Abstract
Groundwater withdrawal at very fast rate poses threat on existing groundwater resources in different parts of the world. This reduction in groundwater levels significantly disturbed the natural aquifer flow rate and thereby different hydrogeochemical processes, which may further impair the groundwater quality. The groundwater quality in rural area of Chhattisgarh State is degraded, and the problem of saline water poses health risk to people. In this research investigation, suitability of groundwater of Bemetara District, Chhattisgarh, India, has been evaluated for drinking purpose through water quality index (WQI) method and principal component analysis (PCA). Total 116 groundwater samples were collected during the pre-monsoon (June 2019) and post-monsoon season (December 2019) and analyzed for physicochemical parameters. Total dissolved solids ranged from 250 to 10,440 mg/L and 289 to 3583 mg/L during pre-monsoon and post-monsoon, respectively, and 55% of the total samples exceeded acceptable BIS limit in pre-monsoon, while about 66% samples exceeded in post-monsoon season. SO42− concentrations varied from 3 to 5734 mg/L during pre-monsoon and 4.5 to 2002 mg/L during post-monsoon, respectively. Total 28% samples in pre-monsoon and 18% samples in post-monsoon season exceeded the maximum permissible BIS limit (400 mg/L) of SO42− ion in the study area. On the basis of WQI, the quality of groundwater varies from “Excellent water” to “Good water” category. The groundwater of northeastern part of the district is not suitable for drinking, and therefore, it is recommended to treat this groundwater before human consumption with special reference to SO42− contamination. PCA inferred that four components are sufficient to explain the variance in chemistry of groundwater that is mainly governed by dissolution of gypsum mineral, other rock–water interaction and anthropogenic activities. Further, water quality was improved in the direction of groundwater flow in the study area, establishing a direct relationship between groundwater flow and water quality of the Bemetara District. This study provides very useful database to design sustainable groundwater management plan for the district.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Untreated waste discharge leads to degradation of the surface water quality, and therefore, water supply for different sectors like agricultural, industrial and domestic needs has been fulfilled by groundwater resources. But the rate of usage of groundwater has resulted into declining groundwater levels, which is very critical to available resources, and this decline level reaches up to 80 m in zone of depression (Chen et al. 2005). This reduction in groundwater levels may significantly altered the groundwater flow conditions (Wang et al. 2008; Zhang et al. 1997, 2000; Fan 1998; Xia et al. 2004). Therefore, different studies have been taken to investigate the aquifer flow conditions in respect to the sustainable utilization of groundwater resources (USGS 1999; CGWB Report 2019). This decline in groundwater level leads to change in the aquifer hydrogeochemical processes (CGWB Report 2019).
Groundwater quality depends on important process like atmospheric precipitation, runoff and inland surface water. Moreover, the groundwater quality is degraded due to disposal of industrial waste and mining activities (Rodell et al. 2009; Chopra and Gopal 2014; Malyan et al. 2019). Further, groundwater quality in a region is influenced by physical and chemical parameters that are strongly affected by natural processes such as water chemistry in the recharged area, water intermixing, groundwater recharge, aquifer discharge and recharge, and water flow path (Singh et al. 2017; Azhdarpoor et al. 2019; Soleimani et al. 2018). The spatial distribution and zoning of NO3− and F− concentration and their health risk assessment in drinking groundwater of Shiraz metropolitan area in the southwest of Iran and Behbahan City were studied by application of Monte Carlo simulation, sensitivity analysis and geographic information system (Badeenezhad et al. 2019, 2021).
The most convenient method to describe the quality of drinking water resources is the Water Quality Index (WQI). The first attempt to develop a WQI was made in 1948, when scientific community found a correlation between pollution load and certain group of organisms (fish, plant and benthic community) (Alves et al. 2014). Later, Horton developed WQI technique in 1965 (Horton 1965). After that, National Sanitation Foundation (NSF) of United States developed WQI in 1970 which is widely used (Brown et al. 1970). Therefore, WQI is not new tool, but has been extensively used across the world to determine the water quality (Abbasi and Abbasi 2012).
Different water quality indices have been developed and used for the evaluation of water quality for drinking purposes (Horton 1965; McDuffie and Haney 1973; Nemerow and Sumitomo 1970; Brown et al. 1970; Landwehr 1976; Parti et al. 1971; Dinius 1972; Dee et al. 1973). A number of studies have been carried out by different workers for assessing the quality of different water resources of India using WQI, viz. Sajitha et al. (2016), Reza and Gurdeep (2010), Ishaku et al. (2011), Kumar et al. (2014), Saxena et al. (2017), Acharya et al. (2018) and Vijayachandran et al. (2018).
Principal component analysis (PCA) is a useful tool to investigate the chemical relationship between different water quality parameter and thereby predicting the dominating parameters (Sharma and Jain 2006). PCA is a multivariate statistical technique that has been widely used to reduce the dimensionality of large data (Vega et al. 1998; Duan et al. 2016; Zhang et al. 2016). The goal of PCA is to describe the majority of the data sets in a few principal components (PCs), and these PCs are the linear combination of observed data with maximum variations with minimum loss of the actual information (Baghanam et al. 2020). PCA has been applied to explain water quality variable in several studies (Vermonden et al. 2009; Daou et al. 2016; Zhang et al. 2016; Abdelaziz et al. 2020; Chai et al. 2021). Huang et al. (2007) used PCA to explain the storm water quality data for pattern recognition and identification of pollution sources from different urban surface-type catchments.
Sharma and Jain (2006) evaluated groundwater quality of Jodhpur District, Rajasthan (India), using multivariate technique and concluded that F−, NO3−, pH and K+ have significant influence on the quality of the aquifer. Marin Celestino et al. (2019) used PCA to investigate hydrogeochemical processes in a wastewater-irrigated region (central Mexico) and reported that groundwater chemistry was dominated by three processes: salinization, mineralization and groundwater contamination.
Chai et al. (2021) investigated the source assessment of pollution in the Fen River for different seasons. Giakwad et al. (2020) evaluated the groundwater quality of western coast of Maharashtra, India, with the use of PCA technique. Abdelaziz et al. (2020) studied groundwater quality index based on PCA in Wadi El Natrun, Egypt, and used PCA to reduce the complexity of the data and identified the group of parameters (Na+, SO42−, Cl−, strontium, Ca2+ and molybdenum) that control the groundwater quality.
