Abstract
In the present study rhizospheric bacteria were isolated from sand dune-dwelling Artemisia princeps, Chenopodium ficifolium, Oenothera biennis, and Echinochloa crus-galli and evaluated the ability of the bacterial isolates to produce jasmonic acid (JA) and abscisic acid (ABA) under NaCl-induced salt stress. We observed that 7 of 126 bacterial isolates were capable of producing siderophores, gibberellic acid (GA), indole-3-acetic acid (IAA), phosphate solubilisation, organic acids e.g., quinic acid, succinic acid, acetic acid and butyric acid. A bioassay of the seven selected isolates on rice showed that the isolate AK1 significantly promoted rice growth. Moreover, AK1 produced IAA and ABA in broth spiked with elevated levels of NaCl (100 mM, 200 mM, 300 mM, and 400 mM). The isolate AK1 was further investigated for plant growth promotion and mitigation of NaCl-induced salt stress in soybean grown under 100 mM, 200 mM, and 300 mM stress. Application of AK1 upregulated the expression of GmLAXs, and GmST genes in plants exposed to salt stress as compared to uninoculated plants. Interestingly, the bacteria-treated soybean showed significant increase in growth attributes with or without salinity stress. The endogenous ABA and JA level of inoculated soybean plants declined under elevated salt stress, thus showing an enhanced stress mitigation. A similar ameliorative trend was observed for total proteins, polyphenol oxidase, and peroxidase activity under saline conditions. The isolate AK1 was identified as Arthrobacter woluwensis AK1 based on its 16S rDNA gene sequencing and subsequent phylogenetic analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Abiotic stresses severely impact the production and cultivation of agricultural crops (Jamil et al. 2011; Shahbaz and Ashraf 2013). Soil salinity is among the most devastating abiotic stressors and has decreased cultivated lands, plant growth, germination rate, crop productivity, yield quality, and associated microbial communities (Shrivastava and Kumar 2015; Wang et al. 2009). Estimates suggest that salinity severely affects over 20 and 33% of cultivated and irrigated lands worldwide, respectively (Singh and Jha 2016). More than 800 million hectares of land are affected by various levels of salinity. Salinity levels increase 10% annually due to poor irrigation practices, the weathering of native rocks, low precipitation, and high surface evaporation (Jamil et al. 2011). By the mid-twenty-first century, salinity might have caused a 50% loss of agricultural lands (Singh and Jha 2016).
Soybean is a salt-sensitive glycophyte, can easily be in influenced by salt stress at any stage of its developments leads to sever adversities (Phang et al. 2008). Salt stress generates osmotic stress due to reduced water availability in soil. Moreover, salt stress also leads to ionic stress triggered by imbalance of solutes in cytosolic solution (Conde et al. 2011). Salt stress significantly decrease almost all plant growth attributes e.g., root shoot length, leaf size chlorophyll content, biomass, quantity of internodes, protein contents and seed quality (Phang et al. 2008). The accessibility of the whole soybean genome sequence gives abundant chances to uncover the fundamental mechanisms of salt tolerance. Salt stress tolerance genes such as GmLAX3, and GmST1 are involved in cell signalling and eventually regulated downstream transcription factors and the effector genes for salt stress responses. GmST1, is responsible for the salts stress tolerance in plant at both adults and seedling stages. Moreover, GmST1 expression in plants increases sensitivity against the stress related plant hormones such as ABA and JA and tends to decrease the endogenous ABA and JA in plants exposed to salts stress. GmST1 also improved tolerance to drought and salt stress by decreasing ROS in plants. LAX gene is involved in stress response in plants and expression of LAX is important for vascular development in plants (Péret et al. 2012). LAX is responsible for the control of vascular patterning and xylem differentiation in plant (Fàbregas et al. 2015). In addition, a part of xylem differentiation, LAX regulates lateral root (LR) development by LR emergence and initiation (Swarup et al. 2008).
There are extensive efforts worldwide to address the issue of salinity, such as developing salt-resistant crops, using salt stress-mitigating chemicals, planting and cultivating halophytes, reducing salts in soils through leaching, and remediating salt-affected soils using organic matter conditioners (Albacete et al. 2015; Lakhdar et al. 2011; Zeng et al. 2014; Zhu and Gong 2014). However, salinity remains a problem and challenge for scientists to develop a less expensive, easily adaptable, and sustainable approach (Paul and Lade 2014). Recently, several beneficial microbes have been used to alleviate salt stress and improve crop growth in rice (Nakbanpote et al. 2014), maize (Marulanda et al. 2010), radish (Kaymak et al. 2009), wheat (Egamberdieva 2008; Upadhyay et al. 2012), canola (Siddikee et al. 2010), lettuce (Mayak et al. 2004), and tomato (Mayak et al. 2004; Tank and Saraf 2010). Such studies showed that PGPRs might be a possible solution to salinity, as they proved beneficial to plant growth and development under saline conditions.
Soybean is grown on an estimated 6% of the world’s arable land. Since the 1970s, soybean production has had the highest increase compared with other major crops and is utilized in various products including tofu, soy sauce, bean paste, soybean oil, and soy milk. Soybeans are sensitive to salinity. The symbiotic interaction between legumes and rhizobia is particularly sensitive to salt stress, which causes cell dehydration and ion accumulation. PGPRs are free microorganisms that colonize the rhizosphere of plants and provide beneficial effects (Kloepper et al. 2004; Lugtenberg and Kamilova 2009; Mayak et al. 2004), prevent detrimental effects of phyto-pathogens (Bloemberg and Lugtenberg 2001; Orhan 2016), while producing phyto-hormones such as indole-3-acetic acid and (IAA) and gibberellic acids (GAs), enhancing nitrogen fixation, and producing siderophores (Lucy and Reed 2004; Mayak et al. 2004; Orhan 2016; Richardson and Simpson 2011).
