Abstract
Dark septate endophytes (DSE) are root-associated fungi and can form plant-DSE symbioses to enhance the growth and abiotic stress resistance of plants. Although DSE exist in many non-mycorrhizal plants, the growing effects of DSE on non-mycorrhizal plants are currently poorly documented. This study investigated how DSE from mycorrhizal plants affect non-mycorrhizal plant growth especially under drought stress. In this study, the non-mycorrhizal plant Isatis indigotica was inoculated with DSE isolated from the roots of mycorrhizal plant Glycyrrhiza uralensis that grows in desert and farmland environments to evaluate plant performance and active ingredient content under different water treatments by measuring growth parameters and physiological indexes. The results indicated that seven DSE colonized the roots of I. indigotica regardless of watering regime. Inoculation with Acrocalymma vagum, Edenia gomezpompae and Darksidea alpha enhanced plant biomass, root surface area and epigoitrin content, and reduced oxidative damage from drought stress. Under drought stress condition, DSE inoculation caused remarkable increases in photosynthesis and revealed positive effects on SOD activity and proline content, which was reliance on fungus species. Correlation analyses indicated that root biomass was negatively correlated with soil pH and available phosphorus, and net photosynthetic rate was negatively correlated with soil organic carbon and available phosphorus. Therefore, DSE isolated from mycorrhizal plants could enhance the growth and active ingredient content of I. indigotica. The link between DSE and non-mycorrhizal plant highlights a potential application for DSE strains in dryland medicinal plant cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Drought, one of the most frequent and severe abiotic stress factors, can limit plant growth and productivity by decreasing the water content in plants and greatly reducing soil nutrient availability (Osakabe et al. 2014; Xue et al. 2019). The response of plants to drought stress can be either self-regulated or established by microbial symbiosis (Zuo et al. 2020). Endophytic fungi, as important root microbes, can form symbiotic relationships with almost every plant in natural ecosystems (Nelson et al. 2018), and these relationships are conducive to faster growth and stronger drought resistance in plants (de Vries et al. 2020). Previous studies have shown that arbuscular mycorrhizal fungi (AMF) can enhance host plant productivity and tolerance under drought stress by transferring soil nutrients to the host plant and increasing its antioxidant enzyme activity (de Vries et al. 2020; Grümberg et al. 2014). However, typical non-mycorrhizal plants (such as Brassicaceae, Cyperaceae, and Chenopodiaceae) are rarely colonized by AMF because they lack essential symbiosis genes or because they can establish only weak AMF symbiosis (Anthony et al. 2020; Delaux 2017; Regvar et al. 2003; Veiga et al. 2013). Even in artificial culture, arbuscules that enhance nutrient exchange were rarely found on non-mycorrhizal plants. Hence, exploring the potential uses of endophytic fungi, including dark septate endophytes (DSE) that have ecological functions similar to those of AM fungi, is crucial for enhancing health and productivity of non-mycorrhizal plants in arid environments (Hiruma et al. 2018).
Dark septate endophytes (DSE) are conidial or sterile ascomycetous fungi that colonize the living root tissues of healthy plants, and these fungi are characterized by dark septate hyphae and melanized microsclerotia (Mandyam and Jumpponen 2005). Microsclerotia are considered to be vegetative propagules with thick melanized walls which protect plants during stress until conditions are favorable for germination (Barrow and Aaltonen 2001). Melanin produced by DSE during the growth process is believed to maintain the structural rigidity of cell wall and may improve the adaptability of DSE to various stressors (Agustinho and Nosanchuk 2017; Berthelot et al. 2020; Potisek et al. 2021; Fernandez and Koide 2013; Yuan et al. 2021; Zhan et al. 2011). Related reports have shown that DSE have a positive impact on host plant function depending on host-symbiont combinations (Newsham 2011). DSE strains can adjust the root morphological structure and expand root networks to improve water absorption in host plants (Hill et al. 2019; He et al. 2019b). In addition, DSE have been found to have the ability to enhance plant growth and stress resistance (Li et al. 2019a; Liu and Wei 2019). Hou et al. (2020) found that DSE can increase the biomass and root vitality of non-hosts and thereby improve their heavy metal stress resistance. Li et al. (2018) reported that DSE isolated from desert ecosystems enhanced drought resistance in non-host plants. However, the effects of DSE from different habitats on the growth and drought resistance of non-mycorrhizal plants are unclear.
Isatis indigotica Fortune (Brassicaceae) is a traditional medicinal plant which widely distributed in northern and central China (Zhang et al. 2019; Zhao et al. 2017). Its dried roots are rich in active ingredients such as epigoitrin and is used clinically to prevent and treat influenza (Luo et al. 2019; National Pharmacopoeia Commission 2015). Although cultivation techniques for medicinal plants have been developed, the deterioration of cultivation areas caused by drought poses a great challenge to the growth and sustainable development of medicinal plant cultivation (Wang et al. 2020a, 2020b). Thus, improving the yield and quality of medicinal materials under drought conditions has become an urgent problem to be solved. Han et al. (2021) found that typical DSE colonisation structures were observed in the roots of 25 medicinal plants including I. indigotica and G. uralensis. Zhang et al. (2012) found that inoculation with DSE increased the total biomass of Lycium barbarum by 39.2%. He et al. (2019b) found that DSE colonization can increase the glycyrrhizic acid and glycyrrhizin contents of G. uralensis under drought stress conditions. As DSE could affect on medicinal plants growth, it is significant to evaluate the potential application of DSE in medicinal plants.
Studies have shown that DSE can colonize mycorrhizal and non-mycorrhizal plants and have a positive effect on the growth of mycorrhizal plants; however, DSE impact on the growth of non-mycorrhizal plants, especially those under drought stress are currently poorly documented (Mandyam et al. 2013; Cosme et al. 2018). The present study aimed to explore the effect of DSE on the growth and active ingredient content of non-mycorrhizal plant I. indigotica under drought stress in order to expand their potential for application in the cultivation of medicinal plants experiencing water deficit conditions. We examined the performance of I. indigotica inoculated with DSE under well-watered and drought stress conditions. Specifically, we answered the following questions: (1) Do DSE isolated from mycorrhizal plant G. uralensis roots in arid and farmland habitats colonize the roots of I. indigotica under well-watered and drought stress conditions? (2) Do DSE contribute to the growth and active ingredient contents of I. indigotica under drought stress conditions? If so, (3) how do DSE affect I. indigotica growth under drought stress conditions?