The groundwater quality in rural area of the Chhattisgarh State is degraded, and the problem of saline water (EC ~ 2000–4500 µS/cm) poses a threat to the people. There is a serious problem of saline water in 113 villages of district Bemetara of Chhattisgarh State, and 1200 to 2600 mg/L of TDS was observed in the groundwater of problematic villages of district Bemetara, which may cause health problem, viz. digestion, high blood pressure, heart attack and kidney problems (CGWB Report 2015). In some parts, the value of SO42− is observed up to 800 ppm which causes gastrointestinal disorders among the inhabitants of the area (Mukherjee and Gupta 2010). According to the groundwater quality assessment by Central Ground Water Board, a central agency of government of India, the SO42− concentration found up to 763 ppm in Bemetara village of Bemetara District in 2014–2015 (CGWB Report 2015). For the alternate sources, the residents of these villages are using contaminated water from ponds, rivers and drains in the area. In a study of health risk evaluation of uranium in groundwater of Bemetara District, uranium levels in water samples range from 1.15 to 83.5 µg/L and 0.68 to 96.08 µg/L during pre-monsoon and post-monsoon, respectively, with few samples exceeding the safe limit of 30 µg/L prescribed by WHO (2011), and there is no harmful effect by radiological risk, but chemical risk can affect human health (Sahu et al. 2020). Dahariya et al. (2020) studied the contamination, sources and environmental hazard of groundwater in Bemetara District of Chhattisgarh, India, and reported WQI (406 ± 82) values which clearly demarcate groundwater unsuitability for drinking purposes.
In view of the above scenario of degraded groundwater quality in the district Bemetara of Chhattisgarh State, the aim of the present investigation is (1) to monitor the quality of groundwater for drinking purpose using WQI; (2) to monitor spatial and seasonal variation of important water quality parameters using the application of GIS software; and (3) to identify several factors responsible for degradation of quality of groundwater using principal component analysis.
Study area
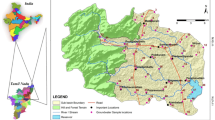
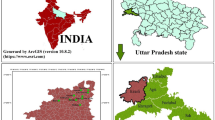
Bemetara District is newly formed district of Chhattisgarh State, India, and covering area of 2854.81 km2 (Fig. 1). It lies in between 21° 22′ and 22° 03′ North latitude and 81° 07′ and 81° 55′ East longitude. Bemetara District has huge quantity of mineral deposits, namely sandstone, limestone (low grade), dolomite and quartzite. Dolomite and limestone mineral were found high in whole district. The study area has a dry and wet tropical climate. The temperature varies from 10 to 48 °C, where the maximum temperature is reached in the month of May and June and minimum temperature fall in January. Bemetara District has flat topography, and totally six rivers flow in the direction of slope of district (north to east), namely Shivnath, Kharun, Surahi, Haff, Sakari and Phonk rivers. Bemetara District geologically comes under Meso- to Neoproterozoic rock sequence of Chhattisgarh supergroup. This Chhattisgarh supergroup is divided further into different groups; our study area falls under Raipur group which comprises four types of geological formation (Maniyari formation, Hirri formation, Tarenga formation and Chandi formation). The reddish brown and purple non-calcareous shale containing gypsum form the characteristics of Maniyari formation, under most of the district area occurred (District Survey Report Bemetara Chhattisgarh 2016).
Hydrogeology of the study area
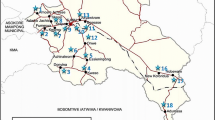
The hydrogeological formation of study area mainly consists of arenaceous–argillaceous–calcareous rocks and is enriched by limestone/dolomite and calcareous shale. The groundwater in these formations occurs under water table, semi-confined and confined conditions. The weathered, cavernous and fractured part of the formation constitutes the aquifer in the area and has great potential in regards to groundwater yield and thereby development groundwater in the district. Geology and hydrogeology of the study area shown in Figs. 2 and 3, respectively. The gypsum karsts occurring in the Maniyari formation of this province are more productive. Though gypsum is more soluble than calcite, their alternative assemblage with thinly laminated shale provides special condition that favors dissolution of gypsum laminae causing roof collapses to create larger openings. However, all the formations in the district are productive (CGWB Report 2015).
Material and methods
Water sampling and analytical techniques
In total, 116 samples were collected from groundwater sources of 51 locations of the district Bemetara, viz. open wells, dug wells, borewells and handpumps, during pre-monsoon (June 2019) and post-monsoon (Dec. 2019), which are extensively being used for drinking water purpose and analyzed for physicochemical parameters using standard methods (APHA 2005). Groundwater samples were taken from one open well from each of 51 locations, but borewell samples were also collected from seven locations along with open well. Before collecting samples, handpumps/borewells were pumped for 5 min to get represented water sample and the sampling bottle was rinsed with the same water. In situ parameters were analyzed on site like pH and electrical conductivity using Hach, USA make HQ40d portable handheld multimeter. Other parameters like major cation and anion were analyzed using Metrohm ion chromatograph. The ionic balance error (IBE) test was performed (Eq. 1) (Freeze and Cherry 1979) and was below 5% for all samples, which support the data accuracy and reliability of the analysis. Total alkalinity and HCO3− were determined by autotitrator of SI analytical instrument, a Xylem brand.
Water quality index (WQI)
WQI is an important method that is used for the evaluation of quality of water for drinking purposes (Subba Rao 1997; Avvannavar and Shrihari 2008; Mishra and Patel 2001; Badeenezhad et al. 2020). BIS (2012) and WHO (2011) set standard limit to different water quality parameters for drinking purposes, and some of these are incorporated into calculation of WQI. Here, we took ten parameters (Table 1) for calculating WQI and each parameter is assigned a weight (wi) depending upon the importance of overall quality of water.
The assigned weight ranges from 1 to 5, where 5 is the highest and 1 is the lowest weight (Srinivasamoorthy et al. 2008; Vasanthavigar et al. 2010). In the next step, the relative weight (Wi) is calculated by Eq. 2 as follows:
where Wi is the relative weight, wi is the assigned weight of each parameter and n is the total number of parameters.