Recent studies on plant-microbe interactions suggest the use of microorganisms for the reclamation of saline soils and to rescue plant growth and productivity from the detrimental effects of salt stress. We hypothesized that the rhizospheres of sand dune-dwelling plants might present promising stress-evading and plant growth-promoting rhizobacteria. It was also assumed that the bacterial isolates living in sand dunes might be capable of single or multiple growth traits and of withstanding varying concentrations of salinity stress. We isolated PGPR from the rhizosphere of sand dune plants and characterized their potentialities. On the basis of initial screening, promising isolates with multiple beneficial traits were tested for IAA and ABA production in broths spiked with varying levels of NaCl. Further experiments were designed to elucidate the interactive mechanism of the selected isolate AK1 with soybean under severe imposed salinity stresses. To this objective, we evaluated the growth attributes, antioxidant activities, and phytohormonal levels of soybean.
2 Materials and methods
2.1 Bacterial strains isolated from sand dunes
We isolated bacteria from the rhizosphere of plants inhabiting sand dunes at Pohang beach, Korea. The plant root samples, along with rhizospheric soil, were individually packed in sterilized polythene zip-bags and stored in an ice box (0–6 °C) for safe transportation to the laboratory. The rhizospheric soil (1 g) collected from the roots of individual plants was used for serial dilution (10−1 until 10−9) using saline water (0.85%). The bacteria were isolated by directly plating serial dilutions on LB agar media and incubated at 28 °C until the appearance of bacterial colonies. The bacterial colonies were purified by streaking on LB agar media. After 24 h of incubation, the pure colonies were judged for morphological characteristics such as size, colour, shape, and growth pattern for apparent identification and differentiation of bacterial isolates.
2.2 Screening of bacterial isolates for indole acetic acid (IAA) production
The production of IAA by bacterial isolates was initially confirmed through Salkowski reagent (Patten 2002). A 2 mL supernatant of each bacterial isolate grown in LB media with and without tryptophan was added to 1 mL Salkowski’s reagent (50 mL 35% HClO4, 1 mL 0.5 M FeCl3) for 30 min in the dark; after 30 min, the development of a pink colour indicated IAA production.
2.3 Screening of bacterial isolates for phosphate solubilisation
Phosphate solubilisation was determined following the method of (Katznelson and Bose 1959). Plates containing trypticase soya agar medium supplemented with (Ca3 (PO4)2 were inoculated with 1 μL LB pure bacterial cultures. Plates were incubated at 30 °C and observed daily for 7 days until the formation of transparent “halos” around each colony.
2.4 Screening of bacterial isolates for siderophores production
Bacterial isolates were spot inoculated on chromeazurol ‘S’ agar plates (Schwyn and Neilands 1987). After 72 h of incubation at 28 °C, the cultures were analysed for the appearance of orange halos in contrast to blue background.
2.5 Screening bioassay for plant growth promotion
To test the growth stimulating potential and gibberellin production, experiments were carried out on the mutant rice cultivar Waito-C (GA-deficient). The sterilized seeds of rice cultivar ‘Waito-C’ were inoculated with selected bacterial isolates (109 cfu/mL) for 6 h in a shaking incubator. The seeds used in the control were mock treated in autoclaved water under the same condition. The inoculated seeds were sown in 0.8% agar media for 6 days under a controlled environment (14/10 h light/dark, 28/24 °C, 70% relative humidity, 250 μmol/m−2 s−1 light intensity). Plant growth characteristics were recorded at 2, 4, and 6 days after sowing.
2.6 Molecular identification of promising bacterial isolate AK1
Based on the best performance during the screening experiments, isolate AK1 was selected for further experimentation and identification. The genomic DNA was isolated following the standard protocol of (Sambrook and Russell 2001). The 16S rDNA gene was amplified and sequenced using the 27F (5′-AGA GTT TGA TC(C/A) TGG CTC AG-3′) and 1492R (5′-CGG (T/C)TA CCT TGT TAC GAC TT-3′) primers, as reported in (Khan et al. 2014). The BLAST search program of NCBI GenBank database/EzTaxon was used to determine the nucleotide sequence homology of the targeted bacterial isolate. For phylogenetic analysis, the neighbour joining (NJ) method was adopted using MEGA v. 6.1 (Tamura et al. 2013). The highly related sequences with the highest homology, highest query coverage, and lowest E-values were selected for alignment with ClustalW. The phylogenetic analysis was performed by constructing a NJ using 16S rDNA gene sequences from AK1 and related strains.
2.7 Isolate AK1 produces IAA and ABA (in vitro) under saline conditions
Isolate AK1 was grown in LB media (trypton 10 g, yeast extract 5 g, pH 7.0 ± 0.2, with L-tryptophan autoclaved for 15 min at 121 °C) spiked with elevated NaCl (100 mM, 200 mM, 300 mM, and 400 mM) for 3 days. The aim was to examine the ABA and IAA production dynamics under saline conditions. The culture media was centrifuged at 5000×g for 15 min to separate the cells from the culture broth. The culture broth was analysed for ABA and IAA contents.
For IAA analysis, samples were extracted, dried, and methylated with diazomethane following the method of (Ullah et al. 2013). The IAA content in the broths with elevated NaCl levels was calculated from the peak areas of IAA and compared to the corresponding known standards, as revealed by the gas chromatography mass spectrometry (GC-MS) in selected ion monitoring mode (SIM).
For ABA, the pH of the bacterial broth (without bacterial cells) was adjusted to 2.5 using 6 N HCl. Before partitioning the deuterated [(±)-3, 5, 5, 7, 7, 7-d6]-ABA, an internal standard was added to the CF and then partitioned with ethyl acetate (EtOAc). Further quantification and detection of ABA was conducted following the protocol of (Qi et al. 1998).
