Abstract
This study has been carried out to determine the uranium concentration associated with physicochemical parameters and water quality index during pre-monsoon and post-monsoon of Bemetara district to correlate the quality of water for public health. The correlation matrix has been applied for the determination of the correlation value to find out the relationship of uranium with water quality parameters. The uranium levels in water samples range from 1.15 to 83.5 µg/L and 0.68 to 96.08 µg/L during pre-monsoon and post-monsoon, respectively. The uranium concentration of few samples exceeds the safe limit of 30 µg/L prescribed by World Health Organization 2011. A positive correlation of uranium concentration with total hardness and total dissolved solids during both monsoons has been observed. The lifetime cancer risk varied from 0.07 × 10−6 to 5.06 × 10−6 and 0.04 × 10−6 to 5.82 × 10−6 in pre-monsoon and post-monsoon period, respectively, which is lower than the maximum permissible limit (< 10−3). The corresponding values of hazard quotient of 21 samples in pre-monsoon and 16 samples in post-monsoon were found to be greater than unity, which indicates a significant risk due to chemical toxicity. The observed results clearly showed that there is no harmful effect by radiological risk, but chemical risk can affect human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is well known that drinking water should be free of harmful chemicals. But in the twentieth century, the groundwater has become polluted due to the addition of toxic substances because of rapid industrial development. As a result, it is not suitable for the purpose of drinking and adversely affects the health of citizens. Several studies have shown that the major pollutants present in groundwater of Indian states include sulfate, salinity, fluoride, nitrates, arsenic, and heavy metals (Garg and Singh 2013). The quality of groundwater is generally classified by physical features, chemical composition, and biological factors. These quality parameters reflect inputs from natural sources, i.e., the air, soil, water, rock, and weathering, as well as different anthropogenic activities such as land clearance, mining, acid rain, agriculture, ignition of fuels, and domestic and industrial wastes. These parameters change generally because of pollution, seasonal fluctuation, groundwater extraction, etc. Monitoring of water quality levels is in this way essential to survey the levels of contamination, to evaluate its portability for human consumption, also to evaluate the potential hazard to the environment and for the supportable administration of these resources (Appelo and Postma 2005; Amadi et al. 2012; Abam 2001; Shittu et al. 2008).
As a result, uranium is available in natural water sources in assessable concentrations. Human ingestion of natural uranium is mainly due to food and water. The contribution of drinking water is approximately 85%, while the food contributes about 15% of the total ingested uranium (Mittal et al. 2017). Naturally occurring radioactive metal uranium found in rocks, air, soil, and water. Uranium concentration in groundwater depends on lithology, geomorphology, and other geographical conditions of the region (Sridhar Babu et al. 2008). In addition, the physicochemical parameters of water in a particular area also influence the uranium concentration (Singh et al. 2003; Yasovardhan et al. 2013). Despite the toxicity, uranium concentration is not regularly measured as an indicator of drinking water quality. Therefore, the assessment of uranium concentration in drinking water is very important. The uranium is characterized as a carcinogenic element by the United States Environmental Protection Agency (USEPA) and recommended that the complete absence of uranium in drinking water should be a safe limit only for carcinogenic exposure in 1991. Currently, the World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) have proposed the maximum contaminated level (MCL) of 30 µg/L for uranium in drinking water (USEPA 2012; WHO 2011). Instead of radiological toxicity of uranium, there are major adverse health effects due to its chemical toxicity (WHO 1998; Jakhu et al. 2016). Its adverse impacts on kidney are well studied (Domingo 1995). The primary sources of ingestion are food and water, after that, it is preferably accumulated in the kidneys, liver, and bones (Kurttio et al. 2005). 66% of ingested uranium is rapidly eliminated through urine, while the remainder is distributed and stored in the kidneys (12–25%), bone (10–15%), and soft tissue (Wrenn et al. 1985). The calculation of radiological and chemical risks of uranium is really very important because of its adverse effect on human health.
