroscopy results further confirmed the formation of different phases of TiNTs and the peak shifting as a result of NiO addition. The photoelectrochemical current (PEC) densities were recorded from zero to 1 V (vs. Ag/AgCl/3.5 M KCl) under 1 sun illumination in water without incorporating additional ions in it. TiNTs annealed at 450 °C showing anatase phase were found to be most promising for the PEC measurements in water. The current densities for the aforementioned TiNTs were enhanced by ~ 80 times with respect to dark measurement conditions. NiO addition using 0.2 M precursor concentration for 450 °C annealed anatase TiNTs produced a further fourfold increase in PEC densities under light with respect to its unmodified form. This is in contrast to ~ 1.6-fold and ~ 1.9-fold increase in PEC densities with respect to their unmodified forms for NiO-modified low-temperature anatase TiNTs and predominantly rutile-phase TiNTs, respectively. The NiO loading using 1.6 M precursor concentration in anatase TiNTs annealed at 450 °C exhibit PEC densities ~ sevenfold higher with respect to their unloaded form. This work highlights the roles of annealing temperature and NiO addition in TiNTs (particularly anatase phase) for achieving enhanced PEC densities for water splitting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the fast-depleting resources of fossil fuels and their environmental impact the quest for clean and unending source of energy is paramount (Kudo and Miseki 2009). The production of hydrogen from splitting the water molecule using sunlight is a worthy answer to this quest. However, a suitable photocatalyst for solar photoelectrochemical water splitting is necessary (Kudo and Miseki 2009). Among many potential photocatalysts, the utility of TiO2 has been known since Honda–Fujishima first reported its use as a photo electrode for the water splitting (Fujishima and Honda 1972). TiO2 has been synthesized in different size and shapes in the form thin films (Bousoulas et al. 2014), nanoparticles (Ali et al. 2013), nanowires (Kumar et al. 2010), nanotubes (Rasheed et al. 2018) and nanobelts (Zhou et al. 2011). For photocatalytic applications, large-surface area materials are needed (Kudo and Miseki 2009). Highly ordered TiO2 nanotubes (TiNTs) have shown promise for solar photocatalytic water splitting applications because of its high surface-to-volume ratios, improved charge transport ability and high chemical stability (Rasheed et al. 2018; Zhang et al. 2010a). Additionally the TiO2 nanotubes may be synthesized in different crystallographic forms. However, the obstacles faced by TiO2 are its large band gap (that is 3.02 eV for rutile phase and 3.20 eV for anatase phase of TiO2) and recombination between photogenerated electrons and holes (Wunderlich et al. 2004; Kudo and Miseki 2009). For enhanced photoreactivity of TiO2 for sunlight and prevention of charge carrier’s recombination it has been modified with different materials to alter its band structure. For this purpose addition of metals (Cu, Ni, Co, V, Cr, Fe, Mn, Mo, Nb, Au, Ru, Ag, and Pt) (Anpo 1997, 2000; Wunderlich et al. 2004; Yamashita et al. 2002), non-metals (S, N, F, C, B, and I) (Umebayashi et al. 2002; Sakthivel et al. 2004; Ho et al. 2006; Murphy 2008; Szkoda et al. 2016) and metal oxides (AgO, ZnO, NiO) (Umebayashi et al. 2002; Sakthivel et al. 2004; Ho et al. 2006; Murphy 2008; Szkoda et al. 2016; Xiao 2012; El-Sabban et al. 2013; Sim et al. 2013) and graphene oxide (Chen et al. 2017) has been explored.
Among aforementioned materials, NiO–TiO2 nanotubes combination has not been studied in detail for solar photoelectrochemical water splitting. NiO is a p-type semiconductor which has high hole concentration, and is frequently used with various n-type semiconductors as a cocatalyst (Hu et al. 2014; Sim et al. 2013; Ku et al. 2011; Zhang et al. 2010b; Guo et al. 2010). By forming a p–n junction, it can promote both the transfer of interfacial carriers and the separation of e−/h+ pairs (Kudo and Miseki 2009). To the best of our knowledge there is no study on the solar water splitting without the ion-assisted aqueous electrolyte. Almost all solar photoelectrochemical studies for hybrid TiO2 materials including NiO–TiO2 rely on ion-assisted water splitting, that is aqueous solution consisting of one of 1 M KOH, 3 M KOH, 1 M NaOH, methanol, or ethanol is used (Sun et al. 2013; Han et al. 2017; Guo et al. 2010; Liu et al. 2015; Xu et al. 2012; Mohapatra et al. 2007; Wang et al. 2011; Sreethawong et al. 2005). However, there is no report of comparative photocurrent responses in water without the addition of any of the aforementioned ions. Moreover, the role of annealing temperature as well as NiO addition to TiNTs and their impact on photoelectrochemical current density responses for water splitting in water has not been reported before.
