Abstract
Aquaculture is an important food producing sector to fulfill nutritional food demand of a continuously growing population. However, disease outbreak has become a major problem in aquaculture which cause huge economic loss to aquaculture industries. The use of expensive chemotherapeutic drugs for treatment have negative impacts on the aquatic environment. So there is a growing concern to find other safe, non-antibiotic based and eco-friendly alternative for the treatment of the diseases. The use of probiotics is a promising alternative approach for the control of infectious agents and treatment of diseases. The benefits of probiotic include stimulation of growth, improved digestion, enhanced immune response and recuperate the water quality as well. Probiotics concoct the fish to fight against various pathogens and improves the overall health as they show anti-bacterial, anti-fungal and anti-viral properties. The use of probiotics in aquaculture is a recent trend and its efficacy in aquatic environment has not been studied extensively. This review paper provides the current knowledge of the use of probiotics in aquaculture, selection criteria, types of probiotics used in aquaculture, their mode of action and administrative methods of probiotics in aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aquaculture is an important and rapidly growing sector as it plays an important role to achieve global protein food demand compared to capture fisheries and terrestrial farmed meat. The role of aquaculture to improve the socio-economic status of any region is highly appreciable because it is not only limited to the source of essential nutrients but it also generates various employment opportunities (Araujo et al. 2015; Handbook on Fisheries Statistics 2014). India ranks second in the world after China in fish production through aquaculture with a contribution of 6.3% of the global aqua production, which is very less as compared to that of China (60.5%) (Chavan 2018; Mo et al. 2018). Fishes are dominant in aqua products, and around 200 fish species are produced for their commercial value (Swapna et al. 2010).
With the increasing intensification and commercialization of aquaculture production, diseases have become a hurdle in the fish farming industry (Hai 2015). The most common disease causing bacterial pathogens among aquaculture are gram-negative such as, Aeromonas, Flavobacterium, Pseudomonas, Vibrio and Yersinia species. These pathogens are etiological agents of various diseases like, enteric red mouth disease, furunculosis, hemorrhage, septicemia, vibriosis and so on (Hamid et al. 2017; Cascales et al. 2016; Patra et al. 2016; Wiklund 2016; Ronneseth et al. 2017). The use of chemotherapeutic drugs has served as an option to cure common diseases prevailing in fish farming (Hambali and Akhmad 2000).
In aquaculture, chemotherapeutic agents like antibiotics and chemicals are the classical cure for microbial infection. However, the extensive usage of these chemotherapeutic drugs leads to their accumulation in aquatic habitat and results in harmful consequences such as emergence of antibiotic-resistant bacteria, accumulation of antibiotic residues in the flesh, kill the beneficial microbes of the gastrointestinal tract and alterations in microbiota (effect on non-target microbes) of the aquatic environment (Munoz-Atienzal et al. 2013; Azevedo et al. 2015). Therefore, the use of antibiotics as chemotherapeutic drugs in aquaculture has become risky (Balcazar et al. 2008; Mancuso et al. 2015; Balcazar et al. 2006a, b). The quest for better alternatives to prevent infection and replace the antibiotics has been a major concern now-a-days.
A promising emerging alternative approach to prevent fish diseases is the use of probiotics, which helps fishes to fight against pathogens by various mechanisms. The importance of probiotics used in aquaculture is not only limited to gastrointestinal tract, but it also plays a major role in the improvement of overall health of an organism (Mehrabi et al. 2018) such as: it acts as growth promoter (Gobi et al. 2018), prevents the diseases (Meidong et al. 2018), enhances the immune response (Havenaar and Marteau 2018; Ramesh and Souissi 2018) and improves the water quality by modifying microbial community of water and sediments (Verschuere et al. 2000; Deng et al. 2018). In ponds, nitrogenous contaminants like ammonia and nitrate have become a serious concern. Previous reports show that the use of Lactobacillus species as probiotics removes the nitrogenous waste from the ponds and use of Bacillus species improves the water quality by converting organic carbon to slime (Ma et al. 2009; Verschuere et al. 2000; Kolndadacha et al. 2011). Khattab et al. 2005 have reported the use of Micrococcus luteus as probiotics which resulted in increased growth performance and improved feed conversion ratio (FCR) in Tilapia (Oreochromis niloticus). Sakata 1990 and Ringo et al. 1995 have demonstrated the role of Bacteroides species, Clostridium species, Agrobacterium species, Brevibacterium species and Microbacterium species as nutritional sources to the host by supplying fatty acids and vitamins.
This review aims to provide useful knowledge about the use of probiotics in aquaculture, selection criteria, most commonly used probiotic strains, their possible mode of action and administrative methods in aquaculture.
1.1 Definition and brief history of probiotics
In 1907, Elie Metchnikoff, a Russian analyst observed that Bulgarian workers had a long life as they consumed fermented milk products. Later in 1965, Lilly and Stillwell explained the concept of probiotics, as a substance which accelerates the growth of good microbes. Parker (1974) gave the definition of probiotics as “organisms and substances, which add to intestinal balance”. Fuller (1992) refined the definition as, “A live microbial feed supplement that beneficially affects the host by improving its intestinal microbial balance”. This definition signifies that the living bacterial cells are an imperative part of potential probiotics and also clarifies the confusion created by the use of term “substance”. WHO (2001) has termed probiotics as live microbes, which when administered in sufficient amount, confer a health benefit to the host.
Probiotics protect the host organism from pathogenic bacteria by liberating metabolites like bacteriocins and different organic acids. These metabolites hinder the adhesion of different pathogens and also inhibit them by limiting the available resources such as nutrients and space (Servin and Coconnier 2003; Vine et al. 2004). Probiotics have the potential to improve the host’s defenses, including the innate and acquired immunity system. This is important for the prevention and treatment of infectious diseases and also to cure inflammation in the digestive tract. Probiotics also have a direct influence on other microbes, either commensal or pathogenic, which is very important for the prevention, treatment and restoration of the bacterial equilibrium inside the gut of the host (Oelschlaeger 2010). The use of probiotics in humans, pigs, steers and poultry has already been studied, but the use of probiotics in aquaculture is relatively a new concept (Daniel 2017; Chua et al. 2017; Jiang et al. 2017; Harimurti and Hadisaputro 2015; Uyeno et al. 2015).
2 Selection criteria for probiotics
The main purpose of probiotics is to establish or to maintain a relationship between beneficial and harmful bacteria which is usually present in the intestine or gut of fish (Thirumurugan and Vignesh 2015; Olsson et al. 1992). Effective probiotics should possess certain qualities which are specified underneath: (Olsson et al. 1992; Merrifield et al. 2010; Pandya 2016; Gatesoupe 1999; Ouwehand et al. 1999a; Ouwehand et al. 1999b; Holzapfel and Schillinger 2002; Fuller 1989).
- 1.
The probiotics should have a beneficial effect on the growth, development and protection of fish against various pathogenic bacteria.
- 2.
The probiotic bacteria should not have any harmful effect on the host.
- 3.
The probiotics should not have the ability of drug resistance, they should have the ability to keep up the hereditary traits.
- 4.
For the utilization of probiotics as an efficient feed, they should exhibit following properties:
Acid and bile tolerance
Resistance to gastric juices
Adherence to digestive system surface
Antagonism towards pathogens
Stimulation of the immunity
Increase in the gut motility
Survival in mucous
Production of enzymes and vitamins
- 5.
They should have good sensorial properties, fermentative action, tolerance towards freeze-drying and viability in feed during packaging and storing process.
