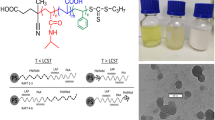
Abstract
Reactive oligomers of styrene–maleic anhydride having controlled architecture were prepared using reversible addition fragmentation chain transfer (RAFT) polymerization. The co-oligomers were characterized by FTIR, Raman, NMR and GPC. The arrangement of co-monomers (i.e. the architecture) in the co-oligomeric RAFT agents could be controlled by changing the molar feed ratio of styrene to maleic anhydride in the range of 1–5.25 under controlled polymerization conditions. Non-alternating (homopolymer) blocks of styrene could be suppressed even at high mole ratios > 1 and the resultant co-oligomeric RAFT agents consisting of mainly alternating (SMS) and semi-alternating (SSM) sequences of styrene and maleic anhydride were obtained. These macro RAFT agents behaved as efficient reactive surfactants and their self-aggregation behavior was greatly influenced by their architecture. These could be successfully used for surfactant-free nanoencapsulation of active hydrophobic materials, such as n-octadecane, indicating their suitability for various applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are multifaceted products used in the chemical industry (de Paz Banez et al. 2000). They have vital role in consumer products, including paints, adhesives, detergents, pharmaceuticals and many more (Baglioni et al. 2014; Gupta et al. 2016; Kooij et al. 2015). Recently, a lot of attention is being given towards investigation of new macromolecular reactive surfactants. These are low molecular weight living co-oligomers and possess several advantages over conventional surfactants.(Luo and Gu 2007) The adsorption of such co-oligomers at interface is kinetically irreversible, which prevents agglomeration (ageing) of emulsion (Baskar et al. 2000). The colloidal stability provided through macromolecular chains allows one-pot synthesis of nano particle suspension (Manguian et al. 2005; Sirohi et al. 2017). The length of hydrophilic head and hydrophobic tail has vital role to play in properties of such co-oligomers. Synthesis of these copolymers also requires control of macromolecular characteristics for which controlled radical polymerization (CRP) is an important tool (Benoit et al. 2000; Davies et al. 2005; Durand and Marie 2009; Lessard and Maric 2009; Manguian et al. 2005; Parida et al. 2012; Park et al. 2003; Stoffelbach et al. 2007). Among several CRP techniques, reversible addition fragmentation chain transfer (RAFT) polymerization is versatile as it is simple, efficient and can be used in heterogeneous conditions (Davies et al. 2005; Hill et al. 2015; Klumperman 2010; Lu et al. 2007; Moad et al. 2009; Pham et al. 2003; Xiong et al. 2004). It also gives scope for incorporation of functionality without disturbing the control of polymerization.
RAFT polymerization has been used to synthesize well-defined block co-oligomers of styrene (Sty) and maleic anhydride (MAn) consisting of alternating segments of Sty and MAn and non-alternating segments of Sty as a tail (Bapat et al. 2012). We have hypothesized that if arrangement of co-monomers (i.e., architecture) in macromolecular surfactant can be altered during polymerization while suppressing the formation of homopolymer tail, it might be possible to influence their self-assembling behavior and improve their suitability for nanoencapsulation. However, study on this aspect has not been reported to the best of our knowledge. In an effort to examine the effect of composition and structure of co-oligomers on self-assembling behavior, different co-oligomers of Sty and MAn were prepared by RAFT polymerization and their suitability in applications for surfactant-free nanoencapsulation was investigated. The use of SM co-oligomer as an emulsifier in miniemulsions and a RAFT agent for controlled synthesis of nanocapsules containing octadecane was explored.
