Abstract
Three different varieties of finger millets (VL-315, VL-324, and VL-347) cultivated in Uttrakhand, India, were used to extract high purity starch using the alkali soaking approach and investigated physicochemical and structural properties. VL-315, VL-324, and VL-347, contain 78 ± 0.35%, 79 ± 0.35%, and 87 ± 0.35% starch, respectively, of which 39.03 ± 0.35%, 37.2 ± 0.35%, and 33.5 ± 0.35% are the amylose contents, respectively. Chemical composition analysis exhibited the level of ash and moisture content in the dry basis of 0.0031 ± 0.01% to 0.035 ± 0.05%, and 12.52 ± 0.8% to 12.92 ± 0.2%, respectively. The solubility and swelling range of VL-315 is 1.3–4.3% and 16.54–10.3 (g/g), respectively, which significantly differ from VL-324 and VL-347. XRD analysis revealed that extracted starch showed a typical A-type crystalline network with a crystallinity range of 17.7–19.3%, which remarkably influenced retro gradation tendencies of starch. SEM demonstrated that extracted starch granules are polyhedral shape with a smooth surface. Finger millet starch has enormous potential in the development of starch-based edible film and coating on food items. In the present work, extracted finger millet starch was studied with the aim of developing a thin and flexible food packaging film. From the results, it was observed that the fabricated films had excellent functional properties, including solubility, swelling index, and water vapor permeability, which could eliminate petroleum-based packaging materials, and gives food materials an extra shelf life, and improve overall food quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Finger millet is an annual herbaceous plant that provides grains recognized as ragi in India. It is one of the main cereals consumed in the southern region of India because of its abundance and low cost. Seed color varies from light brown to dark brown; generally, seeds have a round shape with 1–2 mm diameter, are known worldwide due to its gluten-free and boast high protein, fiber, and antioxidant contents. Therefore, it is presumed as an excellent grain in terms of nutritional values such as 5–8% proteins, 10–15% dietary fiber, 1–2% other extractives, and 65–75% carbohydrates (Chandra et al. 2016). It also contains the right amount of minerals, such as calcium (344 mg) and potassium (408 mg). High nutritional value makes finger millet superior to other cereals, namely rice, and wheat. It is grown mostly in the marginal regions where other seeds fail to yield sustainable production. About 37% of the total millet production in the world is cultivated in India. It is mainly grown in Karnataka, Maharashtra, and Uttrakhand (Balasubramanian et al. 2011). Starch is a universal polysaccharide that is abundantly available in finger millet seeds. Typically, the size of the starch granule varies between 1 and 120 mm and is present in different shapes, such as polygonal, round, and lenticular (Kumar et al. 2019b). Starch consists of two polymeric components with varied contents such as amylose and branched amylopectin depending on the environmental condition, plant varieties, and sources (Pérez-Pacheco et al. 2014). It exhibits excellent water absorption capacity and undergoes gel formation during heating. Therefore, currently, the requirements of starch in various applications such as adhesive, diluents, gelling agents, thickener, emulsion stabilizer, fillers and bulking and suspending agents has significantly surged in food and non-food industries. Besides, starch nanoparticles are also used as a carrier in the drug delivery system. The composition and physicochemical properties of some millet have mostly been explored. Random studies on different types of millets are not properly documented to hinder the further development and application at industrial scales. Besides, various factors, such as soil and genetic and environmental conditions, also influence millet properties and structure that have not been appropriately documented (Rathore et al. 2019). The precise understanding of finger millet starch properties will encourage demand in industries. Various sources of starch have been explored to meet the increasing demand in food industries. The present study focused on finding new non-conventional sources of starch to meet the demand for food and non-food packaging industries. In this regard, finger millets are possible alternatives and recognized starch sources due to their high availability throughout India. However, limited and discrete studies on millets are found. Increasing environmental awareness and healthy lifestyle motivate the research scholar and academician to look for eco-friendly and biodegradable food packaging and coating materials (Shah et al. 2016). In the present time, various natural polymers, including starch, cellulose, gums, and proteins, have been documented for the development of eco-friendly biodegradable food packaging and coating (Vieira et al. 2011). The primary purpose of these packaging materials is to protect food products during transportation and storage. It also improves food quality and shelf life by controlling the migration rate of moisture and gases through the material. The main focus of the present work is to provide detailed and complete knowledge about the finger millet and then, based on the current understanding of finger millet starch, focused on the development of transparent, flexible, and thin films with desired properties. The transparent packaging films show customers a realistic picture of food materials within the package to improve the reliability of the overall food materials. This study provides a detailed characterization of the basic properties of finger millet starch, including thickness, moisture content, swelling index, solubility, and moisture barrier. It then evaluates the use fullness of finger millet starch in the development of flexible thin packaging films.