Chemical parameters and their calculated relative weight (Wi) are given in Table 1.
The next quality rating value (qi) is calculated by Eq. 3:
where qi is the quality rating, Ci is the concentration of parameter in water sample (ppm) and Si is the BIS value for each parameter in ppm.
And finally WQI is calculated by Eqs. (4) to (5):
where SIi = subindex of ith parameter; qi = rating based on the concentration of ith parameter; n = number of parameters.
Five WQI classes and water types can be categorized accordingly as given in Table 2.
Data processing
An univariate and multivariate normal distribution are required for the best outcomes of the statistical multivariate methods like PCA (Zhou et al. 2007; Oppong and Agebedra 2016; Marin et al. 2018). Shapiro–Wilk’s (Shapiro and Wilk 1965) and Royston’s tests (Royston 1983) were used to verify the univariate and multivariate normality conditions, respectively. The Spearman’s rank correlation was used in this multivariate analysis because water quality data were non-normal distribution, and this correlation method is best suitable for reducing deviation of variables (Marin Celestino et al. 2019). Based on Royston’s test, the dataset has a non-normal distribution. To achieve a normal-like distribution, the original set of variables was transformed using a logarithmic transformation (natural logarithm). To approach the best conditions of the multivariate analysis, feature scaling on the database was done using standardization (or Z-score normalization). Standardization minimizes the variance in variables and protects dissimilarity metrics such as the Euclidean distance from being severely influenced (Davis and Sampson 1986). Each variable was normalized to its Z score, which was determined using Eq. (6):
where Zi is the standardized Z score, Xi is each variable’s value, and mean and S are each variable’s mean value and standard deviation, respectively. The Kolmogorov–Smirnov (K–S) test was used to assess how well the transformed variables were adjusted to the normal log distribution (Rizvi et al 2015; Muangthong and Shrestha 2015; Marin Celestino et al. 2019; Castillo et al. 2021). The precision and acceptability of the data for PCA were assessed by using the Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests. KMO is a metric for determining the sampling’s adequacy by identifying the proportion of shared variation that might be linked by unknown factors (Marin Celestino et al. 2019; Castillo et al. 2021). The KMO and Bartlett’s sphericity test results are described in the results and discussion section.
Principal component analysis (PCA)
In PCA, the standardized and normalized data as discussed in earlier section are used. Factors are produced through an eigenvalue analysis of the correlation matrix. These factors are vector which shows interorthogonality within a multidimensional space defined by a number of variables in the analysis (Sharma and Jain 2006). Unlike the original variable, the factors are uncorrelated with each other. They are described by means of their correlation with (or “loading” on) the original variables and ranked in order of the amount of the total variance they explain. A loading close to one indicates a strong relationship between factor and the variable, whereas a zero loading indicates no relationship (Davis and Sampson 1986; Sharma and Jain 2006). Among the whole factors, first few factors explain the bulk of total variance and the remaining factors are not used in the analysis. These retained factors were then rotated using Varimax method. Varimax rotation tried to attain a simple structure, whereby factor loading is close to one or zero. This helps in the interpretation of the factors that either does or does not include a particular variable. Principal component and factor analysis are applied on the groundwater of the district Bemetara, and the whole analysis has been carried out using software RStudio Vs 1.4.1106.
Results and discussion
Hydro-chemical characteristics of groundwater of the study area
In total, 116 samples were collected from the study area during the year 2019–2020 from the abstraction sources in collaboration of Water Resources Department (WRD), Govt. of Chhattisgarh, Raipur. The minimum, maximum and average values of all the chemical parameters for both the seasons are given in Table 3.
The pH of all collected samples is alkaline in nature and varies from 6.2 to 7.7 and 6.1 to 7.1 during pre-monsoon season (PRS) and post-monsoon season (PMS), respectively. Almost all samples are found within BIS (2012) and WHO (1996) standard limits of 6.5–8.5.
In Bemetara District, total dissolved solid (TDS) in groundwater ranges from 250 to 10,440 mg/L and 289 to 3583 mg/L during PRS and PMS, respectively. About 55% samples exceeded the acceptable limit (ALT) but lies below the prescribed maximum permissible limit (MPL) of 2000 mg/L in PRS and about 66% samples were found above ALT but within 2000 mg/L in PMS. The maximum value of TDS 10440 mg/L was observed in the groundwater of the village Kunra of the block Nawagarh. The spatial distribution map of TDS for PRS and PMS is shown in Fig. 4.
CO3−, HCO3− and hydroxides are mainly responsible for alkalinity in water system. The alkalinity value varied from 83 to 280 mg/L and 52 to 415 mg/L during PRS and PMS, respectively. About 50% samples exceeded ALT but under the prescribed MPL of 600 mg/L in PRS, and about 59% samples were found above ALT but are under 600 mg/L in PMS. None of the sample crossed the prescribed limit of 600 mg/L during PRS and PMS, respectively (Fig. 5).
The presence of bivalent ions mainly Ca2+ and Mg2+ with their compounds cause hardness in water. Total hardness value ranges from 119 to 3267 mg/L and 116 to 2124 mg/L during PRS and PMS, respectively. About 50% samples cross the ALT of 200 mg/L but lie below the MPL of 600 mg/L, and 38% samples exceeded the prescribed MPL 600 mg/L during PRS. During the PMS, about 67% samples are within the prescribed MPL and 28% samples cross the prescribed MPL. The spatial distribution maps of hardness are shown in Fig. 6 for both the seasons. It is observed from the spatial distribution maps of both the season that the northeastern part of the study area is highly contaminated and the value of hardness crossed 3000 ppm and the maximum value of hardness 3267 mg/L was observed in the groundwater of the village Bitkuli of the block Bemetara.
In groundwater of the study area, the values of Ca2+ range from 26 to 569 mg/L and 26 to 648 mg/L during PRS and PMS, respectively. About 24% samples crossed the prescribed MPL of 200 mg/L during the PRS and 17% samples crossed the prescribed MPL during the PMS. The less number (7%) of samples crosses the MPL in the PMS because of the dilution effect as compared to PRS. The value of Mg2+ varies from 11 to 488 mg/L during PRS and 12 to 259 mg/L during PMS. Only 12% samples exceed prescribed MPL 100 mg/L during PRS and 7% exceed above 100 mg/L during PMS.