In the present work highly ordered TiNTs are synthesized by anodization. The as-prepared TiNTs are annealed in air at different temperatures of 340 °C, 450 °C, and 670 °C and modified with NiO using different precursor concentrations. The effect of annealing temperature and NiO precursor concentration is investigated by the artificial solar illumination-driven photocatalytic water-splitting responses and structural characterization. It is found that anatase TiNTs modified with NiO using 1.6 M precursor concentration show highest photoelectrochemical water splitting current densities.
Materials and methods
Materials
Titanium sheet (Good fellow England, sheet thickness: 0.2 mm, purity: 99.6%), ethylene glycol (Sigma-Aldrich, 98+%) ammonium fluoride (Sigma-Aldrich, 98+%), ammonium hydroxide (Sigma-Aldrich, analytical grade) nickel chloride hexahydrate (RDH), analytical-grade ethanol (BDH) and hydrochloric acid (Sigma-Aldrich) were used in their as-received form.
Synthesis of TiNTs by anodization
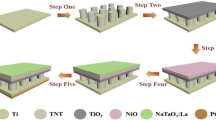
The 1.5 cm × 1.0 cm strips were cut from titanium (Ti) sheet with thickness of 0.01 cm. Titanium strips were cleaned in diluted analytical-grade hydrochloric acid (HCl) and de-ionized (DI) water. These strips were then sonicated in an ultrasonication bath for 15 min in a mixture of ethanol and DI water. Subsequently, the strips were rinsed with DI water and dried using air blower. The cleaned titanium strips were then anodized at room temperature in an electrolyte having 70 mg ammonium fluoride, 36 ml ethylene glycol and 1 ml of DI water using two-electrode configuration. Anodization was carried out for 3 h using a DC power supply at 40 V. Titanium strip (working electrode) was connected to the positive terminal and graphite (counter electrode) was connected to negative terminal of the power supply. The resultant TiO2 nanotubes were then separately annealed in air at 340 °C, 450 °C and 670 °C for 2 h with a heating rate of 8 °C/min and cooling rate of 2 °C/min.
Modification of TiNTs with NiO
The annealed TiNTs were then successively soaked in ethanol-based solutions of 0.2 M NiCl2⋅6H2O and 0.2 M NH4OH for 30 s each in one cycle. This soaking process was repeated for 20 cycles. Additionally, the TiNTs annealed at 450 °C were soaked in 1.6 M solution of NiCl2⋅6H2O in ethanol followed by its soaking in 1.6 M ethanol-based solution of NH4OH for same amount of time and cycles. The soaking process in these precursor solutions results in the formation of Ni(OH)2 on the TiNTs. To obtain NiO from Ni(OH)2 the modified nanotubes were again heated in air at 340 °C for 2 h with a heating rate of 8 °C/min and cooling rate of 2 °C/min. Overall, seven different combinations of TiNTs samples were prepared, i.e., the TiNTs annealed at 340 °C, 450 °C and 670 °C, their corresponding 0.2 M precursor loaded TiNTs in addition to the 1.6 M precursor loaded TiNTs annealed at 450 °C.
Characterization
The structural analysis has been performed by BRUKER D8-Discover X-ray diffraction unit with monochromatic Cu Kα1 radiation (λ = 1.54060 Å, accelerating voltage 40 kV, current of 40 mA). The TiNTs samples were scanned in 2θ range of 20.0°–70.0° with step size of 0.04° and scanning rate of 1 /min. The scanning electron microscopy was performed by FESEM TESCAN, MAIA3 in secondary electron imaging mode. Raman spectroscopy was performed using a MST-4000A (DONGWOO OPTRON, CO., Ltd.), equipped with He–Cd laser having an excitation wavelength of 442 nm.
The photocatalytic water-splitting experiments involved the dark and photocurrent density measurements in 30 ml distilled water. The measurements were performed using three-electrode configuration such that TiO2 or TiO2–NiO nanostructured films were used as working electrode, gold was used as a counter electrode while the Ag/AgCl was used as a reference electrode. These samples were illuminated by a solar simulator (Keithlink, China) equipped with Xe lamp having luminous intensity of 1000 W/m2. Current density vs. voltage curves were obtained using Gamry instruments Potentiostat/Galvanostat/ZRA reference 3000. The data were collected in the voltage range of − 0.2 to + 1.0 V (against Ag/AgCl electrode) at a scan rate of 15 mV/s at room temperature.
