Abstract
Nothing is published about the arbuscular mycorrhizal fungi (AMF) of the Azores archipelago, either with regard to individual species, or at the community level. This study, based on identification through spore morphological characteristics, compares the AMF community structure of semi-natural and intensively managed pastures. Thirty-nine glomeromycotan fungal spore types were detected in soil, with species in the genera Acaulospora, Ambispora, Archaeospora, Claroideoglomus, Entrophospora, Gigaspora, Paraglomus, Sclerocystis, Scutellospora sensu Morton and Msiska (Mycorrhiza 20 483–496, 2010) and Rhizophagus. The two most representative groupings were the glomoid spore types and Acaulospora with 13 and 10 species respectively, followed by Scutellospora with 3. The glomeromycotan fungal richness was similar for both intensive and semi-natural pastures, with 28 spore types in the former and 23 in the latter but their composition differed. Semi-natural pastures were dominated by species from Acaulospora and Scutellospora, particularly S. calospora and A. cf. myriocarpa, while for intensively farmed pastures, species with glomoid spores, and members of the two genera Claroideoglomus and Paraglomus were found most frequently and abundantly. Spore densities of the most commonly found groupings — Acaulospora, Claroideoglomus, Scutellospora and the glomoid spores were correlated with soil chemical properties, suggesting that soil characteristics influence the AMF communities. These results indicate that intensity of pasture management may not influence AMF richness but is probably an important factor influencing their composition and abundance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arbuscular mycorrhizal fungi (AMF) are symbiotic associations between plants and obligate symbiotic fungi of the phylum Glomeromycota thought to have originated 400–500 million years ago (Schülbler et al. 2001). In this intimate association the fungi provide their hosts with minerals nutrients (particularly inorganic phosphate) and water (Querejeta et al. 2007), and increase resistance to biotic and abiotic stresses, including pathogens (Pozo and Azcon-Aguilar 2007), water limitation and environmental pollutants (Hildebrandt et al. 2007). In turn, the fungi obtain carbon in organic compounds from their photoautotrophic partners (Smith and Read 2008).
These beneficial effects of AMF are important in natural ecosystems, although it has been suggested that they may be less functionally important in high-input agriculture (Barea and Jeffries 1995; Galvez et al. 2001). Agricultural practices such as intensity of cultivation, nature and quantity of applied fertilisers and plant protection strategies have considerable impacts on soil and soil organisms (Miller et al. 1995; Boddington and Dodd 2000). The community structure of AMF is no exception (Sieverding 1989; Douds and Millner 1999; Oehl et al. 2003; Li et al. 2007; Liu et al. 2012), but it has proved difficult to identify any consistent effects attributable to different management practices such as tillage, fertiliser input, pesticide use and crop rotation (Gosling et al. 2006; Gai et al. 2006). Populations of AMF can be affected by disturbance or change of host plant use, and Jansa et al. (2002; 2003) reported that tillage affected significantly the community structure of AMF, decreasing the sporulation of some members of the Diversisporales. Also Oehl et al. (2004) found that differences between conventional versus organic farming were reflected in both species richness and composition. Although they detected no change in abundance of species of Glomus (in the broad sense that the genus name was used before Schübler and Walker 2010: see below) between farming systems, the same pattern was not observed in relation to spore abundances of Acaulospora and Scutellospora species, which were more abundant in organic systems. These findings suggest that conventional management practices diminish local differences in community composition, resulting in homogenization and domination by AMF generalist species.
AMF respond to a range of environmental factors such as pH and nutrient status (Helgason and Fitter 2009; Dumbrell et al. 2010). It has been showed in several studies that pH it is an important factor influencing the AMF species composition (Wang et al. 1985; Ezawa et al. 2001; Oehl et al. 2010). Some AMF species (e.g., some former ‘Glomus’) are said to prefer alkaline and neutral soil, while others (e.g., Acaulospora) are thought to sporulate more abundantly in acid soils (Gai and Liu 2003; Oehl et al. 2010). Differences in AMF composition between conventional and organic systems can also be explained by the nutrients applied which lead to different levels of soil phosphorus (P) (Eason et al. 1999; Oehl et al. 2004). Higher available soil-P can reduce AMF formation, either through a direct effect on external hyphal growth or indirectly associated with host P status (Sanders 1975). The genus ‘Glomus’ due to its large ecological plasticity is generally dominant in intensively managed sites (Oehl et al. 2003, 2004), being for this reason classified as a P-tolerant genus (Johnson 1993; Bhadalung et al. 2005).
Land use changes are important factors impacting biodiversity in island ecosystems. The native forests of the Azores archipelago had been mostly destroyed since human occupation and converted into agricultural land (Martins 1993; Borges et al. 2008). During the second half of the 20th century, after failure of different monoculture crops such as wheat (Triticum spp.), woad (Isatis tinctoria) and orange (Citrus spp.), milk production has grown in importance (Martins 1993; Borges and Brown 1999), and intensively managed pastures now dominate the landscape in the archipelago, covering 67.7 % of the arable land. There are also semi-natural pastures comprising 11.2 % of the total area (Garcia and Furtado 1991), most of which are located at high altitude in recent infertile acid volcanic soils. The intensively managed pastures are dominated by introduced grasses and legumes, whereas native legumes such as Lotus uliginosus, grasses, rushes, sedges and ferns are more frequent in the less intensively managed semi-natural localities (Borges and Brown 1999). Both kinds of pasture are dominated by Holcus lanatus, a non-indigenous plant with a frequency more than 50 % (Dias 1996; Silva and Smith 2006; Kueffer et al. 2010). In Terceira Island it is very common, being the third most frequent exotic plant (Silva and Smith 2006) in all kinds of soil.