2 Materials and methods
2.1 Fungal and plant materials
Root samples of G. uralensis were collected in the arid area of Northwest China and the farmland area of Anguo city, China in September 2019. Roots were surface-disinfected by sequential washes in 75% ethanol for 5 min and 10% sodium hypochlorite for 5 min, after which they were rinsed several times in sterilized water and then dried on sterile filter paper. Finally, root segments were placed on potato dextrose agar (PDA) culture medium with antibiotic supplements (ampicillin and streptomycin sulfate) in Petri dishes (Zuo et al. 2020). The sterilized root samples were cultured in the dark at 27 °C and were observed daily. Meanwhile, the distilled water (200 µL) left in the final step were coated on PDA medium as a contrast. The dark hyphae growing from the cut ends of root segments were transferred to new PDA plates and kept in the dark at 27 °C (Li et al. 2018).
Fresh hyphae (approximately 50 mg) were scraped from each colony, and DNA was extracted by the fungal genomic DNA extraction kit (Solarbio, China) according to the manufacturer’s instructions. The primers ITS4 (5’-TCCTCCGCTTATTGATATGC-3') and ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3') were used to amplify the internal transcribed spacer (ITS) region by polymerase chain reaction (PCR). PCR reaction system (20 µL): 2×Es Taq Master Mix 10 µL (10 mM Tris-HCl, pH 8.3; 50 mM KCl; 1.5 mM MgCl2; 250 µM each dNTP; 0.05 U Polymerase/µL), each primer 0.5 µL (10 µmol/L), DNA template 3.5 µL (50–100 ng/µL), ddH2O 5.50 µL. PCR was performed in a Life ECO™ thermocycler (BIOER, China) with the following program: pre-denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, primer annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The purified PCR products were sequenced. The sequences were analyzed using BLAST tool in NCBI with the option “type strain material” to determine taxonomic identifications. Clustal X (v.1.81) was used to complete sequence alignment. Finally, MEGA 6.0 was used for phylogenetic analysis and construction of maximum likelihood trees (Hou et al. 2020; Xie et al. 2017). DNA sequences were deposited in GenBank under the accession numbers MW042345 (AV1), MW042346 (PC), MW042347 (EG), MW042348 (DA), MW042350 (BN), MW042351 (PT) and MW042354 (AV2). The isolates were preserved at -80 °C in the culture collection of the Laboratory of Plant Ecology, Hebei University, China. Meanwhile, all isolates were cultured on the PDA medium for 2 weeks under dark conditions at 27 °C for subsequent experiments.
Mature seeds of I. indigotica were collected from the Anguo Medicine Planting Site in Hebei Province, China. Seed surface was sterilized with 75% ethanol for 3 min, then with 2.5% sodium hypochlorite for 10 min, and then rinsed with sterile water for three times. The sterilized seeds were placed on water-agar (10 g/L) medium in Petri dishes for germination at 27 °C (Hou et al. 2020).
2.2 Plant growth experiment
The experiment was conducted using a completely random design in an 8 x 2 factorial arrangement. The variables for I. indigotica cultivation were inoculated with the various DSE (a non-inoculated control, AV1, PC, EG, DA, BN, PT and AV2) and the water conditions (well-watered and drought stress). Each treatment consisted of five replicates, with two plants in each pot, for a total of 80 experimental pots.
The sterilized seedlings were planted in sterile pots (diameter 8 cm, height 24 cm; 2 seedlings per pot) containing 400 g (200 g of soil mixed with 200 g of river sand) of culture substrate that had been autoclaved at 121 °C for 120 min (He et al. 2020; Hou et al. 2020). The culture substrate contained 21.57 mg/g of organic matter, 130 mg/kg of ammonium nitrogen, and 7.90 mg/kg of available phosphorus. For the DSE-inoculated treatments, fungal discs (5 mm in diameter, 1 disc per plant) were excised from a 14-day-old PDA culture medium and placed 1 cm below each plant roots (Li et al. 2019b). The control treatments were inoculated with discs excised from a 14-day-old PDA culture medium without fungus. All inoculation processes were performed on a sterile, ultra-clean workbench. All pots were placed in a growth chamber with a photoperiod of a 14 h/10 h, temperatures of 27 °C/22 °C (day/night), and an average air relative humidity of 60%.
One month after sowing, half of the seedlings (in both the inoculation and control treatments) were maintained under well-watered conditions (70% field water capacity), while the other half were under drought stress (35% field water capacity). The I. indigotica seedlings were harvested at 90 days after sowing.
2.3 Plant growth parameters
Before harvesting, the chlorophyll content (Chl) of the third mature leaf from the top of the plants was measured using the SPAD-502 Chl meter (Konica Minolta Sensing, Osaka, Japan). The absorbance of the leaves was measured at wavelengths of 650 nm and 940 nm. A “SPAD number” was calculated from these two transmission values. The net photosynthetic rate (Pn) of the third leaf from the top was measured by the portable photosynthesis measurement system (LI-6400XT, Li-COR, Lincoln, United States). Then, plant shoots and roots in each pot were harvested separately. The root system of each plant was gently washed to remove any adhering sandy soil. The cleaned root samples were placed in clear plexiglass trays containing deionized water and scanned with a scanner (EPSON Perfection V800 Photo, Japan). The total root length, average root diameter, root surface area, and root volume were measured using the WinRHIZO image analysis system (Regent Instruments, Quebec, QC, Canada).
The root samples were collected after scanning to analyse DSE colonization, indole-3-acetic acid (IAA) content and epigoitrin content. The leaves were used to assess plant physiological parameters, such as superoxide dismutase (SOD) activity and malondialdehyde (MDA) content. The remaining roots and shoots were dried at 70 °C for 48 h to calculate the plant biomass. Soil samples from each replicate were air-dried (15 °C to 25 °C) and then stored at 4 °C until the analyses of soil physicochemical properties.
2.4 Determination of proline contents and antioxidant enzyme activity
The proline content was determined based on the method of Bates et al. (1973). Fresh leaves (0.5 g) were homogenized in 3% sulfosalicylic acid solution (5 mL). The homogenate was heated in a boiling water bath for 10 min, then filtered with filter paper. The filtrate (2 mL) was reacted with glacial acetic acid (2 mL) and acid-ninhydrin (2 mL) heated for 30 min at 100 °C. The reaction was terminated in an ice bath. The reaction mixture was extracted with 4 mL of toluene and then centrifuged at 3000 r/min for 5 min. Finally, the absorbance was read at 520 nm using a spectrophotometer.