Materials and methods
Materials
The finger millet varieties, VL-315, VL-324, and VL-347, were procured from ICAR-Vivekananda Parvatiya Krishi Anusandhan Sansthan, Almora, Uttrakhand India. Sodium hydroxide, potassium hydroxide, iodine reagent were purchased from Loba Chem (Mumbai, India).
Methods
Starch isolation
Finger millet starch was extracted according to the alkali socking approach. Initially, finger millet (100 gm) was washed in running water and then soaked in 0.25% w/v of sodium hydroxide at 15 ± 1 °C for 24 h. Soaked finger millets were grounded using the Sujata blender (India) for 3 min. Muslin cloth and decantation methods were used to isolated starch from slurry. Isolated starch was cleaned using regular and distilled water to remove the impurities. The purified starch was then dried for 24 h at 35 ± 1 °C.
Moisture content (MC)
Initially, the weights of the dried crucibles were measured (w1). Then, the starch samples were spread on the dried crucibles and dried at 105 ± 1 °C for 24 h. After drying, the weight of all crucibles was measured again (w2). MC was determined using Eq. (1)
where w1 and w2 are the initial (g) and final weight of crucibles (g), respectively.
Ash content
Initially, the crucibles were heated at 450 ± 5 °C for 24 h to eliminate impurities and weight (M1). 5 g of starch samples were placed in the crucibles and heated in a furnace at 450 ± 5 °C for 4 h. After that, crucibles were carefully removed to avoid the ash loss and weighed again (M2). The ash content was determined according to Eq. (2)
whereas M1 and M2 are the initial (g) and final weight of crucibles (g), respectively.
Swelling index
The swelling index was evaluated at 60 ± 1 °C, 70 ± 1 °C, 80 ± 1 °C, and 90 ± 1 °C according to the reported method with small changes (Tejavathi et al. 2020). Starch samples (0.1 g) were mixed in10 ml of distilled water. Now, starch mixtures were heated with continuous mixing at various temperatures mentioned above for 30 min and then centrifuged at 3000 rpm for 15 min. The supernatant part was isolated and dried at 130 ± 1 °C for 24 h. The rest of the sediment left at room temperature for 5 min and then weighed. The swelling index of starch samples was calculated using Eq. (3)
Solubility
The solubility of isolated finger millet starch was determined using the approach documented by (Punia et al. 2017) with minor changes. The starch sample (0.5 g) was dispersed in water and heated with continuous mixing at 60 ± 1 °C, 70 ± 1 °C, 80 ± 1 °C, and 90 ± 1 °C for 30 min and then centrifuged at 3000 rpm for 15 min. The supernatant part was carefully isolated. The 5 g supernatant part was poured on a petri dish and then dried a preheated oven at 130 ± 1 °C for 4 h and weighed. Starch solubility was calculated using Eq. (4).
FTIR analysis
FT-IR spectra of finger millets using the potassium bromide disc approach were obtained using the Shimadzu FT-IR instrument (Shimadzu Corporation, Kyoto, Japan). FTIR spectra were obtained by scanning at a wavelength between 400 and 4000 cm–1 with a resolution of 4 cm (Kumar et al. 2020).
SEM analysis
The morphology of the isolated finger millet granules was studied using a Scanning Electron Microscope (SEM) operated at an acceleration potential of 15 kV.
XRD analysis
The crystallinity of the finger millets was examined using XRD Diffractometer (Phillips PW1710) operated at 40 kV and 25 mA. Rate (4°/min) was used to scan the samples Development of the starch film.
The Flexible films were prepared from extracted finger millet starch using a solution casting method. The finger millet starch (3% w/v) and sorbitol (25% w/w) were mixed into 100 ml of distilled water. The starch mixture was heating with continuous stirring at 90 ± 1 °C for 30 min using a hot plate. The starch solution was left for 15 min to remove the air bubbles. A fixed amount (15 ml) of the starch solution was evenly distributed onto a petri dish. The film solution dried at 25 ± 1 °C for 24 h.
Film thickness
Thickness was taken at six different positions on the film surface using a digital micrometer (Mitutoyo, Japan) with an accuracy of 0.001 mm.
Moisture content (MC)
MC was evaluated using oven drying known weight of samples with size (2 cm × 2 cm) at 105 °C for 24 h. The variations in the weight of the samples were determined using Eq. (5)
whereas, w1 and w2 are the initial weight (g) and dried weight of samples (g), respectively.
Swelling Index (SI)
SI of the film was determined using the gravimetrical method. The samples with size (2 cm × 2 cm) were dried in a preheated oven (105 ± 1 °C) for 24 h and weighed (m1). The dried sample with was dipped in the beaker filled 15 ml distilled water for 5 min. Excess water of the swelled sample was removed using tissue paper and weighed (m2). SI was determined using Eq. (6)
whereas, m1 and m2 are the weight of dried (g) and wet samples (g), respectively.