Results and discussion
XRD analysis
Figure 1 shows the XRD patterns of TiNTs annealed at different temperatures. The XRD pattern for TiNTs annealed at 340 °C (Fig. 1a) shows the characteristic peak of anatase phase at 25.37°. This peak grows in intensity as the annealing temperature is increased to 450 °C (Fig. 1b). The sharpness and intensity of the anatase phase peaks indicate the formation of crystalline TiO2 nanotubes. In addition to these peaks the XRD pattern in Fig. 1a, b also shows hexagonal phase peaks belonging to titanium strip. TiO2 peaks in Fig. 1a, b can be indexed according to JCPDS card no. 01-084-1286 (tetragonal anatase phase of TiO2) and titanium peaks can be assigned to JCPDS card no. 01-089-2959 (hexagonal phase of titanium) respectively. When annealing temperature is increased to 670 °C, the crystal structure transformation starts as seen in Fig. 1c and highlighted by appearance of new XRD peaks at 27.50°, 36.34°, 39.50°, 41.54°, 44.20°, 54.62°, 56.92°, and 69.26°. These peaks can be indexed to the rutile phase of TiO2 using JCPDS card no. 01-077-0442. As the anatase phase peaks are still visible in the XRD pattern shown in Fig. 1c, therefore, TiNTs annealed at 670 °C would be referred to as predominantly rutile-phase TiNTs.
The crystallite size for each temperature phase is calculated by Scherrer’s formula as expressed below:
where D is the crystallite size, K is called shape factor having value 0.89, λ is the wavelength of the incident X-rays, θ is the half of the mean value of corresponding peak. β is the FWHM of the peak due to refection from (101) plane of anatase and (110) plane of rutile as well which are the most prominent peaks in the overall XRD patterns. The calculated crystallite size for 340 °C (low-temperature anatase phase), 450 °C (high-temperature anatase phase), 670 °C (predominantly rutile-phase) TiNTs is 27.10 nm, 29.30 nm, and 35.60 nm, respectively. Therefore, it can be concluded that as the annealing temperature is increased the crystallite size also increases.
Figure 2 shows the plot for the TiNTs annealed at different temperatures modified with NiO using 0.2 M precursor concentration. With the addition of NiO the width of the peak at about 38° (seen in Fig. 1) clearly increases as depicted by Fig. 2. This is due to the appearance of cubic phase NiO peak at ~ 37.60°, which can be indexed to (111) plane according to JCPDS card no. 01-078-0643. All the samples shown in Fig. 2 for modified TiNTs, the peaks of NiO are weak due to use of low precursor concentration of 0.2 M.
Figure 3 shows the XRD patterns for TiNTs annealed at 450 °C to obtain anatase phase (Fig. 3a), which is subsequently modified with NiO using different precursor concentrations of 0.2 M and 1.6 M, respectively (Fig. 3b, c). It is clear from the comparison of Fig. 3b with Fig. 3a that the addition of NiO to anatase TiNTs using 0.2 M precursor concentration, result in the increase of width of the composite peak centered at about 38° accompanied by the appearance of a shoulder peak at ~ 37.60°. It is worth noting that this shoulder is absent in Fig. 3a. Further enhancement in intensity of the composite peak centered around 38° is observed in Fig. 3c due to the increase in precursor concentration to 1.6 M for modification of anatase phase TiNTs with NiO.
Scanning electron microscopy (SEM)
The SEM images in Fig. 4 show the morphology of TiO2 nanotubes annealed at different temperatures before (Fig. 4a, c, e) and after (Fig. 4b, d, f) addition of NiO using 0.2 M precursor concentration. Figure 4a reveals that the average diameter of the TiNTs annealed at 340 °C is ~ 120 nm and the average wall thickness is ~ 8 nm. By increasing the annealing temperature to 450 °C, the diameter of the nanotubes decreases to ~ 100 nm and the wall thickness increases to ~ 12 nm as shown in Fig. 4c. At this temperature, the nanotubes are well ordered and vertically oriented. With further increase in annealing temperature to 670 °C, the nanotubes start to get distorted and lose their order. At this temperature, the average diameter of the nanotubes decreases to ~ 40 nm and the average wall thickness increases to ~ 20 nm as depicted in Fig. 4e. This morphological disorder is due to the emergence of rutile phase as seen in XRD pattern in Fig. 1c. Figure 2b, d and e show the effect of NiO addition by reacting 0.2 M of NiCl2⋅6H2O and NH4OH on the morphology of nanotubes annealed at different temperatures. It can be seen from these SEM images that some of the NiO nanoparticles are covering the opening of nanotubes while most of them are agglomerated on its walls.
Raman spectroscopy of TiNTs
Raman spectra were collected to study the structural characteristics of different temperature phases and to investigate the effect of NiO loading on TiNTs. The Raman spectra of TiNTs annealed at 340 °C and 450 °C as depicted in Fig. 5a, b matched with standard anatase phase modes reported in literature (Frank et al. 2012). The TiNTs annealed at 670 °C show additional Eg and A1g modes which correspond to the rutile phase (Fig. 5c) according to reported results (Frank et al. 2012).