SOD activity was measured by the photochemical reduction method (Elavarthi and Martin 2010). Fresh leaves (0.5 g) were homogenized in 5 mL of 50 mM potassium phosphate buffer (pH 7.8) containing chilled 0.2 mM EDTA and 2% (w/v) polyvinylpyrrolidone and placed in an ice bath. The homogenate was centrifuged at 15000 rpm for 30 min to collect the supernatant for enzyme assays. One unit of SOD activity is defined as the amount of enzyme causing a 50% inhibition in the photochemical reduction of nitroblue tetrazolium at a wavelength of 560 nm, determined using the spectrophotometer (752 N model, Shanghai INESA Instrument Analytical Instruments Co., Ltd., China).
The MDA content was measured by the thiobarbituric acid (TBA) method (Peever and Higgins 1989). Fresh leaves (0.5 g) were homogenized in 5 mL of 10% trichloroacetic acid and centrifuged at 12000×g for 10 min. Then the supernatant (2 mL) was added to 0.5% TBA (2 mL). The mixture was heated in a boiling water bath for 15 min and then rapidly cooled. After centrifugation at 12000×g for 10 min, the absorbance was determined at wavelengths of 450, 532 and 600 nm using the spectrometer. The MDA content was calculated following the formula:
2.5 Determination of indole-3-acetic acid content and active ingredients in roots
Fresh roots (100 mg) were homogenized in 1 mL of 0.01 mol/L precooled phosphate buffer solution (pH=7.2). The homogenate was centrifuged at 3000×g for 20 min at 4 °C to collect the supernatant that was used to determine the IAA content with an IAA ELISA kit (Mlbio, Shanghai, China). The absorbance was measured using the Epoch 2 microplate reader (BioTek, Winooski, USA) at 450 nm.
The epigoitrin content was determined by high-performance liquid chromatography (HPLC) (National Pharmacopoeia Commission 2015). The dried root samples were ground into powder and passed through a 40-mesh sieve. A 1 g sample was decocted in 50 mL of boiling water for 2 h and filtered through a 0.45 µm filter. The 10 µL aliquot of the filtrate was subjected to separation by HPLC using a reversed-phase C18 symmetry column (4.6 mm × 250 mm, pore size 5 µm; Waters Corp., Milford, MA, USA). The mobile phase was prepared from a 0.02% aqueous phosphoric acid water solution (phase A) and methanol (phase B). Isocratic elution was performed with 70% A-30% B. The flow rate was 1.0 mL/min. The analysis time was 20 min. The eluted compounds were detected spectrophotometrically at 245 nm using a 2998 PDA photodiode array detector. The epigoitrin was purchased from the China National Institutes for Food and Drug Control.
2.6 DSE root colonization
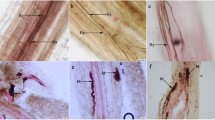
To assess whether the roots of I. indigotica were colonized by DSE, the method described by Phillips and Hayman (1970) was used to observe the fungal structures in the roots. Fresh root segments (0.5 cm) were cleared in 10% (w/v) potassium hydroxide (100 °C, 1 h) and then stained in 0.5% (w/v) acid fuchsin (90 °C, 20 min). Fifty root segments in each sample were randomly selected and then observed by microscopy at 20× and 40× magnification.
2.7 Soil physicochemical properties
Soil organic carbon (SOC) was estimated by the combustion method (Heiri et al. 2001). Soil pH was measured with pH 3000 meter (STEP Systems Gmbh, Germany). Soil ammonium nitrogen (SAN) was measured by the Smartchem 200 (Alliance, France) analyser (Xie et al. 2017). Soil available phosphorus (SAP) was determined by sodium bicarbonate extraction–molybdenum blue method (Olsen et al. 1954).
2.8 Statistical analysis
Two-way variance analysis (ANOVA) was performed to examine the effects of DSE inoculation, water conditions, and their interactions on the plant growth parameters, photosynthetic parameters, antioxidant parameters, and epigoitrin and soil nutrient contents. Tukey’s and T tests were used to compare the mean values (P < 0.05). SPSS 21.0 software was used for the above analyses. Redundancy analysis (RDA) was utilized to examine the correlation between physicochemical parameters and the growth parameters of I. indigotica using CANOCO 4.5.
3 Results
3.1 Identification of endophytic fungi
Based on morphology (Supplementary Fig. S1) and the comparison of fungal sequences in GenBank database and molecular phylogeny analysis (Supplementary Fig. S2), six DSE isolated from arid region were identified as Acrocalymma vagum (AV1), Paraphoma chlamydocopiosa (PC), Edenia gomezpomplae (EG), Darksidea alpha (DA), Brunneochlamydosporium nepalense (BN), Preussia terricola (PT). Meanwhile, one DSE isolated from farmland region were identified as Acrocalymma vagum (AV2). Thereinto, Acrochaetium vagum (AV1, AV2) is a common species isolated from arid and farmland areas.
3.2 Plant biomass production
After harvesting, DSE hyphae and microsclerotia were observed microscopically in all the tested root samples of I. indigotica plants under all treatments (Supplementary Fig. S3). The root biomass, shoot biomass, total biomass and root-shoot ratio of I. indigotica were significantly affected by DSE inoculation, water conditions, and their interaction (Table 1). The root biomass and root shoot ratio under drought condition were significantly higher than those under well-watered conditions (Fig. 1a, c). Under well-watered conditions, inoculation with AV1, DA, PT and AV2 enhanced the root biomass, shoot biomass, total biomass and root-shoot ratio; inoculation with EG promoted the shoot biomass and total biomass compared with those of the control plants (Fig. 1, Supplementary Fig. S4a). Under drought stress, inoculation with all DSE strains except BN resulted in an increase in the root biomass, shoot biomass and total biomass, whereas inoculation with AV1, PC, DA, PT and AV2 increased root-shoot ratio compared with those of the control plants (Fig. 1, Supplementary Fig. S4a).