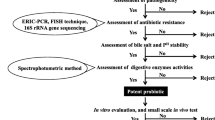
Bacteria isolated from different sources are subjected to screening through multiple steps in order to assess their potential as ideal probiotics. The screening process involves gram staining, indexing, in-vitro evaluation of antagonistic properties, acid tolerance, bile tolerance, susceptibility to drugs and biofilm formation. Figure 1 shows the sequential screening process for the selection of isolated bacteria as probiotics. Successful fulfillment of all criteria qualify them as potential probiotic fit for use in the aquaculture.
3 Probiotics & aquaculture
Usually the aquatic probiotics are commercially available in two major forms- dry and liquid. Dry forms have higher shelf life and are mixed with water or feed which is given to the host. On the other hand, the liquid form of probiotics, usually preferred in egg hatcheries is directly blended with the feed or added to the tanks (Decamp and Moriarty 2007). The liquid forms of probiotics are reported to show better and positive results due to their lower density than spore and dry form probiotics (Nageswara and Babu 2006).
The aquatic probiotics can be further categorized into two classes based on their mode of administration. First one involves the mixing of probiotic bacteria with feed supplement for the enhancement of useful bacteria inside the gut. Second class involves the addition of probiotic directly to the water so that they can consume nutrients available in the water and inhibit the proliferation of pathogens. These two categories of probiotics were used in finfish and shrimp aquaculture (Nageswara and Babu 2006; Sahu et al. 2008).
The probiotics isolated from different natural sources such as gastrointestinal tract (GIT), stomach, gill, kidney, gonads and other internal organs are called putative probiotics. In contrast, the commercial sources (non-putative) comprise of those which are already synthesized and commercially available in the market. The most frequently used probiotic microorganisms belong to Bacillus, Lactobacillus and Bifidobacterium genus (Nwanna 2015). Various species of Lactobacillus, Bifidobacterium and Streptococcus reported for use in aquaculture as probiotics, include L. acidophilus, L. casei, L. fermentum, L. gasseri, L. plantarum, L. salivarius, L. rhamnosus, L. johnsonii, L. paracasei, L. reuteri, L. helveticus, L. bugaricus, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium lactis, Bifidobacterium longum, Saccharromyces species, Saccharromyces boulardii, S. thermophiles and S. cremoris (Nwanna 2015).
Various aquatic probiotics have been reported which show activity not only against bacterial pathogen, but also against fungus and virus to improve growth and immunity of the host.
3.1 Antibacterial activity
Many probiotics used in aquaculture are well-known for their antibacterial property against known pathogens. Lactococcus lactis RQ516 probiotic shows inhibitory action against Aeromonas hydrophila when given to Tilapia (Oreochromis niloticus) (Zhou et al. 2010). Also L. lactis probiotic has antibacterial activity against two pathogens-Yersinia rukeri and Aeromonas salmonicida that affects the fish growth (Balcazar et al. 2007a, b). Leuconostoc mesenteroides has the potential to inhibit the fish pathogens found in Nile tilapia (O. niloticus) (Zapata and Lara-Flores 2012). According to reports, Bacillus subtilis considerably reduces the motile Aeromonads, total Coliforms and Pseudomonads found in ornamental fishes (Ghosh et al. 2008; Newaj-Fyzul and Austin 2015). Lactic acid bacteria such as Lactobacillus acidophilus, Lactobacillus buchneri, Lactobacillus fermentum, Lactococcus lactis, and Sterptococcus salivarius were isolated from Spanish mackerel (Scomberomorus commerson) intestine and were capable to inhibit the Listeria innocua growth (Moosavi-Nasab et al. 2014). Many Lactobacilli species isolated from the intestine of Anguilla species, Clarias orientalis, Labeo rohita, Oreochromis species and Puntius carnaticus showed significant antimicrobial activity against Aeromonas and Vibrio species (Dhanasekaran et al. 2008).
3.2 Antiviral activity
In recent years, the antiviral activity of probiotics has gained attention (Lakshmi et al. 2013), but the exact mechanism of action through which probiotic bacteria show antiviral effects is still unknown. However the in-vitro analysis reveals that the inhibition of viruses can occur by secretion of extracellular enzymes produced by the bacteria. For example, Aeromonas species, Corynebacterium, Pseudomonas and Vibrio species show the antiviral activity against the IHNV (Infectious hematopoietic necrosis virus) (Kamei et al. 1988; Zorriehzahra et al. 2016). Feeding of probiotic strain Bacillus megaterium has increased the resistance against WSSV (white-spot syndrome virus) in the shrimp, Litopenaeus vannamei (Li et al. 2009). The research has reported that probiotics strains Bacillus and Vibrio species are effective against WSSV and efficiently protect Litopenaeus vannamei (Balcazar 2003). Application of Lactobacillus as probiotic, either as a single strain or as a mixture with Sporolac resulted in better resistance against lymphocystis viral disease which is found in Paralichthys olivaceus (olive flounder) (Harikrishnan et al. 2010).
3.3 Antifungal activity
Only few studies have been reported about the antifungal activity of probiotics. Aeromonas strain A199 from Anguilla australis (eel) culture water, had high inhibitory property against Saprolegnia species (Lategan et al. 2004). In another study, Pseudomonas species M162, Pseudomonas species M174 and Janthinobacterium species M169 have increased the immunity against saprolegniasis in Oncorhynchus mykiss (rainbow trout) (Zorriehzahra et al. 2016). In 2012, Nurhajati et al. demonstrated that Lactobacillus plantarum FNCC 226 showed inhibitory potential in catfish (Pangasius hypophthalamus) against Saprolegnia parasitica A3 (Nurhajati et al. 2012).
The reported probiotic strains used in aquaculture can either be obtained commercially or isolated from different fish species. A detailed summary of different probiotics, their sources and the beneficial effects on the host are given in Table 1.
4 Mode of action
Probiotics have a special mode of action to protect the host from intestinal issues. The probiotic microorganisms hinder the establishment of different pathogenic bacteria by a process called colonization resistance. Probiotic microorganisms secrete a variety of inhibitory substances which inhibit Gram +ve and Gram –ve microscopic organisms. Principally, these inhibitory secretions are acetic acid, lactic acid, H2O2, bacteriocins and so on. These secretions decrease the number of pathogens by inhibiting the formation of virulence substances (Nwanna 2015). Oelschlaeger (2010) explained the mode of action of probiotics in a simple way in which probiotics modulate the acquired immune system as well as innate immunity to prevent host gut from disease causing pathogens and to treat against various digestive tract inflammations. Next possible action is that, they directly affect the pathogenic bacteria present in the gut, thus, resulting in the restoration of the probiotics in the gut. Finally they target various toxins produced by the microbial population resulting in their detoxification and inactivation in the gut (Oelschlaeger 2010). Thus, all modes of action of probiotics are directly associated with gut micro-biota (Wolf 2006; Pandiyan et al. 2013). Probiotic secrete antagonistic compounds which help to improve the immunity and enhance the growth of fish. It also helps to improve the water and soil quality. The mode of action of probiotics is shown in Fig. 2.
Mode of action of probiotics. Modified from Chauhan and Singh (2018)
Some possible well-known mechanisms by which probiotic bacteria protect the host organism against intestinal disorders are as follows:
4.1 Competition for space / blocking of adhesion sites
The activity of probiotics is visualized by the aggressive hindrance for the attachment sites on intestinal epithelial layer (Nwanna 2015). The mechanism of action by which probiotic bacteria struggle for the adhesion site is called ‘competitive inhibition’. The ability of bacteria to colonize the gut and adhere to the epithelial surface and subsequently inhibit the adhesion of pathogens is desirable criteria in the selection of probiotics (Balcazar et al. 2006a, b; Lazado et al. 2011). Lactobacillus prevent the adhesion of the pathogenic bacteria such as Escherichia coli, Klebsiella species and Pseudomonas aeruginosa on intestinal cells of the host (Nwanna 2015). Probiotic adhesion can be non-specific due to the presence of physiochemical agents or specific due to the adhesion of the probiotics either on the surface of adherent bacteria or the receptor molecules on the epithelial cells (Salminen et al. 1996; Lazado et al. 2015).