Detailed analysis of the Raman spectra in Fig. 5a corresponding to TiNTs annealed at 340 °C shows that the strongest Eg(1) mode lies at 147.40 cm−1. In this spectrum other peaks observed at 202.10 cm−1, 391.70 cm−1, 512.30 cm−1 and 630.20 cm−1 correspond to Eg(2), B1g(1), B1g(2) + A1g, and Eg(3), respectively. The Eg modes [Eg(1), Eg(2), and Eg(3)] are related with the stretching vibrations of titanium and oxygen atoms relative to each other (Frank et al. 2012). B1g(1) is titanium atom vibration mode while B1g(2) and A1g are oxygen atom vibration modes (Frank et al. 2012). As the annealing temperature is increased to 450 °C, the strongest Eg(1) as well as Eg(2) mode shifts to lower wavenumber of 146.30 cm−1 and 196.30 cm−1, respectively, as shown in Fig. 5b. The Raman peaks related to B1g(1), B1g(2) + A1g, and Eg(3) modes shift to higher wavenumbers of 392.60 cm−1, 513.20 cm−1 and 632.30 cm−1, respectively. As the annealing temperature is further increased to 670 °C, Eg and A1g modes associated with rutile phase appear at 442.20 cm−1 and 606.30 cm−1, respectively, as depicted in Fig. 5c. These peaks are related with oxygen atom vibrations and are in accordance with the rutile-phase Raman modes reported in literature (Frank et al. 2012). Furthermore, modes associated with anatase phase do not vanish reconfirming the mixed-phase character of TiNTs at this temperature. Additionally the Eg(1) and Eg(2) modes are shifted to further lower wavenumbers of 144.40 cm−1 and 195.10 cm−1, respectively. On the other hand, the B1g(1), B1g(2) + A1g, A1g and Eg(3) exhibit a Raman shift to higher wavenumbers of 393.20 cm−1, 514.10 cm−1 and 633.80 cm−1, respectively. This means that with the annealing temperature the energy of these modes is increased. The inset of the Fig. 5 highlights the fact that as the annealing temperature increases, the red shift for strongest mode Eg(1) can be clearly observed. The red shift in Eg(1) mode of TiO2 has been related to the increase in crystallite size due to change in temperature and/or phase (Balaji et al. 2006). Based on the XRD data reported here, the crystallite size for TiNTs annealed at 340 °C, 450 °C and 670 °C has been calculated as 27.10 nm, 29.30 nm, and 35.60 nm, respectively. Hence, an increase in crystallite size is observed with increase in annealing temperature. Therefore, the Eg(1) mode peaks shown in the inset of Fig. 5 are shifted to lower wavenumbers. Based on the Raman spectroscopy results, it can be concluded that the TiNTs annealed at 340 °C and 450 °C exhibit anatase phase while those annealed at 670 °C show mixed rutile and anatase phase structure reconfirming the XRD results.
Figure 6 shows that due to the addition of NiO (using a reaction between 0.2 M precursor and ammonium hydroxide) into different temperature phases of TiO2 nanotubes, the mean positions of most of the peaks exhibit a blue or a red shift with increase in its FWHM. This elucidates that with the addition of NiO the TiNTs show lower crystallinity than their pristine counterparts. In NiO-modified anatase phase TiNTs shown in Fig. 6a, b there is slight blue shift of 0.40 and 0.30 cm−1 in the strongest Eg(1) mode in comparison with Fig. 5a, b, respectively. Moreover, there is also slight blue shift observed in the B1g(1) mode of Raman spectra in Fig. 6a, b. However, Eg(3) mode related to aforementioned spectra show a clear blue shift compared to Raman spectra of unmodified anatase TiNTs. A similar comparison reveals a red shift in case of Eg(2) and B1g(2) + A1g modes (Fig. 6a, b). Moreover, comparison of B1g(2) + A1g mode in Figs. 5 and 6 reveals a red shift in the Raman spectra with addition of NiO. According to the literature, when NiO is added to TiO2, a Ni2+ ion replaces Ti4+ in TiO2 structure (Chen et al. 2016). This results in the creation of oxygen vacancy to maintain charge neutrality. These oxygen vacancies are mainly responsible for observed red shift in the B1g(2) + A1g mode. On the other hand, Eg(1), B1g(1), and Eg(3) modes observed in Fig. 6c exhibit a blue shift with respect to Raman spectra given in Fig. 5c. Additionally, rutile-phase modes (Eg and A1g) depicted in Fig. 6c also show a blue shift with the addition of NiO. That means that oxygen atoms’ vibrations occur at higher frequencies compared to pristine rutile modes. The observed blue shift in these modes is attributed to the incorporation of NiO in the TiO2 structure (Chen et al. 2016).
