Abstract
Bemisia tabaci is a complex of cryptic species of whitefly distributed worldwide; they are serious agricultural pests and vectors of plant viruses. Whiteflies are commonly infected by endosymbiotic bacteria, but the infection profiles among genetic groups of B. tabaci are highly complex. Here we analyzed the genetic variation of B. tabaci and endosymbiont infection patterns in Java, Indonesia. Specifically, adult B. tabaci were collected from four provinces and 43 partial cytochrome oxidase subunit I gene sequences were determined to identify the genotypes. Results showed that B. tabaci was grouped into three different cryptic species, Asia I, Asia II 5, and Asia II 7, at rates of 90.70%, 6.98%, and 2.32%, respectively. The dominant group, Asia I, was distributed throughout the island, whereas Asia II 5 and Asia II 7 were detected only in West Java. In these cryptic species, the infection rates of the secondary endosymbionts Arsenophonus, Cardinium, Hamiltonella, Rickettsia, and Wolbachia were 37.21%, 72.09%, 37.21%, 88.37%, and 90.70%, respectively. Arsenophonus and Cardinium were detected two subgroups (A1 and A2; C2 and C4), but Hamiltonella, Rickettsia, and Wolbachia were detected a one subgroup (H1, R1, and W1). The A1 and A2 subgroups were distributed in a mixed manner across the entire island; however, the C2 and C4 subgroups were distributed differentially in West Java and in Central and East Java, respectively. Multiple infections were common and their patterns were highly variable in each cryptic species. In particular, Hamiltonella was detected in Asia I and Asia II 5 but never coinfected with Arsenophonus in the same individual. Overall, this study shows that Asia I is the dominant genetic B. tabaci group on Java Island and that infection by endosymbionts occurs in a highly complex and sometimes geographically related manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The sweet potato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a phloem-feeding insect that causes damage to crops through direct and indirect feeding. The species is globally distributed in tropical, subtropical, and temperate regions (Brown et al. 1995; De Barro and Hart 2000). It is polyphagous, feeding on more than 100 plant species belonging to 89 families, in particular species belonging to Compositae, Cruciferae, Cucurbitaceae, Solanaceae, and Leguminosae families (Perring 2001; Berry et al. 2004; Li et al. 2011). Moreover, B. tabaci indirectly devastates crop plants by transmitting plant viruses such as Begomovirus, Crinivirus, Closterovirus, Ipomovirus, and Carlavirus (Jones 2003).
Bemisia tabaci is listed as one of the world’s 100 worst invasive alien species due to its unique ability to travel to and establish, evolve, and dominate in new locations (Lowe et al. 2000). Bemisia tabaci was first reported in Indonesia in 1938 as the vector of leaf curl disease in tobacco plants; the disease is caused by Begomovirus, which was transmitted in this case from the weed species Ageratum spp., Synedrella spp., and Eupatorium odoratum found in Sumatra and Java (Hidayat et al. 2017). Thereafter, B. tabaci spread throughout the country, particularly from 1994 to 1999 (De Barro et al. 2008). The spread of the species was associated with the invasion of Begomovirus and incidence of Pepper yellow leaf curl Indonesia virus (PepYLCIV). As a vector, B. tabaci spread PepYLCIV from Central Thailand to the western part of Sumatra and subsequently to the southern parts of Java and Bali.
Bemisia tabaci shows high genetic diversity in its various globally distributed genetic groups and it is now considered a cryptic species complex. De Barro et al. (2011) suggested that the nucleotide sequence of the mitochondrial cytochrome oxidase subunit I gene (COI) could be used as a barcode to classify B. tabaci populations. Based on a 3.5% pairwise divergence in COI sequences within B. tabaci species, 44 distinct species have been reported as follows: Africa, Asia I, Asia I-India, Asia II 1–12, Asia III, Asia IV, Asia V, Australia, Australia/Indonesia, China 1–5, Indian Ocean, Ru, Middle East Asia Minor (MEAM) I-II, Mediterranean (MED), MEAM K, New World (NW) 1–2, Japan 1–2, Uganda, Italy 1, and Sub-Saharan Africa 1–5. Two new species, Asia II 13 and Spain 1, were also recently reported (Kanakala and Ghanim 2019).
Several studies have been conducted in Indonesia to identify the genetic characteristics of B. tabaci (Hidayat et al. 2008; Dinsdale et al. 2010; Firdaus et al. 2013; Srinivasan et al. 2013; Rahayuwati et al. 2016; Shadmany et al. 2019). As an example, Hidayat et al. (2008) investigated the biotype of B. tabaci collected between 2004 and 2005 using the random amplified polymorphism DNA PCR method and reported that B. tabaci genetic types belonged to B (MEAM1) and non-B biotypes. In further studies, COI sequence analysis revealed some different indigenous genotypes in Indonesia, such as Asia I, Asia II 6, Asia II 7, Asia II 12, and Australia/Indonesia. Asia I is the dominant B. tabaci genotype found across Indonesia (Srinivasan et al. 2013; Rahayuwati et al. 2016), whereas Asia II 6 (KJ716440) has been detected in Cirebon in West Java (Shadmany et al. 2019), Asia II 7 in the western region of Kalimantan, and Asia II 12 in the western region of Java (Firdaus et al. 2013). The Australia/Indonesia genetic group has been identified in Indonesia (Dinsdale et al. 2010). Because of climate change and increased global transportation of agricultural products, it is important to evaluate the ongoing change in the genetic diversity of B. tabaci on a regular basis.