4.2 Production of inhibitory substances
The probiotic bacteria produce inhibitory substances which have bacteriostatic or bactericidal influences on pathogenic microbes (Servin 2004) like hydrogen peroxide, bacteriocins, lysozymes, siderophores, proteases, and many others (Panigrahi and Azad 2007; Tinh et al. 2008). Some bacteria produce volatile fatty acid (acetic, butyric, lactic and propionic acid) and organic acid, as a result of which there is a decrease in pH of gastrointestinal tract. Hence, it inhibits the proliferation of opportunistic pathogens (Tinh et al. 2008). A compound named indole (2,3-benzopyrrole) has inhibitory potential against various pathogens i.e. Vibrio anguillarum, Aeromonas salmonicida, Edwaedsiella tarda and Yersinia ruckeri (Gibson 1998; Lategan et al. 2006; Abbass et al. 2010).
4.3 Competition for nutrients
The survival of any microbial population depends on its ability to compete for nutrients and available energy with other microbes in the same environment (Verschuere et al. 2000). While struggling for nutrients, probiotics can out-contend the pathogens by utilizing all the available nutrients that would have been consumed by pathogenic microorganisms. This mechanism would restrict the pathogen’s presence in the intestinal tract because without nutrients the bacteria cannot survive (Nwanna 2015). For example, siderophores are low-molecular-weight iron-chelating agents that dissolve precipitated iron or extract it from the iron complexes, thus making it available for bacterial growth (Neilands 1981). Siderophore-producing bacteria can be used as probiotics because they can sequester ferric iron in an iron-low environment, hence, making it unavailable for the growth of pathogenic bacteria (Tinh et al. 2008). A culture supernatant of Pseudomonas fluorescens which was grown in iron-limited conditions inhibits the growth of Vibrio anguillarum. It has been shown that P. fluorescens can competitively inhibit the growth of fish pathogen Aeromonas salmonicida by competing for the available free iron (Gram et al. 1999; Smith and Davey 1993).
4.4 Improving water quality
According to the studies, use of Gram +ve bacteria (Bacillus species) as probiotics to improve the water quality has been reported. It was concluded that the Gram positive bacteria, especially Bacillus species are more efficient in conversion of organic matter into CO2, slime or bacterial biomass. The studies suggest better performance of Gram positive bacteria over Gram negative bacteria. It is also suggested that the farmers can control the accumulation of dissolved and particulate organic carbon during the growing season by maintaining high level of probiotics in the production pond (Balcazar et al. 2006a, b; Mohapatra et al. 2013). The probiotic bacteria possess significant algicidal activity and affects several species of microalgae (Fukami et al. 1997). The nitrifying probiotic bacteria are beneficial as they increase the number of microbial species in water and improve the water quality by eliminating ammonia and nitrate toxicity (Zorriehzahra et al. 2016; Mohapatra et al. 2013). Also, after the use of probiotics, other parameters like temperature, pH, dissolved oxygen, ammonia and hydrogen sulfide in rearing water were found to be of better quality. Thus, probiotics maintain a positive and healthy environment for shrimp and prawn larval culture in aquatic system (Aguirre-Guzman et al. 2012; Banerjee et al. 2010).
4.5 Disruption of quorum sensing
Quorum Sensing (QS) is a bacterial regulatory mechanism, which is responsible to control the expression of various biological macromolecules such as, the virulence factors in a cell density-dependent manner. In this mechanism, bacteria regulate the gene expressions by producing, releasing and recognizing small signal molecules called auto-inducers (Chu et al. 2014). Many bacteria are using this system to communicate and regulate a diverse array of physiological activities (Miller and Bassler 2001). Disruption of the QS system of pathogens has been proposed as a new anti-infective strategy in aquaculture (Defoirdt et al. 2004; Zorriehzahra et al. 2016).
Since N-Acyl homoserine lactones (AHLs) are the main family of QS auto-inducers used in Gram negative bacteria, their Biodegradation prove to be an efficient way to interrupt QS,. Bacillus sp. QSI-1 is an efficient quorum quencher on virulence factors production and biofilm formation of fish pathogen Aeromonas hydrophila (Chu et al. 2014). Bacillus sp. QSI-1 reduced the accumulation of AHLs but did not affect the growth of A. hydrophila YJ-1. It has been found that the supernatant of QSI-1 showed significant inhibition of protease production (83.9%), hemolytic activity (77.6%) and biofilm formation (77.3%) in YJ-1. In biocontrol experiment, QSI-1 significantly reduced the pathogenicity of A. hydrophila strain YJ-1 in zebrafish (Danio rerio). The fish fed with QSI-1 were observed to have a relative percentage survival of 80.8%. The results indicated that AHLs degrading bacteria should be considered as an alternative for antibiotics in aquaculture for the bio-control of bacterial fish diseases (Chu et al. 2014). Probiotic bacteria such as Lactobacillus, Bifidobacterium and Bacillus cereus strains degrade the signal molecules of pathogenic bacteria by enzymatic secretion or production of auto-inducer antagonists (Brown 2011). Medellin-Pena et al. (2007) showed that Lactobacillus acidophilus secretes a molecule that inhibits the QS or interacts with bacterial transcription of Escherichia coli O157 gene.
5 Methods of administration of probiotics
Various methods have been put forth to regulate the use of probiotics in aquaculture. They can be added in feed, resulting in the colonization on the surface of intestinal tract. In prawns, the most common regulatory method for administration of probiotics is through water/oral routine (Huang et al. 2006). But most of the probiotics are designed in such a way that they can be mixed with the feed additives to show high efficiency against pathogens (Austin et al. 1992; Gildberg and Mikkelsen 1998; Hai et al. 2009; Gomes et al. 2009). The probiotics such as Lactobacillus rhamnosus were reported to improve the fecundity of Danio rerio (Gioacchini et al. 2010). Other methods such as addition of probiotics directly into water or in bacterial suspension were also reported (Queiroz and Boyd 1998; Gibson et al. 1998; Ringo and Vadstein 1998; Cha et al. 2013; Hansen and Olafsen 1989; Sung et al. 1994; Itami et al. 1998).
Probiotics can be used individually or in a combination of different strains (Havenaar et al. 1992; Salinas et al. 2005; Kesarcodi-Watson et al. 2008; Kesarcodi-Watson et al. 2012). Previous reviews on probiotics have focused on the utilization of sole culture species, and it is speculative whether combination two or more cultures of probiotic strains would be useful. Mixed probiotic strains are more efficient than probiotics based on a single strain (Verschuere et al. 2000; Hai et al. 2009). Multi-species and multi-strain probiotics enhance the defense mechanism against various infectious diseases (Kesarcodi-Watson et al. 2012; Timmerman et al. 2004). A recent study compared the activity of mixed strain of Lactobacillus acidophilus and B. subtilis in Nile tilapia in which serum bactericidal activity and hematocrit values were higher in comparison to sole strain (Aly et al. 2008a, b). Similar studies were conducted to modulate immunity against Streptococcus iniae by a mixture of Lactobacillus plantarum and Lactococcus lactis in Japanese flounder (Beck et al. 2015). In the growth and survival of Labeo rohita, multi strain probiotics have been efficiently used which enhanced fry and hatchling stages (Jha et al. 2015).