Although AMF are generally considered of great ecological importance, nothing is published about the diversity and composition of communities in the Azores. Traditional studies on AMF diversity are based mainly on spore morphology (Walker 1992). However, taxonomic identification of AMF spores collected directly from the field is quite difficult because they are often unidentifiable due to degradation or parasitism by other organism, and thus do not necessarily reflect the AMF populations in soil (Clapp et al. 1995). Additionally, fungal spore diversity differs seasonally, with some fungi sporulating in late spring and others at the end of summer. However, an advantage is that spore numbers are indicators over a longer time period, i.e. over months, which allow us to describe AMF community structures based on spore morphology (Douds and Millner 1999; Oehl et al. 2003), with the exception of putative non-sporulating AMF species. However, the extraction of spores from soil is a rapid and non-expensive method, and it may reveal taxa not easily detected by molecular methods (e.g. Gollotte et al. 2004; Gamper et al. 2009; Krüger et al. 2009). The use of successive trap cultures and subsequent extraction and study of spores takes much longer but also reveals significantly greater diversity than a ‘one-off’ spore extraction (Oehl et al. 2004).
We assessed the qualitative and quantitative differences of the AMF community, based on spore morphological characterisation in semi-natural and intensively managed pastures in Terceira Island, the third largest island of the Azorean archipelago. We predict that both species richness and abundance of AMF will be lower in intensively managed pastures, and species composition should differ between semi-natural and intensively managed pastures.
2 Materials and methods
2.1 Study sites and sampling procedure
This study was conducted in the Terceira Island, the third island of the Azorean archipelago in terms of size (402 km2). The Azores archipelago have a temperate oceanic climate characterised by high levels of relative atmospheric humidity that could reach 95 % at high altitude native forests and ensures slight thermal variations throughout the year (Azevedo et al. 1999). The average temperature is 17.5 ° C in low altitudes, while the maximum temperature is in August and minimum in February (Azevedo 1996). The pluviometric regime reaches its peak in January-February and minimum and July (Azevedo 1996): Angra do Heroísmo (47 m): 969 mm year (140 mm in January and 40 mm in July) and Serra de S. Bárbara (1.023 m): 3.000 mm year (Borges 1997).
The sampling areas were cattle-grazed upland pastures of two different types: semi-natural pastures with low grazing intensity and frequency (managed for more than 50 years, with a relatively high diversity of grasses and forbs; see Borges and Brown (2001) and intensively managed pastures with high grazing intensity and frequency (managed for more than 30 years, characterised also by a depauperate vascular flora of five or fewer dominant species).
The semi-natural pastures, Pico Galhardo (PX) (38° 41’ 51.71” N 27° 13’ 25.34” W) with a area of 4.8 ha, and Terra Brava (TB) (38° 41’ 59.74” N Longitude 27° 12’ 41.37” W) with a area of 12.4 ha (Fig. 1), are included in the Natura 2000, a site of communitarian interest (SIC- Serra de Santa Barbara e Pico Alto), and are part of the protected area Terceira Island Park. They are at high-altitude, and therefore located in the called “cloud-zone forest” (Sjögren 1990), where the rainfall (vertical and horizontal) is high, leaving the air saturated with moisture and the soil waterlogged. In these conditions, anoxia phenomena occur in the soil, decreasing the mineralisation of soil organic matter (Borges 1997). They are dominated by the perennial grasses Holcus lanatus and Agrostis castellana, have a high floristic diversity (Dias 1996; Borges 1997), often including other grasses such as Anthoxanthum odoratum, Lolium multiflorum, Holcus rigidus and Poa trivialis and non-forage species, including Lotus uliginosus, Rumex acetosella ssp. angiocarpus, Potentilla anglica, Hydrocotyle vulgaris, Plantago lanceolata, Lobelia urens, Cerastium fontanum, Conyza bonariensis, Anagallis arvensis, Hypochoeris radicata, Ranunculus repens, Pteridium aquilinum and Juncus effusus (Dias 1996; Borges 1997). The traditional silvopastoral system is practiced in these pastures where bullfight cattle are given free range. Fertilization is not allowed and the number of cattle is limited. The intensively managed pastures, Agualva 1 (RP1) at 38° 45’ 27.18” N 27° 11’ 41.55” W, with an area of 6.4 ha, and Agualva 2 (RP2) 38° 45’ 24.44” N 27° 11’ 42.24” W, with a area of 1.2 ha, (Fig. 1). These pastures resulted of the conversion of native forest to wood production and, finally, to permanent pastures. The pastures show different topographic conditions resulting of the conversion process, which limit the management practices, and change the intensity of grazing (Borges and Brown 1999). They are surrounded by exotic eucalyptus plantations. Though dominated by H. lanatus and Lolium perenne they may also have high populations of Trifolium repens (Borges 1997; Dias 1996) P. lanceolata, Cyperus esculentus, Mentha suaveolens, Cerastium fontanum and Rumex conglomeratus (Dias 1996; Borges 1997). They are characterised by closed fields in large dimensions with wooded fencerows of Cryptomeria japonica, (Borges 1997). The grazing sytem is rotative characterised by heifers and beef cattle with a stocking rate average of 2.5 cows/ha during the late spring and early autumn (Borges 1997). Every year is applied Nitrogen (NH4NO3 with 27 % N, 81 kg ha-1) to both pastures after each period of grazing with approximately 5 weeks between the next grazing. Every 5 years the pastures are tilled and re-sow with annual grasses such us Lolium perenne.
2.2 Sample collection
A total of 40 soil samples with associated roots were randomly collected in the target areas in August 2007, of which twenty were in semi-natural pastures (PX and TB) and the remaining twenty in the intensively managed pastures (RP1 and RP2). Approximately 2 kg of soil was collected from the rooting zone of the dominant plant species, H. lanatus, to a depth of 20 cm. The soil samples were air-dried, passed through a 2-mm sieve and stored at 4 °C before analysis.