2.8 Isolate AK1 interaction with soybean under elevated salt stress
Seeds of soybean CV. Tae-Kwang were procured from the Soybean Genetic Resource Centre, Kyungpook National University, tested for viability, and used in the current study. Seeds were surface sterilized by treating with 2.5% sodium hypochlorite for 30 min, followed by thorough rinsing with autoclaved double-distilled water. Seeds were germinated for 10 days in germination trays, and uniform plants were obtained. The sterilized germination trays and pots were filled with autoclaved horticulture soil (121 °C, 15 psi for 15 min). The composition of horticultural soil was as follows: peat moss (10–15%), perlite (35–40%), coco peat (45–50%), zeolite (6–8%), and NH4+ ∼ 0.09 mg/g, NO3− ∼ 0.205 mg/g, P2O5 ∼ 0.35 mg/g, and K2O ∼ 0.1 mg/g. Randomly selected uniform rice seedlings were grown (V1 stage) individually in plastic pots (10 cm × 9 cm) for 21 days; the bases were used to prevent contamination through the leaching of irrigation water. The experimental design was: (1) Control, normal soybean without isolate AK1, (2) soybean plants inoculated with isolate AK1, (3) 100 mM salt stress treated soybean with or without isolate AK1, (4) 200 mM salt stress and with or without isolate AK1, (5) 300 mM salt stress, with or without isolate AK1. The growth chamber conditions were as follows: day/night cycle 14 h at 28 °C/10 h at 25 °C and 60–70% relative humidity. Bacterial cells dissolved in 35 mL sterilized double-distilled water were applied three times to ensure complete infection in the first treatment at the time of transplantation and two times consecutively at 1-week intervals. The harvested cells were then washed with 0.8% NaCl solution and dissolved in autoclaved double-distilled water adjusted to an optical density of 0.5. The analysed plant growth attributes include chlorophyll contents (SPAD-502 Minolta, Tokyo, Japan), shoot and root length, and fresh biomass at the time of harvest. For endogenous plant hormonal analysis, the soybean plants were immediately stored in liquid nitrogen and then freeze-dried for 1 week (Virtis Freeze Dryer, Gardiner, NY, USA).
2.9 RNA Extraction and RT-PCR analysis
GmLAX3, and GmST1 are among the key genes involved in salts stress response in the plants. Expression of the genes are an important parameter to assess the effect of salts stress response in the plants. In order to evaluate the expression of GmLAX3, and GmST1 in stressed and control plants. Leaves of soybeans were crushed in liquid nitrogen and total RNAs were extracted from the leaves using TRIzol™ Reagent (Thermo Scientific; USA) according to the manufacturer’s instruction. RNA was quality was monitored on 1% agarose gel. Total 5 μg of extracted RNA for each sample was used for construction of cDNA using SuperScript® III (Invitrogen; USA) according to the manufacturer’s instructions. The resulted cDNA (1 μl) was used for PCR using Taq polymerase (New England BioLabs; Ipswich, MA, USA) with 30 cycles of amplification using set of primers for GmLAXs, GmST and actin. Whereas actin was used as reference to monitor the expression level of selected genes.
2.10 GA detection in bacteria through GC/MS-SIM
Bacterial isolate AK1 was cultured, centrifuged at 10,000×g and filtered through 45 μm filter. The culture filtrate (CF) was used for analysis of different types of GAs through GC/MS SIM. Before column chromatography, deuterated GA internal standards ([17, 17-2H2] GA1, GA3, GA4, GA7, GA8, GA12 and GA20) were added to the CF. Quantification of GAs was performed according to the method described by Lee et al. (1998). The extracts were run through a C18 column (90–130 μm; Alltech, USA) to get 48 fractions. For each GA, 1 μL of aliquot was injected in GC/MS (Table 1). The GAs from CF (GA4, GA8, GA12 and GA20) were calculated from the peak area ratios. The retention time was determined using hydrocarbon standards to calculate the KRI (Kovats retention index) value.
2.11 Quantification of endogenous phytohormones
The endogenous ABA was extracted and quantified following (Qi et al. 1998). The freeze-dried whole plant parts (500 mg) were extracted and analysed via column chromatography along with a deuterated internal standard [(±)– 3,5,5,7,7,7–d6]–ABA. After methylation, the semi-pure fractions were injected into a gas chromatograph-mass spectrophotometer coupled with a selected ion monitor (Agilent Technologies, Palo Alto, CA, USA) to simultaneously monitor the ions of m/e 162 and 190 for Me-ABA and 166 and 194 for Me-[2H6]-ABA. The experiment was repeated in triplicate.
Endogenous JA was extracted and quantified from the freeze-dried samples (500 mg) of all treatments following the protocol of (McCloud and Baldwin 1997). The samples were passed through a series of extraction steps with an internal standard [9,10-2H2]-9,10-dihydro-JA (20 ng). The resultant semi-pure fraction was esterified with excess diazomethane and was analysed using GC–MS (6890 N network GC system and the 5973 network mass selective detector; Agilent Technologies) in the selected ion mode. The ion fragment was monitored at m/z 83 amu corresponding to the base peaks of JA and [9,10-2H2]-9,10-dihydro-JA. The endogenous JA content was estimated from the peak areas compared with the respective standards. The experiment was repeated in triplicate.