The measurement of uranium levels and annual effective dose in drinking water samples due to uranium ingestion on behalf of the health risk is the main objective of this study. The physicochemical parameters, i.e., pH, TDS, EC, total hardness, calcium, magnesium, alkalinity, chloride, sulfate, nitrate, phosphate, and fluoride, were also measured to find out the correlation if any, with the analyzed uranium concentration in water samples. The water quality index (WQI) is also analyzed to assess the quality of water. This study is useful to evaluate the present quality of groundwater and determining the suitability of groundwater for various purposes depending on various geochemical processes and WQI. Therefore, this study will work in the future as a basis for improving the quality of groundwater.
2 Geology of the study area
Bemetara district is one of the newly born districts of Chhattisgarh states. The district is moderately populated and situated in the central part of the Chhattisgarh State with 2854.81 km2 area. Its latitude 21° 22′ to 22° 03′ N and longitude 81° 07′ to 81° 55 E. The Shivnath River flows toward the east of the city of Bemetara, and the southern side has dense forests. Bemetara has a tropical wet and dry climate; from March to June, the temperature remains normal throughout the year. It is bounded by Mungeli districts in the north and Durg districts in the south, Rajnandgaon and Kabirdham district in the west, and Baloda-bazar and Raipur district in the East. Bemetara district is an important region for limestone deposits in Chhattisgarh state. The minor minerals are low-grade limestone, sandstone, quartzite, soil, rivers, and are found in very large quantities. The Chhattisgarh is famous for its quality rice and named rice bowl of India. Bemetara district is the main key area for quality rice production due to its natural geology and hydrogeology as physiographically Bemetara district is having almost flat topography. In Bemetara district, rocks formation is with limestone formation toward calcareous shale with gypsum inter-bands. It is also represented as pebble bed. Groundwater prospect map is shown in Fig. 1 (District survey report 2016).
3 Experimental techniques
3.1 Sampling
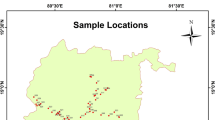
Samples were collected from 50 various locations with the help of the grid map from bore wells and supply systems which are used for drinking purpose. Sampling locations are shown by the green dots in Fig. 2. Airtight plastic bottles were used for the collection of samples, which were pre-rinsed with distilled water and clean properly with the water of sampling area at the time of sampling. After collecting water samples, before analyzing them for uranium concentration and physicochemical parameters, they were filtered by using 0.45-micron Whatman filter paper. Sampling was done during the pre-monsoon and post-monsoon period in the month of May 2017 and October 2017, respectively.
3.2 Measurement of uranium in samples
LED fluorimeter (Quantalase LF-2A) has been used to analyze the uranium levels in water samples. It can accurately measure the concentration range of 0.5–1000 μg/L with a precision of ± 5%. The fluorescence yields fluctuate for different complexes of uranium. Therefore, a fluorescence-enhancing reagent (fluren, an inorganic reagent) was added to the sample so that all the complexes could be converted into fluorescence yield in the same form. Five milliliters of the sample with 0.5 mL of 10% fluren was taken in a cuvette (produced using ultra-low fluorescence fused silica), and uranium concentration was recorded on the instrument. Samples were analyzed by standard addition method to avoid any matrix effect.
3.3 Physicochemical analysis of sample water
pH, electrical conductivity (EC), and total dissolved solids (TDS) estimations were carried out by using Hanna Multiparameter instrument no. HI 5521 and HI 5522. The total hardness and calcium hardness were estimated by EDTA method by using Eriochrome Black-T and Patton and Reeder’s indicator, respectively, and magnesium hardness has been estimated by subtracting calcium hardness from total hardness. The chloride concentration is determined by Mohr’s methods by adding silver nitrate to give precipitation of silver chloride. The total alkalinity due to bicarbonate has been determined by titration with standard solution of HCl and methyl orange indicator. The content of sulfate, phosphate, and nitrate ions are evaluated by using UV–Visible spectrophotometer 117. Fluoride concentration is measured by ion selective electrode by using Orion 4 Star Thermo scientific.