Figure 7 gives a comparison of Raman spectra for pure (annealed at 450 °C) and NiO-modified anatase phase with increasing precursor concentration of 0.2 M and 1.6 M. It can be seen that the position of Raman modes associated with anatase phase is changing with addition of NiO. The positions of Eg(1), Eg(2), B1g(1), and Eg(3) modes in Fig. 7a, b reveal a blue shift in the vibrational frequencies with respect to spectrum of pristine anatase TiNTs shown in Fig. 7a. Moreover, B1g(2) + A1g mode related to oxygen atom vibration shows a red shift with the increasing concentration of NiO compared with anatase phase. The summary of Raman spectroscopy results for each sample is given in Table 1.
Photocatalytic water splitting
The photocurrent densities measured in distilled water in dark as well as light were analyzed by categorizing it into three groups. The first group of data shows the effect of annealing temperature on photocurrent densities of TiNTs. The second set highlights the effect of NiO addition using 0.2 M precursor concentration in the TiNTs annealed at different temperatures. The third group of data shows the role of NiO loading using different precursor concentration on the photocurrent responses of TiNTs annealed at 450 °C.
The temperature effect on the water-splitting photoelectrochemical current (PEC) density responses is depicted in Fig. 8. In the presence of 1 sun illumination, TiNTs annealed at 340 °C (low-temperature anatase phase) the PEC density increases with increasing voltage and at 1.0 V vs. Ag/AgCl (3.5 M) a photocurrent density of 55.05 µA/cm2 is observed. Under similar conditions the TiNTs annealed at 450 °C (high-temperature anatase phase) show the PEC density of 80.70 µA/cm2, while the TiNTs annealed at 670 °C (predominantly rutile-phase) achieve the PEC density of 36.35 µA/cm2. The current densities under dark conditions for TiNTs annealed at the aforementioned temperatures give the value of ~ 2.00 µA/cm2. It is therefore clear from the plots in Fig. 8 as to why anatase structure (particularly the 450 °C phase) is favored over other polymorphs for photocatalytic and solar cell applications (Carp et al. 2004).
The reason for superior photocatalytic activity for anatase phase of TiO2 is its lower charge carrier recombination rate in comparison with the other phases (Carp et al. 2004). Furthermore, higher surface adsorption capacity and higher Fermi level also makes its photoreactivity better (Zhang et al. 2010a). Higher photoreactivity of TiO2 has been attributed to crystallinity, surface area, and the number of the surface-active sites available (Carp et al. 2004). Therefore, the role of particle size is of prime importance. The average size of anatase crystallites in our case is ~ 29 nm, while for predominantly rutile phase the crystallite size is ~ 35 nm based on the XRD data using Scherrer’s formula. The higher grain size of predominantly rutile phase might be the cause of their lower photocatalytic current densities. On the other hand the lower current densities for low-temperature anatase phase of TiNTs (annealed at 340 °C) may be due to the reduced crystallinity at this temperature.
The effect of NiO addition using 0.2 M precursor concentration in TiNTs annealed at different temperatures is shown in Fig. 9. It can be clearly seen that under 1 sun illumination and at a 1.0 V applied voltage vs. Ag/AgCl, the PEC density for NiO-modified anatase phase TiNTs annealed at 450 °C is 320.40 µA/cm2. In comparison to the unmodified TiNTs this corresponds to about 400% (or fourfold) increase in PEC density. On the other hand NiO addition using the 0.2 M precursor concentration in TiNTs annealed at 340 °C reach a PEC density of 87.60 µA/cm2 at 1.0 V vs. Ag/AgCl. This corresponds to a 59% increase in PEC density compared to unmodified TiNTs annealed under similar conditions. Moreover, 0.2 M NiO precursor addition in TiNTs annealed at 670 °C achieve a PEC density of 68.10 µA/cm2 at the aforementioned voltage. This value is 87% higher than the corresponding value obtained without the addition of NiO. Therefore, it can be concluded that addition of NiO greatly enhances the photocurrent density for TiNTs annealed at 450 °C.
Figure 10 reveals that by increasing the precursor concentration for NiO in TiNTs annealed at 450 °C tremendously increases the photocurrent densities. Finally, for 1.6 M precursor concentration for NiO the photocurrent density reaches a value of 537.65 µA/cm2 at 1.0 V vs. Ag/AgCl in the presence of light. This corresponds to an almost 667% (or 6.7-fold) increase with respect to unmodified TiNTs annealed at 450 °C. In dark, the photocurrent densities of all the responses shown in Fig. 10 are ~ 2 µA/cm2.