Endosymbiotic bacteria are common in several plant-sapping insects such as whiteflies (including B. tabaci), aphids, and hoppers; they establish mutual relationships with the insects that improve their survival (Baumann 2005). Consequently, endosymbionts influence the growth and development of their hosts, as well as their physiological and ecological adaptation to their environment (Baumann 2005; Rosell et al. 2010). In general, endosymbionts can be primary or secondary (Baumann 2005): primary endosymbionts cause permanent infection and provide essential nutrients (which are lacking from plant juices) to their hosts; secondary endosymbionts have a facultative relationship with their hosts, providing fitness benefits in reproduction, host plant specialization, and tolerance to pathogenic microbial infection and/or challenging environmental conditions (Montllor et al. 2002; Oliver et al. 2003; Tsuchida et al. 2004; Sintupachee et al. 2006; Chiel et al. 2009; Kaiser et al. 2010; Feldhaar 2011).
Bemisia tabaci possesses the primary endosymbiont Portiera aleyrodidarum along with at least seven secondary endosymbionts, i.e., Arsenophonus, Cardinium, Fritschea, Hamiltonella, Hemipteriphilus, Rickettsia, and Wolbachia (Gottlieb et al. 2006; Bing et al. 2013a, 2013b; Zchori-Fein et al. 2014). Secondary endosymbionts are known to affect various biological characteristics of B. tabaci, including reproduction (Zchori-Fein et al. 2001; Hunter et al. 2003; Zchori-Fein and Perlman 2004; Himler et al. 2011), survival (Liu et al. 2007; Kontsedalov et al. 2008; Gottlieb et al. 2010; Thierry et al. 2011), insecticide resistance (Kontsedalov et al. 2008), and capacity for disease transmission to plants (Gottlieb et al. 2010). In particular, secondary endosymbionts, such as Hamiltonella, play important roles in the transmission of plant viruses, including the Tomato yellow leaf curl virus that causes severe disease in tomato plants (Czosnek and Ghanim 2011). Therefore, endosymbionts essentially modulate the development of their host and produce environmental effects (Thao and Baumann 2004).
The infection patterns of secondary endosymbionts within B. tabaci are highly complex, but some specific relationships occur among different genetic groups (Gueguen et al. 2010; Kanakala and Ghanim 2019; Wang et al. 2019). Moreover, these infection patterns are highly variable according to geographic regions (Gueguen et al. 2010; Zchori-Fein et al. 2014). For instance, the infection profiles of both MEAM1 and MED, which are globally invasive cryptic species, vary greatly across geographic regions (Chiel et al. 2007; Gueguen et al. 2010; Skaljac et al. 2010, 2013; Chu et al. 2011; Park et al. 2012; Bing et al. 2013a, 2013b). The dynamic diversity of endosymbiont infection patterns can be influenced by vertical, horizontal, or maternal transmission (Baumann 2005; Pan et al. 2012). Although the infection patterns of MEAM1 and MED have been investigated in several countries, data on the endosymbiont profiles of indigenous cryptic species in Asian countries are limited.
Therefore, in the present study, the genetic groups of B. tabaci were identified by sequencing the mitochondrial COI gene from individuals collected in four provinces of Java, Indonesia. Moreover, data on the endosymbionts of these individuals were collected by sequencing 16S/23S rDNA genes and analyzing the infection profiles of the endosymbionts in each genetic group of B. tabaci.
2 Materials and methods
2.1 Collection of whiteflies
Whiteflies were collected from ten regencies in four different provinces (West Java, Central Java, East Java, and Special Region of Yogyakarta) of Java, Indonesia (Fig. 1). Adult whiteflies were collected from various crop plants (chili pepper, cucumber, eggplant, luffa, melon, tomato, and yard long bean) and ornamental plants (angel’s trumpet and chrysanthemum) using an aspirator. They were subsequently preserved in 70% ethanol in a 1.5-ml microtube and separated based on crop plants and locations. Preserved samples were stored at −20 °C until further analysis.
The collection sites and distribution of three cryptic species of Bemisia tabaci (Asia I, Asia II 5, and Asia II 7) and their secondary endosymbionts in Java, Indonesia. The gray areas show collection sites. The colored symbols represent the cryptic species and secondary endosymbionts (as per the key)
2.2 DNA extraction
Total genomic DNA was extracted from individual B. tabaci specimens using a PureLink Genomic DNA Mini Kit (Invitrogen; supplied by Thermo Fisher Scientific, USA). Individual whitefly samples were extracted by homogenizing insects in 180 μl of genomic digestion buffer and 20 μl of proteinase K (50 μg/mL). DNA samples were extracted and purified using a genomic spin column as described in the kit instructions. The total DNA samples were then stored at −20 °C or directly used in the PCR assay.
2.3 PCR amplification and sequencing
A fragment of the COI gene was initially amplified based on the universal primer pair LCO-1490/HCO-2198; the same DNA was then amplified using the specific primer pair C1-J-2195/L2-N-3014. Secondary endosymbiont sequences were amplified using 16S rDNA or 23S rDNA primers. PCR was performed on a 30-μl mixture containing 2 μl of each primer (10 pmol/μl), 2 μl of DNA template (40 ng), 9 μl of distilled H2O, and 15 μl of PCR premix (Solgent Co., Daejeon, Korea). The reaction mixtures were amplified under particular annealing temperature conditions (Table 1) in a Veriti 96-Well Thermal Cycler (Applied Biosystems, Foster City, CA). PCR products were purified from the gel using the Wizard® PCR Preps DNA Purification System (Wizard® SV Gel, Promega Co., Madison, WI, USA). They were then sequenced either directly or by cloning into the T-Blunt Easy Plasmid Vector (Promega Co., Madison, WI, USA), by the Solgent Sequencing Facility (Solgent Co., Daejeon, Korea).
