Abstract
Phosphorous (P) availability is a major concern in European agriculture where reserves are limited. In the case of pea (Pisum sativum L.), one of the most important legumes in the human diet, P has specific effects on nodulation and N2 fixation. Therefore, when biofertilization schemes are considered for pea cropping, it is very important to include symbiotic dinitrogen-fixing bacteria as well as phosphate-solubilizing bacteria (PSB). In this study sixteen PSB were isolated from the rhizosphere of two pea cultivars in two French soils with different characteristics. They were phenotypically and genotypically diverse displaying 9 different Two Primers-Random Amplified Polymorphic DNA (TP-RAPD) patterns. The 16S rRNA gene analysis of representative strains showed that they belong to four highly divergent phylogenetic groups. Most of the PSB strains belonged to the genus Pseudomonas and were closely related to Pseudomonas baetica, P. lutea, P. azotoformans, P. jessenii and P. frederiksbergensis. Other strains from the genus Burkholderia were closely related to B. caledonica and those from the genus Rhizobium to R. grahamii. The single strain of genus Bacillus was close to Bacillus toyonensis. Some phylogenetic groups to which our PSB strains belong are widely distributed in plant rhizospheres in different countries and continents. This is particularly interesting in the case of strains from the phylogenetic group of P. fluorescens which includes PSB strains with high ability to solubilize phosphate indicating that they may be used as biofertilizers in many soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pea (Pisum sativum L.) is one of the most important legumes in human diet. Its benefits for human health are derived from its protein, fiber, starch, vitamins, mineral and phytochemical contents, and these have been widely studied (Dahl et al. 2012). Moreover, pea is one of the legumes included in the last Common Agricultural Policy (CAP) reform to be used in greening practices (Hodge et al. 2015) since legumes are a source of biologically fixed nitrogen which helps to reduce the use and application of chemical nitrogen fertilizers and hence of the fossil fuels used to produce them (Bedoussac et al. 2015). Both fossil fuel and P sources are projected to be in serious decline by the end of the 21st century (Mohr et al. 2015; Withers et al. 2015), and their continued availability is a major concern in European countries where reserves are very limited. Therefore, plant biofertilizers are not only useful to produce healthier foods (García-Fraile et al. 2012; Flores-Félix et al. 2015), but they also constitute an alternative to chemical fertilization to provide essential elements for plant growth. Legumes are able to fix atmospheric N in symbiosis with rhizobia and, in the case of some legumes such as soybean, their inoculation with these bacteria has been performed for decades in order to supply N to plants (Catroux et al. 2001). However, legumes fixing atmospheric nitrogen in symbiosis have more P requirements than plants receiving nitrogen fertilisers (Graham and Vance 2000; Gyaneshwar et al. 2002), and particularly in pea P has specific effects on N2 fixation and nodule initiation, growth, development, and metabolic function (Jakobsen 1985). Therefore, biofertilization of pea with symbiotic N-fixing bacteria may be as important as with P solubilizing bacteria (PSB), which play a crucial role in soil P cycle and in the P-uptake by plants (Richardson and Simpson 2011). PSB have been identified on the basis of analysis of their rrs genes in several plant rhizospheres (Peix et al. 2003; Peix et al. 2004; Chung et al. 2005; Islam et al. 2007; Naik et al. 2008; Browne et al. 2009; Oliveira et al. 2009; Peix et al. 2009; Selvakumar et al. 2009; Collavino et al. 2010; Liu et al. 2011; Narveer et al. 2014; Xiang et al. 2011; Liu et al. 2014; Acevedo et al. 2014; Ruangsanka 2014), including those of some legumes, such as Lotus (Castagno et al. 2011), Glycine max (Li et al. 2008; Ndungu-Magiroi et al. 2012), Phaseolus vulgaris (Kumar et al. 2012a) and Vigna unguiculata. (Toro et al. 2013), but to the best of our knowledge there are no published studies on PSB from the rhizosphere of pea.
Therefore, the aims of this study were the phenotypic characterization of PSB strains isolated from the rhizosphere of pea in two French agricultural soils with different crop histories, the identification of these strains based on analysis of their 16S rRNA genes and on analysis of their phylogenetic relationships with previously reported PSB bacteria isolated from the rhizospheres of various plants in other countries and continents.
2 Materials and methods
2.1 Plant and soil conditions
The plants used for the experiment were peas cv. Frisson, which is a winter variety traditionally used in France. In order to study the occurrence and diversity of PSB associated with these plants two soils with different characteristics were chosen. Soil 1 (Epoisses) is a calcareous clay-loam soil from the East of France (Côte d’Or), in which pea was cultivated in 1982, and after which no legume has been cultivated. Soil 2 (La Bruyère) is a sandy soil which also comes from Eastern France (Côte d’Or), and it was last cultivated with pea in 2002. The samples were taken from different places in each plot, in the first 0–20 cm depth, sieved through a 5 mm mesh and homogenized. Jars of 2.5 L capacity were placed in a greenhouse containing 5 pea plants per pot. They were grown for 3 weeks.