2.12 Polyphenol oxidase and peroxidase analysis
The activity of antioxidant enzymes, such as peroxidase (POD) and polyphenol oxidase (PPO) was analysed following the protocol outlined by (Kar and Mishra 1976), with slight modifications. Leaf samples (400 mg) were ground using a chilled mortar and pestle. The samples were then homogenized with 0.1 M potassium phosphate buffer (pH 6.8) and centrifuged at 4 °C for 15 min at 5000 rpm in a refrigerated centrifuge. A part of the supernatant was used to determine total protein contents whereas, apart was used to estimate enzymatic activities. The protein contents were estimated by Bradford assay (Bradford 1976) at OD 595 nm on a SHIMADZU spectrophotometer (Kyoto, Japan). The reaction mixture for the assay of POD contained 0.1 M potassium phosphate buffer (pH 6.8), 50 μl pyrogallol (50 μM), 50 μl H2O2 (50 μM), and 100 μl of the sample crude extract. The reaction mixture was incubated for 5 min at 25 °C, followed by the addition of 5% H2SO4 (v/v) to stop the enzymatic reaction. The level of purpurogallin formed was determined by the absorbance at 420 nm. While for the assay of polyphenol peroxidase (PPO) activity, the same reaction mixture containing the same components as that of POD excluding H2O2 was used, and the resulting assay was measured at 420 nm. One unit of POD and PPO was directly measured by an increase of 0.1 units of absorbance.
2.13 Analysis of organic acids through HPLC
Culture of AK1 was centrifuged at 10,000 rpm for 10 min and supernatant was filtered through 0.45 μm filter. Exactly 20 μl of CF was injected in HPLC injector in the operation condition of 0.001 N H2SO4 of mobile phase. The mobile phase was adjusted at isochratic flow at the rate of 0.6 mL/min. The column was operated at 25 °C and the concentrations of the organic acids were determined with help of RI detector. The values were determined with comparison of standards.
2.14 Statistical analysis
All experiments were repeated in triplicate, and the data collected from each repetition were pooled together. The data were then analysed using a two-way ANOVA followed by Bonferroni Post Hoc test (significance level ≤ 0.05). Each treatment was replicated 10 times throughout the study. A completely randomized design was used to compare the plant growth-regulating effects of PGPR on rice germination and soybean plants. The graphical presentation and statistical analyses were carried with the help of Graphpad Prism 5 (USA).
3 Results
3.1 Bacterial isolation and initial screening for Waito-C rice
The rhizosphere of Artemisia princeps, Chenopodium ficifolium, Oenothera biennis, and Echinochloa crus-galli – which inhabit the sand dunes on the eastern sea coast at Pohang – was yielded 126 bacterial isolates (Table 1). All isolates exhibited single or multiple plant growth-promoting traits, such as IAA and siderophore production and phosphate solubilisation. However, of the 126 bacterial isolates, only seven demonstrated all three traits (Table 2, Fig. 1a). On the basis of multiple PGP traits, these seven isolates were further analysed in rice. Our results on rice growth recorded at 2, 4, and 6 days after treatment revealed that the isolate AK1 significantly increased rice seedling growth as compared to control and other PGPRs.
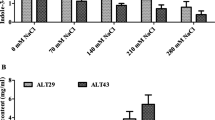
a Promotion of rice growth as affected by selected bacterial isolates. b ABA contents detected in the broth culture (BC) of isolate AK1. The isolate AK1 was grown in BC spiked with elevated concentrations of NaCl (100 mM, 200 mM, 300 mM, and 400 mM) and ABA was measured in ng/100 mL of CB. c IAA contents detected in the broth culture (BC) of isolate AK1. The isolate AK1 was grown in BC spiked with elevated concentrations of NaCl (100 mM, 200 mM, 300 mM, and 400 mM) and IAA was measured in μg/mL of CB. Columns with error bars represent the mean ± SD (n = 10). The data were analysed using a two-way ANOVA followed by Bonferroni test (significance level ≤ 0.05, n = 10). * indicates significant differences among the treatments
3.2 Phytohormones production of isolate AK1 under in-vitro abiotic stress
Characterization of isolate AK1 under varying salinity concentrations (0 mM, 100 mM, 200 mM, 300 mM, and 400 mM) revealed ABA and IAA production in the broth. Analysis of the cultured broth spiked with various salinity concentrations showed that isolate AK1 secreted ABA under both normal and stressed conditions. However, the ABA contents steadily increased with an increase in NaCl-induced salt stress (Fig. 1b). ABA production might be advantageous for species growing in restrictive soil conditions, such as salinity and drought (i.e., the synthesis of ABA could help alleviate stress in the plants). Contrary to the ABA contents in the broth, IAA levels significantly declined under NaCl-induced salt stress as compared to the control (Fig. 1c).
3.3 Organic acid detection in AK1
Some organic acids with low molecular weight, such as quinic acid, succinic acid, acetic acid, and butyric acids, were secreted by AK1 (Fig. 2a). Lactic and malic acids were not detected in culture medium. The key secreted organic acids of AK1 were succinic and acetic acids with the concentration up to 2.5 μg/mL. Quinic acid was detected at the concentration of about 1.5 μg/mL whereas, butyric acid was produced at the concentration of 0.5 μg/mL. In addition, the results revealed (Fig. 2b) that the CF of AK1 the presence of different types of bioactive GAs e.g., GA4, GA8, GA2 and GA20. The GC-MS-SIM analysis showed the existence of GA ion signals in correlation with [2H2] GA standards. The concentrations of the GAs were calculated by comparing their mass spectra and Kovats retention indices (KRI) with those available from a spectral library.
3.4 Isolate AK1 induces plant growth promotion
The present results demonstrated a significant increase in the plant length and biomass of isolate AK1-treated plants with or without salt stress. It was also absorbed that soybean growth was simultaneously inhabited with the increase in salt concentration. The plant-microbe interaction has reverted salinity stress as determined by a significant increase in root and shoot length and weight, particularly at concentrations of 200 mM and 300 mM NaCl (Fig. 3a-d). However, no significant difference was found for root and shoot length and weight between soybean plants inoculated with or without isolate AK1 under 100 mM NaCl. The SPAD value indicated that chlorophyll contents were significantly higher in plants inoculated with isolate AK1 than in respective control treatments (Fig. 3a-d).