The effects of DSE inoculation and water conditions on the root biomass (a), total biomass (b), and root-shoot radio(c) of I. indigotica. The error bars represent the standard error of the mean. Different letters above the error bars indicate a significant difference at P<0.05 by Tukey’s test. * means a significant difference between WW and DS. *P<0.05, **P<0.01 and ***P<0.001. WW, well-watered conditions; DS, drought stress conditions. C indicates non-inoculated control. AV1, PC, EG, DA, BN, PT, AV2 indicate plants inoculated with Acrocalymma vagum, Paraphoma chlamydocopiosa, Edenia gomezpompae, Darksidea alpha, Brunneochlamydosporium nepalense, Preussia terricola, Acrochaetium vagum
3.3 Photosynthetic parameters in leaves
DSE inoculation, water conditions and the interaction between DSE inoculation and water conditions had significant effects on SPAD and Pn values (Table 1). The SPAD value is greater under drought stress than under well-watered conditions. The Pn of control plants was lower under drought stress than under well-watered condition, while the Pn of plants inoculated with AV1, EG, BN and AV2 was higher than that under well-watered condition (Fig. 2). Under well-watered conditions, inoculation with AV1, PC, EG, DA, PT and AV2 increased the SPAD value, whereas inoculation with PC, DA, PT and AV2 increased the Pn value compared with those of the control plants (Fig. 2). Under drought stress, inoculation with DSE expect EG and BN had significant increases in SPAD values, and inoculation with all the tested DSE caused significant increases in Pn compared with those of the control plants (Fig. 2).
The effects of DSE inoculation and water conditions on the SPAD values (a) and net photosynthetic rate (b) of I. indigotica. The error bars represent the standard error of the mean. Different letters above the error bars indicate a significant difference at P<0.05 by Tukey’s test. * means a significant difference between WW and DS. *P<0.05, **P<0.01 and ***P<0.001. WW, well-watered conditions; DS, drought stress conditions. C indicates non-inoculated control. AV1, PC, EG, DA, BN, PT, AV2 indicate plants inoculated with Acrocalymma vagum, Paraphoma chlamydocopiosa, Edenia gomezpompae, Darksidea alpha, Brunneochlamydosporium nepalense, Preussia terricola, Acrochaetium vagum
3.4 Root morphological traits
The interaction between DSE inoculation and water conditions were significant for the root length, root diameter, root surface area and root volume of I. indigotica (Table 1). Except for AV2, the root length is greater under drought treatment than under well-watered treatment. Root diameter of plants inoculated with AV1, EG and AV2 under drought treatment was increased compared with that of the plants inoculated with well-watered conditions. Except for DA and AV2, the root surface area is larger under drought treatment than under well-watered treatment (Fig. 3). Under well-watered conditions, inoculation with DA and AV2 promoted the root length, surface area and volume (Fig. 3a, c, Supplementary Fig. S4b). PT inoculation induced increases in root length and surface area (Fig. 3a, c). Inoculation with EG only led to greater root length compared with those of the control plants (Fig. 3a). Under drought stress, inoculation with AV1, EG and AV2 resulted in higher root length than those observed in the control plants (Fig. 3a). Inoculation with AV1, EG, DA and AV2 promoted the root surface area (Fig. 3c).
The effects of DSE inoculation and water conditions on the root length (a), root diameter(b) and root surface area(c) of I. indigotica. The error bars represent the standard error of the mean. Different letters above the error bars indicate a significant difference at P<0.05 by Tukey’s test. * means a significant difference between WW and DS. *P<0.05, **P<0.01 and ***P<0.001. WW, well-watered conditions; DS, drought stress conditions. C indicates non-inoculated control. AV1, PC, EG, DA, BN, PT, AV2 indicate plants inoculated with Acrocalymma vagum, Paraphoma chlamydocopiosa, Edenia gomezpompae, Darksidea alpha, Brunneochlamydosporium nepalense, Preussia terricola, Acrochaetium vagum
3.5 Antioxidant enzyme activities in leaves
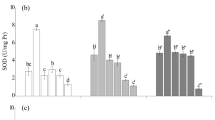
DSE inoculation, water conditions and their interaction had significant effects on SOD activity in I. indigotica (Table 1). Under drought stress, inoculation with AV1, PC and BN increased SOD activity, whereas inoculation with all DSE except BN decreased MDA content compared to that in the control plants (Fig. 4a, b).
The effects of DSE inoculation and water conditions on the SOD activity (a), MDA content (b), proline content (c) and epigoitrin content (d) of I. indigotica. The error bars represent the standard error of the mean. Different letters above the error bars indicate a significant difference at P<0.05 by Tukey’s test. * means a significant difference between WW and DS. *P<0.05, **P<0.01 and ***P<0.001. WW, well-watered conditions; DS, drought stress conditions. C indicates non-inoculated control. AV1, PC, EG, DA, BN, PT, AV2 indicate plants inoculated with Acrocalymma vagum, Paraphoma chlamydocopiosa, Edenia gomezpompae, Darksidea alpha, Brunneochlamydosporium nepalense, Preussia terricola, Acrochaetium vagum
3.6 Osmotic substances in leaves
DSE inoculation, water conditions and the interaction between DSE inoculation and water conditions significantly affected the proline content of I. indigotica (Table 1). And the proline content was increased under drought stress compared with that of plants under well-watered conditions (Fig. 4c). Under well-watered conditions, inoculation with AV1, EG, BN and AV2 increased the proline content compared with that in the control plants (Fig. 4c). Under drought stress, PC, EG, DA, PT and AV2 inoculation increased the proline content compared to those in the control plants (Fig. 4c).
3.7 IAA and active ingredient contents in the roots
The interactions between DSE inoculation and water conditions were significant for the IAA and epigoitrin contents in the roots of I. indigotica (Table 1). The epigoitrin content of control plants under drought stress was lower than that in the well-watered treatment, but the epigoitrin content of plants inoculated with PC, DA and BN in drought treatment was increased compared with that of plants under well-watered conditions (Fig. 4d). Under well-watered conditions, inoculation with AV1, PC, BN and AV2 increased the epigoitrin content compared with that in the control (Fig. 4d). Under drought stress, inoculation with PT increased the IAA content in the plants, and inoculation with all DSE resulted in higher epigoitrin content than that in control plants (Supplementary Fig. S4b, Fig. 4d). The epigoitrin content with AV2 inoculation was significantly lower than that with AV1 inoculation (Fig. 4d).
3.8 Soil physicochemical properties and correlation analyses
The interaction between DSE inoculation and water conditions had significant effects on soil pH and SOC, SAN, and SAP contents (Table S1). The pH value is greater under well-watered treatment than under drought treatment, indicating that drought decreased the soil pH. Under drought stress, inoculation with AV1, EG, DA, BN, PT and AV2 decreased the SOC content (Fig. S5b). Inoculation with AV1, PC, DA and BN resulted in an increase in the SAN content (Fig. S5c). Inoculation with all tested DSE except AV2 resulted in a lower SAP content than that observed in the control soil (Fig. S5d).