2.2 Isolation of total and phosphate solubilizing bacteria (PSB)
Isolation and counts of total bacteria were performed in 3 different culture media to obtain the maximum culturable number and diversity of PSB. Medium A was a complex medium containing different C sources, with the following composition: 0.07 g l−1 K2HPO4, 0.2 g l−1 KH2PO4, 0.1 g l−1 MgSO4.7H2O, 0.05 g l−1 CaCl2.2H2O, 0.02 g l−1 FeCl3, 0.5 g l−1 NH4NO3, 1 g l−1 mannitol, 1 g l−1 glucose, 1 g l−1 sucrose, 1 g l−1 sodium acetate, 1 g l−1 sodium L-lactate, 100 mg l−1 yeast extract, 0.5 mg l−1 biotin, 0.5 m gl−1 thiamine hydrochloride, 0.5 mg l−1 calcium pantothenate and 15 g l−1 agar. Medium B was Tryptone Soy Agar (TSA, Becton-Dickinson Co.) diluted 1:10, and Medium C was based on cold soil extract (Lilley et al. 1996) and was prepared with extracts from soil 1 and from soil 2, separately (C1, C2). The three media were supplemented with cycloheximide (50 μg ml−1) to avoid fungal growth. Serial dilutions of both soils were performed by suspending 10 g of soil in 90 ml sterile water, and after shaking well for 30 min, decimal dilutions from 10−1 to 10−6 were obtained. From each one of them 200 μl were inoculated onto Petri dishes containing the three culture media and spread thoroughly with sterile glass balls. The plates were incubated at 28 °C for 7 days, and then counted in the dilution plates showing from 30 to 300 colonies.
PSB were selected in YED-P plates containing 1 % glucose, 0.5 % yeast extract, 0.2 % tricalcium phosphate and 2 % agar (Peix et al. 2001), by replica plating as indicated earlier (Lederberg and Lederberg 1952). The plates were incubated at 28 °C for 7 days, and the presence of PSB was detected by the halo produced surrounding the colonies. The strains showing high ability of phosphate solubilization by means of their halo diameter being 15 mm or larger according to De Freitas et al. (1997) were isolated and stocked in glycerol solution at −80 °C.
2.3 Phenotypic characterization
Physiological and biochemical tests were performed using the API 20NE system (BioMérieux, France) following the manufacturer’s instructions. The strains Pseudomonas fluorescens DSM 50108 (1147555), Burkholderia cepacia LMG 1222T (1067577) and Rhizobium radiobacter ATCC 19358T (1467744) were used as positive controls. The data obtained were coded in binary form and Jaccard’s coefficient was calculated to construct a similarity matrix. A dendrogram was obtained using the unweighted pair group method with arithmetic mean (UPGMA). Profile codes were obtained from the results of the tests using the APILAB database system (https://apiweb.biomerieux.com/jsp/ident/index.jsp).
2.4 Two Primers-Random Amplified Polymorphic DNA (TP-RAPD) pattern analysis
To obtain TP-RAPD patterns, PCR was performed using an AmpliTaq Gold reagent kit (Perkin-Elmer Biosystems, California, USA) and the primers 879 F and 1522R at a final concentration of 2 μmol l−1 (Rivas et al. 2002). PCR conditions were as follows: pre-heating at 95 °C for 9 min; 35 cycles of denaturing at 95 °C for 1 min; annealing at 50 °C for 1 min and extension at 72 °C for 2 min, and a final extension at 72 °C for 7 min. Electrophoresis was performed on 1.5 % agarose gels at 6 V cm−1.
2.5 16S rRNA gene sequence analysis
The 16S rRNA genes were nearly full-length sequenced as described by Rivas et al. (2007). The sequences obtained were compared with those from the EzTaxon-e database (Kim et al. 2012). Sequences were aligned using the Clustal W software (Thompson et al. 1997). The distances were calculated according to Kimura’s two-parameter method (Kimura 1980). A phylogenetic tree was inferred using the neighbour-joining method (Saitou and Nei 1987). Bootstrap analysis was based on 1000 re-samplings. The MEGA 5 package (Tamura et al. 2011) was used for all analyses.