2.4 Phylogenetic analysis
All sequences of whiteflies and secondary endosymbionts were aligned using ClustalW, implemented in BioEdit v7.2.5, before being analyzed using Mega-X (Kimura 1980; Kumar et al. 2018). A phylogenetic tree was constructed using the maximum likelihood method with Kimura 2-parameter model. The phylogenies were tested using 1000 bootstraps replications (Felsenstein 1985). The whitefly sequences were assigned to species based on >3.5% pairwise sequence divergence (Dinsdale et al. 2010). All whitefly and secondary endosymbiont sequences have been submitted to the GenBank database (see results for details).
2.5 Genetic analysis
The genetic structure of the B. tabaci COI sequences was analyzed using DnaSP v5.10, by which the sequence polymorphism, singleton variable sites, parsimony informative sites, number of haplotypes, and haplotype and nucleotide diversity were characterized (Librado and Rozas 2009). The haplotype network of B. tabaci sequences was analyzed using a minimum spanning network relationship among the cryptic species via popART software.
3 Results
3.1 Identification of B. tabaci cryptic species in Java, Indonesia
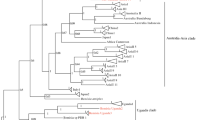
In total, 43 whiteflies from different populations were identified using COI universal and specific primer pairs (Table 1). Based on the maximum likelihood phylogram of previously known sequences in the GenBank database, the phylogenetic analysis of the determined COI sequences showed that B. tabaci was grouped into three different cryptic species, namely Asia I, Asia II 5, and Asia II 7 (Fig. 2) at rates of 90.70%, 6.98%, and 2.32%, respectively (Table 1). Asia I was distributed throughout the island; Asia II 5 was identified in Bogor and Bandung, West Java, and was newly detected in Indonesia; and one Asia II 7 sample was identified in Bogor, West Java. In terms of host plant, Asia I was collected from various crop plants, including chili (Capsicum sp.), cucumber (Cucumis sativus), cutleaf groundcherry (Physalis sp.), eggplant (Solanum melongena), luffa (Luffa acutangula), melon (Cucumis melo), tomato (Solanum lycopersicum), and yard long bean (Vigna unguiculata subsp. sesquipedalis), as well as from ornamental plants, including angel’s trumpet (Brugmansia sp.) and chrysanthemum (Chrysanthemum sp.). In contrast, Asia II 5 was collected from jicama/Mexican yam bean (Pachyrhizus erosus), common bean (Phaseolus vulgaris), and tomato (Solanum lycopersicum), whereas Asia II 7 was collected only from Capsicum sp. (Table 2).
Pairwise divergence in the COI nucleotide sequences among the three genetic groups varied at both intraspecific and interspecific levels. Intraspecific variation was higher in Asia I (0.12%–3.25%) than in Asia II 5 (0.12%–0.25%). Among the three groups, interspecific variation between Asia I and Asia II 5 (17.35%–20.33%) was higher than that between Asia I and Asia II 7 (15.18%–17.76%) (Table 3), while interspecific variation between Asia II 5 and Asia II 7 was 11.19%–11.33%. All B. tabaci sequences were submitted to NCBI GenBank (accession numbers: MN918040 to MN918082).
3.2 Genetic diversity and differentiation of B. tabaci cryptic species in Java, Indonesia
Table 4 shows the genetic diversity of haplotype sequence polymorphism and divergence, with Asia II 7 being excluded because only one sample was collected. The number of polymorphic sites was higher in Asia I (28 polymorphic sites, 27 singleton variable sites, and 1 parsimony informative site) than in Asia II 5 (2 polymorphic sites and 2 singleton variable sites). In total, 9 haplotypes were detected from 43 analyzed sequences: Asia I had five haplotypes, whereas Asia II 5 had three, and Asia II 7 had one. Haplotype diversity was higher in Asia II 5 (1) than in Asia I (0.24); however, nucleotide diversity was higher in Asia I (0.00182) than in Asia II 5 (0.00163).
The relationship among the haplotypes of each cryptic species was determined by minimum spanning network analysis (Fig. 3). The three cryptic species were diversified into nine haplotypes that were highly distant from each other. Among the five haplotypes of Asia I, the H6 haplotype occupied the center of network position. The single Asia II 7 haplotype was found between Asia I and Asia II 5.
3.3 Infection of B. tabaci secondary endosymbionts and phylogenetic analysis
The infection profiles of five secondary endosymbionts showed significant variation in each cryptic whitefly species (Table 5). Overall, Wolbachia had the highest infection rate (90.70%), followed by Rickettsia (88.37%), Cardinium (72.09%), Arsenophonus (37.21%), and Hamiltonella (37.21%). Arsenophonus infected Asia I but not Asia II 5 and Asia II 7 (Table 5, Fig. 4). Strikingly, Arsenophonus was detected in all samples collected from East (Malang) and Central (Pati) Java, only a few samples (9.3%) from Central Java (Yogyakarta and Magelang), but none of the samples from West Java (Fig. 1).
Recently, Kanakala and Ghanim (2019) summarized overall patterns of endosymbionts in B. tabaci at a subgroup level; two Arsenophonus (A1 and A2), four Cardinium (C1 – C4), one Hamiltonella (H1), three Rickettsia (R1, R2, and R3) and two Wolbachia (supergroups O and B). Based on Kanakala and Ghanim (2019), divergence and distribution of endosymbionts in Java Island were analyzed at the subgroup level. Our analysis of the phylogenetic tree revealed two subgroups of Arsenophonus, A1 and A2, in Java (Fig. 4). Interestingly, our four samples of East (Malang and Poncokusumo) and Special Region of Yogyakarta (Sleman), Java, were clustered into A2 subgroup but were separated from 11 subclades named as A2 (a-k) in the study of Kanakala and Ghanim (2019). Cardinium was detected in all three cryptic species and was distributed across all areas of Java. Two subgroups of Cardinium, C2 and C4, were detected. The C2 subgroup was found in all three cryptic species, whereas C4 was found only in Asia I (Table 5; Fig. 5). While most of the C2 subgroup were found in West Java, the C4 subgroup was primarily found in Central and East Java (Fig. 1). Only one subgroup of Hamiltonella (H1) and Rickettsia (R1) was detected in Asia I and Asia II 5, respectively (Table 5; Figs. 6 and 7). However, H1 and R1 had different geographic distributions: H1 was distributed in some areas of Central (Brebes) and West Java (Cianjur and Bandung) but not in East Java (Malang) or other areas of Central Java (Magelang and Pati); R1 was distributed in all areas of Java (Fig. 1). Wolbachia was detected in all three cryptic species of B. tabaci. All whiteflies were infected by a single Wolbachia subgroup, W1, which belonged to the supergroup B and was distributed in all areas of Java (Table 5; Fig. 8). All secondary endosymbiont sequences analyzed in this study were submitted to NCBI GenBank.