The RDA explained 99.1% of the variation in plant growth indicators. Axis 1 of the RDA explained 90.5% of the variation, whereas axis 2 further explained 8.6% of the variation. The pH value was negatively correlated with root biomass, proline and MDA content. The SOC content was negatively correlated with Pn. The SAP content was negatively correlated with root biomass and Pn (Fig. 5).
Redundancy analysis (RDA) of the growth indicators of I. indigotica and the explanatory variables when inoculated with seven DSE species. SOC=soil organic carbon; SAN=soil available nitrogen; SAP=soil available phosphorus; RB=root biomass; RD=root diameter; IAA=indole-3-acetic acid; SOD=superoxide dismutase; MDA=malondialdehyde; Pn=net photosynthetic rate
4 Discussion
This study reported the effects of DSE isolated from mycorrhizal plants on the growth of the non-mycorrhizal plant I. indigotica under drought stress for the first time. Typical DSE hyphae and microsclerotia were observed in the roots of all the inoculated plants, which indicated that these DSE are effective colonizers of the roots of I. indigotica regardless of the water conditions. Existing studies have obtained variable results regarding the effect of DSE inoculation on plant growth under drought stress (Santos et al. 2017; Zhang et al. 2017). Here, the responses of I. indigotica to DSE inoculation ranged from negative to positive. Specifically, inoculation with AV1, EG, DA, PT and AV2 had positive effects on the root biomass, shoot biomass and total biomass under both water conditions. This implies that the response of non-mycorrhizal plants to DSE colonization is dependent on the identity of the DSE strain. In addition, I. indigotica inoculated with AV1 from a desert site achieved greater total biomass than that inoculated with AV2 from a farmland site under drought stress. The reason for this phenomenon may be that surviving in a desert site increases DSE drought tolerance. These results further clarify that the species and source of DSE may affect their interactions with non-mycorrhizal plants (Li et al. 2019b).
The root system is the primary organ that senses variations in soil moisture (Verma et al. 2019). Previous research has shown that DSE can regulate plant root structure and improve plant fitness to drought stress (Han et al. 2020; Li et al. 2018; Li et al. 2019b; Liu and Wei 2019). Meanwhile, AV1, EG, DA and AV2 inoculation also increased the root surface area under drought stress. Deep, massive root systems can be used to absorb water and nutrients, which ultimately influences plant growth (Alvarez-Flores et al. 2014; Hund et al. 2008). Therefore, the changes in root morphology and structure induced by DSE may benefit plant drought adaptation (Awad et al. 2018; González-Teuber et al. 2017). Existing studies suggested that endophytic fungi can produce phytohormones to benefit the host plant in combatting the adverse effects of abiotic stresses (Qiang et al. 2019). IAA is a major endogenous auxin in plants that is essential for root growth and development (Xu et al. 2018). Several DSE species were found to produce IAA and to thereby promote plant growth and stress tolerance (Priyadharsini and Muthukumar 2017; Qiang et al. 2019). Our results showed that DSE can participate in the adaptation of the non-mycorrhizal plant I. indigotica to drought by regulating its IAA metabolism (Han et al. 2020; Wu et al. 2020).
Previous research has found that drought stress directly affects the photosynthesis process by damaging the photosynthetic organs of plants, hindering chlorophyll synthesis, and ultimately reducing the Pn (Pinheiro and Chaves 2011; Xia et al. 2018). Inoculation with these DSE increased the SPAD values and Pn under drought stress. He et al. (2019b) found that the DSE inoculation enhanced the chlorophyll content of G. uralensis under drought stress compared with that of control plants. Our results support the conclusion that DSE inoculation can improve photosynthesis in non-mycorrhizal plants and further confirmed the role of DSE in improving photosynthesis in plants (Ahmadvand and Hajinia 2018; Hosseini et al. 2017). In this study, inoculation with all the tested DSE promoted the epigoitrin content of I. indigotica. These findings are similar to those of previous studies showing that inoculation with DSE promoted plant active ingredient content (He et al. 2019a; He et al. 2019b; Zhu et al. 2015; Zubek et al. 2012). Consequently, DSE can act as promoters to influence the growth and medicinal quality of non-mycorrhizal medicinal plants, especially those in drought ecosystems (He et al. 2019a; He et al. 2019b).
Proline is a stress-related amino acid that functions as an osmoregulator and a reactive oxygen species (ROS) scavenger; higher levels of proline accumulate in plants in response to drought stress (Hayat et al. 2012; Pal et al. 2018). Here, inoculation with PC, EG, DA, PT and AV2 increased the proline content of I. indigotica compared to that of control plants, suggesting that the inoculated plants had a higher osmotic adjustment capacity under drought stress. Valli and Muthukumar (2018) found that DSE inoculation could enhance proline accumulation in tomato shoots under water-limited conditions. These results indicate that DSE inoculation can regulate proline accumulation to facilitate osmotic regulation, leading to enhanced tolerance to dehydration under water deficits (Hosseini et al. 2017; Zang et al. 2015). MDA is generally considered to be the product of cell membrane lipid peroxidation (Liu et al. 2017), and inoculation with all DSE in this study except BN decreased the MDA content under drought stress. This result indicates that DSE inoculation provides I. indigotica with a mechanism for avoiding oxidative damage under drought conditions (Huang et al. 2017). SOD serves as an effective ROS scavenger to avoid the oxidative damage caused by drought stress (Wu et al. 2006), and inoculation with AV1 and PC enhanced the SOD activity of I. indigotica in this study. This result indicates that in the DSE-I. indigotica association, SOD might play an active role in the detoxification of ROS stress (Baltruschat et al. 2008).