Experimental
Materials
Styrene (Sty) (Merck, Germany) was washed with 10% aqueous solution of NaOH (CDH, India) and then with water, dried over anhydrous Na2SO4 (CDH, India), filtered and distilled under reduced pressure and stored at − 10 °C prior to use. Benzyl chloride (AR grade, Merck, India) was used as received. Magnesium turnings (99.9%, Acros Organics, USA), diethyl ether (specially dried, Merck, India), iodine balls (99.5%, Fischer Scientific, India), carbon tetrachloride and p-toluene sulphonic acid (p-TSA) were procured from Merck, India and used as received. Azobisisobutyronitrile (AIBN) (CDH, India) was recrystallized in methanol (AR grade, Merck, India) prior to use. Maleic anhydride (MAn) (AR grade, Sigma Aldrich), potassium per sulphate (KPS) (Merck, India), n-octadecane, (90%, Spectrochem, India), n-hexane (Spectrochem, India), and ammonia solution (25% w/v) (Merck, India), methanol (Merck, India) were used as obtained. The n-octadecane was used as phase change material with transition temperature of 298 K and latent heat of fusion 215 J/g. An aqueous ammonia solution (0.5%) was freshly prepared from 25% (w/v) ammonia solution (Merck, India). Carbon disulphide (Merck, India) was kept on anhydrous sodium sulphate prior to use.
Co-oligomer preparation
RAFT agent, phenylethyl phenyl dithioactetate (PEPDA) was synthesized as per the literature (Quinn et al. 2001) and was stored at − 10 °C prior to use. 1H NMR (CDCl3, 400 MHz, 25 °C): δ 1.7 (d, 3H), 4.2 (s, 2H), 5.1 (q, 1H), 7.3 (m, 10H). 13C NMR (CDCl3, 400 MHz, 25 °C): δ 20 (CH3CH), 54 (CHC6H5), 57 (CH2C6H5), 128 m (Ar 10C), 233 (SC(=S)CH(CH3)).
Styrene–maleic anhydride co-oligomers were synthesized in a 50 ml two-necked round bottom glass flask equipped with a condenser and a magnetic stirring bar (Scheme S1 of Supporting Information). For co-oligomer preparation (for example, 50 mol% of styrene in feed, SM-50) styrene, maleic anhydride, RAFT agent and AIBN were taken in mole ratio of 50:50:1: 50:50:2:0.4 in the flask. The mixture was degassed by purging N2, the polymerization was carried out at 60 °C for 130 min. The synthesized co-oligomer was soluble in the monomer. The co-oligomer was then precipitated in methanol. The obtained co oligomer was further purified by dissolving in acetone followed by precipitation in methanol to remove impurities. Co-oligomers with 66 (SM-66), 75.2 (SM-75) and 83.6 (SM-84) mol% of styrene feed were also prepared in the similar fashion (Table S1 Supporting Information). 1H NMR (CD3COCD3, 400 MHz, 25 °C): δ/ppm 1.0–2.4 (CHC6H5/CH2C6H5), 3.1–4.0 (2H, (CO)2O), 5.1 (q, 1H), 7.5 (m, 10H), IR (neat, cm−1): 703, 1029, 1222 1453, 1494, 1601, 1776, 1857, 2939, 3064.
The monomer conversion was kept low to restrict the gradient concentration using RAFT polymerization. Molecular weights and PDIs were determined using gel permeation chromatography system fitted with a RI detector (Waters 2414) and Styragel® HR 3 and HR 4 columns using THF as mobile phase and series of polystyrene standards (molecular weight 690–176,000 g/mol) were used for molecular weight calibration.
Experimental molecular weights were compared with the theoretical molecular weights estimated as per the following relation and are given in Table 1.
where Mn (Theo) is the theoretical number average molecular weight of polymer, MRAFT is the molecular weight of RAFT agent (phenylethyl phenyldithioacetate), WM is the weight of monomer (g), WRAFT is the weight of RAFT agent (g), Conv. is the conversion (weight of polymer (g)/weight of monomers (g)).