The value of Cl− ranges from 10 to 1080 mg/L and 12 to 652 mg/L during PRS and PMS, respectively. More than 90% samples lie within the prescribed ALT of 250 mg/L during both the seasons. NO3− content in the study area ranges from 0 to 194 mg/L and 0 to 569 mg/L during PRS and PMS, respectively. About 93% of the samples of the study area fall within MPL of 45 mg/L and 7% of samples even crossed the MPL during PRS and about 67% of the samples of the study area fall within the prescribed MPL of 45 mg/L and 33% of samples even crossed the prescribed MPL during PMS. F− in the groundwater of Bemetara District ranges from 0.06 to 2.4 mg/L and 0 to 1.04 mg/L during PRS and PMS, respectively. Almost all samples lie under the prescribed ALT of 1.0 mg/L during both the seasons.
SO42− is generally found as soluble salts of Ca2+, Mg 2+ and Na + in the groundwater. During the PRS, the concentration of SO42− ranges from 3 to 5734 mg/L and 4.5 to 2002 mg/L during PMS. BIS sets 200 mg/L as the ALT and 400 mg/L as MPL for SO42− in drinking water. In Bemetara District about, 55% of the samples are below the prescribed ALT of 200 mg/L and 28% samples exceeded the value of MPL of 400 mg/L during the PRS, while 67% samples lie below the ALT and 19% sample exceeded prescribed MPL of 400 mg/L during PMS. The spatial distribution of SO42− in the study area is shown in Fig. 7 for PRS and PMS. The northeastern area of the study area is highly contaminated as shown in the spatial distribution maps for both the seasons, and the maximum value of SO42− 5735 mg/L was observed in the groundwater of the village Kunra of the block Nawagarh.
Depthwise variation of groundwater quality of the study area
Depthwise distribution of groundwater samples has been arranged in three categories, i.e., shallow aquifer (0–20 m), medium aquifer (21–40 m) and deep aquifer (> 40 m). In PRS, about 31% samples from the shallow aquifer (SA), no sample from medium and 2% sample from deep aquifer lie under the ALT of TDS, i.e., 500 mg/L (BIS 2012), while about 49% samples from the SA, 2% samples each from medium and deep aquifer exceeded limit of TDS, i.e., 2000 ppm (BIS 2012), and only 14% samples from SA exceeded the MPL of TDS. In case of PMS, about 26% samples from the SA, no sample from the medium and deep aquifer fall within the ALT, while about 64% samples from SA, 2% samples from deep aquifer exceeded the ALT but under the MPL of TDS and 9% samples from SA exceeded the MPL of TDS (Table 4).
During PRS, about 48% samples from the SA, 2% samples from medium and no sample from deep aquifer fall within ALT of alkalinity, i.e., 200 mg/L (BIS 2012), while about 46% samples from the SA, 0% samples from medium aquifer and 3% samples from deep aquifer lie under the limit of MPL of alkalinity, i.e., 600 mg/L (BIS 2012) and no samples from SA, medium aquifer and deep aquifer exceeded the MPL of alkalinity. In case of PMS, about 41% samples from the SA, no sample each from medium and deep aquifer fall within the ALT, while only 57% samples from SA and 2% samples from deep aquifer lie above ALT but under the MPL of alkalinity and none of samples from SA, medium aquifer and deep aquifer exceeded the MPL of alkalinity (Table 5).
In PRS, about 12% samples from the SA, 0% samples each from medium and deep aquifer fall within the ALT of hardness, i.e., 200 mg/L (BIS 2012), while about 48% samples from the SA, 0% samples from medium aquifer and 2% samples from deep aquifer lie below the MPL of hardness, i.e., 600 mg/L (BIS 2012), and only 38% samples from SA exceeded the MPL of hardness. In case of PMS, about 5% samples from the SA, no sample from the medium and deep aquifer fall within the ALT, while about 66% samples from SA, 2% samples from deep aquifer crossed the ALT but are under MPL of hardness and 27% samples from SA exceeded the MPL of hardness (Table 6).
In PRS, about 53% samples from the SA, 0% samples from medium and 2% samples from deep aquifer fall within the ALT of SO42−, i.e., 200 mg/L (BIS 2012), while about 17% samples from the SA, 0% samples from each medium aquifer and deep aquifer lie under MPL of SO42−, i.e., 400 mg/L (BIS 2012), and 26% samples from SA, 2% samples from medium aquifer and 0% samples from deep aquifer exceeded the MPL of SO42−. In PMS, about 66% samples from the SA, no sample from the medium and 2% samples from deep aquifer fall within the ALT, while only 14% samples from SA lie in between ALT and MPL of SO42− and 19% samples from SA exceeded the MPL of SO42− (Table 7).
From the above discussion, it may be concluded that almost all collected groundwater samples belong to shallow aquifer and the significant amount of collected contaminated with higher SO42− contamination. Further, water quality at different depths at the same site of few locations has been studied and it was observed that higher concentrations of different water quality parameters were generally observed at higher depths below the ground, which is due to more residence time of groundwater in the deeper aquifer (Fig. 8).
Water quality index
Hydro-chemical data of groundwater of Bemetara District were processed for determination of WQI and thereby quality check for drinking purposes. To assess water quality, sampling station was selected as to cover all Bemetara District areas and all sampling points shown in Fig. 1. Ten parameters, i.e., HCO3−, TDS, SO42−, Cl−, NO3−, Ca2+, Mg2+, F−, Na+ and K+, were taken into account to calculate WQI for each station and for both the season. Finally, single numeric value represents the type of water according to WQI classes given in Table 2. WQI has been computed for 58 groundwater samples for PRS and PMS (Table 8). The values of WQI range from 26.24 to 1001.59 for PRS and 25.64 to 538.63 for PMS.
It has been observed from Table 7 that 32.27% samples fall under “Good water” category and 27.58% samples found to be “Excellent water” for PRS. Cumulative “Excellent water” and “Good water” category represents about 60% of the samples in PRS. In case of PMS, 46.55% samples fall under “Good category” and 15.52% samples represent “Excellent water.” About 62% samples represent combination of two categories, i.e., “Excellent water” and “Good water.” All five WQI categories distribution in percentage for PRS and PMS has been provided in Supplementary material as Fig. S1.