Related studies have shown that plant symbiotic fungi usually play a vital role in the exchange of nutrients between plants and soil and can change the physical and chemical properties of soils (Zuo et al. 2020). In this study, soil pH under well-watered treatment is higher than that drought treatment, whereases pH was negatively correlated with root biomass, proline content and MDA content. These results indicated that drought-induced changes included pH decrease (Palomo et al. 2013) and MDA content increase. Meanwhile, DSE inoculation increased proline content to reduce oxidative damage and increased root biomass to promote plant growth (Hosseini et al. 2017; Li et al. 2019b; Qiang et al. 2019). The SAN content of control treatment was higher under well-watered treatment than under drought treatment, while the SAN content after inoculation with AV, PC and DA was lower under well-watered treatment than under drought treatment. Meanwhile, inoculation with AV1, PC and DA increased the SAN content under drought stress, indicating that DSE could convert soil organic nitrogen into ammonia nitrogen, making nitrogen more freely available to roots (Hill et al. 2019; Upson et al. 2009; Vergara et al. 2017). The main explanation for these results may be that DSE produce a series of enzymes to mineralize soil organic nutrients into effective forms to increase the size of the plant-available nutrient pool and promote plant growth (He et al. 2019a; Surono 2017). The SOC content was negatively correlated with Pn. The SAP content was negatively correlated with root biomass and Pn. These results suggest that DSE inoculation increased Pn and root biomass, thus enlarged the contact area between plant roots and soil, allowing the plants to absorb more nutrients and leading to soil nutrient depletion (Chiu and Paszkowski 2019; Priyadharsini and Muthukumar 2017; Surono 2017). Therefore, DSE can contribute to I. indigotica growth under drought stress conditions by enhancing plant photosynthesis, upregulating antioxidant systems or stimulating the accumulation of osmotic substances, and altering soil physicochemical properties.
5 Conclusions
In this study, we found that seven DSE isolated from the mycorrhizal plant G. uralensis could effectively colonize the roots of the non-mycorrhizal plant I. indigotica and that the seven DSE displayed considerable functional differences in plant growth. The responses of plants to DSE ranged from negative to beneficial depending on the fungal species and the water conditions. Interestingly, under drought stress, the beneficial effects of the A. vagum strain isolated from a desert ecosystem were better than those of the A. vagum strain isolated from a farmland ecosystem. Our results supplement previous findings that endophytes can enhance drought resistance of plants and emphasize the importance of using DSE in medicinal plant cultivation under drought stress. As I. indigotica plays an important role in the cultivation of medicinal plants, the DSE–I. indigotica association should be further field-tested to determine its ability to suppress drought stress in dryland agriculture.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Agustinho, DP, Nosanchuk JD (2017) Functions of fungal melanins. Reference Module in Life Sciences Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-12-809633-8.12091-6
Ahmadvand G, Hajinia S (2018) Effect of endophytic fungus Piriformospora indica on yield and some physiological traits of millet (Panicum miliaceum) under water stress. Crop Pasture Sci 69:594–605. https://doi.org/10.1071/CP17364
Alvarez-Flores R, Winkel T, Anh NTT, Joffre R (2014) Root foraging capacity depends on root system architecture and ontogeny in seedlings of three Andean Chenopodium species. Plant Soil 380:415–428. https://doi.org/10.1007/s11104-014-2105-x
Anthony MA, Celenza JL, Armstrong A, Frey SD (2020) Indolic glucosinolate pathway provides resistance to mycorrhizal fungal colonization in a non-host Brassicaceae. Ecosphere 11
Awad W, Byrne PF, Reid SD, Comas LH, Haley SD (2018) Great plains winter wheat varies for root length and diameter under drought stress. Agron J 110:226–235. https://doi.org/10.2134/agronj2017.07.0377
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schafer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510. https://doi.org/10.1111/j.1469-8137.2008.02583.x
Barrow JR, Aaltonen RE (2001) Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. Roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza 11:199–205. https://doi.org/10.1007/s005720100111
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Berthelot C, Zegeye A, Gaber DA, Chalot M, Franken P, Kovacs GM, Leyval C, Blaudez D (2020) Unravelling the role of melanin in Cd and Zn tolerance and accumulation of three dark septate endophytic species. Microorganisms 8:537 https://doi.org/10.3390/microorganisms8040537
Chiu CH, Paszkowski U (2019) Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harb Perspect Biol 11:a034603. https://doi.org/10.1101/cshperspect.a034603
Cosme M, Fernandez I, Van der Heijden MGA, Pieterse CMJ (2018) Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci 23:577–587. https://doi.org/10.1016/j.tplants.2018.04.004
de Vries FT, Griffiths RI, Knight CG, Nicolitch O, Williams A (2020) Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 368:270–274. https://doi.org/10.1126/science.aaz5192
Delaux PM (2017) Comparative phylogenomics of symbiotic associations. New Phytol 213:89–94. https://doi.org/10.1111/nph.14161
Elavarthi S, Martin B (2010) Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol Biol 639:273–281. https://doi.org/10.1007/978-1-60761-702-0_16
Fernandez CW, Koide RT (2013) The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol 6:479–486. https://doi.org/10.1016/j.funeco.2013.08.004
González-Teuber M, Urzúa A, Plaza P, Bascuñán-Godoy L (2017) Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol 219:231–240. https://doi.org/10.1007/s11258-017-0791-1
Grümberg BC, Urcelay C, Shroeder MA, Vargas-Gil S, Luna CM (2014) The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean. Biol Fertil Soils 51:1–10. https://doi.org/10.1007/s00374-014-0942-7