Characterization
The composition of co-oligomers was analyzed using 13C NMR (1H NMR was not used here because of overlapping of peaks). 13C NMR was performed on a 400 MHz (JEOL, Tokyo Japan) spectrometer using CDCl3 and acetone-D6 as solvents. The composition analysis of styrene–maleic anhydride co-oligomers was carried out by 13C NMR using relative area of carbonyl peak (AC=O) with respect to total area of aromatic carbon peaks (quaternary carbon (AQuat C) and other aromatic carbons (Aar) using the following equation):
Triad compositional analysis of co-oligomers was carried out in accordance to the reported method (Buchak and Ramey 1976; Butler et al. 1989; Ha 1999) using 13C NMR with 4 s relaxation delay and 20,000 acquisitions.
Modulated differential scanning calorimetry (MDSC) of co-oligomers was carried out under inert atmosphere using Q200 DSC from TA Instruments (New Castle USA). All samples were equilibrated at 0 °C for 5 min and then heated up to 200 °C at a ramp of 5°C/min. The samples were then cooled from 200 to 0 °C at a ramp of 5°C/min and finally heated again up to 250 °C at a ramp of 2 °C/min (the values of second cycle were used for reported results).
Surface active character of obtained amphiphilic co-oligomers was determined by their micelle forming capacity. The critical micelle concentration was derived using a method reported in the literature (Nakahara et al. 2005). Stock solutions of co-oligomers were prepared in DI water, which were then ammonolyzed using 0.5% ammonia solution (w/v) and pyrene was used as the fluorescent probe. Fluorescence spectroscopy of solutions was conducted on FluoroMax-4 spectrometer (Horiba scientific, Kyoto Japan) to obtain emission spectra (300–500 nm) at excitation wavelength of 334 nm using a slit width of 2 nm. Pyrene has tendency to exhibit five solvent dependent vibrational bands. The ratio of intensity (I3/I1) of first (at 373 nm) and third (at 383 nm) vibrational bands strongly relies on polarity of solvent environment of pyrene (Figure S1 of Supporting Information). The ratio of peak intensities at 383 nm (I3) and 373 nm (I1) obtained from the emission spectra of pyrene was plotted as a function of logarithm of co-oligomer concentration. At CMC, the above ratio shows a sudden increase in its value because of inclusion of pyrene inside micelles (Figure S2(a) of Supporting Information). This sudden change was determined by drawing two tangents below and above the change point and their interception point was taken as the CMC value (Figure S2(b) of Supporting Information). Particle-size distribution of self-aggregates of ammonolyzed co-oligomers (surfactants) was analyzed by dynamic light scattering instrument (Zetasizer Nano ZS, 4 mW He–Ne laser, Malvern, Worcestershire UK). The intensity size distributions of self-aggregates were obtained using nonnegative least squares algorithms.
Preparation of n-octadecane loaded polystyrene capsules using SM co-oligomer
For preparation of polystyrene/n-octadecane nanocapsules (NCs), aqueous solution of ammonia (0.5% w/v) was added to a mixture of styrene (1.0 g), macro RAFT agent (0.1 g), n-octadecane (0.25 g) and deionized water (2.0 g). The mixture was initially stirred for 20 min at 60 °C then subjected to ultrasonication for 10 min at 50 °C. To complete the hydrolysis of anhydride units, the mixture was further stirred at 60 °C for 60 min and finally sonicated for 15 min. The obtained miniemulsion was transferred to 25 ml two necked round bottom flask and kept in N2 purging for 15 min. The polymerization was initiated by injecting aqueous solution of potassium per sulphate and the reaction was further carried out for 5 h at 68 °C. The obtained latex was freeze-dried, washed with hot water and dried under vacuum to obtain nanocapsule powder. The diameter of the nanocapsules and core was measured using ImageJ software (NIH, USA). Diameter of 100 nanocapsules was measured and an average value with standard deviation has been reported for the sample. The shell thickness was calculated by subtracting the diameter of core from the capsule diameter and has been reported with standard deviation.
Modulated differential scanning calorimetry (MDSC) of nanocapsules was carried out under inert atmosphere using Q200 DSC from TA Instruments (New Castle USA). The sample was equilibrated at − 20 °C for 2 min and then heated to 60 °C at 10 °C/min under nitrogen atmosphere.