2.6 Effect of carbon sources on phospahte solubilization
The effects of carbon sources on phosphate solubilization was tested following the methodology of Mardad et al. (2014) on the National Botanical Research Institute’s phosphate growth medium (NBRIP) medium containing 1 % glucose, 0.5 % Ca3(PO4)2, 0.5 %; MgCl2 · 6H2O, 0.5 %; 0.025 % MgSO4 · 7H2O, 0.02 % KCl, and 0.01 % (NH4)2SO4. For the comparison, the glucose present in the medium was replaced by galactose or fructose and the bacterial cultures were incubated for 7 days at 28 °C and 160 rpm. Autoclaved uninoculated batch cultures were included as negative controls. The P released from tricalcium in the supernatants obtained after centrifugation at 10,000 rpm for 10 min was analyzed by inductivelycoupled plasma-atomic emission spectrometry (ICP-AES) using a Varian 720-ES. The results were presented as a percentage of P released with respect to the control (glucose-containing NBRIP).
2.7 Pea inoculation assays with Rhizobium strains
The plant assays of Rhizobium isolates were performed on peas as was previously described (Ramírez-Bahena et al. 2009). The strain Rhizobium leguminosarum DSM 30132T (currently R. pisi)T which is able to nodulate pea but not to solubilize phosphate in YED-P plates was used as a reference. The negative controls were unninoculated pea plants. Seeds were sown into pots containing sterile vermiculite plus 0.2 % (w/w) tricalcium phosphate as substrate. After germination, inoculations were performed by adding 1 ml of a 106 cfu ml−1 bacterial suspension to each seedling. Nitrogen- and soluble phosphorous-free Rigaud and Puppo (1975) solution alternately with sterile distilled water was used to water the plants. Then they were placed in a plant growth chamber with mixed incandescent and fluorescent lighting (400 microeinsteins m−2 s−1; 400 to 700 nm), programmed for a 16 h photoperiod, day-night cycle, with a constant temperature varying from 25 to 27 °C, and 50 to 60 % relative humidity. Five replicates per treatment were set and the plants were harvested 6 weeks after planting. P content was analyzed by ICP-AES. Nitrogen was determined by combustion using a Leco CN-628 analyzer. Data were analyzed by one-way analysis of variance, and mean values compared by Fisher’s Protected LSD test (Least Significant Differences) (P ≤ 0.05).
3 Results and discussion
3.1 Isolation of phosphate solubilizing bacteria
A total of 16 PSB strains showing solubilization halos of at least 15 mm diameter were isolated. From them, 5 were found in the soil from Epoisses, and 11 in the soil from La Bruyère. From the first 5 strains, 2 where first isolated in medium B, 2 in soil extract medium (C1), and 1 strain in medium A. For the 11 strains from La Bruyère, 7 were isolated in the soil extract based medium (C2), 2 in medium A and 2 in medium B. The counts of total bacteria for the soil from Epoisses were 15.1 × 106, 31.9 × 106 and 35.7 × 106 CFU g−1 of soil in media A, B and C1 respectively. For the soil from La Bruyère total bacterial counts were 30.2 × 106, 32.0 × 106 and 29.1 × 106 CFU g−1 of soil in media A, B and C2 respectively. As can be seen, the number of PSB isolated was very low compared to the total microbiota counts obtained. These results are in agreement with previous studies where the number of isolates showing a high ability to solubilize phosphate in vitro is sometimes quite low compared to the total microbiota of the soils analysed (Alexander 1977). It is remarkable that the counts of total microbiota varied with the different culture media used for the isolation, the differences being dramatic in the case of the Epoisses soil sample inoculated in medium A, in which the number of colonies isolated was half of that with the other media.
3.2 Phenotypic characterization
From all PSB strains isolated in this study only one of them, PSB7, was a Gram positive rod, and the presence of spores was observed after Gram staining. The remaining strains were Gram negative non-sporulating rods. The phenotypic characterization was performed by using the API 20NE system whose usefulness for this purpose was previously shown in different bacterial groups including Gram negative and Gram positive sporulating bacteria (Rivas et al. 2007). Nevertheless, as expected, the Gram positive sporulated strain PSB7 showed an API 20NE profile (1457765) that was not coincident with any of the species held in the APILAB database. Although this system is inadequate for identification of most rhizospheric soil bacteria (Peix et al. 2003), some rhizospheric species are included in the APILAB database and so, in the case of Gram negative strains, we compared the codes obtained with those of Apiweb. The strains P. fluorescens DSM 50090T (code 1147555), B. cepacia LMG 1222T (code 1067577) and R. radiobacter ATCC 19358T (code 1467744) used as positive controls were correctly identified using this system. Biocoding for the API 20NE tests resulted in 0177555 for the strains PSB1, PSB11, PSB13 and PSB14, 0157555 for the strains PSB3, PSB4, PSB5 and PSB6, 1047557 for strain PSB8 and 0447455 for strain PSB2. All these biocodes were related to that of P. fluorescens in the APILAB database with similarities ranging from 79.1 to 99.9 % (see Table 1). The code 1367577 obtained for strains PSB10 and PSB15 showed 99.6 % similarity with B. cepacia and the codes 0667344 and 0667744 from strains PSB12 and PSB16, respectively, match with that of R. radiobacter with 96.1 % and 99.7 % similarity, respectively. In none of these cases was a good identification obtained at species level, but that at genus level was correct.