2.3 Soil analyses
The soils of semi-natural and intensive pastures are sandy loams (Pinheiro 1990). The soil analyses (Table 1) were performed on samples collected in August 2007. Ten samples from each site were pooled and analysed at the University of Azores Soil Laboratory (CITA-A). Potassium (K), calcium (Ca) and magnesium (Mg) were extracted with sodium acetate (1/5) at pH 7, and determined using a Varian ICP atomic emission spectrophotometer. Soil pH was measured from a soil and water paste (1:2.5 v/v), and available phosphorus (P) (Olsen and Sommers 1982) by atomic absorption spectrometry after extraction with a 0.5 M NaHCO3 solution at pH 8.5. Total soil nitrogen (N) (Kjehldahl) content (Allen 1989) and organic matter (OM) (by dry-ashing) were also measured.
2.4 Establishment of trap cultures
Open pot trap cultures (Gilmore 1968) were established with 1-week-old Zea mays seedlings in fresh soil from each site mixed with autoclaved sand and volcanic soil “bagacina” (2:1:1 v/v/v). Ten pots containing 1.5 kg of the mixture were prepared from each of 10 field soil samples per site. Host plant seeds were surface sterilised by immersion in alcohol (96 %) for 30 s, and 4 % bleach for 2 min, followed by two rinses in sterile distilled water, sown in pots on sterilised sand soil, and germinated in the greenhouse. Four seedlings were transplanted to each pot, and maintained in a greenhouse for 5 months. All pots were watered every 2 days with distilled water, and no nutrient solution was added.
2.5 Spore extraction and morphological identification
This work was a preliminary study of AMF diversity and composition based on spores extracted from trap cultures, because identification from field-collected samples is hampered by such factors as parasitism, degradation through age and environmental alteration (e.g., discoloration) and low spore numbers. Discrepancies between data from trap cultures and field samples are likely to be the result of the conditions in the pot cultures, which may favour certain AMF taxa (Brundrett et al. 1999; Oehl et al. 2003, 2004; Hijri et al. 2006; Wang et al. 2008). Oehl et al. (2004) reported that three of 35 species recorded at a field site failed to produce spores from such traps. Also, Hijri et al. (2006) noted that Paraglomus was never detected in trap cultures, although it occurred frequently in the field site experiment. However, the opposite was observed in relation to Archaeospora trappei which only be detected in trap cultures (Hijri et al. 2006). Consequently, trap culture may provide a different picture of AMF communities than analysis of field-collected roots, which could contribute to a lack of consistent differences across the two managements practices investigated. However, trap cultures may give a better indication of what was actually alive in the soil, whereas spores from soil might represent dead leftovers from species that were present in the past, but have died out.
Glomeromycotan spores were extracted from 50 g of air-dry soil of each sample from field soil and trap cultures by wet sieving and sucrose centrifugation (Walker et al. 1982) and were stored at 4 °C in autoclaved water pending examination. Different spore types were initially separated under a stereo-microscope, and then examined through a compound microscope in a 1:1 mixture of polyvinyl alcohol lacto-glycerol (PVLG) and Melzer’s reagent. They were then classified into known species, or spore types that could not be placed in a current species, based on colour, size, surface ornamentation, hyphal attachment, reaction to Melzer’s reagent, and wall structure. Counts were made for of the total number of spores of each type.
There is considerable confusion and controversy with regard to the taxonomy of the Glomeromycota. There is a revision based largely on molecular evidence (Schübler and Walker 2010; Krüger et al. 2012), and another erecting a large number of genera (Oehl et al. 2011a), particularly for the genus Glomus in a broad sense, based largely on the appearance of the subtending hypha of the spore and the nature of the occlusion of spore contents. These two views are largely incompatible, and we have chosen to follow the former. Consequently, we use the genus ‘Glomus’ (which we therefore continue to place in inverted commas) in a broad sense, including the species that are listed in Schüßler and Walker (2010) as ‘Species of uncertain position in Glomus sensu lato’, and we similarly follow the narrow definitions of Rhizophagus, and Sclerocystis as circumscribed in that publication, and the germination shield bearing genera (termed ‘the Scutellospora group’ herein) in the Gigasporaceae as presented in Redecker et al. (2013).
2.6 Data analyses
Spore density, richness, and occurrence were based on characterisation of spores extracted from the trap cultures, because most of them extracted directly from field soil were in low number and parasitised by other organism, which could hinder accurate identification, thus all results after this point are relate to trap cultures.
Spore density (number per 50 g dried soil), frequency of occurrence (number of samples containing particular taxon) and species richness (number of taxa found in a particular land use type) were calculated. We conducted a two-dimensional ordination of the four sites using nonlinear multidimensional scaling (MDS) in the software Community Analysis Package v. 4.0 (CAP 4) (Seaby et al. 2004). This method has some advantages over correspondence analysis (CA) and Principal component analysis (PCA) because it can be performed with any similarity measure and is independent of judgment by the researcher. Bray-Curtis distances were computed to model dissimilarities. A log-linear saturated model was constructed to examine the relationships among the land use types.
Pearson correlation coefficients were calculated for chemical properties of each soil type and the total AMF spore density or the AMF spore density of the more common groups (Acaulospora, Claroideoglomus, ‘Glomus’ and the Scutellospora group).
The sampling followed a hierarchical nested design, with field sites nested within land use type. Variation of species richness between land uses and field sites within each land use was analysed by Nested ANOVA (Minitab version 13.31, 2000). Variation of total AMF spore density and of the commonest genera (Acaulospora, Claroideoglomus, ‘Glomus’ and the Scutellospora group) was similarly analysed. All data were tested for normality to fulfil the assumptions of Nested ANOVA.