Effect of isolate AK1 on growth attributes of soybean grown with or without NaCl (100 mM, 200 mM, and 300 mM: a shoot length, b root length, c shoot weight, d root weight, and (e) chlorophyll contents. Columns with error bars represent the mean ± SD (n = 10). The data were analysed using a two-way ANOVA followed by Bonferroni test (significance level ≤ 0.05, n = 10). * indicates significant differences among the treatments
Under normal condition, the inoculation of isolate AK1 in soybean plants significantly increased shoot length and weight and root weight compared to the control (Fig. 3a–d). However, insignificant differences were observed for root length and chlorophyll contents of isolate AK1-inoculated and non-inoculated soybean plants (Fig. 3b, e).
3.5 Endogenous ABA and JA content of soybean
The current study observed an increase in ABA levels of the inoculated and non-inoculated soybean plants in a NaCl dose-dependent manner. However, the inoculation of isolate AK1 demonstrated its stress-mitigating capacity, as it significantly reduced the endogenous ABA contents compared to the non-inoculated plants under 100 mM, 200 mM, and 300 mM salt concentrations. In the control treatments, no significant differences were found for endogenous ABA levels of isolate AK1 treated and control soybean plants (Fig. 4a). Moreover, the results showed that elevated salt stress triggered a significant increase in the endogenous JA contents of soybean. However, application of AK1 reduced significantly (p < 0.5) the JA contents in plants as compared to uninoculated plants (Fig. 4b).
Quantification of endogenous ABA and JA contents in soybean. a The effect of isolate AK1 on the ABA contents of soybean plants grown under elevated NaCl (100 mM, 200 mM, and 300 mM) and ABA was measured in ng/g of DW of whole plant. b The effect of isolate AK1 on JA contents of soybean plants grown under elevated NaCl (100 mM, 200 mM, and 300 mM) and JA contents were measured in ng/g of DW of whole plant. Columns with error bars represent the mean ± SD (n = 10). The data were analysed using a two-way ANOVA followed by Bonferroni test (significance level ≤ 0.05, n = 10). * indicates significant differences among the treatments
3.6 Expression profile of GmST1 and GmLAX3 inoculated with AK1 under salt stress
Role of GmST1 and GmLAX3 was determined in comparative expression levels in soybean plant exposed to salt stress and un-stressed plant. Actin was used as reference in trail of experiment. The results revealed that both GmST1 and GmLAX3 genes were expressed in plants exposed to 100, 200 and 300 mM of salt inoculated with AK1 as compared to plant exposed to the stress with inoculation of AK1. However, both of GmST1 and GmLAX3 expression levels were comparatively same in inoculated and uninoculated with AK1 plants grown in 0 mM of salt stress, used as control (Fig. 5).
3.7 Effect of isolate AK1 on total proteins and antioxidative activities of soybean
The total protein contents of soybean increased with the NaCl concentration gradient (Fig. 6a). However, the total protein contents significantly declined in plants treated with isolate AK1 as compared to non-inoculated plants under varying NaCl levels of 100 mM, 200 mM, and 300 mM salt concentrations. Under normal conditions, the sole application of isolate AK1 induced significantly higher protein contents in soybean plants as compared to non-inoculated plants (Fig. 6a).
Quantification of total proteins, polyphenol oxidase, and peroxidase in soybean. The effect of isolate AK1 on (a) total protein content, b polyphenol oxidase (PPO), and (c) peroxidase (POD) activities of soybean grown in elevated NaCl (100 mM, 200 mM, and 300 mM). Columns with error bars represent the mean ± SD (n = 10). The data were analysed using a two-way ANOVA followed by Bonferroni test (significance level ≤ 0.05, n = 10). * indicates significant differences among the treatments
The presence of NaCl causes oxidative stress in plants; therefore, we examined the behaviour of antioxidant enzymes (Fig. 6b and c). Our results demonstrated that salt stress significantly increased the activity of PPO in non-inoculated plants, as compared to the isolate AK1-inoculated plants (Fig. 6b). Additionally, isolate AK1 decreased the concentration of PPO in soybean plants under all NaCl-induced stress levels (100 mM, 200 mM, and 300 mM). However, no significant difference was observed for PPO activity between isolate AK1-inoculated and non-inoculated control treatments (Fig. 6b).
The activity of peroxidase increased in soybean plants similarly to that of total protein content treated with elevated NaCl levels (Fig. 6c). Under varying salinity stress, isolate AK1 significantly decreased the POD activity of soybean as compared to the non-inoculated soybean plants (Fig. 6c). However, a single application of isolate AK1 significantly enhanced the POD activity compared to the control soybean plants (Fig. 6c).
3.8 Molecular identification of isolate AK1
The novel plant growth-promoting bacterial isolate AK1 was identified as a new strain of Arthrobacter woluwensis based on 16S rDNA sequencing; this was confirmed by the phylogenetic analysis of the bacterial isolate (Fig. 7). The result of the BLAST search revealed that the bacterial isolate AK1 had maximum sequence homology with Arthrobacter woluwensis and was thus named Arthrobacter woluwensis AK1. The sequence was submitted to NCBI GenBank and was assigned the accession number MF276646.
