Abstract
An alkenyl (butene)-functionalized amphiphilic macromolecular reversible addition fragmentation chain transfer (macro-RAFT) agent was designed as both surfactant and polymerization mediator for ab initio emulsion and miniemulsion polymerization of styrene (St), intending to synthesize polymer nanoparticles with alkenyl-enriched surfaces. However, the pendent alkenyl units exhibited unexpectedly higher reactivity in ab initio emulsion polymerization than they did in solution polymerization. Their copolymerization with St resulted in depletion of alkenyl groups, positive deviation of molecular weights, broad molecular weight distributions, and limiting conversions. Miniemulsion polymerization was superior to ab initio emulsion polymerization in its ability to prepare alkenyl-functionalized nanoparticles. Consumption of alkenyl groups happened, but only within the nucleation period. In spite of positive deviation of molecular weights and broad molecular weight distributions, the final particles were characteristics of alkenyl functionalities confined to the particle surfaces. Thus, PSt nanoparticles with alkenyl-enriched surface were obtained via miniemulsion polymerization.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles (NPs) are promising platforms for biomedical applications due to their unique physicochemical properties [1]. Extensive studies demonstrated that surface properties, such as charge and hydrophobicity, should be well considered when designing NPs as drug delivery vehicles featuring high drug loading efficiency, controlled biodistribution and low cytotoxicity [2]. However, for this purpose, a detailed understanding of how NP surfaces with various properties affect biological systems is essentially required for creating NPs with such useful therapeutic functions. Up to now, numerous model nanosystems, such as inorganic NPs (Au [3], Fe3O4 [4], SiO2 [5] etc), carbon materials (fullerene [6], nanotubes [7], graphene [8] etc.), have been developed and investigated as potential cancer therapeutics.
In comparison to those inorganic and carbon-based NPs, polymeric nanoparticles have attracted more interest owing to their structural versatilities including size, compositions and surface functionalities [9]. These surface functionalities include hydroxyl [10], aldehyde [11,12,13,14,15,16,17], carboxylic acid [18,19,20], amino [21,22,23],epoxy [24], sulfhydryl [25], chloromethyl [26, 27], and vinyl groups [28]. Among them, the vinyl group is especially useful because of the broad applications in highly efficient radical coupling [29,30,31,32,33], postmodification [34], and transformations into other useful functionalities [35]. It further suggested that these reactions offered straightforward ways to modulate polymeric particle properties such as hydrophilicity [36], surface charge [37], immunogenicity [38], biodistribution [39] and intracellular bioavailability.
Polymeric particles with vinyl groups have generally been prepared by following approaches. (1) Dispersion or seeded dispersion (co)polymerization of divinylic monomers [40]. (2) Precipitation polymerization of divinylic monomers [41]. Although the above approaches have been successful adopted in preparing vinyl-functional polymeric particles, some drawbacks of these methods have been identified. First, the resulting particles are much larger than 1 μm, which are usually opsonized and accumulate in the liver and spleen, with possibility of aggregation and capillary occlusion [9]. In addition, in the cases of both a batch process and a semi-batch process, synthesis of polymeric particles with controlled distribution and content of vinyl groups on the surface is very difficult to achieve. For example, a second addition of monomer generated new particles [28] with sharply different vinyl group content. It resulted in the difficulty in precise location of post-functional groups, and therefore modulating the surface properties of particles.
Recently, it has been demonstrated that well-designed amphiphilic macromolecular RAFT (macro-RAFT) agents can be applied as both reactive surfactant and polymerization mediator in RAFT (mini)emulsion polymerization [40,41,42,43,44,45]. In this case, polymerization would start at the interface of minidroplets or micelles, and then polymer chains would grow inwards [42,43,44,45,46,47]. In addition, since the molecular architecture and composition of the macro-RAFT agent can be easily adjusted, the content of particle surface functionalities can thus be tuned via delicately designed macro-RAFT agents [42, 48]. On the other hand, in the scope of nanoparticle surface functionalization, thiol-ene click reaction has emerged as an extremely powerful tool [49], due to its versatility, high efficiency, high tolerance to the presence of air/oxygen and moisture, and a wide range of ene and thiol groups (or compounds) available for thiol-ene reaction [50]. Although these results suggested that RAFT (mini)emulsion polymerization in combination with thiol-ene reaction could be utilized to engineer the nanoparticles, there are still some aspects concerning the selection of ene groups that deserve careful consideration: (1) The reactivity of the ene groups during thiol-ene reactions. It is very important for the thiol-ene systems with balanced shelf-life stability and high efficiency [51]. (2) The relative reactivity of the multiple vinyl groups during radical polymerization [9]. The significant difference in reactivity between the clickable ene units and styrenyl- or acrylate-based monomers provides particles with well-defined molecular characteristics, including desirable functionality in a site-specific manner and predictable molecular weight. Up to now, the reactivity of various types of enes, such as norbornene, vinyl ether, allyl ether, alkene, acrylate, styrene, with thiols has been reported in literatures [50]. However, the effects of ene structure on the basic (mini)emulsion polymerization performance are much less investigated. There are only sporadic reports of synthesis of clickable polymeric nanoparticles with cyclohexene group-enriched surface [48].