Photoelectrochemical current density curves recorded in water for TiO2 nanotubes (TiNTs) annealed at 450 °C. (a) Shows all the responses in dark conditions. Under 1 sun illumination condition for (b) Unmodified TiNTs (c) NiO-modified TiNTs using 0.2 M precursor concentration and (d) NiO-modified TiNTs 1.6 M precursor concentration
Based on the definition in the literature (Iandolo et al. 2015), the onset potential has been defined as the potential at which PEC density value is 55 µA/cm2. This value is about 1.5 times the value of PEC density recorded at 1 V vs. Ag/AgCl in water for TiNTs annealed at 670 °C. This sample showed the lowest PEC density among all samples. The onset potential values for all samples tested in light for water splitting based on the definition above are given in Table 2.
For the confirmation of photoelectrochemical water-splitting response, current density vs. time scan under the chopped illumination (using 1 sun simulator source) was performed. This scan was carried out for TiNTs annealed at 450 °C modified with NiO using 0.2 M precursor concentration and is shown in Fig. 11.
The potential of a photocatalyst used for water splitting is primarily judged by determining its efficiency with which light energy is transformed into chemical energy. The photoconversion efficiency can be defined as the ratio of maximum energy output in the form of hydrogen to the energy provided in the form of light.
η is the percent efficiency, Jp is the photocurrent density (A/cm2), \(E_{{{\text{rev}}}}^{0}=1.229\;{\text{V}}\) is the standard reversible potential required for the water-splitting reaction vs. NHE, Eapp is the total applied potential (Eapp = Emeas = Eaoc), Eaoc is the photoanode potential which is measured with respect to the Ag/AgCl (reference electrode) at open circuit conditions, Ilight is the power density of incident light used (Zhang et al. 2010a). In our case Ilight is 1000 W/m2. Based on this equation the photocurrent efficiencies in distilled water have been enhanced from 0.03% for pure TiNTs annealed at 450 °C to 0.13% for the NiO TiNTs modified with 1.6 M precursor concentration. It is worth noting that the photoconversion efficiencies reported here are based on the measurements done in distilled water. There are no reports in literature for the photoconversion efficiencies in water without having additional ions in it. Furthermore, in case of water-splitting measurements in KOH, NaOH or methanol solutions the reported photoconversion efficiencies are an order of magnitude higher than the one reported here (Guo et al. 2010). The comparison of photoconversion efficiency (PCE) at 1 V (vs. Ag/AgCl) for all samples is given in the Table 3.
The enhancement of photocatalytic current is explained as follows. TiO2 is an n-type material with the band gap of about 3.23 eV and NiO is a p-type material with a band gap of ~ 3.6 eV (Chen et al. 2015). When NiO nanoparticles and TiO2 TiNTs come in contact with each other a p–n junction is formed due to the alignment of their Fermi levels. It requires ~ 1.98 eV energy for the holes in the valence band of TiO2 to jump into the valence band of NiO. On the other hand the energy required for electrons to migrate from conduction band of TiO2 into conduction band of NiO is ~ 2.64 eV. During applied bias, the drift of holes from TiO2 would be higher than the drift of electrons. Therefore, the number of holes available in the valence band of TiO2 at any time during applied bias would be lower than number of electrons in the conduction band. Due to this fact, the recombination probability of photogenerated e−/h+ pairs will be smaller (Chen et al. 2015). This mechanism is depicted in Fig. 12.
Conduction mechanism in NiO/TiNTs p–n junctions adapted from (Chen et al. 2015)
Conclusions
In brief, self-organized TiO2 nanotubes (TiNTs) have been synthesized by electrochemical anodization at 40 V for 3 h in an electrolyte containing ethylene glycol, ammonium fluoride, and de-ionized water. TiNTs were then subsequently annealed at 340 °C, 450 °C, and 670 °C for 2 h. One set of annealed TiNTs was then loaded with 0.2 M precursor for NiO. The anatase-phase TiNTs obtained by annealing at 450 °C were also loaded with NiO using 1.6 M precursor concentration. The photocatalytic water-splitting current densities recorded in water without the use of additional ions. NiO loading using 0.2 M precursor concentration showed highest (~ fourfold) increase in photocurrent density annealed TiNTs at 450 °C (anatase phase). A 6.67-fold increase in photocurrent density was observed in water for same TiNTs modified with 1.6 M NiO precursor concentration. This increase in water-splitting current density is attributed to band alteration as a result of junction formation between p-type NiO and n-type TiNTs.