Synbiotics is the combination of probiotics with various plant products and prebiotics (Salminen et al. 1998; Van Hai and Fotedar 2009). It has been reported in many studies that synbiotics improves the microbial supplementation in the gastrointestinal tract of the host organism (Gibson and Roberfroid 1995). The feeding of synbiotic Enterococcus faecalis and mannan-oligosaccharide (MOS) showed better FCR (food conversion ratio) as compared to feeding of probiotic and prebiotic individually (Rodriguez-Estrada et al. 2009). The application of probiotics, prebiotics and synbiotics have improved the survival of aquatic organisms against pathogenic bacteria. The survivability were found to be maximum in the probiotic treatment followed by prebiotic and synbiotic (Daniels et al. 2013; Decamp and Moriarty 2007).
The enrichment of live feed with probiotics as encapsulation has developed into an interesting idea. In this technique, the probiotic bacteria can remain viable or even proliferate on the live feed. Therefore, probiotics can be delivered by the live feed to the host in a very efficient manner (Hai 2015). Various live feeds have been reported so far such as copepods (Sun et al. 2013), rotifer (Gatesoupe 1997) and Artemia species (Daniels et al. 2013; Gatesoupe 1994; Van Hai et al. 2010), in which probiotics were encapsulated. This approach of enrichment of live feed with probiotics has proved to be effective over other conventional methods. Van Hai et al. 2010 have reported an effective enrichment of Artemia nauplii using a combination of Pseudomonas synxantha and Pseudomonas aeruginosa for Penaeus latisulcatus (western king prawn). Similarly, Sun et al. 2013 have reported that the copepod (Pseudodiaptomus annandalei) is an appropriate vector of probiotic (Bacillus species) as live feed for Epinephelus coioides larvae.
The probiotics can be administered in either form- as live or dead strains. Various reports are available for the use of probiotics in either form. The comparison of live and dead forms reveals an interesting observation. Live probiotics provide immunity to the host in most of the cases and in a few cases certain inactivated probiotics also do the same. Hence, the use of probiotics in live or heat killed forms are case specific and cannot be generalized. For instance, Sharifuzzaman et al. 2011; Arijo et al. 2008; Panigrahi et al. 2011; Ramesh et al. 2015 have reported the use of viable probiotics with better results. Sharifuzzaman and Austin 2010; Arijo et al. 2008 have demonstrated the role of live probiotic cells Kocuria SM1 by production of cross-reactive antibodies in rainbow trout to protect against infections due to Vibrio anguillarum, V. ordalii and V. harveyi. Similarly, live cells of Bacillus licheniformis and Bacillus pumilus exhibited an enhanced expression of lysozyme activity and respiratory burst in rohu species (Ramesh et al. 2015). Panigrahi et al. 2011 states that higher expression of immune genes (TNF, TGF-b, IFN and Ig) is responsible for better immunity. The expression of these immune genes is induced using live probiotic cells (live-spray and freeze-dried) compared to the heat-killed ones. The phagocytic activity was found to be higher in rainbow trout, when they were fed with live cells of probiotic bacteria Lactobacillus rhamnosus JCM1136 as compared to heat-killed cells (Panigrahi et al. 2005).
Also, in some cases the supplementation of cell-free supernatant and heat-killed probiotics stimulated innate immunity of the fish (Irianto and Austin 2003). But they offer poor protection to rainbow trout and Chinese drum (Miichthys miiuy) against pathogens, V. anguillarum, Streptococcus iniae, Aeromonas hydrophila and Lactococcus garvieae (Brunt and Austin 2005; Pan et al. 2008). When Nile tilapia was nourished with both dead and live probiotics against Edwardsiella tarda disease, dead probiotics were found ineffective as compared to viable probiotics (Taoka et al. 2006).
The administration duration of probiotic bacteria is also considered a very significant factor. According to research, the time-period for application of the potential probiotic can be as short as 6 days or as long as 5 months or even 8 months (Joborn et al. 1997; Aubin et al. 2005; Aly et al. 2008a, b). Prolonged administration of probiotics can induce immune-suppression of continuous responses of nonspecific immune systems (Sakai 1999). Supplementation of probiotic bacteria has demonstrated to give short-term benefits. However, they were not detected inside the gastrointestinal tract over a period of 1–3 weeks (Robertson et al. 2000; Kim and Austin 2006; Balcazar et al. 2007a, b). Short-term supplementation has turned out to be effective, while the data on long-term effectiveness is not available (Brunt et al. 2007; Newaj-Fyzul et al. 2007; Pieters et al. 2008; Wu et al. 2015; Skjermo et al. 2015). Feeding of probiotics (Shewanella xiamenensis and Aeromonas veronii) tograss carp for about 28 days reduced the cumulative mortality when challenged with Aeromonas hydrophila (Wu et al. 2015). Aubin et al. 2005 checked the recovered amount of probiotics over a time period and observed that recovery levels were found to be higher after 20 days than 5 months. The frequency of administration of probiotics also play a very important role in maintaining the effectiveness and function of probiotics,. A daily application of probiotics is better than thrice a week during the culture period (Guo et al. 2009).
6 Conclusion
In recent years, the use of probiotics as biological control agent has improved fish performance, water quality, prevention of diseases, enhancement of immune responses and so on. This review concludes that several probiotic strains are highly specific while others are quite selective. Efforts need to be made to streamline the whole range of probiotic strains and categorize them based on their action-specific mechanism. A simple step in this direction is going to make the use of probiotics very efficient, economical and eco-friendly. After proving the worth of probiotics, there is a need to look forward towards designing probiotic strains which are specific and can be used to target specific fish species. The evaluation of optimal conditions for probiotic interaction with the host also holds a lot of scope for further investigation. In the past, there have been instances of failure of in-vivo studies which were conducted based on the positive in-vitro results. We need to detail out the conditions in real samples which may affect their survival, colonization, proliferation and interaction with the host in a particular environment. This will help us to properly screen and test probiotics which will lead to no mismatch in in-vitro and in-vivo observations. Other important scope for future research is to study the fate of probiotics in host organism. The fate of live strains and durability of health effects of probiotics in host organism are uncertain and require further investigation.
After doing much study about the efficacy and action mechanism of probiotics, there are still many doubts which are unclear. Nevertheless, the future should focus on relevant research to develop innovative and suitable approach for administration of probiotic strains in foods and animals. Probiotic strains viability, functionality, host-microbe’s interactions, antioxidant status, antagonistic and synergistic activity or probably side effects of probiotics should be the major concerns of study. In addition, advanced molecular level research is required on probiotic science for a better understanding of the molecular mechanisms and to decode the probiotic unique gene with novel applications.