3.4 Relationships between B. tabaci cryptic species and multiple infections of secondary endosymbionts
All whiteflies, except one individual, (42/43 individuals) were coinfected by two, three, or four secondary endosymbionts in various combinations. Furthermore, multiple infections of three and four secondary endosymbionts were observed at rates of 46.15% and 46.15%, respectively, in Asia I (Table 6). All individuals were infected by at least one secondary endosymbiont but never infected by all five. The Asia II 5 whiteflies were infected by various secondary endosymbionts but never by Arsenophonus. The single individual from Asia II 7 was infected by Cardinium and Wolbachia (Table 6). The coinfection patterns of secondary endosymbionts varied substantially in this study: 17 patterns of coinfection were detected at the subgroup level of each secondary endosymbiont. The highest rate of coinfection was by C4 + H1 + R1 + W1 subgroups (20.51%) in Asia I (Table 6). The coinfection of Cardinium and Rickettsia was common, whereas coinfection with Arsenophonus and Hamiltonella was not detected in any individual (Table 6). Therefore, B. tabaci in Java were infected at high rates by various secondary endosymbionts in a complex combination of infection patterns.
4 Discussion
Previous studies have reported that the genetic diversity of B. tabaci in Indonesia is composed of Asia I, Asia II 6, Asia II 7, Asia II 12, Australia/Indonesia, and the invasive MEAM1 cryptic species. Among these, Asia I is widely distributed across all Indonesian islands (Hidayat et al. 2008; Dinsdale et al. 2010; Firdaus et al. 2013; Srinivasan et al. 2013; Rahayuwati et al. 2016). In contrast, Asia II 6 (KJ716440) is found in Cirebon in West Java (Shadmany et al. 2019), Asia II 7 in the west region of Kalimantan, and Asia II 12 in the west region of Java (Firdaus et al. 2013).
Based on our COI sequence comparison and phylogenetic analysis, we demonstrated that B. tabaci populations in Java consist of three genotypes, i.e., the Asia I, Asia II 5, and Asia II 7 cryptic species. We found that Asia I was distributed throughout Java, whereas Asia II 5 and Asia II 7 were found only in West Java. Moreover, we are the first to identify Asia II 5 in Java; it was collected from three agricultural crops, i.e., P. erosus in Ciampea, Bogor and P. vulgaris and S. lycopersicum in Lembang, Bandung in West Java. However, we did not detect Asia II 6, Asia II 12, Australia/Indonesia, or the invasive MEAM1 cryptic species in this study, despite them having been previously reported in the area (Hidayat et al. 2008; Dinsdale et al. 2010; Srinivasan et al. 2013).
Similar to previous reports, we found that Asia I was the dominant cryptic species in the study area. Indeed, this cryptic species is widely distributed in several Asian countries, including Pakistan, India, Bangladesh, Malaysia, Singapore, Indonesia, Cambodia, Thailand, Vietnam, China, Taiwan, and Japan (Hu et al. 2015; Götz and Winter 2016; Kanakala and Ghanim 2019). Thus, Asia I can be considered the dominant genetic group of B. tabaci in Asia. In the present study, Asia I was clustered with one dominant haplotype, which was found in all the collection sites, and four minor haplotypes, which were dispersed from it. The dominant haplotype found here was more similar to that in China than that in India (unpublished observation). Hu et al. (2015) reported a similar result, i.e., the same dominant haplotype is distributed in China and Indonesia but differentiated into several minor haplotypes.
The Asia II 5 cryptic species identified in this study is newly reported in Indonesia. Asia II 5 has previously been reported in Pakistan, India, Nepal, Bangladesh, Myanmar, and Nauru (Ellango et al. 2015; Khatun et al. 2018; Kanakala and Ghanim 2019; Acharya et al. 2020). Our finding adds to the evidence suggesting that Asia II 5 is widely distributed across the countries of South Asia. We specifically identified Asia II 5 in only three regions of West Java, suggesting that this cryptic species is not abundant on the island. Similar findings have been reported in other countries such as Bangladesh (Khatun et al. 2018); however, Asia II 5 is the major genetic group in Nepal (Acharya et al. 2020).
The Asia II 7 cryptic species has a wide distribution in several Asian countries (Kanakala and Ghanim 2019). To date, it has been identified in Pakistan (Ashfaq et al. 2014; Islam et al. 2018), India (Ellango et al. 2015), Malaysia (Shadmany et al. 2019), Indonesia (Firdaus et al. 2013), China (Qiu et al. 2011), and Taiwan (Hsieh et al. 2006). Asia II 7, also known as the Cv biotype, is a common genetic group of B. tabaci in South China, which prefers ornamental plants over vegetable plant, unlike MEAM1 (B biotype) (Dinsdale et al. 2010; Qiu et al. 2011). However, its distribution is restricted to small areas of other countries. In Indonesia, for example, Asia II 7 was previously reported only in the western region of Kalimantan, a finding similar to that reported in studies on the Malaysian part of the same island (Firdaus et al. 2013). In the present study, we newly identified Asia II 7 in one specific site, a pepper field at Ciampea in Bogor, which is located in the western region of Java. West Java is active in terms of exchange and transportation of agricultural crops and products (Subdirectorate of Horticulture Statistics 2019); therefore, the discovery of Asia II 5 and Asia II 7 in this region suggests that they may have invaded via agricultural products from other Asian countries.