Core content was calculated using following equation
Results and discussion
The mol% of styrene in Sty/MAn mixture varied from 50 to 83.6% (i.e. mole ratio of 1–5.25). It was observed that keeping the conversion below 50% and feed mol% of styrene < 90% could prevent the formation of non-alternating triads in co-oligomers. The co-oligomers corresponding to 83.6 and 75.2 mol% of styrene were soluble in the reaction medium throughout the reaction. For Sty of 50 and 66 mol%, the reaction medium became quite viscous towards the end of polymerization. RAFT polymerization facilitated the formation of well-defined co-oligomers with narrow molecular weight distribution. The number average molecular weight (Mn) of co-oligomers determined by GPC was found to be low in the range of 2000–2200 and PDI in the range of 1.1–1.4 (Table 1). For all the cases, the experimental values were found to be similar to theoretical except for SM-66, where the difference was a little larger.
Composition of co-oligomers
The actual composition of the co-oligomers was determined from the peak ratios of quaternary carbon of phenyl ring and remaining aromatic carbons to that of carbonyl carbon from 13C NMR spectra (Fig. 1). Comparing the values obtained from 13C NMR with the Sty mol% in the feed (Table 2) it was found that the composition of the co-oligomers was greatly influenced by the feed ratio of the monomers. In copolymerization of Sty and MAn, it is generally considered to be an alternating copolymer with composition with equal incorporation of the two monomers. This is because of very low reactivity ratio of Sty and zero for MAn. However, the reactivity ratio of Sty, as reported in the literature (Schoukens et al. 2013) varies from 0.01 to 0.13. It highlights the tendency to incorporate more of styrene moiety when mol% of Sty in the feed is more than 70%. Further, in bulk polymerization, as in the present case, the availability of the two monomers in the vicinity of the growing radicals is likely to get adversely affected due to their poor diffusion in viscous medium. Therefore, as the reaction proceeds and MAn gets depleted and the local concentration of styrene may further increase resulting in higher incorporation of styrene in the growing chains.
The Raman spectra of the four co-oligomers are shown in Fig. 2. The ratio of peak area for styrene at 1604 cm−1 to that of MAn at 1857 cm−1 was considered for determination of co-oligomer composition and values are given in Table 2. The values indicate that incorporation of the two monomers could be regulated by changing the feed ratio for the given set of polymerization conditions.
The composition of styrene obtained from Raman spectra were in fair agreement with corresponding values obtained from 13C NMR (Table 2). Therefore, Raman analysis may also be used for composition analysis of co-oligomers.
Copolymer structure
Microstructure of Sty and MAn copolymer is generally considered to be alternating. However, in our case, RAFT co-oligomers showed varying degree of composition. Such observations indicate a different microstructural arrangement in these types of co-oligomers. Therefore, to have a clear understanding about the monomeric organization, distribution of Sty/MAn monomers in the co-oligomers was analyzed using 13C NMR. The assignment of peaks for different carbons present in the co-oligomers is shown in Fig. 1. The Cb peak represents the quaternary carbon (136.5–148 ppm) position of styrene linked to the backbone chain of co-oligomers. It is sensitive towards distribution of styrene-centered triads in co-oligomers.
Peaks between 136.5 and 141.5, 141.5–145 and 145–148 ppm indicate alternating triad (MSM), semi-alternating (SSM and MSS) and non-alternating (SSS) triad sequences of the co-oligomers, respectively (Buchak and Ramey 1976; Butler et al. 1989; Ha 1999). The up field peaks in δ range of 30–60 ppm related to resonance of methine and methylene carbon of co-oligomers are also sensitive to the triad sequences. Peaks at 33–37, 37–42 and 42–45 ppm correspond to resonance of alternating, semi-alternating and non-alternating triad sequences, respectively. However, the quantitative analysis of triad sequences using peaks of aliphatic region is difficult due to their overlapping with each other (Schoukens et al. 2013). Therefore, the microstructure of co-oligomers has been predicted using sensitivity of Cb peak as shown in Fig. 3.