References
Abareethan M, Amsath A (2015) Characterization and evaluation of probiotic fish feed. Int J Pure Appl Zool 3:148–153
Abbass A, Sharifuzzaman SM, Austin B (2010) Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 33:31–37
Adnan M, Patel M, Hadi S (2017) Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 5:e3085. https://doi.org/10.7717/peerj.3085
Aguirre-Guzman G, Lara-Flores M, Sanchez-Martinez JG, Campa-Cordova AI, Luna-Gonzalez A (2012) The use of probiotics in aquatic organisms: a review. Afr J Microbiol Res 6(23):4845–4857
Al-Faragi JK, Alsaphar SA (2012) Isolation and identification of Bacillus subtilus as (probiotic) from intestinal microflora of common carp Cyprinus carpio L. In Proceeding of the Eleventh Veterinary Scientific Conference 355:361
Aly SM, Ahmed YAG, Ghareeb AAA, Mohamed MF (2008a) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immun 25(1):128–136
Aly SM, Mohamed MF, John G (2008b) Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquac Res 39(6):647–656
Amin M, Adams M, Bolch CJ, Burke CM (2017) In vitro screening of lactic acid bacteria isolated from gastrointestinal tract of Atlantic Salmon (Salmo salar) as probiont candidates. Aquac Int 25(1):485–498
Araujo C, Munoz-Atienza E, Nahuelquin Y, Poeta P, Igrejas G, Hernandez PE, Herranz C, Cintas LM (2015) Inhibition of fish pathogens by the microbiota from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Anaerobe 32:7–14
Arijo S, Brunt J, Chabrillon M, Diaz-Rosales P, Austin B (2008) Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout, Oncorhynchus mykiss (Walbaum), against V. harveyi. J Fish Dis 31(8):579–590
Aubin J, Gatesoupe FJ, Labbe L, Lebrun L (2005) Trial of probiotics to prevent the vertebral column compression syndrome in rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Res 36(8):758–767
Austin B, Baudet E, Stobie M (1992) Inhibition of bacterial fish pathogens by Tetraselmis suecica. J Fish Dis 15(1):55–61
Azevedo RVD, Fosse Filho JC, Cardoso LD, Mattos DDC, Junior V, Vazquez M, Andrade DRD (2015) Economic evaluation of prebiotics, probiotics and synbiotics in juvenile Nile tilapia. Rev Ciênc Agron 46(1):72–79
Balcazar JL (2003) Evaluation of probiotic bacterial strains in Litopenaeus vannamei. Final Report. National Center for Marine and Aquaculture Research Guayaquil Ecuador
Balcazar JL, De Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Muzquiz JL (2006a) The role of probiotics in aquaculture. Vet Microbiol 114(3):173–186
Balcazar JL, Decamp O, Vendrell D, De Blas I, Ruiz-Zarzuela I (2006b) Health and nutritional properties of probiotics in fish and shellfish. Microb Ecol Health Dis 18(2):65–70
Balcazar JL, De Blas I, Ruiz-Zarzuela I, Vendrell D, Calvo AC, Marquez I, Girones O, Muzquiz JL (2007a) Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta). Br J Nutr 97(03):522–527
Balcazar JL, Vendrell D, De Blas I, Ruiz-Zarzuela I, Girones O, Muzquiz JL (2007b) In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet Microbiol 122(3):373–380
Balcazar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Muzquiz JL, Girones O (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278(1):188–191
Banerjee S, Khatoon H, Shariff M, Yusoff FM (2010) Enhancement of Penaeus monodon shrimp postlarvae growth and survival without water exchange using marine Bacillus pumilus and periphytic microalgae. Fish Sci 76(3):481–487
Banerjee G, Nandi A, Ray AK (2017) Assessment of hemolytic activity, enzyme production and bacteriocin characterization of Bacillus subtilis LR1 isolated from the gastrointestinal tract of fish. Arch Microbiol 199(1):115–124
Beck BR, Kim D, Jeon J, Lee SM, Kim HK, Kim OJ, Holzapfel WH (2015) The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 42(1):177–183
Bhatnagar A, Lamba R (2015) Antimicrobial ability and growth promoting effects of feed supplemented with probiotic bacterium isolated from gut microflora of Cirrhinus mrigala. J Integr Agric 14(3):583–592
Bisht GP, Singh UP, Pandey DCFR (2012) Bacillus subtilis as a potent probiotic for enhancing growth in fingerlings of common carp (Cyprinus carpio L.). Indian J Fish 59(3):103–107
Brown M (2011) Modes of action of probiotics: recent developments. J Anim Vet Adv 10(14):1895–1900
Brunt J, Austin B (2005) Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 28(12):693–701
Brunt J, Newaj-Fyzul A, Austin B (2007) The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 30(10):573–579
Cascales D, Guijarro JA, Reimundo P, Garcia-Torrico AI, Mendez J (2016) Genome sequence of the fish pathogen Yersinia ruckeri strain 150, isolated from diseased rainbow trout. Genome Announc. https://doi.org/10.1128/genomeA.01331-16
Cha JH, Rahimnejad S, Yang SY, Kim KW, Lee KJ (2013) Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 402:50–57
Chauhan A, Singh R (2018) Probiotics and their applications in aquaculture. In: Sharma D, Saharan BS (eds) Microbial cell factories, 1edn. Taylor & Francis, New York, pp 321–338
Chavan S (2018) Statistical modeling and forecasting of Total fish production of India: a time series perspective. Int J Curr Microbiol App Sci 7(3):1698–1707
Chen Y, Li J, Xiao P, Li GY, Yue S, Huang J, Mo ZL (2016) Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquac Nutr 22(2):374–381
Chu W, Zhou S, Zhu W, Zhuang X (2014) Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci Rep 4:5446
Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW (2017) Designer probiotics for the prevention and treatment of human diseases. Curr Opin Chem Biol 40:8–16
Daniel N (2017) Status of aquaculture with respect to nutrition and feed. Int J fish Aquat 5(1):333–345
Daniels CL, Merrifield DL, Ringo E, Davies SJ (2013) Probiotic, prebiotic and synbiotic applications for the improvement of larval European lobster (Homarus gammarus) culture. Aquaculture 416:396–406
Decamp O, Moriarty D (2007) Aquaculture species profit from probiotics. Feed Mix 15(1):20
Defoirdt T, Boon N, Bossier P, Verstraete W (2004) Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240(1):69–88
Deng M, Chen J, Gou J, Hou J, Li D, He X (2018) The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture 482:103–110
Dhanasekaran D, Saha S, Thajuddin N, Panneerselvam A (2008) Probiotic effect of Lactobacillus isolates against bacterial pathogens in Clarias orientalis. FU Med Biol 15(3):97–102
Divya KR, Isamma A, Ramasubramanian V, Sureshkumar S, Arunjith TS (2012) Colonization of probiotic bacteria and its impact on ornamental fish Puntius conchonius. J Environ Biol 33(3):551–555
Dutta D, Ghosh K (2015) Screening of extracellular enzyme-producing and pathogen inhibitory gut bacteria as putative probiotics in mrigal, Cirrhinus mrigala (Hamilton, 1822). Int J Fish Aquat 2(4):310–318
Fukami K, Nishijima T, Ishida Y (1997) Stimulative and inhibitory effects of bacteria on the growth of microalgae. Live food in aquaculture 185–191 Springer Netherlands
Fuller (1989) Probiotics in man and animals. J Appl Bacteriol 66(5):365–378
Fuller R (1992) History and development of probiotics. In: Fuller R (ed) Probiotics: the scientific basis. Chapman and Hall, London, pp 1–45
Gatesoupe FJ (1994) Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic vibrio. Aquat Living Resour 7(4):277–282
Gatesoupe FJ (1997) Siderophore production and probiotic effect of vibrio sp. associated with turbot larvae, Scophthalmus maximus. Aquat Living Resour 10(4):239–246