3 Results
3.1 AMF spore types
Thirty-nine AM spore types from among eleven genera were detected in the soil traps from the two land use types studied (Table 1). However, only six of these were detected by extraction directly from soil: Gigaspora sp., Acaulospora laevis, A. cf koskei, Sclerocystis rubiformis, Scutellospora calospora and Cetraspora pellucida (Table 2).
Nineteen species could not confidently be named to species, the majority of them belonging to the genera ‘Glomus’ (13) and Paraglomus (2) followed by Acaulospora, Ambispora, Gigaspora and Racocetra each with just one species not identified to the species (Table 2). The two most representative genera were ‘Glomus’ and Acaulospora with 14 and 10 species respectively, followed by the genera Paraglomus and the Scut. group, each with 3 species (Table 2).
3.2 AMF species richness and composition
Similar numbers of species were found from soil traps from both land uses types. The semi-natural and intensive pastures yielded 23 and 28 glomeromycotan taxa or spore types respectively, and no significant differences were found in species richness between land uses (Nested Anova: F = 1.39, d.f. = 1,2, p = 0.25) or between field sites within each land use (Nested Anova: F = 0.29, d.f. = 1,2, p = 0.75).
In contrast, AMF species composition differed between land uses. Along with the log-linear analysis, the Bray-Curtis-based MDS analysis highlighted differences in community composition for the different tillage systems (Fig. 2). The first axis, clearly separated the semi-natural locations with lower levels of land use (PX; TB), from the intensively managed sites (RP1; RP2), while the second axis show some degree of heterogeneity between the two intensively managed sites (Fig. 2).
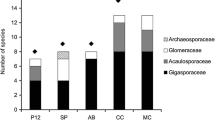
Approximately 30 % of AMF taxa were common to both land uses (A. cf koskei, A. laevis, A. cf. myriocarpa (considered by some authors to be in the genus Archaeospora ( Oehl et al. 2011b) or Ambispora (Blaszkowski 2012), Acaulospora cf. paulinae, glomoid spore types 1 and 2, Paraglomus sp. 1, S. calospora, Cetraspora pellucida, Racocetra sp. Rhizophagus clarus and Sclerocystis rubiformis), demonstrating a ‘generalist’ behaviour (Table 2). Some taxa occurred exclusively in just one land-use type or in just one field site. Most species of Acaulospora, and the only representatives of Ambispora and Gigaspora were confined to the semi-natural pasture, while intensive pasture soil was dominated by Claroideoglomus and ‘Glomus’ species (Table 2). Members of Acaulospora and the Scut. group occurred most frequently (Fig. 3) and were most abundant (Fig. 4) in semi-natural sites, S. calospora and A. cf. myriocarpa being dominant. In contrast, spores from the ‘Glomus’ grouping occurred most frequently in all samples from the intensive pastures (Fig. 3) and were most abundant, representing more than 20 % of the total AMF spores density (Fig. 4). Among the various taxa of this group, glomoid spore type 4 was the most common. Species of Paraglomus were also more frequent and abundant in intensive field sites than in semi-natural ones (Figs. 3 and 4), and Acaulospora spp. were present in almost all samples of intensive pasture (Figs. 3 and 4), with A. cf. paulinae, being the dominant species of this genus in RP2. There were also differences between sites within each land use. In the semi-natural pastures we observed that A. delicata, A. thomii, Archaeospora schenckii, Gigaspora sp. and R. cf. intraradices/irregularis only occurred in PX, whereas A. excavata, A. tuberculata and glomoid spore types 3 and 4 were detected only in TB (Table 2). In the intensive pastures, we also noted that some species like A. elegans, and glomoid spore types 5, 6. 7, 8 and 9 occurred only in RP1 (Table 2). Nine taxa as Glomus globiferum, glomoid spore types 1, 9, 10, 11, and 12, Claroideoglomus lamellosum, Entrophospora infrequens and Paraglomus sp. 2 were detected only in RP2 (Table 2).
3.3 AMF spore density
The total AMF spore densities did not differ among the two studied land uses (Nested Anova: F = 0.17, d.f. = 1, 2, p ≤ 0.683), or between field sites within each land use (Nested Anova: F = 0.64, d.f. = 2,36, p ≤ 0.531). Nevertheless, significant differences were found between land uses for the spore densities of Acaulospora, Claroideoglomus, ‘Glomus’ and the Scut. group (Table 3). The spore density of Acaulospora spp. was higher in the semi-natural pastures than in intensive ones, being the dominant genus in both semi-natural field sites, especially in PX with a relative abundance of 46 % (Fig. 4). The Scut. group spore density was also significantly influenced by management intensity, contributing approximately 40 % (Fig. 4) to that of the semi-natural pastures. In contrast, about 40 % of the AMF spores from the intensive pasture were from the genus Claroideoglomus and the ‘Glomus’ grouping. The glomoid spore density also varied significantly between the two land uses. Spores belonging to Claroideoglomus were detected only in intensively managed pastures.
3.4 Soil nutrient parameters
Total AMF spore density was not significantly correlated with any soil nutrients, but correlations were shown for AMF spore densities amongst the four groups sporulating at the highest densities (Acaulospora, Claroideoglomus, ‘Glomus’ and the Scut. group) (Table 4). The density of Acaulospora spores was positively correlated with N (r = 0.467), Mg (r = 0.408), OM (r = 0.457) and Ca (r = 0.379). Negative and significant correlations occurred between Claroideoglomus spores density and all soil nutrients analysed. The same result was also observed in relation to ‘Glomus’ and the Scut. group spore density except for P, which was not significantly correlated with spore density.