Being different from cycloalkenyl functionalities, alkenyl (butene) units have open-chain structure and exhibit less steric hindrance toward radical addition. This indicates their higher reactivity in some reactions special for the surface post-functionalization of particle nanoparticles, like thiol-ene click reaction [50]. Furthermore, theoretically, butene units are less reactive than styrenyl- or acrylate-based monomers toward radical polymerization. These features make the incorporation of butene groups onto the nanoparticles of great interest, since it not only expands the scope of thiol-ene clickable particles, but also benefits the investigation of the dependence of polymerization selectivity on ene structure in (mini)emulsion systems. In this work, ab initio emulsion and miniemulsion polymerizations of styrene (St) are mediated by a butene-functionalized amphiphilic macro-RAFT agent, intending to prepare polymer NPs with vinyl group-enriched surfaces. The polymerization kinetics in terms of St conversion, alkenyl group conversion, latex polymer molecular weight and distribution are compared between these two methods. Finally, a successful thiol−ene click (TEC) reaction between NPs and water-soluble thiol compound demonstrates that PSt nanoparticles with butene-enriched surface were obtained via miniemulsion polymerization.
Materials and methods
Materials
Styrene (St) and acrylic acid (AA) were purified by vacuum distillation prior to polymerization. Deionized water (conductivity < 4 μS cm−1) was used as received. 4,4′-azobis (4-cyanovaleric acid) (V-501, 98%), potassium persulfate (KPS, > 99%), sodium dodecyl sulfate (SDS, surfactant), n-hexadecane (HD, costabilizer), dithiothreitol (DTT) and Darocur 2959 were used without further purification. The small RAFT agent, 2-{[(dodecylsulfanyl)-carbonothioyl]sulfanyl}propanoic acid (DSCTSPA), was synthesized and purified according to the reference [52]. 4-((but-3-en-1-yloxy) methyl) styrene (BEOMST) is an asymmetric divinyl monomer, which was synthesized as described in the literature [53].
Synthesis of alkenyl-functionalized trithiocarbonate amphiphilic macro-RAFT agent (macro-RAFT agent 1)
The macro-RAFT agent 1 was synthesized by a two-step solution polymerization. The synthetic scheme is shown in Scheme S1 (Supplementary Information section). During the first step, a solution containing AA (9.257 g, 1.28 × 10−1 mol), DSCTSPA (1.500 g, 4.28 × 10−3 mol), V-501 (0.120 g, 4.28 × 10−4 mol), and 1,4-dioxane (25.10 g, 2.85 × 10−1 mol) was added to a flask. The mixture was deoxygenated and preceded with stirring at 80 °C for 2 h. Then, another solution containing BEOMST (4.834 g, 2.57 × 10−2 mol), V-501 (0.120 g, 4.28 × 10−4 mol) and 1,4-dioxane (11.28 g, 1.28 × 10−1 mol) was introduced to the reactor. The second step reaction lasted for further 12 h. AA and BEOMST conversions were measured by gas chromatography (GC, Shimadzu GC-2010 High-Performance Capillary Gas Chromatograph with FID detector) using 1,4-dioxane as the internal standard, and exceeded 98 and 51%, respectively. The product (macro-RAFT agent 1) was collected by precipitation of the mixture in cyclohexane. The macro-RAFT agent 1 was dried in vacuum oven under vacuum at 60 °C. The 1H NMR spectrum in Fig. S1 (Supplementary Information section) confirmed the chemical structure in Fig. 1.
Synthesis of trithiocarbonate amphiphilic macro-RAFT agent without alkenyl groups (macro-RAFT agent 2)
During preparation of macro-RAFT agent 2, we followed the same procedures and recipes for the synthesis of macro-RAFT agent 1, with a replacement of BEOMST with St (4.5 g, 4.32 × 10−2 mol) in the second step. St conversion was 50%. The final product was collected by precipitation of the mixture in cyclohexane and dried under reduced pressure at 60 °C. The procedure is schematically illustrated in Scheme S2 (Supplementary Information section). The 1H NMR spectrum in Fig. S2 (Supplementary Information section) confirmed the chemical structure in Fig. 2.
Ab initio emulsion polymerization of St mediated by macro-RAFT agent (E1, E2, E3 and E4)
E1, E2 and E3 employed the same alkenyl-functionalized macromolecular RAFT agent (macro-RAFT agent 1) concentration with different temperature profiles. E4 is a control run mediated by macro-RAFT agent 2 bearing no alkenyl groups. The detailed recipes and temperature profiles are shown in Table 1 and in the first paragraph of “Results and discussion”, respectively.
Taking E1 as an example, 6.148 g macro-RAFT agent 1 was dissolved in 115 g deionized water. 30 g St was mixed with above aqueous solution. After 20 min, the coarse emulsion was transferred to a 250-mL flask, and deoxygenated with nitrogen for 30 min. Then, the flask was immersed in a thermostated water bath at 70 °C. Polymerization was initiated by injection of 0.108 g KPS dissolved in 2 g water. After 20 min, 0.4 g of NaOH dissolved in 3 g water was added to the flask to enhance the dispersion stability of the emulsion. Samples were taken at regular time intervals to follow monomer conversions, molecular weights and average number of alkenyl groups per polymer chain.