Water quality of groundwater of district Bemetara is found to be good for some part of the district, but the rest of the area is not fit for drinking purposes. To find out the degraded water quality zones in the study area, spatial distribution map has been created using Arc GIS Software Vs 10.4 with the use of IDW technique (Fig. 9). It was evident from Fig. 9 that water quality is good in the southwestern part of the study area, while water quality is poor to unsuitable for drinking in the northeastern side of Bemetara District that needs treatment before direct consumption in case of pre-monsoon season and almost the same pattern was found for post-monsoon season. It may be inferred that quality of groundwater is not suitable for drinking in the northeastern zone of Bemetara District for both the seasons.
Correlation among the chemical parameters
Correlation among the physicochemical parameters for both the seasons has been evaluated using correlation matrix and given in Table 9. The correlation values were grouped into three classes as very strong correlation (r greater than 0.75), moderate correlation (r = 0.50–0.75) and low correlation (r = 0.30–0.50). From the obtained correlation results, a very strong positive correlation is obtained between Ca2+ and SO42− (0.98), Na+ and Cl− (0.87), Na+ and F− (0.86) during the PRS, while Ca2+ and SO42− (0.94) for the PMS. A moderate positive correlation was found between Mg2+and Ca2+ (0.67), Mg2+and SO42− (0.70), Mg2+and F−(0.52), Ca2+ and F− (0.62) during the PRS and Mg2+ with SO42− (0.71), Mg2+ with NO3− (0.68), Mg2+with Cl− (0.64), Mg2+ with HCO3− (0.54) and Na+ with Ca2+(0.59), Na+ with NO3− (0.68), Na+ with Cl−(0.66), Na+ with SO42− (0.65), Na+ with HCO3− (0.54) during the PMS. From the above discussion, it may be concluded that there may be common source of Ca2+, Mg 2+ and SO42− in the groundwater, i.e., dissolution of dolomite or gypsum mineral. Further, SO42−plotted against the Ca2+, Mg2+, Na+ and K+ (Fig. 10) and the best relationship was observed between Ca2+ and SO42− (maximum r2), further supporting the fact that the source of SO42− in the groundwater of the study area may be CaSO4, i.e., gypsum, which is present in Maniyari shale formation of the region.
Principle component analysis
PCA is a multivariate method that reduces the dimensions of a dataset that contain different variables which are interrelated to each other. PCA was used to study the origin of major salts in the groundwater of Bemetara. Before applying PCA, two tests were performed to analyze the statistical interrelation among the parameters. The KMO test value was 0.5, and Bartlett’s sphericity value was 0.00 that confirmed the data are appropriate and suitable for PCA (Zhang et al. 2020). Eigenvalues and cumulative contribution of all principal components for both the seasons are given in Table 10, and varimax rotated component loadings are given in Table 11. Different components incorporated in the explication of sources for both the seasons are presented in Table 12.
In pre-monsoon season, only four principal components (PCs) were taken into account with eigenvalue more than 1 or near to 1, accounting for 87.09% of total variance, and individual percentage of the variation in the data was 50.49%, 17.80%, 10.72% and 8.08%, respectively (Table 10). The first principal component (PC1) was mainly characterized by SO42−, EC, Mg2+, Ca2+, Cl− and F− in groundwater that may be attributed to dissolution of gypsum mineral and weathering of Cl− bearing rocks (Sharma and Kumar 2020; Mullaney et al. 2009). In addition, the F– may be due to rock–water interactions in the aquifer, and therefore, this factor (F1) may be considered to represent the local geogenic process. Also, PC1 shows 50.49% of the total variation in the data. The second principal component (PC2) accounts17.80% of the total variation in the dataset and had loading on NO3−, HCO3− and alkalinity. NO3− in groundwater is mainly contributed from extensive usage of chemical fertilizers (Zhang et al. 2020), and therefore, this factor (F2) was considered to represent the anthropogenic source. The third principal component (PC3) characterized by K+, Mg2+, Cl− and NO3− accounts for 10.72% of the total variation in the data. K+, Mg2+ and Cl− were resulted from the interaction of groundwater and rock materials. The NO3− in groundwater was associated with chemical fertilizers application in farming and could consider this factor (F3) mixed type source, i.e., geogenic and anthropogenic. The fourth principal component (PC4) had been loaded with pH, Na+ and NO3−which accounts for 8.08% of the total variation in the dataset. Na + may be observed in livestock and domestic waste (Zhang et al. 2020) and could enter subsurface system where there are inappropriate management activities of waste. Therefore, this factor (F4) was called as nonpoint source of pollution mainly from agriculture.
In the post-monsoon season, four PCs can explain 87.61% of total variation with individual contributions of 54.30%, 16.43%, 11.13% and 5.76%, respectively (Table 10). EC, hardness, Na+, Ca2+, Mg2+ and SO42− had high loading in PC1. These ions are related to natural geogenic processes as same explained in factor (F1) in pre-monsoon season. In PC2 loading element were pH, HCO3−, SO42− and NO3−, accounting for16.43% of the total variance. NO3 − and SO42− could be resulted from different agricultural activities (Zhang et al. 2020) and consider similar as to source (F4) in the pre-monsoon season, i.e., nonpoint source of pollution. HCO3−, Cl−, NO3− and F− were loading elements that contribute 11.13% of the total variance. NO3− related to anthropogenic source, whereas F− and Cl− are mainly attributed to geogenic source. Therefore, this factor (F3) is considered as mixed type source as explained in the pre-monsoon season, i.e., geogenic and anthropogenic. The fourth principal component (PC4) was mainly characterized by Na+ and Cl−, explaining 5.76% of the total variance. As explained earlier in the pre-monsoon data, this factor (F1) can be regarded as local geogenic processes and salinity.
Variation of water quality along groundwater flow
The investigation of aquifer water flow conditions is very crucial for sustainable water resource management of any region. The movement of groundwater flow interacts with aquifer rock material and carries chemical species in dissolved form (Rakhmatullaev et al. 2010, 2012; Huneau et al. 2011).