3.4 Health risk assessment
Due to the intake of uranium-contaminated drinking water, health risk can be categorized into two parts: radiological risk (carcinogenic) and chemical risk (non-carcinogenic).
3.5 Radiological risk assessment
The conversion factors prescribed by ICRP 72 and WHO are used for the calculation of annual effective dose, cumulative dose, and excess lifetime cancer risk (ICRP 1996; WHO 2004). The unit conversion factor (0.02528 Bq/L = 1 µg/L) is utilized for the calculation of uranium activity concentration. Cumulative dose has been resolved for an average life of 70 y and risk factor of 7.3 × 10−2 Sv−1 utilized for the calculation of cancer risk (WHO 2004; ICRP 1991). The annual effective dose for an adult is calculated by the following equation due to uranium ingestion in 2 L of water.
where D = annual effective dose in µSv/y, A = Uranium activity concentration in Bq/L, F = effective dose per unit intake through ingestion (4.5 × 10−5 mSv/Bq).
3.6 Chemical risk assessment
Hazard quotient (HQ) is used for the calculation of chemical risk. HQ gives the extent of harm produced due to the ingestion of uranium-contaminated water, which is given by Eq. 2. Rfd is a Reference dose = 0.6 in µg/kg/day (Ye-shin et al. 2004) and LADD is given by Eq. 3
where LADD = lifetime average daily dose (µg/kg/day), EPC = exposure point concentration (µg/L), IR = water ingestion rate (L/day), EF = exposure frequency (days/year), ED = total exposure duration (years), AT = average time (days), BW = body weight (kg).
Using therefore,
IR = 2 L/day; EF = 365 days, ED = 70 y, AT = 25,550 (obtained from 70 × 365) and BW = 53 kg (for an Indian standard man) (Dang et al. 1994).
3.7 Water quality index (WQI)
In this study, 11 parameters have been selected for calculating the water quality index. The standards of drinking water quality suggested by World Health Organization (WHO), Indian Council of Medical Research (ICMR), and Bureau of Indian Standards (BIS) have been used for the calculation of WQI (WHO 2011; BIS 2012; ICMR 1975). WQI and drinking water standards with unit weight for pre- and post-monsoon are given in Tables 1 and 2. The water quality index of water is calculated by weighted arithmetic index method (Brown et al. 1972). Quality rating or sub-index (\(q_{n}\)) was given by the following equation:
where qn = quality rating of nth water quality parameters, Vn = observed value of the nth parameter at a given sampling location, Vid = ideal value of the nth parameters in pure water (except the pH (7.0) the ideal value of all parameters is 0), and Sn = standard permissible value of nth parameters.
Quality rating (qn) and nth water quality parameters in contaminated water show a number reflecting the relative of these parameters with respect to its standard permissible value. The unit weight of corresponding parameters is inversely proportional to the prescribed standard value Sn and expressed by the following equation:
Wn = unit weight for the nth parameters, K = proportionality constant.
Water quality index is calculated by aggregated quality ratings linearly with unit weight.