Miniemulsion polymerization of St mediated by macro-RAFT agent 1 (ME 1)
ME1 was carried out at 70 °C, and was mediated by the alkenyl-functionalized macromolecular RAFT agent (macro-RAFT agent 1).
By dissolving water-soluble components (1.5 g of SDS and 6.148 g of macro-RAFT agent) in deionized water (115 g of water) and oil-soluble components (1.5 g of HD) in monomer (30 g of St), we mixed and stirred the separated solutions for 10 min. The resultant emulsion was then homogenized by using a Scientz JY92-II sonifier (amplitude 50%, 500 W) for 99 cycles. Each cycle lasted 10 s with an interval of 5 s. The obtained miniemulsion was then charged to a 250-mL four-neck flask reactor equipped with a condenser, a nitrogen inlet, a sampling port, and a mechanical stirrer. The reactor was purged with nitrogen for 30 min before the reaction started. Finally, the injection of 0.135 g of KPS dissolved in 5 g water was added when its temperature reached 70 °C, giving the zero time of the polymerization. Samples were periodically withdrawn to monitor the conversion of monomer as a function of time and the evolution of molar masses, molar mass distributions, and number of alkenyl groups per polymer chains as a function of monomer conversion.
UV-induced thiol−ene reaction
The reaction was carried out in aqueous phase. DTT (0.064 g, 4.15 × 10−4 mol, thiol compound) and Darocur 2959 (0.093 g, 4.15 × 10−4 mol, photoinitiator) were dissolved in 15 g of polymer latex (2.77 × 10−4 mol of alkenyl group) from experiment ME1. The mixture was transferred to a 10-mL quartz beaker with septa, and irradiated by UV light for 5 h at ambient temperature under magnetic stirring. The radiation source was a YINGFENG YZ T8 40 W × 4 high-pressure mercury arc lamp.
Characterization
Monomer conversion
Monomer conversions during polymerizations were followed gravimetrically.
GPC analysis
Molecular weights and dispersities (Đ) of polymers were determined at 30 °C by GPC (Waters 2707/1525/2414) with three Waters Styragel® columns (Styragel® HR3, HR4 and HR1 (or HR5) using differential refractive index detector. The eluent was tetrahydrofuran (THF) with a flow rate of 1 mL/min. The measurement was calibrated using narrow polystyrene standards (Polymer Laboratory) with molecular weight ranging from 1.2 to 666 kg mol−1.
For RAFT polymerization in miniemulsion and ab initio emulsion, the theoretical number average molecular weights (Mn,thes) were calculated by Eq. (1).
where [RAFT]0 is the initial molar concentration of RAFT agent. MRAFT,GPC, MM, [M]0, and x are molecular weight of the macromolecular RAFT agent measured by GPC, molecular weight of monomer, initial monomer concentration, and monomer conversion, respectively.
NMR analysis
1H NMR spectra were measured on Bruker Avance AV 400 MHz Digital FT-NMR Spectrometer at room temperature (Internal reference: TMS (tetramethylsilane); macro-RAFT agent and polymers: 1% solution in DMSO-d6). The NMR integrations were repeated 5 times on the same spectrum. For ab initio emulsion samples prepared at high conversions, the errors in integration results originated from both smoothing of baseline noise and manual selection of peaks. For the rest samples, the error resulted from manual selection of peaks.
Polymers from ab initio emulsion polymerizations were measured directly. Samples from miniemulsion run were purified before NMR analysis as follows: the latex polymers were milled into small pieces and washed five times with hot water under ultrasound treatment to remove SDS. Then, HD was driven off from the polymers at 115 °C for 8 h under vacuum.
Calculation of average number of pendent alkenyl groups per polymer chain (N)
N was determined as Eq. (2):
where A(=CH2) and A(-CH3) are the areas of = CH2 and –CH3 according to 1H NMR spectrum, respectively.
Particle size and its distribution
The particle size was measured by dynamic light scattering (DLS, Malvern Zetasizer Nano S). Before DLS analysis, each part of the latex samples was diluted with 50 parts of deionized water and kept at 50 °C under 10 mmHg for 15 h to drive off the residual monomer. The z-average particle size was then measured after a sonification treatment.
The particle size and its distribution were also measured on transmission electron microscopy (TEM, JEM-1230, JEOL). The samples were prepared as follow: one drop of the dilution (0.03 g of latex in 20 g water) was placed onto the carbon-coated copper mesh and dried at room temperature. The particle sizes were derived from the TEM image statistic (300~500 particles for each sample were counted). The calculation of volume average particle sizes was based on Eq. (3).
Results and discussion
Table 1 summarizes the experimental recipes used in the current study as well as the resulting particle sizes. E1, E2, E3 and E4 were all ab initio emulsion polymerizations. E1, E2 and E3 employed the same alkenyl-functionalized RAFT agent (macro-RAFT agent 1) concentration with different temperature profiles. These three experiment runs conducted here began at 70 °C. However, for E1, the temperature was kept at 70 °C throughout the reaction. For E2 and E3, 35 min after the injection of the initiator aqueous solution, the temperatures were reduced to 60 °C and raised to 85 °C, respectively, in 5 min period. (Note: The polymerizations achieved around 60% conversion at 35 min.). E4 is a control run mediated by macro-RAFT agent 2 bearing no alkenyl groups, and operated at 70 °C. ME1 was also carried out at 70 °C, but followed a miniemulsion process mediated by macro-RAFT agent 1. No instability issues were observed during all experiment runs.