Displacement of some cryptic species has occurred in several countries. For example, Chu et al. (2010) demonstrated the displacement of MEAM1 (B biotype) by MED (Q biotype) in China. In addition, Ashfaq et al. (2014) reported that both MEAM1 and Asia I had displaced Asia II 1, Asia II 5, and Asia II 7 and expanded their ranges in Punjab and Sindh in Pakistan. Götz and Winter (2016) also showed that MEAM1 invaded Vietnam from South China and replaced the indigenous species Asia II 1. Displacement of cryptic species might be influenced by factors such as variation in life history traits, mating behaviors, and insecticide resistance (Crowder et al. 2010), as well as by genetic variation in whiteflies (Wang et al. 2013) and species diversity and geographic distribution of host plants (Chu et al. 2012). Moreover, a suggested mechanism of biotype formation, i.e., that a mating interaction between two biotypes could produce more males and that the secondary endosymbiont Wolbachia could induce cytoplasmic incompatibility in B. tabaci, may also affect displacement (De Barro and Hart 2000). Another possibility explaining the displacement of B. tabaci complex species is the movement of the species among countries due to human activities such as international trade.
Infection of secondary endosymbionts is common in sap-sucking insects such as B. tabaci (Gueguen et al. 2010; Skaljac et al. 2010). The infection rate of secondary endosymbionts can be affected by various factors, such as biotype, sex, host plant, and geographical location (Pan et al. 2012). In the present study, all individuals of each cryptic species were infected by at least one secondary endosymbiont at various rates. For example, Asia I in Java was infected by all five secondary endosymbionts but at different rates (infection rate from highest to lowest: Wolbachia > Rickettsia > Cardinium > Arsenophonus > Hamiltonella). This infection profile differs from that reported in Bangladesh, where the highest infection rate was from Arsenophonus followed by Cardinium and Wolbachia (Khatun et al. 2019).
Interestingly, we detected Hamiltonella in the Asia I and Asia II 5 cryptic species of Java. Previous studies have shown that Hamiltonella can be found in MEAM1, MED, and NW2 cryptic species but has been absent from Asian cryptic species, including Asia I, Asia II 1, Asia II 3, Asia II 5, Asia II 6, Asia II 7, Asia II 10, and China 1 (Gueguen et al. 2010; Chu et al. 2011; Bing et al. 2013a; Fujiwara et al. 2015; Kanakala and Ghanim 2019; Khatun et al. 2019). According to our phylogenetic analysis, all the Hamiltonella species identified in the present study belonged to the H1 subgroup of Hamiltonella defensa, which infects MEAM1 and MED in China, Japan, and Korea. Our analysis also indicated that the 16S rDNA sequence of Hamiltonella was identical in all the tested individuals but differed by 0.16%–0.64% from that of MEAM1. We can only speculate as to how Asia I and Asia II 5 acquired Hamiltonella. It is possible that Hamiltonella in the Asian indigenous cryptic species has been displaced from MEAM1, which is known to have invaded Indonesia (Hidayat et al. 2008).
Phylogenetic analysis of secondary endosymbionts has revealed that each endosymbiont is genetically diverse and classified into several subgroups (Kanakala and Ghanim 2019). Consistently, we found that both Arsenophonus and Cardinium could be classified as two subgroups, A1 and A2 and C2 and C4, which had 0.74%–9.34% and 0.31%–11.63% 16S rDNA sequence variation, respectively. Of these subgroups, A1 and C4 infected Asia I at a rate three times that of A2 and C2. In contrast, the three other endosymbionts were detected in the B. tabaci of Java as single subgroup only: Hamiltonella H1, Rickettsia R1, and Wolbachia W1.
In our previous study, we suggested that a relationship existed between the genetic variation and geographic distribution of some B. tabaci-infecting endosymbionts (Khatun et al. 2019). For instance, two subgroups of Rickettsia, R1 and R2, were differentially distributed in Bangladesh: R1 was detected in the northern region, whereas R2 was detected in the southern region. In contrast, two subgroups of Arsenophonus (A1 and A2) were widely distributed in a mixed manner. Similarly, the present study revealed that Cardinium is found in all areas of Java, but that its two subgroups are differentially distributed; C2 is found in West Java while C4 is generally found in Central and East Java. However, the two subgroups of Arsenophonus (A1 and A2) are distributed in a mixed manner in East and Central Java only and are absent from West Java. Thus, similar to the cases of Rickettsia and Arsenophonus in Bangladesh, we found that two subgroups of Cardinium were distributed differentially, whereas two subgroups of Arsenophonus were distributed in a mixed manner. A similar geographic distribution for the two subgroups of Rickettsia has been reported in India, with R1 and R2 detected in the northern and central regions, respectively (Singh et al. 2012). Taken together, these results suggest that endosymbiont infection in B. tabaci is highly associated with geographic distribution as well as the genetic group of the host insect.