4 Discussion
4.1 AMF species diversity
Pasture management can affect the structure of AMF communities causing a decline in AMF diversity and producing a shift in the AMF community composition (Boddington and Dodd 2000; Jansa et al. 2003). In the current study we found that, based on spore morphology, there were no differences in AMF diversity in semi-natural and intensives pastures. A similar result was obtained in relation to AMF spore density, which although it was slightly higher in semi-natural systems was not statistically significant. Lack of consistent differences across the two managements practices investigated are opposite to previous reports of lower diversity (Oehl et al. 2004; Gai et al. 2009; Verbruggen et al. 2010) and abundance of AMF spores (Eason et al. 1999; Oehl et al. 2003; Castillo et al. 2006a) in disturbed soils than in less disrupted environments.
Our results were, however, consistent with some other reports. Jansa et al. (2002) found that spore abundance was not significantly affected by soil tillage, and Sjoberg et al. (2004) did not find significant differences in spore densities between semi-natural grassland and ploughed fields. Schalamuk et al. (2006) identified spores of 24 species of AMF in non-tilled and tilled wheat soils in Argentina suggesting that lack of disruption to the hyphal network in the no-tillage plots favoured members of the family Glomeraceae, though current phylogenetic classification (Krüger et al. 2012) shows that those species belong to several families in the order Glomerales.
In Terceira, the influence of human activities on the simplification of ecosystems is quite dramatic with very few endemic arthropods occurring in intensive pastures (Borges and Brown 1999; Cardoso et al. 2009). In fact, it is known that the input of fertilisers and pesticides and several other management practices (e.g., grazing intensity) are important in defining the quality of the pasturelands for AMF (e.g. Gange et al. 1993; Dhillion and Gardsjord 2004). However, the input of fertilisers to Azorean pastureland is lower than average inputs to pastures on the European mainland (Garcia and Furtado 1991), which means that at Terceira, both semi-natural and intensively managed pastures are reasonably benign habitats for AMF (Borges and Brown 1999). Moreover, topographical differences could create different communities within the site, some more inaccessible to grazing and others more intensively grazed, leading to some heterogeneity among pastures of the same land use type (Borges and Brown 1999).
4.2 AMF spores composition
Distinct differences were found in species composition between the two types of land use in our study. Species from the ‘Glomus’ grouping were dominant in intensively managed pastures, perhaps indicating adaptability in adjusting patterns of sporulation to environmental conditions (see also Castillo et al. 2006a; Gai et al. 2009; van der Gast et al. 2011), while species from Acaulospora and Scut. group were dominant in semi-natural pastures. Such differences in species composition could be related to differences in propagative units among the glomeromycotan families (Jansa et al. 2003; Castillo et al. 2006a). Some members of Glomeraceae may have a highly infective extra-radical mycelium that can directly colonise roots (Hart and Reader 2002). Species of Rhizophagus and Funneliformis are known to self-anastomose thus being able to re-establish an interconnected network after mechanical disturbance (Daniell et al. 2001). It is thought that, on the contrary, members of the Gigasporaceae are only capable of propagation via spore dispersal or infection from an intact mycelium and thus would colonise plant roots more slowly than members of Glomeraceae. Thus, under tillage the yearly disruption of the extraradical hyphae of these fungi would reduce their capacity of infection (Jansa et al. 2003; Börstler et al. 2006).
Spore dormancy and specific environmental conditions for spore germination probably slow the rate at which regenerating AMF can colonise plant roots (Hart and Reader 2002). For this reason, prevalence of species with glomoid spores in agricultural soil with repetitive severe physical disturbance, such as ploughing is widely reported (Jansa et al. 2002; Mathimaran et al. 2007; Castillo et al. 2006b; Lee and Eom 2009; Wang et al. 2008).
It is noteworthy that spores of Acaulospora spp. were often found in the intensively managed land use areas and together with those from ‘Glomus’ formed the highest proportion of spores in intensive pastures. Some members of Glomeraceae and Acaulosporaceae families respond to stress by producing more spores, allowing them to persist and dominate disturbed landscapes for longer (Castillo et al. 2006a; Jefwa et al. 2009).
It is also important to highlight the highest abundance and occurrence of Paraglomus species in intensive pastures. This result is consistent with a previous report by Lee and Eom ( 2009) which showed that P. occultum was only detected in the conventional farming while organic farming was dominated by Acaulospora species. However, a different result, based on molecular phylogenetic methods, was described by Gosling et al. (2014), who showed that members of the Paraglomerales were more common in soils under organic management than in sites managed conventionally in England. These authors also showed that distribution of Paraglomus spp. was not related to soil physical characteristics.
4.3 Soil nutrient availability
The effect of land use on AMF communities is probably strongly linked to the changes in soil fertility due to management practices (Titus and Leps 2000; Ezawa et al. 2001). In our study, the density of the four most abundant group - Acaulospora, Claroideoglomus, ‘Glomus’ and the Scut. group - present in the both semi-natural and intensively managed pastures, was correlated with almost all measured soil nutrients. Khanam et al. (2006) also found a positive correlation between soil nutrients levels (N, K, OM) and spore numbers in agricultural crops in Bangladesh. Nevertheless, the opposite has been observed in relation to total AMF spore density, which supported the generalisation that inorganic fertilisers may have a deleterious effect on many glomeromycotan species (Jefwa et al. 2006).