Ab initio emulsion polymerization
Average number of alkenyl groups per polymer chains (N)
Ns were examined by NMR spectroscopy. Figure 3 depicts the evolutions of N with monomer conversion. The raw NMR spectra were collected in Fig. S4 (Supplementary Information section). In E1, N decreased from 3.0 to 1.2 within first 20% conversion, and remained constant at 1.2 as the polymerization proceeded to around 70% conversion. After that, N started to decrease for the second time with conversion. At the end of polymerization, N is only 0.3, indicating 90% percent of alkenyl groups were consumed. Ns in E2 and E3 followed the similar rules as that in E1 before 70% conversion, but they declined for the second time at varied conversions. The conversions were postponed from 60% (E2) to 70% (E1) and then to 85% (E3), as the reaction temperature increased from 60 to 70 °C and then to 85 °C during the late stage.
Evolution of average number of alkenyl groups per polymer chains (N) with monomer conversions during ab initio emulsion polymerization of St mediated by alkenyl-functionalized RAFT agent (macroRAFT-1). The average error in N is 9% at conversions less than 70%, and increases to 17% at higher conversions
Number average molecular weights (Mns) and molecular weight distributions
Figure 4 represents the plots of Mn and Đ values against monomer conversions in E1, E2 and E3, respectively. The theoretical Mns were calculated from Eq. (1), where the contribution from the irreversible termination was ignored. From Fig. 4, the followings are derived:
First, Mns of all experiments positively deviated from the theoretical line since the very beginning of polymerization, and increased linearly with conversion till 70% conversion. During this period, Đs of all experiments decreased gradually with conversion, and finally reached around 1.3. The decrease in Đs has been ascribed to the higher propagation rate in later-born particles than that in earlier-born counterparts [54]. Second, as the polymerization proceeded to conversions higher than 70%, Mns grew sharply and turned to deviate from the theoretical values more significantly. In the meanwhile, Đs grew steeply. A close examination shows that above evolutions of Đ and Mn are temperature-dependent, being similar to those shown in Fig. 3. The turning points were delayed to higher conversions by increased temperature.
Luo et al. proposed that the incomplete conversion of the original macro-RAFT agent would lead to positive deviation of experimental Mns from the theoretical ones [45]. However, this was not responsible for the present results. As shown in Fig. S5 (Supplementary Information section), GPC curves collected from a UV 311 nm (Peak of C=S group) detector revealed that the fraction of macro-RAFT agent 1 involved in the polymerization was very high. Then, a control run coded as E4 was performed. It targeted the same Mn as the former three runs, but was mediated by macro-RAFT agent 2 bearing no alkenyl groups. Being different from its counterparts, E4 produced polymers with predictable Mns (Fig. 5) as well as Đs being rather stable after 70% conversion. As these GPC analysis results are combined with the conversion of alkenyl units in the former three runs (Fig. 3), it was concluded that the positive deviations of Mns in E1, E2 and E3 were due to copolymerization of alkenyl units with St and polymer chain branching. From Fig. S6 (Supplementary Information section), it is clear that a shoulder peak of higher molecular weight appears in the GPC spectra of the high conversion samples, owing to the branched polymer chains. Furthermore, as alkenyl groups connect directly to the hydrophilic PAA blocks in the molecules of macro-RAFT agent 1, the branching points were expected to locate within the particle surface layers.
Polymerization kinetics
Figure 6 presents the conversion-time plots of all ab initio emulsion polymerization runs. All runs ceased within 100 min but at varied conversions. Limiting conversion was observed in E1 and E2 operated finally at 60 and 70 °C, which are 90.0 and 92.5%, respectively. In contrast, monomer conversions exceeded 98% in both E3 operated at 85 °C and E4 mediated by the macro-RAFT agent 2 without alkenyl groups.
In early works, Chen observed the limiting conversion during conventional (non-living) emulsifier-free polymerization of St. The reason was a shell-growth mechanism combining with a monomer-diffusion-controlled polymerization mechanism during high conversion range. Such a mechanism occurred exclusively for latex system with large particles having a diameter larger than 200 nm, because the end-to-end distance of the growing radicals was much smaller than the radius of those particles [55]. In comparison to Chen’s work, an apparent difference was discerned for the current study that the particle sizes of all latex were only around 65 nm and far less than the threshold size (200 nm) to arouse above mechanism.
As indicated, in the high conversion ranges of E1, E2 and E3 (Fig. 4), the polymer branching degrees grew significantly with consumption of alkenyl groups. These acted to increase viscosity strongly within the particle surface layers because of the location of branching points close to the particle surfaces. Consequently, the entry event, when an aqueous radical enters a particle, can be diffusion-controlled and even be prevented [56]. Eventually, monomer conversion plateaued at values around 90% in E1 and E2.