Multiple infections of different endosymbionts are common in various cryptic species of B. tabaci (Gueguen et al. 2010; Zchori-Fein et al. 2014). Our study demonstrated that Asia I, which is the dominant cryptic species in Java, exhibited 15 combinations of multiple infections among 5 endosymbionts at the subgroup level. Contrastingly, Asia II 5 and Asia II 7 had few coinfection combinations due to their small sample size. Relative to the 6–8 coinfection combinations observed for Asia I and Asia II 1 in Bangladesh (Khatun et al. 2019), our findings for Asia I in Java represent a highly diversified multiple infection pattern. In addition, we demonstrated here that coinfection of Hamiltonella and Arsenophonus never occurred in the same B. tabaci individual. This finding is consistent with those of previous studies (Gueguen et al. 2010; Duron 2014). The reason why coinfection with Hamiltonella and Arsenophonus is not observed remains to be elucidated. However, a recent study showed that the secondary symbionts Hamiltonella of B. tabaci and Arsenophonus of Trialeurodes vaporariorum coexist with the primary endosymbiont Portiera in the bacteriocyte, and that they similarly affect the sex ratio of whiteflies by regulating fertilization and supplying B vitamins (Wang et al. 2020). Therefore, the infection patterns of endosymbionts seem to be influenced by the potential roles, e.g., symbiotic or competitive association, of each endosymbiont (Gueguen et al. 2010; Wang et al. 2020).
To summarize, three cryptic species of B. tabaci, namely Asia I, Asia II 5, and Asia II 7, were found in Java. These were infected by different secondary endosymbionts in highly diversified combinations. The infection patterns of each endosymbiont were strongly associated with the geographical distribution of the host species. In particular, the infection of Hamiltonella was identified for the first time in Asian indigenous cryptic species from Java. Overall, this study provides useful insights that enhance understanding of the relationship between Asian cryptic species of B. tabaci and their endosymbionts in Java, Indonesia.
References
Acharya R, Kumar Shrestha Y, Raj Sharma S, Lee KY (2020) Genetic diversity and geographic distribution of Bemisia tabaci species complex in Nepal. J Asia Pac Entomol 23:509–515. https://doi.org/10.1016/j.aspen.2020.03.014
Ashfaq M, Hebert PD, Mirza MS et al (2014) DNA barcoding of Bemisia tabaci complex (Hemiptera: Aleyrodidae) reveals southerly expansion of the dominant whitefly species on cotton in Pakistan. PLoS One 9:e104485. https://doi.org/10.1371/journal.pone.0104485
Baumann P (2005) Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189. https://doi.org/10.1146/annurev.micro.59.030804.121041
Berry SD, Fondong VN, Rey C, Rogan D, Fauquet CM, Brown JK (2004) Molecular evidence for five distinct Bemisia tabaci (Homoptera: Aleyrodidae) geographic haplotypes associated with cassava plants in sub-Saharan Africa. Ann Entomol Soc Am 97:852–859. https://doi.org/10.1603/0013-8746(2004)097[0852:meffdb]2.0.co;2
Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS (2013a) Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci 20:194–206. https://doi.org/10.1111/j.1744-7917.2012.01522.x
Bing XL, Yang J, Zchori-Fein E, Wang XW, Liu SS (2013b) Characterization of a newly discovered symbiont of the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae). Appl Environ Microbiol 79:569–575
Brown JK, Frohlich DR, Rosell RC (1995) The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol 40:511–534. https://doi.org/10.1146/annurev.en.40.010195.002455
Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M (2007) Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res 97:407–413. https://doi.org/10.1017/S0007485307005159
Chiel E, Inbar M, Mozes-Daube N, White JA, Hunter MS, Zchori-Fein E (2009) Assessments of fitness effects by the facultative symbiont Rickettsia in the sweetpotato whitefly (Hemiptera: Aleyrodidae). Ecol Popul Biol 102:413–418. https://doi.org/10.1603/008.102.0309
Chu D, Gao C, De Barro P et al (2011) Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: comparison of secondary symbionts from biotypes B and Q in China. Bull Entomol Res 101:477–486. https://doi.org/10.1017/S0007485311000083
Chu D, Tao YL, Chi H (2012) Influence of plant combinations on population characteristics of Bemisia tabaci biotypes B and Q. J Econ Entomol 105:930–935. https://doi.org/10.1603/ec10373
Chu D, Wan FH, Zhang YJ, Brown JK (2010) Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ Entomol 39:1028–1036. https://doi.org/10.1603/en09161
Crowder DW, Horowitz AR, De Barro PJ et al (2010) Mating behaviour, life history and adaptation to insecticides determine species exclusion between whiteflies. J Anim Ecol 79:563–570. https://doi.org/10.1111/j.1365-2656.2010.01666.x
Czosnek H, Ghanim M (2011) Bemisia tabaci – tomato yellow leaf curl virus interaction causing worldwide epidemics. In: The whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) interaction with Geminivirus-infected host plants. Springer, Dordrecht, pp 51–67
De Barro PJ, Hart PJ (2000) Mating interactions between two biotypes of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) in Australia. Bull Entomol Res 90:103–112. https://doi.org/10.1017/s0007485300000201
De Barro PJ, Hidayat SH, Frohlich D et al (2008) A virus and its vector, Pepper yellow leaf curl virus and Bemisia tabaci, two new invaders of Indonesia. Biol Invasions 10:411–433. https://doi.org/10.1007/s10530-007-9141-x
De Barro PJ, Liu S-S, Boykin LM, Dinsdale AB (2011) Bemisia tabaci : a statement of species status. Annu Rev Entomol 56:1–19. https://doi.org/10.1146/annurev-ento-112408-085504
Dinsdale A, Cook L, Riginos C, Buckley YM, de Barro P (2010) Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am 103:196–208. https://doi.org/10.1603/an09061