It has been reported that when excess of Sty is taken and polymerization is continued for a high conversion, non-alternating Sty blocks (SSS) are added as tail on the alternating structure (Yao et al. 2011). In contrast, in our case, the RAFT polymerization time was regulated (kept up to 130 min), to keep the conversions below 50%. This prevented the formation of non-alternating (SSS) triads. With increase in molar ratio of Sty vs MAn in the feed, the ratio of area of Cb alt/Cb (semi alt + non alt) was found to decrease in the co-oligomers. It was observed that the percentage of non- alternating triad (SSS) was very low in SM-84 co-oligomer, which had the highest mol% of Sty and it was completely absent in the other three co-oligomers (Table 3). These three co-oligomers (SM-75, SM-66 and SM-50) contained only the alternating (MSM) and semi-alternating triads (MSS + SSM). It may be inferred that for the four ratios used in the experiment, the given reaction time was not sufficient for styrene to form non-alternating triads. However, the high enough concentration of Sty in comparison to MAn was able to form semi-alternating triads of Sty. Our results are in agreement with the observation made by Zeng and Shirota (1989) on free radial copolymerization of Sty and MAn. They have reported that poly(styrene-co-maleic anhydride) is not a real alternating copolymer in spite of nearly 1:1 composition of the copolymer formed from a wide range of monomer feed compositions. This has been evaluated using a more sensitive fluorescence spectroscopy technique instead of elemental analysis usually carried out for compositional analysis of such copolymers. They have shown that Sty–Sty diad fractions increased with increase in Sty mole fraction in the feed. Roth et al. (1981) have reported that the copolymerization with excess mole fraction of styrene results in deviation from the alternating structure. This structure has been evaluated using resonance of aromatic carbon attached to polymer chain in 13C NMR.
Thermal behaviour of RAFT co-oligomers
Modulated DSC graphs of the co-oligomers given in Fig. 4 show that they have a single glass transition temperature (Tg). This indicates that the co-oligomers have both the co-monomers incorporated in the chain and they are uniformly distributed without formation of long segments of styrene. The glass transition temperature, Tg was found to decrease linearly from 189 to 110 °C as the concentration of styrene in co-oligomers increased from 59 to 72.6 mol%. This lowering of Tg may be because of lower degree of interaction among chains with lesser number of maleic anhydride units. At the same time, since there are no non-alternating triads, the gradual shift in glass transition temperature is in fact a function of changing concentrations of alternating and semi-alternating structures in the co-oligomers. Their relative content is responsible for the change in their thermal properties.
Self-assembly behaviour of co-oligomers
Co-oligomers synthesized can find applications as reactive surfactants for formation of miniemulsion. To characterize their self-assembly behaviour, the co-oligomers were converted to reactive surfactants by hydrolyzing and ammonolyzing the MAn units to create hydrophilic groups in the co-oligomers. The CMC values for these reactive surfactants were estimated using fluorescence emission spectroscopy as per the reported method described in “Experimental” and detailed in Supporting Information. The observed CMC of the co-oligomers were 0.1584, 0.4410, 0.6300 and 1.1200 mg/ml, for SM-84, SM-75, SM-66 and SM-50, respectively. The co-oligomer architecture was found to influence the CMC values. The CMC values were found to decrease gradually with increase in Sty mol% in the co-oligomers due to increase in semi-alternating triads (MSS) in the co-oligomers resulting in increased hydrophobicity of the surfactants (Fig. 5).
The micelles formed from the surfactants were also characterized by particle size analysis. Figure 6 shows the particle size distribution of micelles formed using different oligomeric surfactants. The peak particle size varied in the range of 15–164 nm.