The above explanation can be further supported by the following experiments: (1) the reaction temperature was increased to 85 °C in E3. (2) E4 employed a macromolecular RAFT agent without pendent alkenyl groups, and produced particles composed of linear polymer chains. The lowered viscosity within both E3 and E4 particle surface layers had little hindrance to radical entry into particles. Therefore, neither E3 nor E4 exhibited limiting conversion in Fig. 6.
Miniemlusion polymerization of St mediated by a macromolecular RAFT agent with pendent alkenyl groups (macro-RAFT agent 1)
Figure 7a shows that consumption of pendent alkenyl groups also happened to miniemulsion polymerization ME1, while it ceased after 30% conversion. As a result, N decreased from 3 to 1.1. At the end of polymerization, nearly 40% of alkenyl units remained. TEC reaction employing a water-soluble thiol compound DTT (Fig. S7, Supplementary Information section) achieved 98.1% (It was calculated from (1–0.04/2.11)) conversion of these alkenyl groups, demonstrating interface confinement characteristics of these alkenyl groups. Therefore, miniemulsion polymerization is superior to ab initio emulsion polymerization in terms of preparation of nanoparticles with alkenyl-functionalized surfaces.
As shown in Fig. 7b, ME1 produced polymers with rather broad Đs that grew steadily from 1.7 to 3.0 with monomer conversion. Mns of the polymers positively deviated from theoretical ones, but the linear relationship between Mns and conversions demonstrates the living characters of the polymerization. As mention above, the positive deviation of experimental Mns was due to the consumption of alkenyl units and polymer chain branching. Figure 7c shows the GPC curves exhibit two-peak distribution. Meanwhile, the particles from ME1 have a very broad particle size distribution and are even bimodal to some extent. (Fig. S3(e)). A combination of these findings suggests the occurrence of particle “swelling” during ME1 [54]. The worst consequence of the “swelling” would lead to the bulk separation layer and lose control in molecular weight [57]. In the case of ME1, although the latex was stable, the molecular weight distributions were very broad. As shown in Fig. 7d, limiting conversion did not occur to miniemulsion polymerization. ME1 achieved full conversion at 90 min. It is likely due to the plasticization of PSt by the costabilizer [58], HD, which benefited the entry of aqueous radicals into particles.
Discussion on the consumption of alkenyl groups
During the early stage of polymerization
In a homogeneous reaction system, the reactive difference between St and pendent alkenyl group is reasonable to be quantitatively compared based on the reactivity ratios (r1, r2) calculated using Alfrey-Price equations [59]. Table 2 presents the predicted reactive difference between the two vinyl groups. These values (r1> > r2 ≈ 0, a high ratio of r1/ r2) in combination with the high molar ratio of St/alkenyl (It is 48 in E1, E2 and E3) imply that the homogeneous polymerization reaction will exhibit high selectivity toward St. This theoretical model coincided well with the recent data from RAFT solution polymerization, that the pendent alkenyl groups would remain totally intact even if the molar ratio decreased to 1.8 [34, 61], which corresponded to 96.3% St conversion in E1, E2, E3 and ME1. These indicate that the consumptions of alkenyl groups in the current study are significantly different from the case of its solution polymerization counterpart.
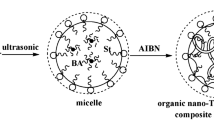
In contrast to solution polymerization, ab initio emulsion and miniemuslion polymerization are both heterogeneous in nature, where the amphiphilic macro-RAFT agent 1 self-assembled into micelles swollen with St and at the St mini-droplet surfaces, respectively. In such two types of nano-objects, the alkenyl units enriched at the interfaces because of their direct connection with the hydrophilic blocks in the RAFT agent molecules. After initiation of polymerization, the entered radicals experienced rapid chain transfer reactions with macro-RAFT agent 1, which released R group radicals that were also orientated at the micelle (E1, E2 and E3) or mini-droplet (ME1) surfaces. This distribution profile allowed alkenyl groups to be in contact with the propagation radicals as well as St for longer time than that in solution [62], and resulted in a significant enhancement of copolymerization of alkenyl units with St. However, in the nucleated particles, RAFT chemistry combing with polymerization demanded the active center of living polymer chain gradually stretched inward away from the alkenyl units, leading to the ceasing of the copolymerization of alkenyl units with St. In Figs. 3 and 7a, the consumption of alkenyl units ceased before 20% conversion, which agreed well with the findings that the nucleation process usually ended at conversions between 20 and 30% [45, 54, 63].
During the late stage of polymerization
Three ab initio emulsion polymerizations resumed consumption of alkenyl units in the high monomer conversion ranges. However, the conversion values were still much lower than the figure estimated for solution polymerization (96.3%). In addition, after the nucleation period, the alkenyl groups remained intact throughout the rest of miniemulsion polymerization (ME1, Fig. 7a). Above observations demonstrate that the resuming consumption of alkenyl units was not a result of growing of alkenyl/St molar ratio with St conversion.