Duron O (2014) Arsenophonus insect symbionts are commonly infected with APSE, a bacteriophage involved in protective symbiosis. FEMS Microbiol Ecol 90:184–194. https://doi.org/10.1111/1574-6941.12381
Ellango R, Singh ST, Rana VS, Gayatri Priya N, Raina H, Chaubey R, Naveen NC, Mahmood R, Ramamurthy VV, Asokan R, Rajagopal R (2015) Distribution of Bemisia tabaci genetic groups in India. Environ Entomol 44:1258–1264. https://doi.org/10.1093/EE/NVV062
Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. https://doi.org/10.1111/j.1365-2311.2011.01318.x
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Firdaus S, Vosman B, Hidayati N, Jaya Supena ED, G.F. Visser R, van Heusden AW (2013) The Bemisia tabaci species complex: additions from different parts of the world. Insect Sci 20:723–733. https://doi.org/10.1111/1744-7917.12001
Folmer O, Black M, Hoeh W, et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. https://www.researchgate.net/publication/15316743_DNA_primers_for_amplification_of_mitochondrial_Cytochrome_C_oxidase_subunit_I_from_diverse_metazoan_invertebrates. Accessed 21 May 2020
Fujiwara A, Maekawa K, Tsuchida T (2015) Genetic groups and endosymbiotic microbiota of the Bemisia tabaci species complex in Japanese agricultural sites. J Appl Entomol 139:55–66. https://doi.org/10.1111/jen.12171
Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E (2006) Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72:3646–3652. https://doi.org/10.1128/AEM.72.5.3646-3652.2006
Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, Fleury F, Zchori-Fein E (2008) Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J 22:2591–2599. https://doi.org/10.1096/fj.07-101162
Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, Sobol I, Czosnek H, Vavre F, Fleury F, Ghanim M (2010) The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol 84:9310–9317. https://doi.org/10.1128/jvi.00423-10
Götz M, Winter S (2016) Diversity of Bemisia tabaci in Thailand and Vietnam and indications of species replacement. J Asia Pac Entomol 19:537–543. https://doi.org/10.1016/j.aspen.2016.04.017
Gueguen G, Vavre F, Gnankine O et al (2010) Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol 19:4365–4376. https://doi.org/10.1111/j.1365-294X.2010.04775.x
Hidayat P, Aidawati N, Hidayat H, Sartiami D (2008) Indicator plant and PCR-RAPD for biotype determination of Bemisia tabaci Gennadius (Hemiptera Aleyrodidae). J Trop Plant Pest Dis 8(1):1–7
Hidayat P, Kurniawan HA, Afifah L, Triwidodo H (2017) Life cycle and life table of the B and non-B biotypes of the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) on chili pepper (Capsicum annuum L.) Purnama. J Entomol Indones 14:143. https://doi.org/10.5994/jei.14.3.87
Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332:254–256. https://doi.org/10.1126/science.1199410
Hsieh C-H, Wang C-H, Ko C-C (2006) Analysis of Bemisia tabaci (Hemiptera: Aleyrodidae) species complex and distribution in eastern Asia based on mitochondrial DNA markers. Ann Entomol Soc Am 99:768–775. https://doi.org/10.1603/0013-8746(2006)99[768:AOBTHA]2.0.CO;2
Hu J, Chen Y-D, Jiang Z-L, Nardi F, Yang TY, Jin J, Zhang ZK (2015) Global haplotype analysis of the whitefly Bemisia tabaci cryptic species Asia I in Asia. Mitochondrial DNA 26:232–241. https://doi.org/10.3109/19401736.2013.830289
Hunter MS, Perlman SJ, Kelly SE (2003) A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc R Soc B Biol Sci 270:2185–2190. https://doi.org/10.1098/rspb.2003.2475
Islam W, Lin W, Qasim M, Islam SU, Ali H, Adnan M, Arif M, Du Z, Wu Z (2018) A nation-wide genetic survey revealed a complex population structure of Bemisia tabaci in Pakistan. Acta Trop 183:119–125. https://doi.org/10.1016/j.actatropica.2018.04.015
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
Kaiser W, Huguet E, Casas J, Commin C, Giron D (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc R Soc B Biol Sci 277:2311–2319. https://doi.org/10.1098/rspb.2010.0214
Kanakala S, Ghanim M (2019) Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS One 14:e0213946. https://doi.org/10.1371/journal.pone.0213946
Khatun M, Jahan S, Lee S, Lee KY (2018) Genetic diversity and geographic distribution of the Bemisia tabaci species complex in Bangladesh. Acta Trop 187:28–36. https://doi.org/10.1016/j.actatropica.2018.07.021
Khatun MF, Shim JK, Lee KY (2019) Genetic diversity and host relationships of endosymbiotic bacteria in the Asian cryptic species of Bemisia tabaci from Bangladesh. Symbiosis 79:75–87. https://doi.org/10.1007/s13199-019-00622-6
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kontsedalov S, Zchori-Fein E, Chiel E, Gottlieb Y, Inbar M, Ghanim M (2008) The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci 64:789–792. https://doi.org/10.1002/ps.1595
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinforma Appl Note 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Li SJ, Xue X, Ahmed MZ et al (2011) Host plants and natural enemies of Bemisia tabaci (Hemiptera: Aleyrodidae) in China. Insect Sci 18:101–120. https://doi.org/10.1111/j.1744-7917.2010.01395.x
Liu SS, De Barro PJ, Xu J et al (2007) Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 318:1769–1772. https://doi.org/10.1126/science.1149887
Lowe S, Browne M, Boudjelas S, de Pooter M (2000) 100 of the world’s worst invasive alien species a selection from the global invasive species database. The Invasive Species Specialist Group (ISSG), Auckland
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27:189–195. https://doi.org/10.1046/j.1365-2311.2002.00393.x
Oliver KM, Russell JA, Morant NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci U S A 100:1803–1807. https://doi.org/10.1073/pnas.0335320100