4 Results and discussion
The measured value of uranium concentration and physicochemical parameters of collected 50 drinking water samples are given in Table 3. The correlation of uranium with other physicochemical parameters during pre- and post-monsoon is shown in Tables 4 and 5. The range of pH in 50 samples is found under the permissible limit of 6.5–8.5 given by WHO 2011 during pre- and post-monsoon, and it is observed that there is no correlation of pH with uranium concentration. The electrical conductivity of water samples is varied from 277 to 4456 µS/cm and 210.9 to 3960 µS/cm with the mean value of 1207 µS/cm and 1079.06 µS/cm and good correlation with uranium during pre- and post-monsoon, respectively. The value of TDS ranges from 138 to 2206 mg/L and 105.5 to 1983 mg/L with the average value of 601.51–540.13 mg/L in pre- and post-monsoon and found a good positive correlation with uranium concentration. The alkalinity in the water samples during pre- and post-monsoon ranged from 45 to 405 mg/L and 40 to 387.5 mg/L and found a positive correlation with uranium concentration. The total hardness of water samples ranged between 100–2170 mg/L during pre-monsoon and 80–1950 mg/L during post-monsoon and a good correlation is found with uranium. The analyzed calcium revealed a ranged between 20–572 mg/L and 12–396 mg/L and good correlated with uranium. The amount of magnesium analyzed in water samples ranged between 12–209 mg/L and 7.2–230.4 mg/L. The correlation of magnesium in pre-monsoon is good and in post-monsoon is a positive correlation (0.4). A chloride concentration was noticed between 24.85–408.25 mg/L and 21.3–383.4 mg/L during pre-monsoon and post-monsoon and positively correlated with uranium in water samples. The amount of nitrate analyzed in samples ranged from 0.54 to 39.96 mg/L and 0.5 to 23.39 mg/L in pre-monsoon and post-monsoon, respectively. The range of sulfate ion in water samples is ranged between 2.77–421.71 mg/L and 2.03–390 mg/L during pre- and post-monsoon, and good correlation is found with uranium. The content of fluoride and phosphate is varied from 0.15 to 1.15 mg/L and 0.05–0.85 mg/L and 0.43 to 1.26 mg/L and 0.2 to 1.24 mg/L during pre- and post-monsoon. The poor correlation of uranium concentration with fluoride and phosphate is found. The range of uranium in water samples during pre-monsoon and post-monsoon is 1.15–83.5 µg/L and 0.68–96.08 µg/L with an average value of 20.85 µg/L and 17.54 µg/L during pre-monsoon and post-monsoon, respectively.
The data of uranium concentration, annual effective dose, lifetime stochastic health effect, lifetime average daily dose, and hazard quotient during pre-monsoon and post-monsoon are summarized in Tables 6 and 7. The annual effective dose from the collected water samples was found to vary from 0.96 to 69.34 µSv/y and 0.56 to 79.79 µSv/y during pre- and post-monsoon, respectively. The annual effective dose was found under the safe limit of 100 µSv/y prescribed by WHO 2004. The lifetime cancer risk varied from 0.07 × 10−6 to 5.06 × 10−6 and 0.04 × 10−6 to 5.82 × 10−6 in pre-monsoon and post-monsoon period with an average value of 1.26 × 10−6 and 1.06 × 10−6. The reported values for cancer risk are low compared to the acceptable level of 10−3 for the radiological risk (Ye-shin et al. 2004). LADD ranged between 0.04–3.15 µg/kg/day and 0.03–3.63 µg/kg/day in this study. The recommended level of lifetime daily dose is 1.0 µg/kg/day by WHO 2011. The value of HQ in both monsoons is 0.07 to 5.25 and 0.04 to 6.04 with an average value of 1.31 and 1.10. The value of Hazard Quotient in 21 water samples and 16 water samples are found to be greater than the safe limit of 1.0 prescribed by WHO (2008) during pre-monsoon and post-monsoon, respectively, which is indicating a major risk due to chemical toxicity.
4.1 Water quality index
Water quality index is very helpful in evaluating, controlling, and managing the quality of water. Water quality index of water samples in pre- and post-monsoon is shown in Figs. 3 and 4. WQI values are classified on the scale prescribed by Brown et al. and Chatterjee and Rajiuddin. All the values of physicochemical factors are in milligram per liter excluding pH and electrical conductivity. All parameters play an important role in determining the quality of water. In pre-monsoon 42% of water samples and in post-monsoon 34% of water samples are excellent in quality. 12% water samples and 28% water samples during pre- and post-monsoon are good in quality; 0.14% of water samples in pre-monsoon and 16% of water samples in post-monsoon are poor in quality. 10% of water samples and 8% of water samples are very poor in quality during both periods. The WQI of 22% samples in pre-monsoon and 14% samples in post-monsoon are above the 100, which indicate that water is unsuitable for drinking purpose. It has been observed that the pollution contents compared to that in post-monsoon is comparatively high in pre-monsoon. The basic data of this investigation will take a long mode in improving the status of water quality, because of being socioeconomically vital.