As mention above, the production of branched polymer chains can act to increase the viscosity within particle surface layers. Limiting conversions thus occurred at around 90% in E1 and E2 as the viscosity grew high enough with polymerization. However, in the case when the viscosity is not high enough to prevent radical entry, the aqueous radicals could enter the particles, but would be trapped within the surface layers. These gave rise to an extended period of time contacting with alkenyl units, and a noticeable enhancement of copolymerization of alkenyl units with St in E1, E2 and E3. These explanations agree well the following experimental results. As the reaction temperatures were increased from 60 °C in E2 to 70 °C in E1 and to 85 °C in E3, the viscosity within particles decreased gradually. The consumptions of alkenyl units were accordingly postponed to higher St conversions in these two runs and E3 achieved full conversion.
The costabilizer in miniemulsion polymerization, HD, is capable of dissolving in PSt [58], thus decreases the viscosity within the particles during miniemulsion polymerization. The aqueous radicals were thus less likely to be restricted to the particle surface layers. Their chain transfer reactions with RAFT agents proceeded smoothly, and released R group radicals stretching inward away from the particle surfaces. Consequently, the occurrence of copolymerization of alkenyl units with St was avoided during the late stage of miniemulsion polymerization.
Finally, these observations for alkenyl-functionalized PSt particles are compared to our previous report on cyclohexenyl-functionalized ones. While the trends for the two pendent vinyl functionalities are the same, the consumption of the alkenyl groups is much more significant as compared to the case of cyclohexene. The potential reason is that cyclic alkene has more than one magnitude higher ratio of r1/r2 (144560) [48] than alkenyl unit (butene, 10,780, Table 2), leading to the remarkably lower reactivity of cyclohexenyl unit than butene unit during copolymerization with St.
Conclusions
The RAFT ab initio emulsion and miniemulsion polymerizations of St mediated by an alkenyl-functionalized amphiphilic macromolecular RAFT agent C12H25-S-(C=S)-S-(BEOMSt3-b-AA30) were investigated. The following conclusions were drawn:
During ab initio emulsion polymerization, the pendent alkenyl (butene) units in the macro-RAFT agent underwent significant copolymerization with St during either the nucleation period or the final stage of polymerization. The system was characteristic of depletion of alkenyl groups, deviation of molecular weights, branching of polymer chains, broadened molecular weight distributions, and even limiting conversions.
Miniemulsion polymerization exhibited improved behaviors, in terms of preparation of nanoparticles with alkenyl-enriched surfaces. The polymerization achieved full conversion. Consumption of alkenyl groups occurred, but only during the nucleation period. The resulting polymers had molecular weights higher than theoretical values and broad molecular weight distributions, but the nanoparticles had alkenyl-functionalized surfaces.
The consumption of alkenyl groups during the nucleation period of these two systems were both due to the long-time contacting among propagating radicals, St, and alkenyl units, which was caused by the self-assembly of macro-RAFT agents into micelles and at the surfaces of mini-droplets, respectively. The depletion of alkenyl units during the final stage of ab initio emulsion polymerizations was due to the aqueous phase radicals being restricted to the particle surfaces as a result of high viscosity within the particles, which enhanced the copolymerization of alkenyl units with St. In contrast, HD decreased the viscosity within the miniemulsion particles and promoted radical entry. It demonstrated that miniemulsion polymerization was a feasible method of synthesizing alkenyl-functionalized particles.
References
Tang DP, Cui YL, Chen GA (2013) Nanoparticle-based immunoassays in the biomedical field. Analyst 138:981–990
Kim ST, Saha K, Kim C, Rotello VM (2013) The role of surface functionality in determining nanoparticle cytotoxicity. Acc Chem Res 46:681–691
Saha K, Agasti SS, Kim C, Li XN, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW, Park SW, Shin JS, Cheon J (2012) A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat Mater 11:1038–1043
Hyeon T, Lee JE, Lee N, Kim T, Kim J (2011) Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res 44:893–902
Zhou GQ, Kang SG, Yang P, Liu Y, Sun BY, Huynh T, Meng H, Zhao LN, Xing GM, Chen CY (2012) Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C-82(OH)(22) and its implication for de novo design of nanomedicine. Proc Natl Acad Sci U S A 109:15431–15436
Liu Z, Yang KL, Robinson JT, Tabakman SM, Dai HJ (2011) Carbon materials for drug delivery & cancer therapy. Mater Today 14:316–323