References
Ali G, Kim HJ, Kum JM, Sung Oh C (2013) Rapid synthesis of TiO2 nanoparticles by electrochemical anodization of a Ti wire. Nanotechnology 24(18):185601. https://doi.org/10.1088/0957-4484/24/18/185601
Anpo M (1997) Photocatalysis on titanium oxide catalysts: approaches in achieving highly efficient reactions and realizing the use of visible light. Catal Surv Jpn 1(2):169–179. https://doi.org/10.1023/A:1019024913274
Anpo M (2000) Use of visible light. Second-generation titanium oxide photocatalysts prepared by the application of an advanced metal ion-implantation method. Pure Appl Chem 72(9):1787–1792. https://doi.org/10.1351/pac200072091787
Balaji S, Djaoued Y, Robichaud J (2006) Phonon confinement studies in nanocrystalline anatase-TiO2 thin films by micro Raman spectroscopy. J Raman Spectrosc 37(12):1416–1422. https://doi.org/10.1002/jrs.1566
Bousoulas P, Michelakaki I, Tsoukalas D (2014) Influence of oxygen content of room temperature TiO2–x deposited films for enhanced resistive switching memory performance. J Appl Phys 115(3):034516. https://doi.org/10.1063/1.4862797
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32(1–2):33–177. https://doi.org/10.1016/J.PROGSOLIDSTCHEM.2004.08.001
Chen J-Z, Chen T-H, Lai L-W, Li P-Y, Liu H-W, Hong Y-Y, Liu D-S et al (2015) Preparation and characterization of surface photocatalytic activity with NiO/TiO2 nanocomposite structure. Materials 8(7):4273–4286. https://doi.org/10.3390/ma8074273
Chen G, Georgieva V, Godfroid T, Snyders R, Delplancke-Ogletree M-P (2016) Plasma assisted catalytic decomposition of CO2. Appl Catal B 190:115–124. https://doi.org/10.1016/J.APCATB.2016.03.009
Chen Q, Zhou M, Zhang Z, Tang T, Wang T (2017) Preparation of TiO2 nanotubes/reduced graphene oxide binary nanocomposites enhanced photocatalytic properties. J Mater Sci Mater Electron 28(13):9416–9422. https://doi.org/10.1007/s10854-017-6683-2
El-Sabban HA, Attia MS, Bakir E, Abdel-Mottaleb SA (2013) Synthesis and photocatalytic performance of AgO–TiO2 and AgI–TiO2 photocatalysts. Egypt J Pure Appl Sci 51:027–032
Frank O, Zukalova M, Laskova B, Kürti J, Koltai J, Kavan L (2012) Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys Chem Chem Phys 14(42):14567–14572. https://doi.org/10.1039/c2cp42763j
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37–38. https://doi.org/10.1038/238037a0
Guo J, Fu W, Yang H, Yu Q, Zhao W, Zhou X, Sui Y et al (2010) A NiO/TiO2 junction electrode constructed using self-organized TiO2 nanotube arrays for highly efficient photoelectrocatalytic visible light activations. J Phys D Appl Phys 43(24):245202. https://doi.org/10.1088/0022-3727/43/24/245202
Han H, Riboni F, Karlicky F, Kment S, Goswami A, Sudhagar P, Yoo J et al (2017) α-Fe2O3/TiO23D hierarchical nanostructures for enhanced photoelectrochemical water splitting. Nanoscale. https://doi.org/10.1039/c6nr06908h
Ho W, Yu JC, Lee S (2006) Synthesis of hierarchical nanoporous F-doped TiO2 spheres with visible light photocatalytic activity. Chem Commun 42(10):1115. https://doi.org/10.1039/b515513d
Hu C, Chu K, Zhao Y, Yang WT (2014) Efficient photoelectrochemical water splitting over anodized P-type NiO porous films. ACS Appl Mater Interfaces. https://doi.org/10.1021/am507138b
Iandolo B, Zhang H, Wickman B, Zorić I, Conibeer G, Hellman A (2015) Correlating flat band and onset potentials for solar water splitting on model hematite photoanodes. RSC Adv 5(75):61021–61030. https://doi.org/10.1039/C5RA10215D
Ku Y, Lin C-N, Hou W-M (2011) Characterization of coupled NiO/TiO2 photocatalyst for the photocatalytic reduction of Cr(VI) in aqueous solution. J Mol Catal A: Chem 349(1–2):20–27. https://doi.org/10.1016/J.MOLCATA.2011.08.006
Kudo A, Miseki Y (2009) Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev 38(1):253–278. https://doi.org/10.1039/B800489G
Kumar A, Madaria AR, Zhou C (2010) Growth of aligned single-crystalline rutile TiO2 nanowires on arbitrary substrates and their application in dye-sensitized solar cells. J Phys Chem C. https://doi.org/10.1021/jp100491h
Liu Q, Ding D, Ning C, Wang X (2015) Reduced N/Ni-doped TiO2 nanotubes photoanodes for photoelectrochemical water splitting. RSC Adv 5(116):95478–95487. https://doi.org/10.1039/c5ra21805e