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180(1):147–165
Ghosh S, Sinha A, Sahu C (2008) Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac Nutr 14(4):289–299
Giannenas I, Karamaligas I, Margaroni M, Pappas I, Mayer E, Encarnacao P, Karagouni E (2015) Effect of dietary incorporation of a multi-strain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 41(1):119–128
Gibson LF (1998) Bacteriocin activity and probiotic activity of Aeromonas media. J Appl Microbiol 85(S1):243S–248S
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Gibson LF, Woodworth J, George AM (1998) Probiotic activity of Aeromonas media on the Pacific oyster, Crassostrea gigas, when challenged with Vibrio tubiashii. Aquaculture 169(1):111–120
Gildberg A, Mikkelsen H (1998) Effects of supplementing the feed to Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immuno-stimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture 167(1):103–113
Gioacchini G, Maradonna F, Lombardo F, Bizzaro D, Olivotto I, Carnevali O (2010) Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 140(6):953–959
Giri SS, Sukumaran V, Sen SS, Vinumonia J, Banu BN, Jena PK (2011) Antagonistic activity of cellular components of potential probiotic bacteria, isolated from the gut of Labeo rohita, against Aeromonas hydrophila. Probiotics Antimicrob Proteins 3(3–4):214–222
Gobi N, Vaseeharan B, Chen JC, Rekha R, Vijayakumar S, Anjugam M, Iswarya A (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Gomes LC, Brinn RP, Marcon JL, Dantas LA, Brandao FR, De Abreu JS, Lemos PEM, McComb DM, Baldisserotto B (2009) Benefits of using the probiotic Efinol® L during transportation of cardinal tetra, Paracheirodon axelrodi (Schultz), in the Amazon. Aquac Res 40(2):157–165
Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen TF (1999) Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl Environ Microbiol 65(3):969–973
Guo JJ, Liu KF, Cheng SH, Chang CI, Lay JJ, Hsu YO, Yang JY, Chen TI (2009) Selection of probiotic bacteria for use in shrimp larviculture. Aquac Res 40(5):609–618
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119(4):917–935
Hai NV, Buller N, Fotedar R (2009) Effects of probiotics (Pseudomonas synxantha and Pseudomonas aeruginosa) on the growth, survival and immune parameters of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquac Res 40(5):590–602
Hambali S, Akhmad R (2000) The use of chemicals in aquaculture in Indonesia. In Use of Chemicals in Aquaculture in Asia: Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia 20-22 May 1996, Tigbauan Iloilo, Philippines (pp. 113–118). SEAFDEC Aquaculture Department
Hamdan AM, El-Sayed AFM, Mahmoud MM (2016) Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J Appl Microbiol 120(4):1061–1073
Hamid NH, Hassan MD, Sabri MM, Hasliza AH, Hamdan RH, Afifah MN, Raina MS, Nadia AB, Fuad MM (2017) Studies on pathogenicity effect of Aeromonas hydrophila infection in juvenile red hybrid tilapia Oreochromis sp. In Proceedings of International Seminar on Livestock Production and Veterinary Technology 532–539
Han B, Long WQ, He JY, Liu YJ, Si YQ, Tian LX (2015) Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 46(2):225–231
Handbook on Fisheries Statistics (2014) August 2014 Dept. of Animal Husbandry, Dairying & Fisheries, Ministry of Agriculture Govt. of India New Delhi
Hansen GH, Olafsen JA (1989) Bacterial colonization of cod (Gadus morhua L.) and halibut (Hippoglossus hippoglossus) eggs in marine aquaculture. Appl Environ Microbiol 55(6):1435–1446
Harikrishnan R, Balasundaram C, Heo MS (2010) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29(5):868–874
Harimurti S, Hadisaputro W (2015) Probiotics in poultry. In Beneficial Microorganisms in Agriculture Aquaculture and Other Areas (pp. 1–19) Springer International Publishing
Havenaar R, Marteau P (1994) Establishing a scientific basis for probiotic R&D. Trends Biotechnol 12(1):6–8
Havenaar R, Ten Brink B, Huis JH (1992) Selection of strains for probiotic use. In Probiotics (pp. 209–224) Springer Netherlands
Holzapfel WH, Schillinger U (2002) Introduction to pre-and probiotics. Food Res Int 35(2):109–116
Huang X, Zhou H, Zhang H (2006) The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol 20(5):750–757
Irianto A, Austin B (2003) Use of dead probiotic cells to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 26:59–62
Itami T, Asano M, Tokushige K, Kubono K, Nakagawa A, Takeno N, Nishimura H, Maeda M, Kondo M, Takahashi Y (1998) Enhancement of disease resistance of kuruma shrimp, Penaeus japonicus, after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture 164(1):277–288
Jha DK, Bhujel RC, Anal AK (2015) Dietary supplementation of probiotics improves survival and growth of Rohu (Labeo rohita Ham.) hatchlings and fry in outdoor tanks. Aquaculture 435:475–479
Jiang T, Li HS, Han GG, Singh B, Kang SK, Bok JD, Kim DD, Hong ZS, Choi YJ, Cho CS (2017) Oral delivery of probiotics in poultry using pH-sensitive tablets. J Microbiol Biotechnol 27(4):739–746
Joborn A, Olsson JC, Westerdahl A, Conway PL, Kjelleberg S (1997) Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain K1. J Fish Dis 20(5):383–392
Kamei Y, Yoshimizu M, Ezura Y, Kimura T (1988) Screening of bacteria with antiviral activity from fresh water salmonid hatcheries. Microbiol Immunol 32(1):67–73
Kang CH, Shin Y, Kim Y, So JS (2016) Isolation of Lactobacillus strains from shellfish for their potential use as probiotics. Biotechnol Bioprocess Eng 21(1):46–52
Kato CD, Kahuma CE, Namulawa VT, Kasozi N (2016) Antibacterial activity of Lactobacillus spp and Lactococcus spp isolated from various parts of pebbly fish, Alestes baremoze. Br Microbiol Res J 17:1–7
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274(1):1–14
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2012) Performance of single and multi-strain probiotics during hatchery production of Greenshell™ mussel larvae, Perna canaliculus. Aquaculture 354:56–63
Khattab YA, Shalaby AM, Abdel-Rhman A (2005) Use of probiotic bacteria as growth promoters, anti-bacterial and their effects on physiological parameters of Oreochromis niloticus. In Proceedings of international symposium on Nile Tilapia in aquaculture 7:156–165)
Kim DH, Austin B (2006) Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol 21(5):513–524
Kolndadacha OD, Adikwu IA, Okaeme AN, Atiribom RY, Mohammed A, Musa YM (2011) The role of probiotics in aquaculture in Nigeria-a review. Cont J Fish Aquat Sci 5(1):8–15
Lakshmi B, Viswanath B, Sai Gopal DVR (2013) Probiotics as antiviral agents in shrimp aquaculture. J Pathogens. https://doi.org/10.1155/2013/424123
LaPatra SE, Fehringer TR, Cain KD (2014) A probiotic Enterobacter sp. provides significant protection against Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) after injection by two different routes. Aquaculture 433:361–366
Lara-Flores M, Olvera-Novoa MA, Guzman-Mendez BE, Lopez-Madrid W (2003) Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 216(1):193–201
Lategan MJ, Torpy FR, Gibson LF (2004) Control of saprolegniosis in the eel Anguilla australis Richardson, by Aeromonas media strain A199. Aquaculture 240(1):19–27
Lategan MJ, Booth W, Shimmon R, Gibson LF (2006) An inhibitory substance produced by Aeromonas media A199, an aquatic probiotic. Aquaculture 254(1):115–124