Feng LY, Qu XG, Wu L (2013) New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv Mater 25:168–186
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41:2545–2561
Pichot C, Charleux B, Llauro MF (1993) Styrene-terminated poly(vinyl alcohol) macromonomers: 2. Free-radical (co)polymerization studies and application to the functionalization of latex particles. Polymer 34:4352–4359
Margel S, Wiesel E (1984) Acrolein polymerization: monodisperse, homo, and hybrido microspheres, synthesis, mechanism, and reactions. J Polym Sci: Polym Chem Ed 22:145–158
Margel S (1984) Characterization and chemistry of polyaldehyde microspheres. J Polym Sci: Polym Chem Ed 22:3521–3533
Rembaum A, Chang M, Richards G, Li M (1984) Structure and immunological properties of polyacrolein formed by means of ionizing radiation and base catalysis. J Polym Sci: Polym Chem Ed 22:609–621
Yan CG, Zhang XM, Sun ZH (1990) Poly (styrene-co-acrolein) latex particles: co-polymerization and characteristics. J Appl Polym Sci 40:89–98
Pichot C, Charleux B (1992) Radical-initiated copolymers of styrene and p-formylstyrene, 1. Solution copolymerization and characterization. Makromol Chem 193:187–203
Charleux B, Pichot C, Fanget P (1992) Radical-initiated copolymers of styrene and p-formylstyrene, 2. Preparation and characterization of emulsifier-free copolymer latices. Makromol Chem 193:205–220
Okubo M, Kondo Y, Takahashi M (1993) Production of submicron-size monodisperse polymer particles having aldehyde groups by seeded aldol condensation polymerization. Colloid Polym Sci 271:109–113
Ceska GW (1974) Carboxyl-stabilized emulsion polymers. J Appl Polym Sci 18:2493–2499
Sakota K, Okaya T (1977) Electrolyte stability of carboxylated latexes prepared by several polymerization processes. J Appl Polym Sci 21:1025–1034
Sakota S, Okaya T (1977) Polymerization behavior and distribution of carboxyl groups in preparation of soap-free carboxylated polystyrene latexes. J Appl Polym Sci 21:1035–1043
Pichot C, Delair T, Charreyre MT, Razafindrakoto V, Wren L (1994) Radically initiated copolymers of styrene with 4-vinylbenzylamine and its trifluoroacetamide derivative, 2. Preparation of latex particles bearing amino groups. Macromol Chem Phys 195:2153–2167
Delair T, Marguet V, Pichot C, Mandrand B (1994) Synthesis and characterization of cationic amino functionalized polystyrene latexes. Colloid Polym Sci 272:962–970
Ganachaud F, Mouterde G, Delair T, Elaissari A, Pichot C (1995) Preparation and characterization of cationic polystyrene latex particles of different arninated surface charges. Polym Adv Technol 6:480–488
Kling JA, Ploehn HJ (1995) Synthesis and characterization of epoxy-functional. J Polym Sci Part A: Polym Chem 33:1107–1118
Delair T, Pichot C, Mandrand B (1994) Synthesis and characterization of cationic latex particles bearing sulfhydryl groups and their use in the immobilization of Fab antibody fragments (1). Colloid Polym Sci 272:72–81
Sarobe J, Forcada J (1996) Synthesis of core-shell type polystyrene monodisperse particles with chloromethyl groups. Colloid Polym Sci 274:8–13
Okubo M, Iwasaki Y, Yamamoto Y (1992) Preparation of micron-size monodisperse polymer microspheres having cationic groups. Colloid Polym Sci 270:733–737
Okubo M, Nakagawa T (1992) Preparation of micron-size monodisperse polymer particles having highly crosslinked structures and vinyl groups by seeded polymerization of divinylbenzene using the dynamic swelling method. Colloid Polym Sci 270:853–858
David RLA, Kornfield JA (2008) Facile, efficient routes to diverse protected thiols and to their deprotection and addition to create functional polymers by thiol-ene coupling. Macromolecules 41:1151–1161
Hawker CJ, Campos LM, Meinel I, Guino RG, Schierhorn M, Gupta N, Stucky GD (2008) Highly versatile and robust materials for soft imprint lithography based on thiol-ene click chemistry. Adv Mater 20:3728–3733
Carioscia JA, Schneidewind L, Ely R, Feeser C, Cramer N, Bowman CN (2007) Thiol–norbornene materials: approaches to develop high Tg thiol–ene polymers. J Polym Sci Part A: Polym Chem 45:5686–5696
Gress A, Volkel A, Schlaad H (2007) Thio-click modification of poly [2-(3-butenyl)-2-oxazoline]. Macromolecules 40:7928–7933
Podešva J, Hrubý M, Spevácek J, Hrdlicková M, Netopilík M (2008) A new chemical modification of liquid polybutadienes: radical addition of aliphatic aldehydes onto pending vinyl groups. J Polym Sci Part A: Polym Chem 46:3919–3925
Ma J, Cheng C, Sun GR, Wooley KL (2008) Well-defined polymers bearing pend-ent alkene functionalities via selective RAFT polymerization. Macromolecules 41:9080–9089
Larock RC, Wiley J (1999) Comprehensive organic transformations, a guide to functional group preparations—second edition. Wiley-VCH, New York
Li Y, Yang C, Khan M, Liu SQ, Hedrick JL, Yang YY, Ee PLR (2012) Nanostructured PEG-based hydrogels with tunable physical properties for gene delivery to human mesenchymal stem cells. Biomaterials 33:6533–6541