3.3 TP-RAPD pattern analysis
Figure 1 and Table 1 shows that the strains isolated in this study displayed 9 different TP-RAPD patterns. Profiles II, IV, V, VI, VIII and IX were shown by just one strain (PSB2, PSB7, PSB8, PSB9, PSB12 and PSB16, respectively). Profile I was presented by 4 strains (PSB1, PSB11, PSB13, PSB14), as was profile III (PSB3, PSB4, PSB5 and PSB6). Finally, two strains presented profile VII (PSB10 and PSB15). These results showed that in spite of the low number of bacteria able to solubilize phosphate present in the pea rhizospheres analysed, they were genetically diverse. Only the strains with the TP-RAPD type III pattern were present in both soils analysed, and they were isolated using two different culture media. The remaining strains were mainly isolated using media containing soil extracts confirming that the culture medium is crucial when isolating bacteria from soil. This results in a different composition and number of bacteria, for which the concept of “culturable bacteria” is dynamic, being a variable number depending on the medium used, as has been already reported by other authors (Lilley et al. 1996; Joseph et al. 2003). Taking into account that TP-RAPD patterns differ in different species (Rivas et al. 2001), they are a good tool for grouping bacteria in order to select representative strains for 16S rRNA gene sequencing because the species presenting the same TP-RAPD pattern have identical 16S rRNA genes (Valverde et al. 2006).
3.4 16S rRNA gene sequence analysis
The current bacterial taxonomy is based on the 16S rRNA gene which is currently sequenced in all type strains of all described species (Woese 2000; Yarza et al. 2013). Over the last decade a new database, named EzTaxon, including only these sequences has been developed in order to avoid bacterial mis-identifications that are very frequent in other databases (Kim et al. 2012). According to the results of this database most strains isolated in this study belong to the Phylum Proteobacteria and concretely to the genera Pseudomonas, Burkholderia and Rhizobium, and only one strain belongs to the Phylum Firmicutes, specifically to genus Bacillus (Table 1). As can be seen in Table 1, most of the strains belong to the genus Pseudomonas. According to these results, a similar genotypic diversity of PSB was found in the two soils analysed and the strains isolated belong to genera considered among the best phosphate solubilizers in the pseudomonads, bacilli and rhizobia groups (Peix et al. 2003; Peix et al. 2004; Chung et al. 2005; Islam et al. 2007; Naik et al. 2008; Browne et al. 2009; Oliveira et al. 2009; Peix et al. 2009; Selvakumar et al. 2009; Collavino et al. 2010; Castagno et al. 2011; Liu et al. 2011; Narveer et al. 2014; Xiang et al. 2011; Kumar et al. 2012b; Ndungu-Magiroi et al. 2012; Liu et al. 2014; Ruangsanka 2014).
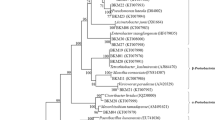
Figure 2 shows a phylogenetic tree including the strains from this study and some closely related species of PSB found in other plant rhizospheres. Four distant phylogenetic groups corresponding to the genera Pseudomonas, Burkholderia, Bacillus and Rhizobium were obtained.
In the phylogenetic group of genus Bacillus, strain PSB7 was closely related to the type strain of B. toyonensis CNCM I-1012T and to one strain isolated from poplar rhizosphere (Liu et al. 2011).
Strain PSB10 belongs to the phylogenetic group corresponding to Burkholderia together with the type strain of B. caledonica LMG 19076T and several PSB strains isolated from the rhizosphere of Ilex paraguariensis in Argentina (Collavino et al. 2010).
Among the pseudomonads, the 16S rRNA gene of strain PSB1 clustered with the type strain of P. baetica a390T. This cluster is close to another one containing the strain PSB8, the type strain of P. jessenii CIP 105274T and two PSB strains isolated from wheat (Triticum aestivum.) and barley (Hordeum vulgare) rhizospheres in Ireland (Browne et al. 2009). The strain PSB3 was closely related to the type strain P. azotoformans IAM 1603T and several PSB strains isolated from wheat and barley rhizosphere in Ireland (Browne et al. 2009). The 16S rRNA gene sequence of strain PSB2 was identical to P. lutea OK2T a PSB species isolated from the rhizosphere of grasses in a Spanish soil (Peix et al. 2004) and they were related to the previous cluster. The strain PSB9 was closely related to the type strain of P. frederiksbergensis JAJ28T and to two PSB strains isolated from wheat and barley rhizosphere in Ireland (Browne et al. 2009). Interestingly the strain PSB3 clustered with strains forming a superior phosphate solubilizing phylogenetic group defined by Browne et al. (2009) that contains the type strain of P. fluorescens and other related species (Fig. 2).