5 Conclusion
The results of the present study indicate that according to the World Health Organization 2011, the uranium level of 22% samples in pre-monsoon and 14% samples in post-monsoon of Bemetara district cross the safe limit, and it is not suitable for drinking purpose. We found such parameters, i.e., pH, electrical conductivity, total alkalinity, sulfate, nitrate, fluoride, and phosphate values are under the permissible limit. In some samples, total dissolved solids, total hardness, calcium, magnesium, and chloride values are higher than the permissible limit set by WHO 2011/BIS 2012. The uranium shows good correlation with electrical conductivity, total hardness, calcium, magnesium, total dissolved solids, chloride, and sulfate in pre-monsoon. In post-monsoon, uranium shows good affinity with electrical conductivity, total hardness, calcium, and total dissolved solids. Correlation matrix for pre- and post-monsoon indicates that this area is highly contaminated with limestone, and the value also proves its presence in groundwater samples of Bemetara district. Uranium shows a negative correlation with pH in both monsoons. The annual effective dose was found under the safe limit than the WHO recommendation level (100 μSv/y). The cancer risks due to consumption of water in the study area are much lesser than the permissible limits. The HQ values of 42% samples and 32% samples during pre- and post-monsoon period, respectively, were found higher than the limit value. Finally, it is concluded that in the study area, chemical risk affects human health; however, radiological risk is in the safe limit. Competitive WQI indicates that the quality of 11 water samples and 7 water samples are unsuitable for drinking purpose during pre-monsoon and post-monsoon, respectively, and is not completely safe for human consumption. It is a severe problem for a large number of people’s lives in Bemetara district due to the high level of indicative parameters above the standards. Water purification system should be established to clean the water in this area. The results showed that for the local public it is necessary to treat the water before using.
References
Abam, T. K. S. (2001). Regional hydrological research perspective in the Niger Delta. Hydrological Sciences Journal, 46, 13–25.
Amadi, A. N., Olasehinde, P. I., Yisa, J., Okosun, E. A., Nwankwoala, H. O., & Alkali, Y. B. (2012). Geostatistical assessment of groundwater quality of coastal aquifers of Eastern Niger Delta. Geosciences, 2, 51–59.
Appelo, C. A. J., & Postma, D. (2005). Geochemistry, groundwater and pollution (p. 251). London: Taylor and Francis.
BIS (Bureau of Indian Standards). (2012). Specification for drinking water. IS: 10500, New Delhi, India.
Brown, R. M., Mccleiland, N. J., Deiniger, R. A., O’ Connor, M. F. A. (1972). Water quality index—Crossing the physical barrier. In: S. H. Jenkis (Ed.), International conference on water pollution research, Jerusalem (Vol. 6, pp. 787–797).
Chatterjee, C., & Rajiuddin, M. (2002). Determination of water quality index (WQI) of a degraded river in Asanol Industrial area, Raniganj, Burdwan, West Bengal. Nature, Environmental and Pollution Technology, 1(2), 181–189.
Dang, H. S., Jaiswal, D. D., Parameswaran, M., & Krishnamony, S. (1994). Physical, anatomical, physiological and metabolic data for reference Indian Man—A proposal. Mumbai: Bhabha Atomic Research Centre.
District Survey Report Bemetara (Chhattisgarh). (2016). As Per Notification No. S.O. 141 (E) New Delhi, 15th January, 2016 of Ministry of Environment, Forest and Climate Change. Directorate of Geology and Mining Mineral Resources Department, Govt. of Chhattisgarh.
Domingo, J. L. (1995). Prevention by chelating agents of metal-induced development toxicity. Reproductive Toxicology, 9(2), 105–113.
Garg, V. K., & Singh, B. (2013). Fluoride signatures in groundwater and dental fluorosis in permanent teeth of school children in rural areas of Haryana State, India. International Journal of Occupational and Environmental Medicine, 4, 107–108.
ICMR (council of medical research). (1975). Manual of standards of quality for drinking water supplies. Indian special report no. 44, 27.