Groundwater level observations from the mean sea level (MSL) were taken from the all selected sampling points in the study area for both the seasons. For the identification of the direction of water flow, spatial contour maps of water levels have been prepared for both the seasons (Fig. 11). Groundwater moves from northeast direction to northwest direction and from northeast to southeast direction during both the seasons of the study area. Further, River Kharun and River Sheonath are flowing along the southeastern boundary, further supporting the direction of groundwater flow direction (Fig. 3). However, from the spatial distribution map of SO42− (Fig. 7), it is evident that the concentration increases from northwest to northeast and southwest to northeast part of the study area. Therefore, it may be inferred from the above discussion that the SO42− decreases with the direction of flow of groundwater (i.e., NE to NW). Also, among all four blocks of the district (Nawagarh, Bemetara, Berla and Saja), two blocks, i.e., Nawagarh and Bemetara, have high SO42− contamination in the study area. High concentration in these blocks may be attributed to existing Maniyari shell formation comprising reddish brown and purple non-calcareous shale with gypsum interbands.
Water quality improves from northeast to northwest part of the district during both the seasons (Fig. 9). As we compared the trend of groundwater flow and water quality (WQI), it was observed that water quality was improved along the flow of groundwater. Therefore, a direct relationship was found between groundwater flow and water quality of the Bemetara District.
Conclusion
For any scheme of drinking water supply in an area, it is mandatory to have the status of water quality of the water resources being used for drinking water supply. Therefore, the evaluation of water quality becomes essential for development of water resource strategies for sustainable water use and to provide database for future planning. Spatial distribution maps were prepared to identify degraded water quality zones, possible sources of pollution and specific parameters not conforming to drinking water quality standards. BIS for drinking water have been violated for physicochemical parameters, viz. TDS, total hardness, Ca2+, Mg2+, SO42− and NO3−. The groundwater quality changes from region to region along the depth of water table, and generally higher concentrations were observed in deeper aquifers. The source of SO42− in the groundwater of the study area may be attributed to dissolution of gypsum as evident from relationship between Ca2+ and SO42− (r2 > 0.8). WQI at different locations has been computed to check the quality of groundwater for drinking purpose and 60% of the samples fall from “Excellent water” to “Good water” category in PRS and about 62% of samples in PMS. About 9% samples were found to be unsuitable for drinking purpose. Spatially, it is revealed that the drinking water sources existing in the northeastern area of the district were found to be contaminated with high SO42− concentration which is not fit for direct public consumption. Multivariate analysis gives insight to different possible potential sources contributing to groundwater pollution in the area and inferred that four components are sufficient to explain the variance in groundwater chemistry mainly controlled by dissolution of gypsum mineral, other rock–water interaction and anthropogenic activities. Water quality was improved in the direction of groundwater flow in the study area, establishing a direct relationship between groundwater flow and water quality of the Bemetara District. This study provides very useful database for policymaker and state government to design sustainable groundwater management plan for the district.
References
Abbasi T, Abbasi SA (2012) Water quality indices. Elsevier, London
Abdelaziz S, Gad MI, El Tahan AHM (2020) Groundwater quality index based on PCA: Wadi El-Natrun, Egypt. J Afr Earth Sci 172:103964
Acharya S, Sharma SK, Khandegar V (2018) Assessment of groundwater quality by water quality indices for irrigation and drinking in South West Delhi, India. Data Brief 18:2019–2028
Al-Hadithi M (2012) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Ratmao–PathriRao watershed, Haridwar District, India. Am J Sci Ind Res 3(6):395–402
Alves MTR, Teresa FB, Nabout JC (2014) A global scientific literature of research on water quality indices: trends, biases and future directions. Acta Limnol Bras 26(3):245–253
APHA (2005) Standard methods for examination of water and wastewater. American Public Health Engineering
Avvannavar SM, Shrihari S (2008) Evaluation of water quality index for drinking purposes for river Netravahi, Mangalore, South India. Environ Monit Assess 143:279–290
Azhdarpoor A, Radfard M, Pakdel M, Abbasnia A, Badeenezhad A, Mohammadi A, Yousefi M (2019) Assessing fluoride and nitrate contaminants in drinking water resources and their health risk assessment in a semiarid region of southwest Iran. Desalin Water Treat 149:43–51
Badeenezhad A, Radfard M, Passalari H, Parseh I, Abbasi F, Rostami S (2019) Factors affecting the nitrate concentration and its health risk assessment in drinking groundwater by application of Monte Carlo simulation and geographic information system. Hum Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2019.1655634
Badeenezhad A, Tabatabaee HR, Nikbakht HA, Radfard M, Abbasnia A, Baghapour MA, Alhamd M (2020) Estimation of the groundwater quality index and investigation of the affecting factors their changes in Shiraz drinking groundwater, Iran. Groundwater Sustain Develop 11:100435
Badeenezhad A, Darabi K, Heydari M, Amrane A, Ghelichi-Ghojogh M, Parseh I, Javanmardi P (2021) Temporal distribution and zoning of nitrate and fluoride concentrations in Behbahan drinking water distribution network and health risk assessment by using sensitivity analysis and Monte Carlo simulation. Int J Environ Anal Chem 1–18
Baghanam AH, Nourani V, Aslani H, Taghipour H (2020) Spatiotemporal variation of water pollution near landfill site: application of clustering methods to assess the admissibility of LWPI. J Hydrol 591:125581