The strains PSB12 and PSB16 formed a cluster together with the type strain of a recently described species of Rhizobium, R. grahamii CCGE 502T. There are no PSB strains of Rhizobium closely related to our strains. Although Mesorhizobium strains are amongst the best phosphate solubilizing rhizobia (Peix et al. 2001; Rivas et al. 2006), surprisingly the Rhizobium strains isolated in this study solubilized phosphate in amounts similar to those found with Pseudomonas species.
The results of the present study showed that the PSB found in the rhizosphere of pea in the two soils studied were genotypically and phenotypically diverse and confirmed previous studies showing that Pseudomonas, Burkholderia and Bacillus are the most active P solubilizers. However our results showed that rhizospheric Rhizobium strains can also be good solubilizers, which contrasts with results that have previously been reported for rhizobial strains isolated from legume nodules that showed a weaker P solubilization than Pseudomonas (Abril et al. 2007; Palomo et al. 2007). Although some different species of PSB were found in the two studied soils highlighting the need for further studies in different geographical locations, the most significant result of this study is that some phylogenetic groups of PSB are widely distributed in plant rhizospheres in different countries and continents. This is particularly interesting in the case of strains from the phylogenetic group of P. fluorescens which includes PSB strains with high ability to solubilize phosphate, indicating that they may be used as biofertilizers in many different soil types.
3.5 Effect of carbon sources on phosphate solubilization
It has been previously shown that carbon sources affect bacterial phosphate solubilization (Hameeda et al. 2006). Glucose and galactose have been reported to be very good carbon sources for phosphate solubilisation in different bacteria (Mardad et al. 2014), whereas fructose showed variable results depending on the bacteria tested (Park et al. 2009; Mardad et al. 2014). Therefore, in this study we analysed the effect of these three carbon sources on the ability of the isolated PSB strains to solubilize phosphate. The results showed that glucose was the best carbon source for phosphate solubilization with all the strains tested (Table 2), confirming the results of other authors (Relwani et al. 2008), and variable results were obtained for the other two carbon sources depending on the bacterial strain tested. Interestingly, all the Pseudomonas strains isolated in this study showed very low percentages of phosphate solubilisation in the presence of fructose with respect to the values obtained in the glucose-based medium, which is in agreement with the results previously found for a strain of Pseudomonas fluorescens (Park et al. 2009).
3.6 Effect of Rhizobium strains on growth and nodulation of pea
The results of the inoculation of the Rhizobium strains PSB12 and PSB16 on pea plants showed that both strains formed effective nodules. Although both induced significantly fewer nodules than the reference strain R. pisi DSM 30132T, their inoculation resulted in plants with significantly higher shoot dry weights (Table 3). The plants inoculated with strain PSB12 and the reference R. pisi strain have significantly higher N concentrations than those inoculated with the strain PSB16, whereas no significant differences were found in the P concentrations among treatments. Nevertheless, the total amounts of these two elements were higher in pea plants inoculated with the strains PSB12 and PSB16 than in those inoculated with the reference strain. These results are in agreement with those previously found for other phosphate solubilizing rhizobia which enhanced the growth of their hosts (Peix et al. 2001; Marciano Marra et al. 2012), and suggest that Rhizobium sp. strains PSB12 and PSB16 have potential to be good biofertilizers for pea since increases ranging from 30 to 50 % were found in shoot dry weight and total content of P and N with respect to those inoculated with the reference strain.