Phylogenetic relationships between 16S rDNA gene sequences from isolate AK1 and related bacterial strains. The sequences were aligned by CLUSTAL W and the phylogenetic tree was constructed by the Neighbour Joing (NJ) method using MEGA6 software. Numbers at branch nodes represent the confidence level of 1000 bootstrap replications
4 Discussion
Alarmingly, salinity stress is one of the major abiotic stressors that has severely affected agriculture production. Salinity stress hinders plant growth and causes primary and secondary yield losses. Previous studies elaborated the spectrum of salinity stress on crops that affects their normal physiological, morphological, and biochemical functions (Shrivastava and Kumar 2015). However, the adverse effects of salt stress on plant growth could be alleviated through the help of PGPRs as shown from the current studies where an increase in biomass and chlorophyll content were recorded compared to control treatments. The beneficial effect of Arthrobacter woluwensis on soybean growth could likely be attributed to the production of IAA, as IAA is a known salinity stress ameliorant (Egamberdieva 2008). A similar study reported that IAA-producing bacteria could enhance plant growth under salt stress (Bianco and Defez 2009), concluding that rhizospheric bacteria inoculation in soybean increased the root growth and root length under salinity as compared to the control.
ABA is a well-known stress phytohormone and highly valued as stress marker in higher plants. A significant decrease in ABA production under the influence of isolate AK1 compared to salt-stressed soybean might improve plant growth and relieve salt stress. ABA production enhances the ability of PGPR with improved capacity of ABA production to alleviate salt stress in plants. (Cohen et al. 2008). The repressive effect of salinity on germination is believed to be related to a decline in endogenous levels of hormones (Debez and Bouzid 2011). The isolate AK1 in this study could be assigned to a third category of beneficial bacteria termed ‘plant stress homeo-regulating rhizobacteria’ (PSHR).
Results of the present study showed that GmST1 and GmLAX3 genes were down regulated under the stress. However, application of AK1 stimulated the expression of GmST1 and GmLAX3 in plants exposed to salt stress. Previously studies reported that under the influence of different abiotic stressors, such as drought and salinity, plants normally go through growth declination followed by death (Le et al. 2012). However, genome-wide transcriptomic analysis of the soybean, revealed that a number of hormone-related genes were expressed differentially in shoot as well as in root under salinity and drought stresses (Song et al. 2016). Auxin such as IAA and ABA are two of the most significant plant hormones, regulating plant growth and plant responses to salt stress. Application of PGP microbes producing auxins and ABA induce the expression of stress response genes such as GmST1 and GmLAX3 and hence tolerance in plant against salt stress (Rahman 2013). numerous studies have reported that IAA might facilitate plant’s adaptions to salt stress and other adverse environmental stressors (Kazan 2013). In addition, Ren et al. (2016) also investigated the role of GmST1 and GmLAX3 in abiotic stress tolerance when overexpressed in plants, which demonstrated that overexpression of the genes in plants produced strong tolerance to salt stress at both seedling and adult stages. The expression of GmST1 and GmLAX3 were regulated through an ABA-dependent pathway in salt tolerance. Taken together, these results assured at least a partial involvement of ABA in GmST1 and GmLAX3-mediated salt tolerance.
The phytohormone jasmonate and its metabolites regulate plant growth and developmental processes and actively contribute to plant defence responses to biotic and abiotic stressors (Pauwels et al. 2009; Turner et al. 2002). This study demonstrated that elevated salt stress triggered a significant increase in the endogenous JA contents of soybean. Moreover, the inoculation of isolate AK1 significantly reduced the JA contents compared to non-inoculated plants under varying levels of salt stress. An increase and decrease in JA biosynthesis in control plants and salt-stressed plants, respectively, shows the importance of JA in stress regulation for soybean. Previous research also showed that both drought and high salinity caused an increase in endogenous JA levels in the leaves and roots of rice (Kiribuchi et al. 2005). However, little information is available on the role of PGPR on JA biosynthesis of associated plants.
The present study revealed that AK1 not only enhanced soybean growth under control environment but also under salt stress. The AK1 produced GA4, which is considered as the most bioactive precursor for GA3 (Khan et al. 2014). The GA4 also exhibit extended stability than GA3 and GA7 during culture fermentation (Albermann et al. 2013). Production of GAs is an important trait enabling endophytes to promote plant growth and mitigate salt stress (Hamayun et al. 2015). The higher amount of GA in endophyte-treated plants under salinity stress elucidates the activation of GAs biosynthesis pathway, while higher production of GA4 confirms plant growth maintenance during stress condition. Thus, by maintaining GAs and, therefore, growth under stress conditions, the endophyte is having a beneficial effect on the plant long-term survival. There are many previous reports showing the ameliorative effects of GAs on plant growth under abiotic stress (Siddiqui et al. 2008; Arteca 2013).
Our results revealed that AK1 produced different types of low molecular weight organic acid. Production of organic acids cause lowering the pH which leads to phosphate solubilization. In the rhizosphere, bacteria secrete organic acids which results in phosphate solubilization from insoluble complexes, making it available for plant uptake (Richardson and Simpson 2011). Harmful effects of salt stress on several plants such as pepper, soybean, cucumber, and rice have been reported in previous studies by Wang et al. (2003), Hasegawa et al. (2000), Munns and Tester (2008), Khan et al. (2014), suggested the formation of ROS, which cause severe damage to cell structures. However, a defence system that is activated under stressed conditions consists of several ROS-scavenging enzymes, such as POD and PPO. These antioxidant enzymes have the ability to remove the free radicals produced during abiotic stress conditions in the cell (Abogadallah 2011). Inoculation of AK1 to soybean significantly elevation of antioxidant enzyme activities (PPO, and POD) under salinity compared to control plants. Inoculated plants might reduce ROS through PPO, and POD activities and improved all plant growth attributes such as shoot and root lengths as well as biomass of the plants when compared to salinity treatment alone. Increased root, shoot lengths and biomass in inoculated plants have been reported in the result of inoculation of many endophytic genera such as Burkholderia, Azoarcus, Gluconobacter, Pantoea, Klebsiella, Rahnella, Herbaspirillum, and Pseudomonas (Elbeltagy et al. 2001; Hurek et al. 2002; Iniguez et al. 2004; Feng et al. 2006; Momose et al. 2009; Botta et al. 2013).