BIS (2012) Indian standard drinking water-specification, 2nd revision, IS:10500:2012. Bureau of Indian Standards, New Delhi
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index: do we dare? Water Sewage Works 117(10):339–343
Castillo JLU, Ramos JA, Leal DA, Cruz M, Martínez AC, Marín Celestino AE (2021) Identification of the dominant factors in groundwater recharge process, using multivariate statistical approaches in a semi-arid region. Sustainability 13(20):11543. https://doi.org/10.3390/su132011543
Chai N, Yi X, Xiao J, Liu T, Liu Y, Deng L, Jin Z (2021) Spatiotemporal variations, sources, water quality and health risk assessment of trace elements in the Fen River. Sci Total Environ 757:143882
Chen ZY, Nie ZL, Zhang ZJ, Qi JX, Nan YJ (2005) Isotopes and sustainability of ground water resources, North China Plain. Ground Water 43(4):485–493
Chopra RPS, Gopal K (2014) Assessment of ground water quality in Punjab. J Earth Sci Climate Change 5:243
Dahariya NS, Yadav A, Chakradhari S, Patel KS, Nicolás J, Yubero E, Martín-Ramos P (2020) Contamination, sources, and environmental hazards of groundwater in Bemetara District, Chhattisgarh, Central India. J Hazard Toxic Radioact Waste 24(1):05019005
Daou C, Nabbout R, Kassouf A (2016) Spatial and temporal assessment of surface water quality in the Arka River, Akkar, Lebanon. Environ Monit Assess 188(12):1–10
Davis JC, Sampson RJ (1986) Statistics and data analysis in geology. Wiley, New York
Dee N, Baker J, Drobny N, Duke KM, Whitman I, Fahringer D (1973) An environmental evaluation system for water resource planning. Water Resour Res 9(3):523–535. https://doi.org/10.1029/WR009i003p00523
Dinius SH (1972) Social accounting system for evaluating water. Water Resour Res 8(5):1159–1177. https://doi.org/10.1029/WR008i005p01159
District Survey Report Bemetara Chhattisgarh (2016) Directorate of Geology & Mining, Mineral Resources Department, Govt. of Chhattisgarh, Raipur (India)
Duan W, He B, Nover D (2016) Water quality assessment and contamination source identification of the eastern Poyang Lake Basin using multivariate statistical methods. Sustainability 8(2):133–148
Fan PF (1998) Groundwater evolution and prediction in North China Plain. Acta Geosci Sin 19(4):346–352
Freeze RA, Cherry JA (1979) Groundwater Prentice-Hall Inc. Eaglewood Cliffs, NJ
Gaikwad S, Gaikwad S, Meshram D, Wagh V, Kandekar A, Kadam A (2020) Geochemical mobility of ions in groundwater from the tropical western coast of Maharashtra, India: implication to groundwater quality. Environ Dev Sustain 22(3):2591–2624
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Admin 37(3):300
Huang J, Du P, Ao C, Ho M, Lei M, Zhao D, Wang Z (2007) Multivariate analysis for stormwater quality characteristics identification from different urban surface types in Macau. Bull Environ Contam Toxicol 79(6):650–654
Huneau F, Dakoure D, Celle-Jeanton H, Vitvar T, Ito M, Traoré S, Le Coustumer P (2011) Flow pattern and residence time of groundwater within the south-eastern taoudeni sedimentary basin (Burkina Faso, Mali). J Hydrol 409(1–2):423–439
Ishaku JM (2011) Assessment of groundwater quality index for Jimeta-Yola area, Northeastern Nigeria. J Geol Min Res 3(9):219–231
Krishan G, Chopra RPS (2015) Assessment of water logging in south western (SW) parts of Punjab, India—a case study from Muktsar district. NDC-WWC J 4:7–10
Krishan G, Lapworth DJ, Rao MS, Kumar CP, Smilovic M (2014) Natural(baseline) groundwater quality in the Bist-Doab catchment, Punjab, India: a pilot study comparing shallow and deep aquifers. Int J Earth Sci Eng 7:16–26
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2014) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5(4):335
Landwehr JM (1976) A statistical view of a class of water quality indices. Water Resour Res 15(2):460–468
Malyan SK, Singh R, Rawat M, Kumar M, Pugazhendhi A, Kumar A, Kumar V, Kumar SS (2019) An overview of carcinogenic pollutants in groundwater of India. Biocatal Agric Biotechnol 21:101288
Marín Celestino AE, Martínez Cruz DA, Otazo Sánchez EM, Gavi Reyes F, Vásquez Soto D (2018) Groundwater quality assessment: an improved approach to k-means clustering, principal component analysis and spatial analysis: a case study. Water 10(4):437
Marín Celestino AE, Ramos Leal JA, Martínez Cruz DA, Tuxpan Vargas J, De Lara Bashulto J, Morán Ramírez J (2019) Identification of the hydrogeochemical processes and assessment of groundwater quality, using multivariate statistical approaches and water quality index in a wastewater irrigated region. Water 11(8):1702
McDuffie B, Haney JT (1973) A proposed river pollution index. American Chemical Society, Division of Water, Air and Waste Chemistry, New York
Mishra PC, Patel RK (2001) Study of the pollution load in the drinking water of Rairangpur, a small tribal dominated town of North Orissa, Indian. J Environ Eco Plann 5(2):293–298
Mohebbi MR, Saeedi R, Montazeri A, Vaghefi KA, Labbafi S, Oktaie S, Mohagheghian A (2013) Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol Ind 30:28–34
Muangthong S, Shrestha S (2015) Assessment of surface water quality using multivariate statistical techniques: case study of the Nampong river and Songkhram river, Thailand. Environ Monit Assess 187:548
Mukherjee A, Gupta A (2010) Quest for fresh ground water in the area of inland salinity of Chhattisgarh State: safe drinking water for sustainable rural water supply. In: IVth World Aqua Congress-2010, pp 8–10
Mullaney JR, Lorenz DL, Arntson AD (2009) Chloride in groundwater and surface water in areas underlain by the glacial aquifer system, Northern United States. US Geological Survey Reston, VA
Nemerow NL, Sumitomo H (1970) Benefits of water quality enhancement. Syracuse: Syracuse. University Report No.16110 DAJ
Oppong FB, Agbedra SY (2016) Assessing univariate and multivariate normality. A guide for non-statisticians. Math Theory Model 6(2):26–33
Parti L, Pavanello R, Pesarin F (1971) Assessment of surface water quality by single index of pollution. Water Res 5(9):741–751. https://doi.org/10.1016/0043-1354
CGWB Report (2015) Ground water year book of Chhattisgarh, 2014–15. North Central Chhattisgarh region, Raipur, Govt. of India, Ministry of Water Resources, Central Ground Water Board, September