Lazado CC, Caipang CMA, Brinchmann MF, Kiron V (2011) In vitro adherence of two candidate probiotics from Atlantic cod and their interference with the adhesion of two pathogenic bacteria. Vet Microbiol 148(2):252–259
Lazado CC, Caipang CMA, Estante EG (2015) Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish Shellfish Immunol 45(1):2–12
Li J, Tan B, Mai K (2009) Dietary probiotic bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture 291(1):35–40
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science147(3659):747–748
Liu XF, Li Y, Li JR, Cai LY, Li XX, Chen JR, Lyu SX (2015) Isolation and characterisation of Bacillus spp. antagonistic to Vibrio parahaemolyticus for use as probiotics in aquaculture. World J Microbiol Biotechnol 31(5):795–803
Liu H, Wang S, Cai Y, Guo X, Cao Z, Zhang Y, Xie Z (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 60:326–333
Ma CW, Cho YS, Oh KH (2009) Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11. Aquaculture 287(3):266–270
Maeda M, Shibata A, Biswas G, Korenaga H, Kono T, Itami T, Sakai M (2014) Isolation of lactic acid bacteria from kuruma shrimp (Marsupenaeus japonicus) intestine and assessment of immunomodulatory role of a selected strain as probiotic. Mar Biotechnol 16(2):181–192
Mancuso M, Rappazzo AC, Genovese M, El Hady M, Ghonimy A, Ismail M, Reda R, Cappello S, Genovese L, Maricchiolo G (2015) In vitro selection of bacteria and isolation of Probionts from farmed Sparus aurata with potential for use as probiotics. Int J Animal Biol 1(4):93–98
Medellin-Pena MJ, Wang H, Johnson R, Anand S, Griffiths MW (2007) Probiotics affect virulence-related gene expression in Escherichia coli O157: H7. Appl Environ Microbiol 73(13):4259–4267
Mehrabi F, Khalesi M, Hazaie K (2018) Effects of pre-and probiotics on growth, survival, body composition, and hematology of common carp (Cyprinus carpio L.) fry from the Caspian Sea. Turk J Fish Aquat Sci 18(4):597–602
Meidong R, Khotchanalekha K, Doolgindachbaporn S, Nagasawa T, Nakao M, Sakai K, Tongpim S (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellfish Immunol 73:1–10
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RT, Bogwald J, Castex M, Ringo E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1):1–1)18
Metchnikoff E (1907) The prolongation of life. Optimistic studies. William Heinemann, London
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55(1):165–199
Mo WY, Man YB, Wong MH (2018) Use of food waste, fish waste and food processing waste for China's aquaculture industry: needs and challenge. Sci Total Environ 613:635–643
Mohapatra S, Chakraborty T, Kumar V, DeBoeck G, Mohanta KN (2013) Aquaculture and stress management: a review of probiotic intervention. J Anim Physiol Anim Nutr 97(3):405–430
Moosavi-Nasab M, Abedi E, Moosavi-Nasab S, Eskandari MH (2014) Inhibitory effect of isolated lactic acid bacteria from Scomberomorus commerson intestines and their bacteriocin on Listeria innocua. Iran Agric Res 33(1):43–52
Mukherjee A, Dutta D, Banerjee S, Ringo E, Breines EM, Hareide E, Ghosh K (2016) Potential probiotics from Indian major carp, Cirrhinus mrigala. Characterization, pathogen inhibitory activity, partial characterization of bacteriocin and production of exoenzymes. Res Vet Sci 108:76–84
Munoz-Atienzal E, Gomez-Sala B, Araujo C, Campanerol C, del Campo R, Hernandez PE, Herranz C, Cintas LM (2013) Antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol 13(1):15
Nageswara PV, Babu DE (2006) Probiotics as an alternative therapy to minimize or avoid antibiotics use in aquaculture. Fishing Chimes 26(1):112–114
Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ringo E, Ray AK (2017) Probiotic potential of autochthonous bacteria isolated from the gastrointestinal tract of four freshwater teleost. Probiotics Antimicrob Proteins 9(1):12–21
Neilands JB (1981) Iron absorption and transport in microorganisms. Annu Rev Nutr 1(1):27–46
Newaj-Fyzul A, Austin B (2015) Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. J Fish Dis 38(11):937–955
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt J, Austin B (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103(5):1699–1706
Nurhajati J, Aryantha INP, Indah DG (2012) The curative action of Lactobacillus plantarum FNCC 226 to Saprolegnia parasitica A3 on catfish (Pangasius hypophthalamus Sauvage). Int Food Res J 19(4):1723–1727
Nwanna LC (2015) Use of probiotics in aquaculture. Appl Trop Agr J 15(2):76–83
Oelschlaeger TA (2010) Mechanisms of probiotic actions–a review. Int J Med Microbiol 300(1):57–62
Olsson JC, Westerdahl ALLAN, Conway PL, Kjelleberg STAFFAN (1992) Intestinal colonization potential of turbot (Scophthalmus maximus) and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol 58(2):551–556
Ouwehand AC, Kirjavainen PV, Gronlund MM, Isolauri E, Salminen SJ (1999a) Adhesion of probiotic micro-organisms to intestinal mucus. Int Dairy J 9(9):623–630
Ouwehand AC, Kirjavainen PV, Shortt C, Salminen S (1999b) Probiotics: mechanisms and established effects. Int Dairy J 9(1):43–52
Padmavathi P, Sunitha K, Veeraiah K (2012) Efficacy of probiotics in improving water quality and bacterial flora in fish ponds. Afr J Microbiol Res 6(49):7471–7478
Pan X, Wu T, Song Z, Tang H, Zhao Z (2008) Immune responses and enhanced disease resistance in Chinese drum, Miichthysmiiuy (Basilewsky), after oral administration of live or dead cells of Clostridiumbutyrium CB2. J Fish Dis 31(9):679–686
Pandiyan P, Balaraman D, Thirunavukkarasu R, George EGJ, Subaramaniyan K, Manikkam S, Sadayappan B (2013) Probiotics in aquaculture. Drug invent 5(1):55–59
Pandya D (2016) Benefits of probiotics in Oral cavity–a detailed review. Ann Int Med Dental Res 2(5):1–17
Panigrahi A, Azad IS (2007) Microbial intervention for better fish health in aquaculture: the Indian scenario. Fish Physiol Biochem 33(4):429–440
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243(1):241–254
Panigrahi A, Viswanath K, Satoh S (2011) Real-time quantification of the immune gene expression in rainbow trout fed different forms of probiotic bacteria Lactobacillus rhamnosus. Aquaculture Res 42(7):906–917
Park Y, Lee S, Hong J, Kim D, Moniruzzaman M, Bai SC (2017) Use of probiotics to enhance growth, stimulate immunity and confer disease resistance to Aeromonas salmonicida in rainbow trout (Oncorhynchus mykiss). Aquaculture Res 48(6):2672–2682
Parker RB (1974) Probiotics, the other half of the antibiotics story. Anim Nutr Health 29:4–8
Patra A, Sarker S, Banerjee S, Adikesavalu H, Biswas D, Abraham TJ (2016) Rapid detection of Flavobacterium columnare infection in fish by species-specific polymerase chain reaction. J Aquac Res Dev 7:1–4
Pieters N, Brunt J, Austin B, Lyndon AR (2008) Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 105(3):723–732
Queiroz JF, Boyd CE (1998) Effects of a bacterial inoculum in channel catfish ponds. J World Aquacult Soc 29(1):67–73
Rajikkannu M, Natarajan N, Santhanam P, Deivasigamani B, Ilamathi J, Janani S (2015) Effect of probiotics on the haematological parameters of Indian major carp (Labeo rohita). Int J Fish Aquat Stud 2(5):105–109
Ramesh D, Souissi S (2018) Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquaculture Res 49(1):367–377
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45(2):268–276