5 Conclusions
Plants are exposed to biotic and abiotic stressors throughout their lifespan, and their productivity mostly depends on their capacity to cope with unfavourable environmental conditions. Our current findings support our hypothesis that the isolation of PGPR from the rhizosphere of plants inhabiting stress-prone environments might yield novel bacterial isolates of a halophilic and halo-tolerant nature, capable of supporting plant growth and the alleviation of salt stress under saline conditions. In this study, we isolated a novel halo-tolerant PGPR A. woluwensis from plants living in a harsh sand dune environment. This study improves our understanding of the physiological mechanisms operating inside microbes, while facing abiotic stressor as shown from ABA and IAA contents detected in the bacterial culture broth. The bacterial inoculation to soybean further revealed the ameliorative role of PGPR in regulating salt stress via manipulating ABA and JA signalling pathways. We conclude that A. woluwensis AK1 could be an eco-friendly bio-fertilizer based on its ameliorative qualities under saline conditions. Such bacterial isolates might be useful in formulating new inoculants with combinations of different mechanisms of action, leading to a more efficient way for bio-control strategies to improve cropping systems.
References
Abogadallah GM (2011) Differential regulation of photorespiratory gene expression by moderate and severe salt and drought stress in relation to oxidative stress. Plant Sci 180:540–547. https://doi.org/10.1016/j.plantsci.2010.12.004
Albacete A, Martínez-Andújar C, Martínez-Pérez A, Thompson AJ, Dodd IC, Pérez-Alfocea F (2015) Unravelling rootstock×scion interactions to improve food security. J Exp Bot 66:2211–2226. https://doi.org/10.1093/jxb/erv027
Albermann S, Elter T, Teubner A, Krischke W, Hirth T, Tudzynski B (2013) Characterization of novel mutants with an altered gibberellin spectrum in comparison to different wild-type strains of Fusarium fujikuroi. Appl Microbiol Biotechnol. 97:7779–7790
Arteca RN (2013) Plant Growth Substances: Principles and Applications. Springer Science & Business Media, Berlin
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107. https://doi.org/10.1093/jxb/erp140
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Botta AL, Santacecilia A, Ercole C, Cacchio P, Del Gallo M (2013) In vitro and in vivo inoculation of four endophytic bacteria on L-ycopersicon esculentum. New Biotechnol 30:666–674
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cohen AC, Bottini R, Piccoli PN (2008) Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul 54:97–103. https://doi.org/10.1007/s10725-007-9232-9
Conde A, Chaves MM, Geros H (2011) Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol 52:1583–1602. https://doi.org/10.1093/pcp/pcr107
Debez ACW, Bouzid S (2011) Effect du NaCl et de regulatoeurs de croissance sur la germination d’ Atriplex halimus L. Cah Agric 10:135–138
Egamberdieva D (2008) Plant growth promoting properties of rhizobacteria isolated from wheat and pea grown in loamy sand soil. Turk J Biol 32:9–15
Elbeltagy A, Nishioka K, Sato T, Suzuki H, Ye B, Hamada T, Isawa T, Mitsui H (2001) Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl Environ Microbiol 67:5285–5293
Fàbregas N, Formosa-Jordan P, Confraria A, Siligato R, Alonso JM, Swarup R (2015) Auxin in flux carriers control vascular patterning and xylem differentiation in Arabidopsis thaliana. PLoS Genet 11:e1005183. https://doi.org/10.1371/journal.pgen.1005183
Feng Y, Shen D, Song W (2006) Rice endophyte Pantoea agglomerans YS19 promotes host plant growth and affects allocations of host photosynthates. J Appl Microbiol 100:938–945
Hamayun M, Hussain A, Khan SA, Irshad M, Khan AL, Waqas M, Shahzad R, Iqbal A, Ullah N, Rehman G, Kim HY, Lee IJ (2015) Kinetin modulates physio-hormonal attributes and isoflavone contents of Soybean grown under salinity stress. Front Plant Sci 6:377. https://doi.org/10.3389/fpls.2015.00377
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Hurek T, Handley LL, Reinhold-Hurek B, Piché Y, De C, Pavillon C, Laval U, Gk-p C (2002) Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol Plant-Microbe Interact 15:233–242
Iniguez AL, Dong Y, Triplett EW (2004) Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol Plant-Microbe Interact 17:1078–1085
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene Expression Profiling of Plants under Salt Stress. Crit Rev Plant Sci 30:435–458. https://doi.org/10.1080/07352689.2011.605739
Kar M, Mishra D (1976) Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol 57:315–319
Katznelson H, Bose B (1959) Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Can J Microbiol 5:79–85
Kaymak HÇ, Güvenç İ, Yaralı F, Dönmez MF (2009) The effects of bio-priming with PGPR on germination of radish (Raphanus sativus L) seeds under saline conditions. Turk J Agric For 33:173–179
Kazan K (2013) Auxin and the integration of environmental signals in to plant root development. Ann Bot 112:1655–1665. https://doi.org/10.1093/aob/mct229
Khan AL et al (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52:689–695. https://doi.org/10.1007/s12275-014-4002-7
Kiribuchi K et al (2005) Involvement of the basic helix-loop-helix transcription factor RERJ1 in wounding and drought stress responses in rice plants. Biosci Biotechnol Biochem 69:1042–1044. https://doi.org/10.1271/bbb.69.1042
Kloepper JW, Ryu CM, Zhang SA (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266. https://doi.org/10.1094/Phyto.2004.94.11.1259
Lakhdar A, Hafsi C, Debez A, Montemurro F, Jedidi N, Abdelly C (2011) Assessing solid waste compost application as a practical approach for salt-affected soil reclamation. Acta Agr Scand B-S P 61:284–288. https://doi.org/10.1080/09064710.2010.485738