CGWB Report (2019) National Compilation on Dynamic Ground Water Resources of India, 2017, Government of India Ministry of Jal Shakti Department of Water Resources, RD & GR Central Ground Water Board, Faridabad, July 2019
Rakhmatullaev S, Huneau F, Kazbekov J, Le Coustumer P, Jumanov J, El Oifi B, Hrkal Z (2010) Groundwater resources use and management in the Amu Darya River Basin (Central Asia). Environ Earth Sci 59(6):1183–1193
Rakhmatullaev S, Huneau F, Kazbekov J, Celle-Jeanton H, Motelica-Heino M, Coustumer P, Jumanov J (2012) Groundwater resources of Uzbekistan: an environmental and operational overview. Open Geosci 4(1):67–80
Reza R, Singh G (2010) Assessment of ground water quality status by using Water Quality Index method in Orissa, India. World Appl Sci J 9(12):1392–1397
Rizvi NIDA, Katyal DEEKSHA, Joshi VARUN (2015) Assessment of water quality of Hindon River in Ghaziabad and Noida, India by using multivariate statistical methods. J Glob Ecol Environ 3(2):80–90
Rodell I, Velicogna I, Famiglietti JS (2009) Satellite-based estimates of groundwater depletion in India. Nature 460:999–1002
Royston JP (1983) Some techniques for assessing multivariate normality based on the Shapiro-Wilk W. J Roy Stat Soc Ser C (appl Stat) 32(2):121–133
Sahu M, Sar SK, Dewangan R, Baghel T (2020) Health risk evaluation of uranium in groundwater of Bemetara district of Chhattisgarh state, India. Environ Dev Sustain 22(8):7619–7638
Sajitha V, Vijayamma SA (2016) Study of physico-chemical parameters and pond water quality assessment by using water quality index at Athiyannoor Panchayath, Kerala, India. Emerg Life Sci Res 2:46–51
Saxena N, Sharma A (2017) Evaluation of water quality index for drinking purpose in and around Tekanpur Area, MP, India. Int J Appl Environ Sci 12(2):359–370
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52(3/4):591–611
Sharma MK, Jain CK (2006) Multivariate analysis of groundwater quality data of District Jodhpur, Rajasthan (India). J Environ Sci Eng 48(4):271–280
Sharma MK, Kumar M (2020) Sulphate contamination in groundwater and its remediation: an overview. Environ Monit Assess 192(2):1–10
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J Geochem Explor 175:59–71
Soleimani H, Nasri O, Ojaghi B, Pasalari H, Hosseini M, Hashemzadeh B, Kavosi A, Masoumi S, Radfard M, Adibzadeh A, Feizabadi GK (2018) Data on drinking water quality using water quality index (WQI) and assessment of groundwater quality for irrigation purposes in Qorveh & Dehgolan, Kurdistan, Iran. Data Brief 20:375–386
Srinivasamoorthy K, Chidambaram M, Prasanna MV, Vasanthavigar M, John Peter A, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Metturtaluk, Salem district, Tamilnadu, India. J Earth System Sci 117(1):49–58
Subba Rao N (1997) Studies on the water quality index in hard rock terrain of Guntur district, Andhra Pradesh, India. National seminar on hydrology of Precambrian Terrains and hard rock areas, pp. 129–134.
USGS (1999) Sustainability of ground-water resources. US Geological Survey Circular 1186. Denver, Colorado
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
Vega M, Pardo R, Barrado E, Debán L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
Vermonden K, Hermus MA, van Weperen M, Leuven RS, van der Velde G, Smolders AJ, Hendriks AJ (2009) Does upward seepage of river water and storm water runoff determine water quality of urban drainage systems in lowland areas? A case study for the Rhine-Meuse delta. Hydrol Process 23(21):3110–3120
Vijayachandran N, Ravisankar N (2018) Evaluation of groundwater quality of coastal aquifer in Sirkali block of Nagapattinam district. Int Res J Eng Technol 5(2):2222–2226
Wang SQ, Song XF, Wang QX, Xiao GQ, Liu CM (2008) Dynamic features of shallow groundwater in North China Plain. Acta Geograph Sin 63(5):462–472
World Health Organization (1996) The world health report: 1996: fighting disease, fostering development. World Health Organ.
WHO (2011) Guidelines for drinking water, recommendations. World Health Organization, Geneva
Xia J, Liu MY, Jia SF, Song XF, Luo Y, Zhang SF (2004) Water security problem and research perspective in North China. J Nat Resour 19(5):550–560 ((in Chinese))
Zhang ZH, Shi DH, Shen ZL, Zhong ZS, Xue YQ (1997) Evolution and development of groundwater environment in North China Plain under human activities. Acta Geosci Sin 18(4):337–344
Zhang ZH, Shen ZL, Xue YQ, Ren FH, Shi DH, Yin ZZ, Zhong ZS, Sun XH (2000) Groundwater environmental evolution in North China Plain. Geology Press, Beijing ((in Chinese))
Zhang R, Tao J, Lu R, Jin Q (2016) Decoupled ARX and RBF neural network modeling using PCA and GA optimization for nonlinear distributed parameter systems. IEEE Trans Neural Netw Learn Syst 29(2):457–469
Zhang H, Cheng S, Li H, Fu K, Xu Y (2020) Groundwater pollution source identification and apportionment using PMF and PCA-APCA-MLR receptor models in a typical mixed land-use area in Southwestern China. Sci Total Environ 741:140383
Zhou F, Liu Y, Guo H (2007) Application of multivariate statistical methods to water quality assessment of the watercourses in Northwestern New Territories, Hong Kong. Environ Monit Assess 132(1):1–13
Acknowledgements
This work is a part of the Purpose Driven Study (PDS) titled “Groundwater Quality Assessment with Special Reference to SO42- contamination in Bemetara District of Chhattisgarh State and Ameliorative Measures” (PDS No. NIH-29_2017-70) under National Hydrology Project and hereby acknowledged. The authors are highly grateful to the Director, National Institute of Hydrology, Roorkee, for providing the required analytical facilities and support and encouragement for carrying out this work.
Funding
Supported by National Hydrology Project (NHP) financially under approved PDS No. NIH-29_2017-70vide letter No. X-87013/1/2016-NHP/4565-4587 dated 31.08.2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, M.K., Kumar, M., Malik, D.S. et al. Assessment of groundwater quality and its controlling processes in Bemetara District of Chhattisgarh State, India. Appl Water Sci 12, 102 (2022). https://doi.org/10.1007/s13201-022-01608-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01608-4