Pan H, Li X, Ge D, Wang S, Wu Q, Xie W, Jiao X, Chu D, Liu B, Xu B, Zhang Y (2012) Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS One 7:e30760. https://doi.org/10.1371/journal.pone.0030760
Park J, Jahan SMH, Song WG, Lee H, Lee YS, Choi HS, Lee KS, Kim CS, Lee S, Lee KY (2012) Identification of biotypes and secondary endosymbionts of Bemisia tabaci in Korea and relationships with the occurrence of TYLCV disease. J Asia Pac Entomol 15:186–191. https://doi.org/10.1016/j.aspen.2011.10.005
Perring T (2001) The Bemisia tabaci species complex. Crop Prot 20:725–737. https://doi.org/10.1016/S0261-2194(01)00109-0
Qiu BL, Dang F, Li SJ, Ahmed MZ, Jin FL, Ren SX, Cuthbertson AGS (2011) Comparison of biological parameters between the invasive B biotype and a new defined cv biotype of Bemisia tabaci (Hemiptera:Aleyradidae) in China. J Pest Sci (2004) 84:419–427. https://doi.org/10.1007/s10340-011-0367-0
Rahayuwati S, Hendrastuti Hidayat S, Hidayat P (2016) Genetic identity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) from endemic area of Pepper yellow leaf curl Indonesia virus based on mithochondrial cytochrom oxidase I fragment (mtCOI). J Entomol Indones 13:156–164. https://doi.org/10.5994/jei.13.3.156
Rosell RC, Blackmer JL, Czosnek H, Inbar M (2010) Mutualistic and dependent relationships with other organisms. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Dordrecht, pp 161–183
Shadmany M, Boykin LM, Muhamad R, Omar D (2019) Genetic diversity of Bemisia tabaci (Hemiptera: Aleyrodidae) species complex across Malaysia. J Econ Entomol 112:75–84. https://doi.org/10.1093/JEE/TOY273
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701. https://doi.org/10.1093/aesa/87.6.651
Singh ST, Priya NG, Kumar J, Rana VS, Ellango R, Joshi A, Priyadarshini G, Asokan R, Rajagopal R (2012) Diversity and phylogenetic analysis of endosymbiotic bacteria from field caught Bemisia tabaci from different locations of North India based on 16S rDNA library screening. Infect Genet Evol 12:411–419. https://doi.org/10.1016/j.meegid.2012.01.015
Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P (2006) Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb Ecol 51:294–301. https://doi.org/10.1007/s00248-006-9036-x
Skaljac M, Anic K, Hrncic S et al (2013) Diversity and localization of bacterial symbionts in three whitefly species (Hemiptera: Aleyrodidae) from the east coast of the Adriatic Sea. Bull Entomol Res 103:48–59. https://doi.org/10.1017/S0007485312000399
Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M (2010) Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol 10:142. https://doi.org/10.1186/1471-2180-10-142
Srinivasan R, Hsu Y, Kadirvel P, Lin M (2013) Analysis of Bemisia tabaci (Hemiptera: Aleyrodidae) species complex in Java Island, Indonesia based on mitochondrial cytochrome oxidase I sequences. Philipp Agric Sci 96:290–295
Subdirectorate of Horticulture Statistics (2019) Hortucultural statistics. Jakarta
Thao MLL, Baumann P (2004) Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr Microbiol 48:140–144. https://doi.org/10.1007/s00284-003-4157-7
Thierry M, Becker N, Hajri A et al (2011) Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol Ecol 20:2172–2187. https://doi.org/10.1111/j.1365-294X.2011.05087.x
Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303:1989. https://doi.org/10.1126/science.1094611
Wang HL, Lei T, Xia WQ, Cameron SL, Liu YQ, Zhang Z, Gowda MMN, de Barro P, Navas-Castillo J, Omongo CA, Delatte H, Lee KY, Patel MV, Krause-Sakate R, Ng J, Wu SL, Fiallo-Olivé E, Liu SS, Colvin J, Wang XW (2019) Insight into the microbial world of Bemisia tabaci cryptic species complex and its relationships with its host. Sci Rep 9:1–15. https://doi.org/10.1038/s41598-019-42793-8
Wang YB, Ren FR, Yao YL, Sun X, Walling LL, Li NN, Bai B, Bao XY, Xu XR, Luan JB (2020) Intracellular symbionts drive sex ratio in the whitefly by facilitating fertilization and provisioning of B vitamins. ISME 14:2923–2935. https://doi.org/10.1038/s41396-020-0717-0
Wang Y-L, Wang Y-J, Luan J-B, Yan GH, Liu SS, Wang XW (2013) Analysis of the transcriptional differences between indigenous and invasive whiteflies reveals possible mechanisms of whitefly invasion. PLoS One 8:e62176. https://doi.org/10.1371/journal.pone.0062176
Weeks AR, Velten R, Stouthamer R (2003) Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc B Biol Sci 270:1857–1865. https://doi.org/10.1098/rspb.2003.2425
Zchori-Fein E, Brown JK (2002) Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am 95:711–718. https://doi.org/10.1603/0013-8746(2002)095[0711:dopawb]2.0.co;2
Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS (2001) A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. PNAS 98:12555–12560. https://doi.org/10.1073/pnas.221467498
Zchori-Fein E, Lahav T, Freilich S (2014) Variations in the identity and complexity of endosymbiont combinations in whitefly hosts. Front Microbiol 5:1–8. https://doi.org/10.3389/fmicb.2014.00310
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016. https://doi.org/10.1111/j.1365-294X.2004.02203.x
Acknowledgments
We thank Mst. Fatema Khatun and Hwal-Su Hwang at Kyungpook National University in the Republic of Korea for their help with sequencing and molecular analysis. This work was supported by the Research Program for Exportation Support of Agricultural Products, Animal and Plant Quarantine Agency, in the Republic of Korea (Grant #Z-1543086-2017-21-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lestari, S.M., Hidayat, P., Hidayat, S.H. et al. Bemisia tabaci in Java, Indonesia: genetic diversity and the relationship with secondary endosymbiotic bacteria. Symbiosis 83, 317–333 (2021). https://doi.org/10.1007/s13199-021-00752-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00752-w