Le DT, Nishiyama R, Watanabe Y, Tanaka M, Seki M (2012) Differential gene expression in soybean leaf tissue satlate developmental stage sunder drought stress revealed by genome-wide transcriptome analysis. PLoSONE 7:e49522. https://doi.org/10.1371/journal.pone.0049522
Lee IJ, Foster KR, Morgan PW (1998) Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 116:1003–1011
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86:1–25. https://doi.org/10.1023/B:ANTO.0000024903.10757.6e
Lugtenberg B, Kamilova F (2009) Plant-Growth-Promoting Rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Marulanda A, Azcon R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232:533–543. https://doi.org/10.1007/s00425-010-1196-8
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. https://doi.org/10.1016/j.plaphy.2004.05.009
McCloud ES, Baldwin IT (1997) Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 203:430–435. https://doi.org/10.1007/s004250050210
Momose A, Ohtake N, Sueyoshi K, Sato T, Nakanishi Y, Akao S, Ohyama T (2009) Nitrogen Fixation and Translocation in Young Sugarcane (Saccharum officinarum L.) Plants Associated with Endophytic Nitrogen-Fixing Bacteria. Microbes Environ 24:224–230
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakbanpote W, Panitlurtumpai N, Sangdee A, Sakulpone N, Sirisom P, Pimthong A (2014) Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J Plant Interact 9:379–387. https://doi.org/10.1080/17429145.2013.842000
Orhan F (2016) Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz J Microbiol 47:621–627. https://doi.org/10.1016/j.bjm.2016.04.001
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Paul D, Lade H (2014) Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev 34:737–752. https://doi.org/10.1007/s13593-014-0233-6
Pauwels L, Inzé D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14:87–91. https://doi.org/10.1016/j.tplants.2008.11.005
Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S (2012) AUX/LAX genes encode a family of auxin in flux transporters that perform distinct functions during Arabidopsis development. Plant Cell 24:2874–2885. https://doi.org/10.1105/tpc.112.097766
Phang TH, Shao G, Lam HM (2008) Salt tolerance in soybean. J Integr Plant Biol 50:1196–1212. https://doi.org/10.1111/j.1744-7909.2008.00760.x
Qi QG, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ (1998) (+)-abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol 117:979–987. https://doi.org/10.1104/Pp.117.3.979
Rahman A (2013) Auxin: are gulator of colds tress response. Physiol Plant 147:28–35. https://doi.org/10.1111/j.1399-3054.2012.01617.x
Ren S, Lyle C, Gl J, Penumala A (2016) Soybean salt tolerance 1 (GmST1) reduces ROS production, enhances ABA sensitivity, and abiotic stress tolerance in Arabidopsis thaliana. Front Plant Sci 7:445
Richardson AE, Simpson RJ (2011) Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol 156:989–996. https://doi.org/10.1104/pp.111.175448
Sambrook JF, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold SpringHarbor Laboratory Press, New York, pp 19–20
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shahbaz M, Ashraf M (2013) Improving Salinity Tolerance in Cereals. Crit Rev Plant Sci 32:237–249. https://doi.org/10.1080/07352689.2013.758544
Shrivastava P, Kumar R (2015) Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol 20:1577–1584
Siddiqui MH, Khan MN, Mohammad F, Khan MMA (2008) Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J Agron Crop Sci 194:214–224
Singh RP, Jha PN (2016) A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front Plant Sci 7 doi:https://doi.org/10.3389/fpls.2016.01890
Song L, Prince S, Valliyodan B, Joshi T et al (2016) Genome-wide transcriptome analysis of soybean primary root under varying water-deficit conditions. BMC Genomics 17:57. https://doi.org/10.1186/s12864-016-2378-y
Swarup K, Benkova E, Swarup R, Casimiro I, Péret B, Yang Y (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10:946–954. https://doi.org/10.1038/ncb1754
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5:51–58. https://doi.org/10.1080/17429140903125848
Turner JG, Ellis C, Devoto A (2002) The Jasmonate Signal Pathway. Plant Cell 14:s153–s164. https://doi.org/10.1105/tpc.000679
Ullah I, Khan AR, Park G-S, Lim J-H, Waqas M, Lee I-J, Shin J-H (2013) Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci Biotechnol 22:25–31. https://doi.org/10.1007/s10068-013-0044-6
Upadhyay SK, Singh JS, Saxena AK, Singh DP (2012) Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol (Stuttgart, Germany) 14:605–611. https://doi.org/10.1111/j.1438-8677.2011.00533.x
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wang SA, Wu HJ, Qiao JQ, Ma LL, Liu J, Xia YF, Gao XW (2009) Molecular Mechanism of Plant Growth Promotion and Induced Systemic Resistance to Tobacco Mosaic Virus by Bacillus spp. J Microbiol Biotechnol 19:1250–1258. https://doi.org/10.4014/jmb.0901.008
Zeng W, Xu C, Wu J, Huang J (2014) Soil salt leaching under different irrigation regimes: HYDRUS-1D modelling and analysis. Journal of Arid Land 6:44–58. https://doi.org/10.1007/s40333-013-0176-9
Zhu YX, Gong HJ (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472. https://doi.org/10.1007/s13593-013-0194-1
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries(IPET) through Agriculture, Food and Rural Affairs Research Center Support Program.
Funding
This research is funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (716001-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.A., Ullah, I., Waqas, M. et al. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 77, 9–21 (2019). https://doi.org/10.1007/s13199-018-0562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-018-0562-3