References
Abril A, Zurdo-Piñeiro JL, Peix A, Rivas R, Velázquez E (2007) Solubilization of phosphate by a strain of Rhizobium leguminosarum bv. trifolii isolated from Phaseolus vulgaris in El Chaco Arido soil (Argentina). In: Rodríguez-Barrueco C, Velázquez E (eds) First International Meeting on Microbial Phosphate Solubilization. Developments in Plant and Soil Sciences, vol 102. Springer, Germany, pp 135–138
Acevedo E, Galindo-CastanedaT PF, Navia M, Romero HM (2014) Phosphate-solubilizing microorganisms associated with the rhizosphere of oil palm (Elaeis guineensis Jacq.) in Colombia. Appl Soil Ecol 80:26–33. doi:10.1016/j.apsoil.2014.03.011
Alexander M (1977) Microbiology of the rhizosphere. Introduction to soil microbiology. Wiley, USA
Bedoussac L, Journet EP, Hauggaard-Nielsen H, Naudin C, Corre-Hellou G, Jensen ES, Prieur L, Justes E (2015) Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron Sustain Dev 35:911–935. doi:10.1007/s13593-014-0277-7
Browne P, Rice O, Miller SH, Burke J, Dowling DN, Morrissey JP, O’Gara F (2009) Superior inorganic phosphate solubilization is linked to phylogeny within the Pseudomonas fluorescens complex. Appl Soil Ecol 43(1):131–138. doi:10.1016/j.apsoil.2009.06.010
Castagno LN, Estrella MJ, Sannazzaro AI, Grassano AE, Ruiz OA (2011) Phosphate-solubilization mechanism and in vitro plant growth promotion activity mediated by Pantoea eucalypti isolated from Lotus tenuis rhizosphere in the Salado River Basin (Argentina). J Appl Microbiol 110:1151–1165. doi:10.1111/j.1365-2672.2011.04968.x
Catroux G, Hartmann A, Revellin C (2001) Trends in rhizobial inoculant production and use. Plant Soil 230:21–30. doi:10.1023/A:1004777115628
Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, Sa T (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37:1970–1974. doi:10.1016/j.soilbio.2005.02.025
Collavino M, Sansberro PA, Mroginski LA, Aguilar OM (2010) Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol Fertil Soils 46:727–738. doi:10.1007/s00374-010-0480-x
Dahl WJ, Foster LM, Tyler RT (2012) Review of the health benefits of peas (Pisum sativum L.). Br J Nutr 108:S3–S10. doi:10.1017/S0007114512000852
De Freitas JR, Banerjee MR, Germida JJ (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol Fertil Soils 24:358–364
Flores-Félix JD, Silva LR, Rivera LP, Marcos-García M, García-Fraile P, Martínez-Molina E, Mateos PF, Velázquez E, Andrade P, Rivas R (2015) Plants probiotics as a tool to produce highly functional fruits: the case of Phyllobacterium and vitamin C in strawberries. PLoS ONE 10:e0122281. doi:10.1371/journal.pone.0122281
García-Fraile P, Carro L, Robledo M, Ramírez-Bahena MH, Flores-Félix JD, Fernández MT, Mateos PF, Rivas R, Igual JM, Martínez-Molina E, Peix Á, Velázquez E (2012) Rhizobium promotes non-legumes growth and quality in several production steps: towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 7:e38122. doi:10.1371/journal.pone.0038122
Graham PH, Vance CP (2000) Nitrogen fixation in perspective: an overview of research and extension needs. Field Crop Res 65:93–106. doi:10.1016/S0378-4290(99)00080-5
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93. doi:10.1023/A:1020663916259
Hameeda B, Reddy YH, Rupela OP, Kumar GN, Reddy G (2006) Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Curr Microbiol 53:298–302. doi:10.1007/s00284-006-0004-y
Hodge I, Hauck J, Bonn A (2015) The alignment of agricultural and nature conservation policies in the European Union. Conserv Biol 29:996–1005. doi:10.1111/cobi.12531
Islam MT, Deora A, Hashidoko Y, Rahman A, Ito T, Tahara S (2007) Isolation and identification of potential phosphate solubilizing bacteria from the rhizoplane of Oryza sativa L. cv. BR29 of Bangladesh. Z Naturforsch C 62:103–110
Jakobsen I (1985) The role of phosphorus in nitrogen fixation by young plants (Pisum sativum). Physiol Plant 64:190–196. doi:10.1111/j.1399-3054.1985.tb02334.x
Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215. doi:10.1128/AEM.69.12.7210-7215.2003
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi:10.1099/ijs.0.038075-0
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi:10.1007/BF01731581
Kumar A, Kumar A, Devi S, Patil S, Payal C, Negi S (2012a) Isolation, screening and characterization of bacteria from rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res Sci Technol 4:1–5
Kumar P, Dubey RC, Maheshwari DK (2012b) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499. doi:10.1016/j.micres.2012.05.002