Mohapatra SK, Misra M, Mahajan VK, Raja KS (2007) Design of a highly efficient photoelectrolytic cell for hydrogen generation by water splitting: application of TiO2-XC × nanotubes as a Photoanode and Pt/TiO2 nanotubes as a cathode. J Phys Chem C. https://doi.org/10.1021/jp071906v
Murphy AB (2008) Does carbon doping of TiO2 allow water splitting in visible light? Comments on ‘Nanotube enhanced photoresponse of carbon modified (CM)-n-TiO2 for efficient water splitting.’. Sol Energy Mater Sol Cells 92(3):363–367. https://doi.org/10.1016/J.SOLMAT.2007.10.007
Rasheed MA, Ahmad K, Khaliq N, Khan Y, Rafiq MA, Waheed A, Shah A, Mahmood, Ali G (2018) Effect of electrochemical reduction on the structural and electrical properties of anodic TiO2 nanotubes. Curr Appl Phys. https://doi.org/10.1016/j.cap.2018.01.001
Sakthivel S, Janczarek M, Kisch H (2004) Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2. Appl Chem B 108(50):19384–19387. https://doi.org/10.1021/JP046857Q
Sim L, Ching KW, Ng S, Ibrahim, Saravanan P (2013) Preparation of improved p–n junction NiO/TiO2 nanotubes for solar-energy-driven light photocatalysis. Int J Photoenergy 2013:1–10. https://doi.org/10.1155/2013/659013
Sreethawong T, Suzuki Y, Yoshikawa S (2005) Photocatalytic evolution of hydrogen over mesoporous TiO2 supported NiO photocatalyst prepared by single-step sol–gel process with surfactant template. Int J Hydrogen Energy 30(10):1053–1062. https://doi.org/10.1016/J.IJHYDENE.2004.09.007
Sun B, Shi T, Peng Z, Sheng W, Jiang T, Liao G (2013) Controlled fabrication of Sn/TiO2 nanorods for photoelectrochemical water splitting. Nanoscale Res Lett. https://doi.org/10.1186/1556-276X-8-462
Szkoda M, Siuzdak K, Lisowska-Oleksiak A (2016) Non-metal doped TiO2 nanotube arrays for high efficiency photocatalytic decomposition of organic species in water. Phys E 84:141–145. https://doi.org/10.1016/J.PHYSE.2016.06.004
Umebayashi T, Yamaki T, Itoh H, Asai K (2002) Band gap narrowing of titanium dioxide by sulfur doping. Appl Phys Lett 81(3):454–456. https://doi.org/10.1063/1.1493647
Wang G, Wang H, Ling Y, Tang Y, Yang X, Fitzmorris RC, Wang C, Jin Z, Zhang, Li Y (2011) Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. https://doi.org/10.1021/nl201766h
Wunderlich W, Oekermann T, Miao L, Hue NT, Tanemura S, Tanemura M (2004) Electronic properties of nano-porous TiO2- and ZnO-thin films-comparison of simulations and experiments. J Ceram Process Res 5(4):343–354
Xiao F-X (2012) Construction of highly ordered ZnO–TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application. ACS Appl Mater Interfaces 4(12):7055–7063. https://doi.org/10.1021/am302462d
Xu M, Da P, Wu H, Zhao D, Zheng G (2012) Controlled Sn-doping in TiO2 nanowire photoanodes with enhanced photoelectrochemical conversion. Nano Lett. https://doi.org/10.1021/nl2042968
Yamashita H, Harada M, Misaka J, Takeuchi M, Ikeue K, Anpo M (2002) Degradation of propanol diluted in water under visible light irradiation using metal ion-implanted titanium dioxide photocatalysts. J Photochem Photobiol A 148(1–3):257–261. https://doi.org/10.1016/S1010-6030(02)00051-5
Zhang Z, Hossain MF, Takahashi T (2010a) Photoelectrochemical water splitting on highly smooth and ordered TiO2 nanotube arrays for hydrogen generation. Int J Hydrogen Energy 35(16):8528–8535. https://doi.org/10.1016/J.IJHYDENE.2010.03.032
Zhang Z, Shao C, Li X, Wang C, Zhang M, Liu Y (2010b) Electrospun nanofibers of p-type NiO/n-type ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl Mater Interfaces 2(10):2915–2923. https://doi.org/10.1021/am100618h
Zhou W, Du G, Hu P, Li G, Wang D, Liu H, Wang J, Boughton RI, Liu D, Jiang H (2011) Nanoheterostructures on TiO2 nanobelts achieved by acid hydrothermal method with enhanced photocatalytic and gas sensitive performance. J Mater Chem. https://doi.org/10.1039/c1jm10588d
Acknowledgements
PIEAS, NCP, and NILOP are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasheed, M.A., Rahimullah, R., Uddin, S.K. et al. Role of temperature and NiO addition in improving photocatalytic properties of TiO2 nanotubes. Appl Nanosci 9, 1731–1742 (2019). https://doi.org/10.1007/s13204-019-00980-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-00980-z