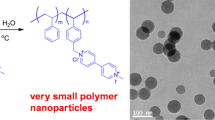
Co-oligomer SM-84, having maximum content of semi-alternating triads (with negligible non-alternating triad) exhibited lowest CMC and formed micelle of smallest size, i.e., 15 nm. With decrease in content of semi-alternating triads, the size of the micelle formed was found to increase to 30, 39 and 164 nm for surfactants of SM-75, SM-66 and SM-50, respectively. CMC values also increased in the same order. It appears that, formation of semi-alternating segments have great influence on the size of the self-assembled micelle of the various oligomeric surfactants.
It has been reported (Luo and Gu 2006) that self-assembling behaviour of styrene–maleic anhydride copolymers is due to alternating and purely non-alternating part (hydrophobic tail) of copolymer. In contrast, we have found that semi-alternating triads can equally influence the formation of different sizes of micelle, indicating their role in self-assembling behaviour of the reactive surfactants. Proposed mechanism for the formation of different micelle is depicted in Fig. 7, where ammonolyzed alternating segments are likely to remain in the aqueous phase and the semi-alternating segments (SSM + MSS) tend to come close to each other facilitating the formation of micelle. As the number of semi-alternating segments increase, the co-oligomers could pack into smaller size giving rise to smaller micelle.
Nanoencapsulation of octadecane using reactive macro RAFT agent
To investigate the feasibility of SM co-oligomers for their use as reactive surface-active agents, SM-84 was selected for nanoencapsulation of OD. SM-84 was used in surfactant-free RAFT miniemulsion polymerization of styrene to prepare NCs. FE-SEM and TEM micrographs of NCs shown in Fig. 8, revealed both filled NCs and unfilled polystyrene particles in the latex obtained from miniemulsion polymerization. The TEM micrographs of latex confirmed the presence of large number of octadecane (OD) filled polystyrene NCs consisting of well-defined core–shell structure with an average diameter of 97 ± 33 nm. The OD is seen as lighter density core surrounded by dense polymeric wall. The average diameter of core of NCs was ~ 31 nm and shell thickness was found to be ~ 17 nm.
The FT-IR spectra of OD and OD NCs shown in Fig. 9 confirmed the presence of styrene, and n-octadecane in NCs. The peaks for aliphatic C–H stretching related to OD appeared at 2920 and 2854 cm−1 while aromatic C-H stretching of styrene was observed at 3000–3100 cm−1. The peak at 698 cm−1 was associated with in-plane rocking vibration of CH2 group of n-octadecane.
DSC thermographs were recorded for the isolated and washed OD NCs to confirm the content of OD in them. These are shown in Fig. 10. The NCs clearly showed melting phase change transition at 28 ± 0.5 °C and crystallization at 23 °C, which are nearly same for the bulk OD. The melting and crystallization were observed to be congruent even when the OD is constrained inside < 100 nm sized NCs. The latent heat of fusion was found to be 43 J/g for the NCs. This corresponds to a core content of ~ 20%.
The results confirmed that reactive macro RAFT agents could be successfully used for nanoencapsulation of hydrophobic materials for various applications.
Conclusions
Oligomeric surfactants based on styrene–maleic anhydride (Sty/MAn) were synthesized using 1-phenylethyl phenyldithioacetate (PEPDA) as the non-retarding RAFT agent and AIBN as initiator. Four different RAFT co-oligomers (SM-50, SM-66, SM-75 and SM-84) were synthesized by varying the mol% of styrene in Sty/MAn mixture from 50 to 83.6%. The molecular weight of co-oligomers of Sty and MAn determined by GPC was found to be low in the range of 2000–2200 and PDI in the range of 1.1–1.4. The composition and architecture of prepared co-oligomers were determined by 13C NMR and Raman spectroscopy. 13C NMR revealed that under control polymerization conditions, the formation of non-alternating triads (SSS) could be suppressed and the relative content of semi-alternating (MSS + SSM) and alternating (MSM) triads could be systematically varied by changing the monomer feed ratio. The self-assembling behaviour of ammonolyzed co-oligomers (surfactants) was analyzed by fluorescence spectroscopy and DLS and it was found that the CMC values and the size of micelle were greatly influenced by the proportion of semi-alternating triads in different oligomeric surfactants. Surfactant SM-84 showed the lowest CMC value and formed the smallest micelle of size 15 nm among all the four co-oligomers. Uniform sized PS/OD nanocapsules were synthesized using SM-84 co-oligomer by RAFT miniemulsion polymerization technique. The OD NCs showed thermal transition similar to the bulk OD with latent heat of fusion of 43 J/g.