ICRP (International Commission on Radiological Protection). (1991). Recommendations of the international commission on radiological protection. Oxford: Pergamon Press; ICRP Publication 60; Ann ICRP 21(1Y3).
ICRP (International Commission on Radiological Protection). (1996). Age-dependent doses to the members of the public from intake of radionuclides part 5: Compilation of ingestion and inhalation coefficients. Oxford: Pergamon Press; ICRP Publication 72; Ann ICRP 26/1.
Jakhu, R., Mehra, R., & Mittal, H. M. (2016). Exposure assessment of natural uranium from drinking water. Environmental Science: Processes & Impacts, 18(12), 1540–1549.
Kurttio, P., Komulainen, H., Leino, A., Salonen, L., Auvinen, A., & Saha, H. (2005). Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environmental Health Perspectives, 113(1), 68–72.
Mittal, S., Rani, A., Mehra, R., Balaram, V., Satyanarayanan, M., & Sawant, S. S. (2017). Assessment of uranium in correlation with physico-chemical properties of drinking water of Northern Rajasthan. Journal of the Geological Society of India, 90, 233–238.
Shittu, O. B., Olaitan, J. O., & Amusa, T. S. (2008). Physicochemical and bacteriological analyses of water used for drinking and swimming purposes in Abeokuta, Nigeria. African Journal of Biomedical Research, 11, 285–290.
Singh, S., Rani, A., Mahajan, R. K., & Walia, T. P. (2003). Analysis of uranium and its correlation with some physic-chemical properties of drinking water samples from Amritsar, Punjab. Journal of Environmental Monitoring, 5, 917–921.
Sridhar Babu, M. N., Somashekar, R. K., Kumar, S. A., Shivanna, K., Krishnamurthy, V., & Eappen, K. P. (2008). Concentration of uranium levels in groundwater. International Journal of Environmental Science & Technology, 5(2), 263–266.
USEPA (United States Environmental Protection Agency). (2012). Drinking water standards and health advisories. Washington DC, U.S.A., EPA 822-S-12-001.
WHO (World Health Organization). (1998). Guidelines for drinking water quality (pp. 72–80). Geneva: WHO.
WHO (World Health Organization). (2004). Guidelines for drinking-water quality (Vol. 1), recommendations, 3rd ed. Geneva: WHO.
WHO (World Health Organization). (2008). Guidelines for drinking-water quality (4th ed., Vol. 1). Geneva: WHO.
WHO (World Health Organization). (2011). Guidelines for drinking water quality (pp. 241–252). Geneva: WHO.
Wrenn, M. E., Durbin, P. W., Howard, B., Lipsztein, J., Rundo, J., Still, E. T., et al. (1985). Metabolism of ingested U and Ra. Health Physics, 48(5), 601–633.
Yasovardhan, N., Basha, A. M., Satyanarayana, S. V., Reddy, G. V., Vishwa, K. P., Padma, P. S., et al. (2013). Seasonal assessment of natural uranium in drinking water around Tummalapalle uranium mining site, Kadapa district, India. International Journal of Recent Scientific Research, 4, 1406–1409.
Ye-shin, K., Hoa-sung, P., Jin-yong, K., Sun-ku, P., Byong-wook, C., Ig-hwan, S., et al. (2004). Health risk assessment for uranium in Korean groundwater. Journal of Environmental Radioactivity, 77(1), 77–85.
Acknowledgements
The authors are extremely thankful and sincerely acknowledge the guidance, help in sampling, encouragement and providing laboratory facilities to the Bhilai Institute of Technology, Durg. We are also thankful to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Project No. 36 (4)/14/90/2014-BRNS, Government of India, for providing financial assistance during this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahu, M., Sar, S.K., Dewangan, R. et al. Health risk evaluation of uranium in groundwater of Bemetara district of Chhattisgarh state, India. Environ Dev Sustain 22, 7619–7638 (2020). https://doi.org/10.1007/s10668-019-00539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-019-00539-6