Lederberg J, Lederberg EM (1952) Replica plating and indirect selection of bacterial mutants. J Bacteriol 63:399–406
Li JH, Wang ET, Chen WF, Chen WX (2008) Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem 40:238–246. doi:10.1016/j.soilbio.2007.08.014
Lilley AK, Fry JC, Bailey MJ, Day MJ (1996) Comparison of aerobic heterotrophic taxa isolated from four root domains of mature sugar beet (Beta vulgaris). FEMS Microbiol Ecol 21:231–242. doi:10.1111/j.1574-6941.1996.tb00350.x
Liu H, Wu XQ, Ren JH, Ye JR (2011) Isolation and identification of phosphobacteria in poplar rhizosphere from different regions of China. Pedosphere 21:90–97. doi:10.1016/S1002-0160(10)60083-5
Liu FP, Liu HQ, Zhou HL, Dong ZG, Bai XH, Bai P, Qiao JJ (2014) Isolation and characterization of phosphate-solubilizing bacteria from betel nut (Areca catechu) and their effects on plant growth and phosphorus mobilization in tropical soils. Biol Fertil Soils 50:927–937. doi:10.1007/s00374-014-0913-z
Marciano Marra L, Fonsêca Sousa Soares C, de Oliveira S, Avelar Ferreira P, Lima Soares B, de Fráguas CR, de Lima J, de Souza MF (2012) Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 357:289–307. doi:10.1007/s11104-012-1157-z
Mardad I, Serrano A, Soukri A (2014) Effect of carbon, nitrogen sources and abiotic stress on phosphate solubilization by bacterial strains isolated from a moroccan rock phosphate deposit. J Adv Chem Eng 1:102. doi:10.4172/2090-4568.1000102
Mohr SH, Wang J, Ellem G, Ward J, Giurco D (2015) Projection of world fossil fuels by country. Fuel 141:120–135. doi:10.1016/j.fuel.2014.10.030
Naik PR, Raman G, Narayanan KB, Sakthivel N (2008) Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol 8:230. doi:10.1186/1471-2180-8-230
Narveer AV, Kumar H, Putatunda C (2014) In vitro phosphate solubilization by Bacillus sp. NPSBS 3.2.2 obtained from the cotton plant rhizosphere. Biosci Biotechnol Res Asia 11:401–406. doi:10.13005/bbra/1288
Ndungu-Magiroi KW, Herrmann L, Okalebo JR, Othieno CO, Pypers P, Lesueur D (2012) Occurrence and genetic diversity of phosphate-solubilizing bacteria in soils of differing chemical characteristics in Kenya. Ann Microbiol 62:897–904. doi:10.1007/s13213-011-0326-2
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimarães CT, Schaffert RE, Sá NMH (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 41:1782–1787. doi:10.1016/j.soilbio.2008.01.012
Palomo JL, García-Benavides P, Mateos PF, Martínez-Molina E, Velázquez E (2007) Two strains isolated from tumours of Prunus persica are able to solubilize phosphate in vitro. In: Rodríguez-Barrueco C, Velázquez E (eds) First international meeting on microbial phosphate solubilization. Developments in plant and soil sciences, vol 102. Springer, Germany, pp 347–349
Park KH, Lee CY, Son HJ (2009) Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett Appl Microbiol 49:222–228. doi:10.1111/j.1472-765X.2009.02642.x
Peix A, Rivas-Boyero AA, Mateos PF, Rodríguez-Barrueco C, Martínez-Molina E, Velázquez E (2001) Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol Biochem 33:103–110. doi:10.1016/S0038-0717(00)00120-6
Peix A, Rivas R, Mateos PF, Martínez-Molina E, Rodríguez-Barrueco C, Velázquez E (2003) Pseudomonas rhizosphaerae sp. nov., a novel species that actively solubilizes phosphate in vitro. Int J Syst Evol Microbiol 53:2067–2072. doi:10.1099/ijs.0.02703-0
Peix A, Rivas R, Santa-Regina I, Mateos PF, Martínez-Molina E, Rodríguez Barrueco C, Velázquez E (2004) Pseudomonas lutea sp. nov., a novel phosphate-solubilizing bacterium isolated from rhizosphere of grasses. Int J Syst Evol Microbiol 54:847–850. doi:10.1099/ijs.0.02966-0
Peix A, Lang E, Verbarg S, Sproeer C, Rivas R, Santa-Regina I, Mateos PF, Martínez-Molina E, Rodriguez-Barrueco C, Velazquez E (2009) Acinetobacter strains IH9 and OCI1, two rhizospheric phosphate solubilizing isolates able to promote plant growth, constitute a new genomovar of Acinetobacter calcoaceticus. Syst Appl Microbiol 32:334–341. doi:10.1016/j.syapm.2009.03.004
Ramírez-Bahena MH, Velázquez E, Fernández-Santos F, Peix A, Martínez-Molina E, Mateos PF (2009) Phenotypic, genotypic, and symbiotic diversities in strains nodulating clover in different soils in Spain. Can J Microbiol 55:1207–16. doi:10.1139/w09-074
Relwani L, Krishna P, Sudhakara RM (2008) Effect of carbon and nitrogen sources on phosphate solubilization by a wild-type strain and uv-induced mutants of Aspergillus tubingensis. Curr Microbiol 57:401–406. doi:10.1007/s00284-008-9212-y