Han L, Zhou X, Zhao YT, Zhu SS, Wu LX, He YL, Ping XR, Lu XQ, Huang WY, Qian J, Zhang LN, Jiang X, Zhu D, Luo CY, Li SJ, Dong Q, Fu QJ, Deng KY, Wang X et al (2020) Colonization of endophyte Acremonium sp. D212 in Panax notoginseng and rice mediated by auxin and jasmonic acid. J Integr Plant Biol 62:1433–1451. https://doi.org/10.1111/jipb.12905
Han L, Zuo YL, He XL, Hou YT, Li M, Li BK (2021) Plant identity and soil variables shift the colonisation and species composition of dark septate endophytes associated with medicinal plants in a northern farmland in China. Appl Soil Ecol 167:104042. https://doi.org/10.1016/j.apsoil.2021.104042
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
He C, Wang WQ, Hou JL (2019a) Characterization of dark septate endophytic fungi and improve the performance of liquorice under organic residue treatment. Front Microbiol 10:1364 https://doi.org/10.3389/fmicb.2019.01364
He C, Wang WQ, Hou JL (2019b) Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front Microbiol 10:2277. https://doi.org/10.3389/fmicb.2019.02277
He C, Cui J, Chen XY, Wang WQ, Hou JL (2020) Effects of enhancement of liquorice plants with dark septate endophytes on the root growth, glycyrrhizic acid and glycyrrhizin accumulation amended with organic residues. Curr Plant Biol 23:100154. https://doi.org/10.1016/j.cpb.2020.10015
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments, reproducibility and comparability of results. J Paleolimnol 25:101–110. https://doi.org/10.1023/A:1008119611481
Hill PW, Broughton R, Bougoure J, Havelange W, Newsham KK, Grant H, Murphy DV, Clode P, Ramayah S, Marsden KA, Quilliam RS, Roberts P, Brown C, Read DJ, Deluca TH, Bardgett RD, Hopkins DW, Jones DL (2019) Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecol Lett 22:2111–2119. https://doi.org/10.1111/ele.13399
Hiruma K, Kobae Y, Toju H (2018) Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: evolutionary origins and host-symbiont molecular mechanisms. Curr Opin Plant Biol 44:145–154. https://doi.org/10.1016/j.pbi.2018.04.009
Hosseini F, Mosaddeghi MR, Dexter AR (2017) Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Bioch 118:107–120. https://doi.org/10.1016/j.plaphy.2017.06.005
Hou LF, Yu J, Zhao LL, He XL (2020) Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front Microbiol 10:3061. https://doi.org/10.3389/fmicb.2019.03061
Huang XF, Zhou DM, Lapsansky ER, Reardon KF, Guo JH, Andales MJ, Vivanco JM, Manter DK (2017) Mitsuaria sp. and Burkholderia sp. from Arabidopsis rhizosphere enhance drought tolerance in Arabidopsis thaliana and maize (Zea mays L.). Plant Soil 419:523–539. https://doi.org/10.1007/s11104-017-3360-4
Hund A, Ruta N, Liedgens M (2008) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant Soil 318:311–325. https://doi.org/10.1007/s11104-008-9843-6
Li X, He XL, Hou LF, Ren Y, Wang SJ, Su F (2018) Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep 8:7896. https://doi.org/10.1038/s41598-018-26183-0
Li X, He XL, Zhou Y, Hou YT, Zuo YL (2019a) Effects of dark septate endophytes on the performance of Hedysarum scoparium under water deficit stress. Front Plant Sci 10:903. https://doi.org/10.3389/fpls.2019.00903
Li X, He C, He XL, Su F, Hou LF, Ren Y, Hou YT (2019b) Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 439:259–272. https://doi.org/10.1007/s11104-019-04057-2
Liu Y, Wei XL (2019) Dark septate endophyte improves drought tolerance of Ormosia hosiei Hemsley & E. H. Wilson by modulating root morphology, ultrastructure, and the ratio of root hormones. Forests 10. 10.3390/f10100830
Liu XM, Xu QL, Li QQ, Zhang H, Xiao JX (2017) Physiological responses of the two blueberry cultivars to inoculation with an arbuscular mycorrhizal fungus under low-temperature stress. J Plant Nutr 40:2562–2570. https://doi.org/10.1080/01904167.2017.1380823
Luo Z, Liu LF, Wang XH, Li W, Jie C, Chen H, Wei FQ, Lu DH, Yan CY, Liu B, Kurihara H, Li YF, He RR (2019) Epigoitrin, an alkaloid from Isatis indigotica, reduces H1N1 infection in stress-induced susceptible model in vivo and in vitro. Front Pharmacol 10:78. https://doi.org/10.3389/fphar.2019.00078
Mandyam K, Jumpponen A (2005) Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
Mandyam KG, Roe J, Jumpponen A (2013) Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol 117:250–260. https://doi.org/10.1016/j.funbio.2013.02.001
National Pharmacopoeia Commission (2015) Chinese pharmacopoeia. China Medical Science and Technology Press, Beijing
Nelson JM, Hauser DA, Hinson R, Shaw AJ (2018) A novel experimental system using the liverwort Marchantia polymorpha and its fungal endophytes reveals diverse and context-dependent effects. New Phytol 218:1217–1232. https://doi.org/10.1111/nph.15012
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793. https://doi.org/10.1111/j.1469-8137.2010.03611.x
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA circular no. 939, US Government Printing Office, Washington DC
Osakabe Y, Osakabe K, Shinozaki K, Tran LS (2014) Response of plants to water stress. Front Plant Sci 5:86 10.3389/fpls.2014.00086
Pal M, Tajti J, Szalai G, Peeva V, Vegh B, Janda T (2018) Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci Rep 8:12839. https://doi.org/10.1038/s41598-018-31297-6
Palomo L, Meile C, Joye SB (2013) Drought impacts on biogeochemistry and microbial processes in salt marsh sediments: a flow-through reactor approach. Biogeochemistry 112:389–407. https://doi.org/10.1007/s10533-012-9734-z
Peever TL, Higgins VJ (1989) Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol 90:867–875. https://doi.org/10.1104/pp.90.3.867
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T Br Mycol Soc 55:158–163. https://doi.org/10.1016/s0007-1536(70)80110-3
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882. https://doi.org/10.1093/jxb/erq340
Potisek M, Likar M, Vogel-Mikus K, Arcon I, Grdadolnik J, Regvar M (2021) 1,8-dihydroxy naphthalene (DHN) - melanin confers tolerance to cadmium in isolates of melanised dark septate endophytes. Ecotoxicol Environ Saf 222:112493. https://doi.org/10.1016/j.ecoenv.2021.112493
Priyadharsini P, Muthukumar T (2017) The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol 27:69–77. https://doi.org/10.1016/j.funeco.2017.02.007
Qiang XJ, Ding JJ, Lin W, Li QZ, Xu CY, Zheng Q, Li YZ (2019) Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil 439:373–391. https://doi.org/10.1007/s11104-019-04028-7