Kai K, Kan CY, Du Y (2005) Synthesis and properties of soap-free poly(methyl methacrylate-ethyl acrylate-methacrylic acid) latex particles prepared by seeded emulsion polymerization. Eur Polym J 41:439–445
Nicolas J, Nicolas J, Bensaid F, Desmaele D, Grogna M, Detrembleur C, Andrieux K, Couvreur P (2008) Synthesis of highly functionalized poly(alkyl cyanoacrylate) nanoparticles by means of click chemistry. Macromolecules 41:8418–8428
Li NW, Binder WH (2011) Click-chemistry for nanoparticle-modification. J Mater Chem 21:16717–16734
Haruma K (2000) Functional polymer microspheres. Prog Polym Sci 25:1171–1210
Goldmann AS, Walther A, Nebhani L, Joso R, Ernst D, Loos K, Barner-Kowollik C, Barner L, Müller AHE (2009) Surface modification of poly(divinylbenzene) microspheres via thiol−ene chemistry and alkyne−azide click reactions. Macromolecules 42:3707–3714
Yang L, Sun P, Yang H, Qi DM, Wu MH (2014) A feasible method of preparation of block copolymer latex films with stable microphase separation structures. Prog Polym Sci 77:305–314
Ferguson CJ, Hughe RJ, Pham BTT, Hawket BS, Gilber RG, Serelis AK, Such CH (2002) Effective ab initio emulsion polymerization under RAFT control. Macromolecules 35:9243–9245
Sprong E, Bruyn HD, Such CH, Hawkett BS (2009) Control of particle morphology in ab initio RAFT mediated emulsion polymerization. Aust J Chem 62:1501–1506
Wang XG, Luo YW, Li BG, Zhu SP (2009) Ab initio batch emulsion RAFT polymerization of styrene mediated by poly(acrylic acid-b-styrene) trithiocarbonate. Macromolecules 42:207–212
Rieger J, Osterwinter G, Bui CO, Stoffelbach F, Charleux B (2009) Surfactant-free controlled/living radical emulsion (co)polymerization of n-butyl acrylate and methyl methacrylate via RAFT using amphiphilic poly(ethylene oxide)-based trithiocarbonate chain transfer agents. Macromolecules 42:5518–5525
Urbani CN, Monteiro MJ (2009) RAFT-mediated emulsion polymerization of styrene in water using a reactive polymer nanoreactor. Aust J Chem 62:1528–1532
Yang L, Xu JQ, Sun P, Shen YF, Luo YW (2014) Ab initio emulsion and miniemulsion polymerization of styrene mediated by a cyclohexenyl-functionalized amphiphilic RAFT agent. Ind Eng Chem Res 53:11259–11268
Breed DR, Thibault R, Xie F, Wang Q, Hawker CJ, Pine DJ (2009) Functionalization of polymer microspheres using click chemistry. Langmuir 25:4370–4376
Lowe AB (2010) Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Poly Chem 1:17–36
Hoyle CE, Lee TY, Roper T (2004) Thiol–enes: chemistry of the past with promise for the future. J Polym Sci Part A: Polym Chem 42:5301–5338
Sprong E, Leswin JST, Lamb DJ, Ferguson CJ, Hawkett BS, Pham BTT, Nguyen D, Such CH, Serelis AK, Gilbert RG (2006) Molecular watchmaking: ab initio emulsion polymerization by RAFT-controlled self-assembly. Macromol Symp 231:84–93
Dong ZM, Liu XH, Lin Y, Li SH (2008) Branched polystyrene with abundant pendant vinyl functional groups from asymmetric divinyl monomer. J Polym Sci Part A: Polym Chem 18:6023–6034
Yang L, Luo Y, Li B (2006) Reversible addition fragmentation transfer (RAFT) polymerization of styrene in a miniemulsion: a mechanistic investigation. Polymer 47:751–762
Chen SA, Lee ST (1992) Limiting conversion for systems of emulsifier-free emulsion polymerization of styrene. Macromolecules 25:1530–1533
Gilbert RG (1995) Emulsion polymerization: a mechanistic approach. Academic Press, London, pp 70–72
Luo YW, Tsavalas J, Schork FJ (2001) Theoretical aspects of particle swelling in living free radical miniemulsion polymerization. Macromolecules 34:5501–5507
DiPaola-Baranyi G, Guillet JE (1978) Estimation of polymer solubility parameters by gas chromatography. Macromolecules 11:228–235
Odian G (2004) Principles of polymerization4th edn. Wiley, New York, pp 466–487
Brandrup J, Immergut EH, Grulke EA (1998) Free radical copolymerization reactivity ratios. Polymer handbook4th edn. Wiley, New Yok, pp II309–II318
Ma J, Cheng C, Wooley KL (2009) Cycloalkenyl-functionalized polymers and block copolymers: syntheses via selective RAFT polymerizations and demonstration of their versatile reactivity. Macromolecules 42:1565–1573
Monteiro MJ (2010) Nanoreactors for polymerizations and organic reactions. Macromolecules 43:1159–1168
Bechthold N, Landfester K (2000) Kinetics of Miniemulsion polymerization as revealed by calorimetry. Macromolecules 33:4682–4689
Acknowledgments
This project is financially supported by Zhejiang Provincial Natural Science Foundation of China (LY18E030008, LY16B060006, LY15E030013 and LY12E03008), the Science and Technology Department of Zhejiang Province (2016C31074, 2017C31033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1615 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Yin, J., Zhao, Q. et al. Differences between ab initio emulsion and miniemulsion polymerization of styrene mediated by an alkenyl-functionalized amphiphilic RAFT agent. Colloid Polym Sci 296, 1615–1625 (2018). https://doi.org/10.1007/s00396-018-4386-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4386-8