References
Baglioni M, Raudino M, Berti D, Keiderling U, Bordes R, Holmberg K, Baglioni P (2014) Nanostructured fluids from degradable nonionic surfactants for the cleaning of works of art from polymer contaminants. Soft Matter 35:6798–6809
Bapat AP, Ray JG, Savin DA, Hoff EA, Patton DL, Sumerlin BS (2012) Dynamic-covalent nanostructures prepared by Diels–Alder reactions of styrene-maleic anhydride-derived copolymers obtained by one-step cascade block copolymerization. Polym Chem 3:3112–3120
Baskar G, Landfester K, Antonietti M (2000) Comblike polymers with octadecyl side chain and carboxyl functional sites: scope for efficient use in miniemulsion polymerization. Macromolecules 33:9228–9232
Benoit D, Hawker CJ, Huang EE, Lin Z, Russell TP (2000) One-step formation of functionalized block copolymers. Macromolecules 33:1505–1507
Buchak BE, Ramey KC (1976) Monomer sequence distribution in styrene-maleic anhydride copolymers. J Polym Sci Polym Lett Ed 14:401–405
Butler GB, Do CH, Zerner MC (1989) The stereochemistry of styrene-maleic anhydride copolymers: 13C-NMR study and PVCILO and INDO/1 calculations. J Macromol Sci Chem 26:1115–1135
Davies MC, Dawkins JV, Hourston DJ (2005) Radical copolymerization of maleic anhydride and substituted styrenes by reversible addition-fragmentation chain transfer (RAFT) polymerization. Polymer 46:1739–1753
De Paz Banez M, Robinson K, Vamvakaki M, Lascelles S, Armes S (2000) Synthesis of novel cationic polymeric surfactants. Polymer 41:8501–8511
Durand A, Marie E (2009) Macromolecular surfactants for miniemulsion polymerization. Adv Colloid Interface Sci 150:90–105
Gupta A, Eral HB, Hatton TA, Doyle PS (2016) Nanoemulsions: formation, properties and applications. Soft Matter 11:2826–2841
Ha NTH (1999) Determination of triad sequence distribution of copolymers of maleic anhydride and its derivates with donor monomers by 13C NMR spectroscopy. Polymer 40:1081–1086
Hill MR, Carmean RN, Sumerlin BS (2015) Expanding the scope of RAFT polymerization: recent advances and new horizons. Macromolecules 48:5459–5469
Klumperman B (2010) Mechanistic considerations on styrene–maleic anhydride copolymerization reactions. Polym Chem 1:558–562
Kooij VD, Hanne M, Sprakel J (2015) Watching paint dry; more exciting than it seems. Soft Matter 32:6353–6359
Lessard B, Maric M (2009) One-step poly (styrene-alt-maleic anhydride)-block-poly (styrene) copolymers with highly alternating styrene/maleic anhydride sequences are possible by nitroxide-mediated polymerization. Macromolecules 43:879–885
Lu F, Luo Y, Li B (2007) A facile route to synthesize highly uniform nanocapsules: use of amphiphilic poly(acrylic acid)-block-polystyrene RAFT agents to interfacially confine miniemulsion polymerization. Macromol Rapid Commun 28:868–874
Luo Y, Gu H (2006) A general strategy for nano-encapsulation via interfacially confined living/controlled radical miniemulsion polymerization. Macromol Rapid Commun 27:21–25
Luo Y, Gu H (2007) Nanoencapsulation via interfacially confined reversible addition fragmentation transfer (RAFT) miniemulsion polymerization. Polymer 48:3262–3272
Manguian M, Save M, Chassenieux C, Charleux B (2005) Miniemulsion polymerization of styrene using well-defined cationic amphiphilic comblike copolymers as the sole stabilizer. Colloid Polym Sci 284:142–150