Regvar M, Vogel K, Irgel N, Wraber T, Hildebrandt U, Wilde P, Bothe H (2003) Colonization of pennycresses (Thlaspi spp.) of the Brassicaceae by arbuscular mycorrhizal fungi. J Plant Physiol 160:615–626. https://doi.org/10.1078/0176-1617-00988
Santos SGD, Silva PRAD, Garcia AC, Zilli JÉ, Berbara RLL (2017) Dark septate endophyte decreases stress on rice plants. Braz J Microbiol 48:333–341. https://doi.org/10.1016/j.bjm.2016.09.018
Surono NK (2017) The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol 28:1–10. https://doi.org/10.1016/j.funeco.2017.04.001
Upson R, Read DJ, Newsham KK (2009) Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 20:1–11. https://doi.org/10.1007/s00572-009-0260-3
Valli PPS, Muthukumar T (2018) Dark septate root endophytic fungus Nectria haematococca improves tomato growth under water limiting conditions. Indian J Microbiol 58:489–495. https://doi.org/10.1007/s12088-018-0749-6
Veiga RS, Faccio A, Genre A, Pieterse CM, Bonfante P, van der Heijden MG (2013) Arbuscular mycorrhizal fungi reduce growth and infect roots of the non-host plant Arabidopsis thaliana. Plant Cell Environ 36:1926–1937. https://doi.org/10.1111/pce.12102
Vergara C, Araujo KEC, Urquiaga S, Schultz N, Balieiro FC, Medeiros PS, Santos LA, Xavier GR, Zilli JE (2017) Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front Microbiol 8:2437. https://doi.org/10.3389/fmicb.2017.02437
Verma H, Borah JL, Sarma RN (2019) Variability assessment for root and drought tolerance traits and genetic diversity analysis of rice germplasm using SSR markers. Sci Rep 9:16513. https://doi.org/10.1038/s41598-019-52884-1
Wang WL, Xu JF, Fang HY, Li ZJ, Li MH (2020a) Advances and challenges in medicinal plant breeding. Plant Sci 298:110573. https://doi.org/10.1016/j.plantsci.2020.110573
Wang X, Liu XQ, Ko YZ, Jin XL, Sun JH, Zhao ZY, Yuan QJ, Chiang YC, Huang LQ (2020b) Genetic diversity and phylogeography of the important medical herb, cultivated Huang-Lian populations, and the wild relatives coptis species in China. Front Genet 11:708. https://doi.org/10.3389/fgene.2020.00708
Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110. https://doi.org/10.1016/j.jplph.2005.09.001
Wu FL, Li Y, Tian W, Sun YD, Chen FY, Zhang YR, Zhai YX, Zhang J, Su HY, Wang L (2020) A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol 40:1080–1094. https://doi.org/10.1093/treephys/tpaa047
Xia C, Christensen MJ, Zhang XX, Nan ZB (2018) Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 424:555–571. https://doi.org/10.1007/s11104-018-3561-5
Xie LL, He XL, Wang K, Hou LF, Sun Q (2017) Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in Northwest China and the influence of edaphic variables. Fungal Ecol 26:135–143. https://doi.org/10.1016/j.funeco.2017.01.007
Xu L, Wu C, Oelmuller R, Zhang WY (2018) Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front Microbiol 9:1646 10.3389/fmicb.2018.01646
Xue ZK, He XL, Zuo YL (2019) Community composition and catabolic functional diversity of soil microbes affected by Hedysarum scoparium in arid desert regions of Northwest China. Arid Land Res Manag 34:152–170. https://doi.org/10.1080/15324982.2019.1637968
Yuan ZL, Druzhinina IS, Gibbons JG, Zhong ZH, Van de Peer Y, Rodriguez RJ, Liu ZJ, Wang XY, Wei HS, Wu Q, Wang JY, Shi GH, Cai F, Peng L, Martin FM (2021) Divergence of a genomic island leads to the evolution of melanization in a halophyte root fungus. ISME J. https://doi.org/10.1038/s41396-021-01023-8
Zang DD, Wang C, Ji XY, Wang YC (2015) Tamarix hispida zinc finger protein ThZFP1 participates in salt and osmotic stress tolerance by increasing proline content and SOD and POD activities. Plant Sci 235:111–121. https://doi.org/10.1016/j.plantsci.2015.02.016
Zhan FD, He YM, Zu YQ, Li T, Zhao ZW (2011) Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J Microbiol Biotechnol 27:2483–2489. https://doi.org/10.1007/s11274-011-0712-8
Zhang HH, Tang M, Chen H, Wang YJ (2012) Effects of a dark-septate endophytic isolate LBF-2 on the medicinal plant Lycium barbarum L. J Clin Microbiol 50:91–96. https://doi.org/10.1007/s12275-012-1159-9
Zhang QM, Gong MG, Yuan JF, Hou Y, Zhang HM, Wang Y, Hou X (2017) Dark septate endophyte improves drought tolerance in Sorghum. Int J Agric Biol 19: 53–60. 10.17957/ijab/15.0241
Zhang DD, Shi YH, Li JY, Ruan DQ, Jia Q, Zhu WL, Chen KX, Li YM, Wang R (2019) Alkaloids with nitric oxide inhibitory activities from the roots of Isatis tinctoria. Molecules 24. https://doi.org/10.3390/molecules24224033
Zhao GH, Li T, Qu XY, Zhang NY, Lu M, Wang J (2017) Optimization of ultrasound-assisted extraction of indigo and indirubin from Isatis indigotica Fort. and their antioxidant capacities. Food Sci Biotechnol 26:1313–1323. https://doi.org/10.1007/s10068-017-0112-4
Zhu ZB, Fan JY, Guo QS, Liu ZY, Zhu GS (2015) The growth and medicinal quality of Epimedium wushanense are improved by an isolate of dark septate fungus. Pharm Biol 53:1344–1351 https://doi.org/10.3109/13880209.2014.982296
Zubek S, Blaszkowski J, Buchwald W (2012) Fungal root endophyte associations of medicinal plants. Nova Hedwigia 94:525–540. https://doi.org/10.1127/0029-5035/2012/0024
Zuo YL, He C, He XL, Li X, Xue ZK, Li XM, Wang SJ (2020) Plant cover of Ammopiptanthus mongolicus and soil factors shape soil microbial community and catabolic functional diversity in the arid desert in Northwest China. Appl Soil Ecol 147. https://doi.org/10.1016/j.apsoil.2019.10338
Funding
This work was financially supported by the National Natural Science Foundation of China (31770561) and the Postgraduate’s Innovation Fund Project of Hebei Province (CXZZBS2020021).
Author information
Authors and Affiliations
Contributions
Min Li and Xueli He conceived and designed the experiments. Min Li, Jiaqiang Liu, Jingya Yang and Yiling Zuo performed the experiments. Min Li, Lifeng Hou and Lili Zhao analysed the data. Min Li and Xueli He wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest/Competing interests
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 11557 kb)
Rights and permissions
About this article
Cite this article
Li, M., Hou, L., Liu, J. et al. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant Isatis indigotica under different water conditions. Symbiosis 85, 291–303 (2021). https://doi.org/10.1007/s13199-021-00813-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00813-0