Ramzani SR, Ismail MM, Daud HM, Abdurofi I (2014) Probiotic application in freshwater prawns; some implication on farm profitability. Ann Biol Res 5(5):64–76
Ravi AV, Musthafa KS, Jegathammbal G, Kathiresan K, Pandian SK (2007) Screening and evaluation of probiotics as a bio-control agent against pathogenic Vibrios in marine aquaculture. Lett Appl Microbiol 45(2):219–223
Ringo E, Vadstein O (1998) Colonization of Vibrio pelagius and Aeromonascaviae in early developing turbot (Scophthalmus maximus L.) larvae. J Appl Microbiol 84(2):227–233
Ringo E, Strom E, Tabachek JA (1995) Intestinal microflora of salmonids: a review. Aquaculture Res 26(10):773–789
Robertson PAW, O'Dowd C, Burrells C, Williams P, Austin B (2000) Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchusmykiss, Walbaum). Aquaculture 185(3):235–243
Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J (2009) Effects of single and combined supplementation of Enterococcus faecalis, mannan oligosaccharide and polyhydroxybutyrate acid on growth performance and immune response of rainbow trout Oncorhynchus mykiss. Aquac Sci 57(4):609–617
Ronneseth A, Castillo D, D'Alvise P, Tonnesen O, Haugland G, Grotkjar T, Engell-Sorensen K, Norremark L, Bergh O, Wergeland HI, Gram L (2017) Comparative assessment of vibrio virulence in marine fish larvae. J Fish Dis 40(10):1373–1385
Sahoo TK, Jena PK, Nagar N, Patel AK, Seshadri S (2015) In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catlacatla. Probiotics Antimicrob Proteins 7(2):126–136
Sahu MK, Swarnakumar NS, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Ind J Microbiol 48(3):299–308
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172(1):63–92
Sakata T (1990) Microflora in the digestive tract of fish and shell-fish. Microbiol Poecilotherms:171–176
Salinas I, Cuesta A, Esteban MA, Meseguer J (2005) Dietary administration of Lactobacillus delbrueckii and Bacillus subtilis, single or combined, on gilthead sea bream cellular innate immune responses. Fish Shellfish Immunol 19(1):67–77
Salminen S, Isolauri E, Salminen E (1996) Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Van Leeuwenhoek 70(2):347–358
Salminen S, Ouwehand AC, Isolauri E (1998) Clinical applications of probiotic bacteria. Int Dairy J 8(5–6):563–572
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28(4):405–440
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17(5):741–754
Sharifuzzaman SM, Austin B (2010) Kocuria SM1 controls vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 108(6):2162–2170
Sharifuzzaman SM, Abbass A, Tinsley JW, Austin B (2011) Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchusmykiss, Walbaum) against Vibrioanguillarum. Fish Shellfish Immunol 30(1):347–353
Sharifuzzaman SM, Rahman H, Austin DA, Austin B (2017) Properties of probiotics Kocuria SM1 and Rhodococcus SM2 isolated from fish guts. Probiotics Antimicrob Proteins:1–9
Skjermo J, Bakke I, Dahle SW, Vadstein O (2015) Probiotic strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture 438:17–23
Smith P, Davey S (1993) Evidence for the competitive exclusion of Aeromonas salmonicida from fish with stress-inducible furunculosis by a fluorescent pseudomonad. J Fish Dis 16(5):521–524
Srisapoome P, Areechon N (2017) Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): laboratory and on-farm trials. Fish Shellfish Immunol 67:199–210
Sun YZ, Yang HL, Huang KP, Ye JD, Zhang CX (2013) Application of autochthonous Bacillus bioencapsulated in copepod to grouper Epinephelus coioides larvae. Aquaculture 392:44–50
Sung HH, Kou GH, Song YL (1994) Vibriosis resistance induced by glucan treatment in tiger shrimp (Penaeus monodon). Fish Pathol 29(1):11–17
Swapna HC, Rai AK, Bhaskar N, Sachindra NM (2010) Lipid classes and fatty acid profile of selected Indian fresh water fishes. J Food Sci Technol 47(4):394–340
Taoka Y, Maeda H, Jo JY, Kim SM, Park SI, Yoshikawa T, Sakata T (2006) Use of live and dead probiotic cells in tilapia Oreochromisniloticus. Fish Sci 72(4):755–766
Thankappan B, Ramesh D, Ramkumar S, Natarajaseenivasan K, Anbarasu K (2015) Characterization of Bacillus spp. from the gastrointestinal tract of Labeo rohita towards to identify novel probiotics against fish pathogens. Appl Biochem Biotechnol 175(1):340–353
Thirumurugan R, Vignesh V (2015) Probiotics: live boon to aquaculture. In: Advances in marine and brackishwater aquaculture. Springer India, pp 51–61
Timmerman HM, Koning CJM, Mulder L, Rombouts FM, Beynen AC (2004) Monostrain, multistrain and multispecies probiotics– a comparison of functionality and efficacy. Int J Food Microbiol 96(3):219–233
Tinh NTN, Dierckens K, Sorgeloos P, Bossier P (2008) A review of the functionality of probiotics in the larviculture food chain. Mar Biol 10(1):1–12
Tovar-Ramırez D, Infante JZ, Cahu C, Gatesoupe FJ, Vazquez-Juarez R (2004) Influence of dietary live yeast on European sea bass (Dicentrarchus labrax) larval development. Aquaculture 234(1):415–427
Uyeno Y, Shigemori S, Shimosato T (2015) Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ 30(2):126–132
Van Hai N, Fotedar R (2009) Comparison of the effects of the prebiotics (Bio-Mos® and β-1, 3-D-glucan) and the customised probiotics (Pseudomonas synxantha and P. aeruginosa) on the culture of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture 289(3):310–316
Van Hai N, Buller N, Fotedar R (2010) Encapsulation capacity of Artemia nauplii with customized probiotics for use in the cultivation of western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture Res 41(6):893–903
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64(4):655–671
Vine NG, Leukes WD, Kaiser H (2004) In vitro growth characteristics of five candidate aquaculture probiotics and two fish pathogens grown in fish intestinal mucus. FEMS Microbiol Lett 231(1):145–152
WHO (2001) Health and nutritional properties of probiotics in food including powder milk with lactic acid bacteria. Medicine Rep 12(10):328–330
Wiklund T (2016) Pseudomonas anguilliseptica infection as a threat to wild and farmed fish in the Baltic Sea. Microbiol Aust 37(3):135–136
Wolf G (2006) Gut microbiota: a factor in energy regulation. Nutr Rev 64(1):47–50
Wu ZQ, Jiang C, Ling F, Wang GX (2015) Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp (Ctenopharyngodon idellus). Aquaculture 438:105–114
Zapata AA, Lara-Flores M (2012) Antimicrobial activities of lactic acid bacteria strains isolated from Nile tilapia intestine (Oreochromis niloticus). J Biol Life Sci 4(1):164–171
Zhou X, Wang Y, Yao J, Li W (2010) Inhibition ability of probiotic, Lactococcus lactis, against A. hydrophila and study of its immunostimulatory effect in tilapia (Oreochromis niloticus). Int J Eng Sci Technol 2(7):73–80
Zorriehzahra MJ, Delshad ST, Adel M, Tiwari R, Karthik K, Dhama K, Lazado CC (2016) Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet Q 36(4):228–241
Acknowledgements
The authors would like to thank to Lovely Professional University, India to provide the database for writing this review and Dr. Rama Gaur Department of Chemistry, School of Chemical Engineering & Physical Sciences, Lovely Professional University, India for her help and guidance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chauhan, A., Singh, R. Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77, 99–113 (2019). https://doi.org/10.1007/s13199-018-0580-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-018-0580-1