Among soil nutrients, phosphorus is considered to reduce the composition and diversity of AMF communities as well as spore and mycelium densities in both temperate and tropical systems (Agwa and Al-Sodany 2003; Oehl et al. 2004; Jansa et al. 2005; Khanam et al. 2006). Kahiluoto et al. (2001) demonstrated reduced AM colonisation of roots and AMF spore density in soil with increasing P fertilisation for several crops on two soils with low and intermediate concentrations of available P. However, in our study no statistically significant correlations were obtained between AMF spore density and this soil nutrient, as found also by Mathimaran et al. (2007) and Rodriguez-Echeverria et al. (2007). It is interesting to note that the P level in the two land use types under study was low and below the level considered prejudicial to AMF. Under P-limited conditions, the investment of a host plant in AMF is expected to increase to maintain the uptake of-limiting nutrients (Johnson et al. 2003). As predicted by the functional equilibrium model, in low-P soil, relative allocation to arbuscules, coils and extraradical hyphae is generally increased by N enrichment (Johnson et al. 2003). Consequently, in these Azorean pastures, we can observe an increase in AMF productivity, species richness and diversity with N, being the most responsive AMF taxa Acaulospora, the Scut. group and Gigaspora. In fact the lower C/N ratio in semi-natural pastures (27.8 vs. 54.6 in intensive pastures) could be explained by a higher mycorrhizal activity and higher cover abundance of legumes in these pastures (Borges and Brown 2001), which promoted the mineralisation process of OM resulting in an increase of the soil N (Atul-Nayyar et al. 2009). The opposite occurs in P-rich soils with N fertilisation application, observing a reduction of AMF community due to the loss of less common AMF species and the increase on abundance of ‘Glomus’ species (Egerton-Warburton et al. 2007).
4.4 Concluding remarks
This is the first published report of glomeromycotan species from the Azores. We conclude that whether pastures were intensively managed or not had no significant effect on AMF spore diversity and total density. However clear differences in AMF species composition were found between land use types. Species of ‘Glomus’ were dominant in intensive pastures, while Acaulospora and the Scut. group predominated in semi-natural pastures. This might be explained through different strategies of colonisation adopted by AMF and by soil nutrient levels. Members of Glomeraceae have a highly infective extra-radical mycelium that could allow immediate colonisation of plant roots, while members of Gigasporaceae are only capable of propagation via spore dispersal, colonising plant roots more slowly than members of Glomeraceae. Moreover, many of the species with glomoid spores form anastomoses between mycelia, and such species will have the ability to re-establish an interconnected network after mechanical disturbance. Therefore, management intensity affected the relative proportion of species from the Glomeraceae and Gigasporaceae.
The spore densities of the four most representative taxonomic groups, ‘Glomus’, Acaulospora Claroideoglomus and the Scut. group, were positively correlated with soil nutrients. Thus, the improvement of soil fertility, especially the availability of K, N, Ca and Mg, appear to have provided favourable environments for mycorrhizal formation and function in these systems. However, a correlation between spore density and soil P content was not found. The low level of soil P content and its implication on absorption of other soil nutrients, particularly N, might also explain some change in the AMF spore composition.
References
Agwa HE, Al-Sodany YM (2003) Arbuscular-mycorrhizal fungi (Glomales) in Egypt. III: Distribution and ecology in some plants in El-Omayed Biosphere Reserve Egyptian. J Biol 5:19–26
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Atul-Nayyar A, Hamel C, Hanson K, Germida J (2009) The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19:239–246
Azevedo E (1996) Modelação do clima insular à escala local. Modelo CIELO aplicado à ilha Terceira. Dissertation, University of Azores
Azevedo EB, Pereira LS, Itier B (1999) Modelling the local climate in island environments: water balance applications. Agr Water Manag 40:393–403
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Hock B, Varma A (eds) Mycorrhiza structure, function, molecular biology and biotechnology. Springer, Heidelberg, Germany, pp 521–559
Bhadalung N, Suwanarit A, Dell B, Nopamornbodi O, Thamchaipenet A, Rungchuang J (2005) Effects of long-term NP-fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant Soil 270:371–382
Blaszkowski J (2012) Glomeromycota. Krakow: W Szafer Institute of Botany, Polish Academy of Sciences
Boddington CL, Dodd JC (2000) The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. I. Field studies in an Indonesian ultisol. Plant Soil 218:137–144
Borges PAV (1997) Fauna de artrópodes (excl. Coleoptera) das pastagens dos Açores (S. Maria, Terceira e Pico) amostrados através da técnica do aspirador entomológico (Outono de 1994). University of Azores
Borges PAV, Brown VK (1999) Effect of island geological age on the arthropod species richness of Azorean pastures. Biol J Linn Soc 66:373–410
Borges PAV, Brown VK (2001) Phytophagous insects and web-building spiders in relation to pasture vegetation complexity. Ecography 24:68–82
Borges PAV, Ugland KI, Dinis FO, Gaspar C (2008) Insect and spider rarity in an oceanic island (Terceira, Azores): true rare and pseudo-rare species. In: Fattorini S (ed) Insect ecology and conservation. Research Signpost, Kerala, India, pp 47–70
Börstler B, Renker C, Kahmen A, Buscot F (2006) Species composition of arbuscular mycorrhizal fungi in two mountain meadows with differing management types and levels of plant biodiversity. Biol Fert Soils 42:286–298
Brundrett MC, Abbott LK, Jasper DA (1999) Glomalean mycorrhizal fungi from tropical Australia. I. Comparison of the effectiveness and specificity of different isolation procedures. Mycorrhiza 8:305–314
Cardoso P, Henriques SS, Gaspar C, Crespo LC, Carvalho R, Schmid JB, Sousa P, Szűts T (2009) Species richness and composition assessment of spiders in a Mediterranean scrubland. J Insect Conserv 13:45–55