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996. doi:10.1104/pp.111.175448
Rigaud J, Puppo A (1975) Indole-3-acetic-acid catabolism by soybean bacteroids. J Gen Microbiol 88:223–228
Rivas R, Velázquez E, Valverde A, Mateos PF, Martínez-Molina E (2001) A two primers random amplified polymorphic DNA procedure to obtain polymerase chain reaction fingerprints of bacterial species. Electrophoresis 22:1086–1089. doi:10.1002/1522-2683()22:6<1086::AID-ELPS1086>3.0.CO;2-6
Rivas R, Velázquez E, Palomo JL, Mateos PF, García-Benavides P, Martínez-Molina E (2002) Rapid identification of Clavibacter michiganensis subspecies sepedonicus using two primers random amplified polymorphic DNA (TP-RAPD) fingerprints. Eur J Plant Pathol 108:179–184. doi:10.1023/A:1015044911913
Rivas R, Peix A, Mateos PF, Trujillo ME, Martinez-Molina E, Velázquez E (2006) Biodiversity of populations of phosphate solubilizing rhizobia that nodulate chickpea in different Spanish soils. Plant Soil 287:23–33. doi:10.1007/s11104-006-9062-y
Rivas R, Garcia-Fraile P, Mateos PF, Martinez-Molina E, Velazquez E (2007) Characterization of xylanolytic bacteria present in the bract phyllosphere of the date palm Phoenix dactylifera. Lett Appl Microbiol 44:181–7. doi:10.1111/j.1472-765X.2006.02050.x
Ruangsanka S (2014) Identification of phosphate-solubilizing bacteria from the bamboo rhizosphere. Sci Asia 40:204–211. doi:10.2306/scienceasia1513-1874.2014.40.204
Saitou N, Nei M (1987) A neighbour-joining method: a new method for reconstructing phylogenetics trees. Mol Biol Evol 44:406–425
Selvakumar G, Joshi P, Nazim S, Mishra PK, Bisht JK, Gupta HS (2009) Phosphate solubilization and growth promotion by Pseudomonas fragi CS11RH1 (MTCC 8984), a psychrotolerant bacterium isolated from a high altitude Himalayan rhizosphere. Biologia 64:239–245. doi:10.2478/s11756-009-0041-7
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The clustal X windows interface: flexible strategies for multiple sequence alignement aided by quality analysis tools. Nucleic Acid Res 25:4876–4882
Toro M, Ramírez-Bahena MH, Cuesta MJ, Velázquez E, Peix A (2013) Pseudomonas guariconensis sp. nov., isolated from rhizospheric soil. Int J Syst Evol Microbiol 63:4413–4420. doi:10.1099/ijs.0.051193-0
Valverde A, Igual JM, Peix A, Cervantes E, Velázquez E (2006) Rhizobium lusitanum sp. nov. a bacterium that nodulates Phaseolus vulgaris. Int J Syst Evol Microbiol 56:2631–2637. doi:10.1099/ijs.0.64402-0
Withers PJA, Elser JJ, Hilton J, Ohtake H, Schipper WJ, van Dijk KC (2015) Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability. Green Chem 17:2087–2099. doi:10.1039/c4gc02445a
Woese CR (2000) Interpreting the universal phylogenetic tree. Proc Natl Acad Sci U S A 97:8392–8396. doi:10.1073/pnas.97.15.8392
Xiang W, Yang Z, Sheng C (2011) Isolation and performance evaluation of halotolerant phosphate solubilizing bacteria from the rhizospheric soils of historic Dagong Brine Well in China. World J Microbiol Biotechnol 27(11):2629–2637. doi:10.1007/s11274-011-0736-0
Yarza P, Spröer C, Swiderski J, Mrotzek N, Spring S, Tindall BJ, Gronow S, Pukall R, Klenk HP, Lang E, Verbarg S, Crouch A, Lilburn T, Beck B, Unosson C, Cardew S, Moore ER, Gomila M, Nakagawa Y, Janssens D, De Vos P, Peiren J, Suttels T, Clermont D, Bizet C, Sakamoto M, Iida T, Kudo T, Kosako Y, Oshida Y, Ohkuma M, R Arahal D, Spieck E, Pommerening Roeser A, Figge M, Park D, Buchanan P, Cifuentes A, Munoz R, Euzéby JP, Schleifer KH, Ludwig W, Amann R, Glöckner FO, Rosselló-Móra R (2013) Sequencing orphan species initiative (SOS): filling the gaps in the 16S rRNA gene sequence database for all species with validly published names. Syst Appl Microbiol 36(1):69–73. doi:10.1016/j.syapm.2012.12.006
Acknowledgments
AP is indebted to Conseil Régional de Bourgogne (France) for fellowship granting and to Gérard Catroux for scientific and personal support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of Gisèle Laguerre
Rights and permissions
About this article
Cite this article
Bahena, M.H.R., Salazar, S., Velázquez, E. et al. Characterization of phosphate solubilizing rhizobacteria associated with pea (Pisum sativum L.) isolated from two agricultural soils. Symbiosis 67, 33–41 (2015). https://doi.org/10.1007/s13199-015-0375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0375-6