Moad G, Rizzardo E, Thang SH (2009) Living radical polymerization by the RAFT process—a second update. Aust J Chem 62:1402–1472
Nakahara Y, Kida T, Nakatsuji Y, Akashi M (2005) New fluorescence method for the determination of the critical micelle concentration by photosensitive monoazacryptand derivatives. Langmuir 21:6688–6695
Parida D, Serra CA, Bally F, Garg DK, Hoarau Y (2012) Intensifying the ATRP synthesis of statistical copolymers by continuous micromixing flow techniques1. Green Process Synth 1:525–532
Park C, Yoon J, Thomas EL (2003) Enabling nanotechnology with self assembled block copolymer patterns. Polymer 44:6725–6760
Pham BT, Nguyen D, Ferguson CJ, Hawkett BS, Serelis AK, Such CH (2003) Miniemulsion polymerization stabilized by amphipathic macro RAFT agents. Macromolecules 36:8907–8909
Quinn JF, Rizzardo E, Davis TP (2001) Ambient temperature reversible addition–fragmentation chain transfer polymerisation. Chem Commun 11:1044–1045
Roth H, Arnold M, Rätzsch M (1981) 13C-NMR-Untersuchungen an Styren-Maleinsäureanhydrid-Copolymeren. Acta Polym 32:277–280
Schoukens G, Martins J, Samyn P (2013) Insights in the molecular structure of low-and high-molecular weight poly (styrene-maleic anhydride) from vibrational and resonance spectroscopy. Polymer 54:349–362
Sirohi S, Singh A, Dagar C, Saini G, Pani B, Nain R (2017) Facile synthesis of microporous SiO2/triangular Ag composite nanostructures for photocatalysis. Appl Nanosci 8:633–643
Stoffelbach F, Belardi B, Santos JM, Tessier L, Matyjaszewski K, Charleux B (2007) Use of an amphiphilic block copolymer as a stabilizer and a macroinitiator in miniemulsion polymerization under AGET ATRP conditions. Macromolecules 40:8813–8816
Xiong Q, Ni P, Zhang F, Yu Z (2004) Synthesis and characterization of 2-(dimethylamino)ethyl methacrylate homopolymers via aqueous RAFT polymerization and their application in miniemulsion polymerization. Polym Bull 53:1–8
Yao Z, Zhang JS, Chen ML, Li BJ, Lu YY, Cao K (2011) Preparation of well-defined block copolymer having one polystyrene segment and another poly(styrene-alt-maleic anhydride) segment with RAFT polymerization. J Appl Polym Sci 121:1740–1746
Zeng W, Shirota Y (1989) Studies on alternating radical copolymerization: analysis of microstructures of styrene-maleic anhydride, styrene-acrylonitrile, and styrene-methyl methacrylate copolymers by fluorescence spectroscopy. Macromolecules 22:4204–4208
Acknowledgements
The authors acknowledge partial financial support provided by Department of Science and Technology, Govt. of India under various research grants. One of the authors (S. Sirohi) would like to thank Bhaskaracharya College of Sciences (University of Delhi) for providing opportunity to pursue Ph.D. under which this work was carried out.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sirohi, S., Jassal, M. & Agrawal, A.K. Surfactant-free nanoencapsulation using reactive oligomers obtained by reversible addition fragmentation chain transfer polymerization of styrene and maleic anhydride. Appl Nanosci 8, 1701–1710 (2018). https://doi.org/10.1007/s13204-018-0845-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0845-2