Castillo CG, Rubio R, Rouanet JL, Borie F (2006a) Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biol Fert Soils 43:83–92
Castillo CG, Borie F, Godoy R, Rubio R, Sieverding E (2006b) Diversity of mycorrhizal plants species and arbuscular mycorrhizal fungi in evergreen forest, deciduous forest and grassland ecosystems of Southern Chile. J Appl Bot Food Qual 80:40–47
Clapp JP, Young JPW, Merryweather JW, Fitter AH (1995) Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. New Phytol 130(2):259–265
Daniell TJ, Husband R, Fitter AH, Young JPW (2001) Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol Ecol 36:203–209
Dhillion SS, Gardsjord TL (2004) Arbuscular mycorrhizas influence plant diversity, productivity, and nutrients in boreal grasslands. Can J Bot 82:104–114
Dias E (1996) Vegetação Natural dos Açores: Ecologia e Sintaxonomia das Florestas Naturais. Dissertation, University of Azores
Douds DD Jr, Millner PD (1999) Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agr Ecosyst Environ 74:77–93
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345
Eason WR, Scullion J, Scott EP (1999) Soil parameters and plant responses associated with arbuscular mycorrhizas from contrasting grassland management regimes. Agr Ecosyst Environ 73:245–255
Egerton-Warburton LM, Johnson NC, Allen EB (2007) Mycorrhizal community dynamics following nitrogen fertilization: A cross-site test in five grasslands. Ecol Monogr 77:527–544
Ezawa T, Smith SE, Smith FA (2001) Differentiation of polyphosphate metabolism between the extra-and intraradical hyphae of arbuscular mycorrhizal fungi. New Phytol 149:555–563
Gai JP, Liu RJ (2003) Effect of soil factors on AMF in the rhizosphere of wild plants. China J Appl Ecol 14:470–472
Gai JP, Christie P, Feng G, Li XL (2006) Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: A review. Mycorrhiza 16:229–23
Gai JP, Christie P, Cai XB, Fan JQ, Zhang JL, Feng G, Li XL (2009) Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau. Ecol Res 24:1345–1350
Galvez L, Douds DD, Drinkwater LE, Wagoner P (2001) Effect of tillage and farming system upon vam fungus populations and mycorrhizas and nutrient uptake of maize. Plant Soil 228:299–308
Gamper H, Walker C, Schüßler A (2009) Diversispora celata sp. nov.: Molecular ecology and phylotaxonomy of an inconspicuous arbuscular mycorrhizal fungus. New Phytol 182:495–506
Gange AC, Brown VK, Sinclair GS (1993) Vesicular-arbuscular mycorrhizal fungi: A determinant of plant community structure in early succession. Funct Ecol 7:616–622
Garcia V, Furtado M (1991) Desenvolvimento agrícola dos ecossistemas insulares açoreanos. In: Dias E, Carretas JP, Cordeiro P (eds) 1ªs Jornadas Atlânticas de Protecção do Meio Ambiente - Açores, Madeira, Canárias e Cabo Verde. Secretaria Regional do Turismo e Ambiente, Angra do Heroísmo, pp 5–8
Gilmore AE (1968) Phycomycetous mycorrhizal organisms collected by open-pot culture methods. Hilgardia 39:87–105
Gollotte A, van Tuinen D, Atkinson D (2004) Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 14:111–117
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agr Ecosyst Environ 113:17–35
Gosling P, Proctor M, Jones J, Bending GD (2014) Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 24:1–11
Hart MM, Reader RJ (2002) Host plant benefit from association with arbuscular mycorrhizal fungi: variation due to differences in size of mycelium. Biol Fert Soils 36:357–366
Helgason T, Fitter AH (2009) Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60:2465–2480
Hijri I, Sykorova Z, Oehl F, Ineichen K, Mader P, Wiemken A, Redecker D (2006) Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol 15:2277–2289
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68:1392146
Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossard E (2002) Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 12:225–234
Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossard E (2003) Soil tillage affects the community structure of mycorrhizal fungi in maize roots. Ecol Appl 13:1164–1176
Jansa J, Mozafar A, Frossard E (2005) Phosphorus acquisition strategies within arbuscular mycorrhizal fungal community of a single field site. Plant Soil 276:163–176
Jefwa JM, Sinclair R, Maghembe JA (2006) Diversity of glomale mycorrhizal fungi in maize/sesbania intercrops and maize monocrop systems in southern Malawi. Agrofor Syst 67:107–114
Jefwa JM, Mung’atu J, Okoth P, Muya E, Roimen H, Njuguini S (2009) Influence of land use types on occurrence of arbuscular mycorrhizal fungi in the high altitude regions of Mt. Kenya. Trop Subtrop Agroecosyst 11:277–290
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae. Ecol Appl 3:749–757
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
Kahiluoto H, Ketoja E, Vestberg M, Saarela I (2001) Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231:65–79
Khanam D, Mridha MAU, Solaiman ARM, Hossain T (2006) Effect of edaphic factors on root colonization and spore population of arbuscular mycorrhizal fungi. Bull Inst Agr Kyushu Univ 29:97–104
Krüger M, Stockinger H, Krüger C, Schüßler A (2009) DNA-based species level detection of Glomeromycota: One PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 183:212–223
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Kueffer C, Daehler CC, Torres-Santana CW, Lavergne C, Meyer J, Otto R, Silva L (2010) A global comparison of plant invasions on oceanic islands. Perspect Plant Ecol Evol Syst 12:145–161
Lee J-E, Eom A-H (2009) Effect of organic farming on spore diversity of Arbuscular Mycorrhizal fungi and Glomalin in soil. Mycobiology 37:272–276
Li LF, Zhang Y, Zhao ZW (2007) Arbuscular mycorrhizal colonization and spore density across different land-use types in a hot and arid ecosystem, Southwest China. J Plant Nutr Soil Sci 170:419–425
Liu Y, Mao L, He X, Cheng G, Ma X, An L, Feng H (2012) Rapid change of AM fungal community in a rain-fed wheat field with short-term plastic film mulching practice. Mycorrhiza 22:31–39
Martins AMF (1993) The Azores - Westernmost Europe: Where evolution can be caught red-handed. Bull Mus Mun Funchal 2:181–198
Mathimaran M, Ruh R, Jama B, Verchot L, Frossard E, Jansa J (2007) Impact of agricultural management on arbuscular mycorrhizal fungal communities in kenyan ferrasol. Agr Ecosyst Environ 119:22-32
Miller RM, Reinhardt DR, Jastrow JD (1995) External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103:17–23
Minitab (2000) Statistical software. Version 13. Minitab Inc.
Morton JB, Msiska Z (2010) Phylogenies from genetic and morphological characters do not support a revision of Gigasporaceae (Glomeromycota) into four families and five genera. Mycorrhiza 20:483–496
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2003) Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe. Appl Environ Microbiol 2816–2824
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2004) Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138:574–583
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, Van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738
Oehl F, Sieverding E, Palenzuela J, Ineichen K, de Silva GA (2011a) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2:191–199
Oehl F, de Silva GA, Goto BT, Sieverding E (2011b) New recombinations in Glomeromycota. Mycotaxon 117:429–434
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Soc. of Agronomy, Madison, Wisconsin, USA, pp 403–427
Pinheiro JF (1990) Estudo dos principais solos da Ilha Terceira (Açores). Dissertation, University of Azores
Pozo MJ, Azcon-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:3932398
Querejeta JI, Allen MF, Alguacil MM, Roldan A (2007) Plant isotopic composition provides insight into mechanisms underlying growth stimulation by AM fungi in a semiarid environment. Funct Plant Biol 34:6832691
Redecker D, Schüβler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza. doi:10.1007/s00572-013-0486-y
Rodriguez-Echeverria S, Hol WHG, Freitas H, Eason WR, Cook R (2007) Arbuscular mycorrhizal fungi of Ammophila arenaria (L.) Link: Spore abundance and root colonisation in six locations of the European coast. Eur J Soil Biol 44:30–36
Sanders FE (1975) The effect of foliar-applied phosphate on the mycorrhizal infections of onion roots. In: Mosse B, Tinker PB (eds) Endomycorrhizas. Academic, London, pp 261–276
Schalamuk S, Velazquez S, Chidichimo H, Cabello M (2006) Fungal spore diversity of arbuscular mycorrhizal fungi associated with spring wheat effects of tillage. Mycologia 98:16–22
Schübler A, Walker C. (2010) The Glomeromycota: a species list with new families and genera. Edinburgh & Kew, UK: The Royal Botanic Garden; Munich, Germany: Botanische Staatssammlung Munich; Oregon, USA: Oregon State University. URL: http://www.amf-phylogeny.com.
Schülbler A, Schwarzott D, Walker C (2001) A new fungal phylum, The Glomeromycota: Phylogeny and evolution. Mycol Res 105:1413–1421
Seaby RMH, Henderson PA, Prendergast JR (2004) Community Analysis Package. Version 4.01. Pisces Conservation Ltd
Sieverding E (1989) Ecology of VAM fungi in tropical agrosystems. Agr Ecosyst Environ 29:369–390
Silva L, Smith CW (2006) A quantitative approach to the study of non-indigenous plants: An example from the Azores Archipelago. Biodivers Conserv 15:1661–1679
Sjöberg J, Persson P, Mårtensson A, Mattsson L, Adholeya A, Alström S (2004) Occurrence of Glomeromycota spores and some arbuscular mycorrhiza fungal species in arable fields in Sweden. Acta Agr Scand B-S P 54:202–212
Sjögren E (1990) Bryophyte flora and vegetation on the island of Graciosa (Azores), with remarks on floristic diversity of the Azorean islands. Arquipélago 8:63–96
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, New York, p 787
Titus JH, Leps J (2000) The response of arbuscular mycorrhizae to fertilization, mowing and removal of dominant species in a diverse oligotrophic wet meadow. Am J Bot 87:392–401
van der Gast CJ, Gosling P, Tiwari B, Bending GD (2011) Spatial scaling of arbuscular mycorrhizal fungal diversity is affected by farming practice. Environ Microbiol 13:241–249
Verbruggen E, Röling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, van der Heijden MGA (2010) Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol 186:968–979
Walker C (1992) Systematics and taxonomy of the arbuscular endomycorrhizal fungi (Glomales) - a possible way forward. Agronomie 12:887–897
Walker C, Mize CW, McNabb HS (1982) Populations of endogonaceous fungi at two locations in central Iowa. Can J Bot 60:2518–2529
Wang G, Stribley D, Tinker P, Walker C (1985) Soil pH and vesicular-arbuscular mycorrhiza. In: Fitter AH (ed) Ecological Interactions in the soil environment: Plants. Microbes and animals - British ecological society special symposium, Blackwell, Oxford, pp 219–224
Wang YY, Vestberg M, Walker C, Hurme T, Zhang X, Lindström K (2008) Diversity and infectivity of arbuscular mycorrhizal fungi in agricultural soils of the Sichuan Province of mainland China. Mycorrhiza 18:59–68
Acknowledgments
Special thanks to Vasco Nunes of Regional Institute of Agrarian Planning for help with the field work, and for providing the aerial photographs. Many thanks to Clara Gaspar of Science Center for her most useful help with statistical analysis and suggestions, and also to Reinaldo Pimentel of Azorean Biodiversity Group (CITA-A) for providing the image software for the paper. We thank the soil laboratory and the chemistry laboratory of the University of the Azores for use of the facilities for spore extraction and chemical analyses of the soil. Many thanks to Raúl Paim for allowing the development of this study in his intensive pastures. CD Melo is indebted to Mery Jaizme-Vega of the Instituto Canario de Investigaciones Agrarias, for the training on extraction methods of AMF species. We gratefully acknowledge financial support for this research from the Portuguese Fundação para a Ciência e a Tecnologia (SFRH/BD/18355/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melo, C.D., Walker, C., Rodríguez-Echeverría, S. et al. Species composition of arbuscular mycorrhizal fungi differ in semi-natural and intensively managed pastures in an isolated oceanic island (Terceira, Azores). Symbiosis 64, 73–85 (2014). https://doi.org/10.1007/s13199-014-0303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-014-0303-1