Solubility (S)
S was determined according to the method documented by (Kumar et al. 2019a). A preheated oven (105 ± 1 °C) was used to dry the sample for 24 h and weighed (mi). An isolated sample with size (2 cm × 2 cm) was immersed in distilled water for 24 h. The undissolved sample was taken out of the water and kept at 25 ± 1 °C for 24 h. The weight of the dried sample was measured (mf). The variation in the weight of the sample was evaluated using Eq. (7)
whereas, mi and mf are the weight of dried (g) and insoluble samples (g), respectively.
Water vapor permeability (WVP)
WVP of the finger millet starch film was evaluated using ASTM E 95-96. The beakers were filled with dried calcium chloride and sealed with the film. Vacuum grease was used to prevent leakage of any moisture from the beaker and film joints. The prepared beakers were placed in an incubator containing distilled water. The incubator was placed in a preheated oven at 25 ± 1 °C. The changes in beakers weight were recorded after a fixed interval of time and determined using Eq. (8)
where \(\frac{{{\Delta }w}}{{\text{T}}}\) is the gain in beaker weight per unit time (g/h), L is film thickness (mm), A is an area of the beaker (cm2), and Pi–Po is the pressure difference (Pa), respectively.
Result and discussion
Proximate analysis
The starch composition, including moisture content, amylose content, ash content, and yield extracted from the three varieties of finger millets (VL-315, VL-324, and VL-347), are illustrated in Table 1. From visual inspection, it appears that the starch extracted from three different finger millet varieties (VL-315, VL-324, and VL-347) is white, and starch yield varied from 63.5 ± 1.2% to 67.9 ± 1.8% on total seed basis. Similarly, starch content 58% and 30%–32% were reported in maize (Lawal et al. 2005) and oats (Hoover et al. 2003). From the results, it was found that the moisture content in three different varieties of the finger millet starch varied widely and ranged between 14.5 ± 0.3% (VL-235) to 24.5 ± 0.5% (VL-247), which were similar to those earlier reported (10.37–11.07%) (Lu et al. 2019). Ash content in extracted starch was noticed in the range of 1.6%-1.9%. The ash content was within the range reported by Suma and Urooj (2015). It seems that the starch obtained from finger millets does not contain any impurities.
Amylose content
Amylose content in the extracted starch dictates most of its usages in the industries. The starch with high amylose content value is the most suitable raw materials in various food and pharmaceutical industries (Vithu et al. 2020). The amylose content of extracted starch is presented in Table 1. The amylose content in three varieties of finger millet starch was 39.03 ± 0.6%, 37.2 ± 0.2%, and 33.5 ± 0.9%, respectively. These amylose content values were much higher than the moth bean starch reported by (Punia et al. 2017) (7.8–22%). Similarly, Liu et al. (2015) reported 33% amylose content in the field peas, which is similar to the present work results. VL-315 showed the highest amylose content value, i.e., 39.03 ± 0.6% compared to VL-347 and VL-324. Further works are required to study the variability in amylose content. Variation in values may be associated with parameters, including genetics, isolated techniques, climatic, and legumes varieties (Zhang et al. 2019).
Swelling index and solubility
The swelling index and solubility of the finger millet starch were studied over the temperature range (60–90 °C). The results are illustrated in Table 2. The solubility of VL-347, i.e., 6.3 ± 0.85% at 90 °C, was higher compared to VL-315 and VL-324. The swelling index and solubility of finger millet starch were higher compared to other legumes starch (Li and Zhu 2018). Solubility was increased rapidly in the temperature range of 70–90 °C. Similarly, Hoover et al. (1997) also reported a rapid increase in the solubility of mung bean starch between 60–90 °C. The high solubility of finger millet starch is highly linked with the strong association of amylopectin chains within the crystalline region compared to starches. The swelling index shows water binding ability of extracted starch, and results indicated that the swelling index of different finger millet starch (VL-315, VL-324, and VL-347) varied and ranged from 8.1 to 17.7 (g/g). Among the three different varieties, the highest swelling index was noticed for VL-315, and the lowest was seen for VL-347. These outcomes are similar to those documented by (Abdel-Rahman et al. 2008). It was noticed that the swelling index of all extracted starch was enhanced with temperature (Table 2). Enhance in swelling index with temperature was more rapid after 70 °C. The increase in swelling index and solubility with rising temperatures can be impacted by various factors such as amylose content, interactions among glucan chains, the structure of both amylose and amylopectin with the melting of starch crystallinity (Simsek et al. 2009).
FTIR analysis
The FTIR spectrum of starches from three different finger millet varieties such as VL-315, VL-324, and VL-347 was investigated to know the variation in the crystallization zone, amorphous zone, and chemical bonds. The FTIR spectrum peaks of the finger millet starch are comparable to the peaks of the standard starch samples, as shown in Fig. 1a. Finger millet starch exhibited broad peaks at 3300–3100 cm−1, representing the vibration of O–H stretching because of hydrogen-bonded hydroxyl groups. The peak at 2900–3000 cm−1 was associated with C-H stretching of the glucose units. The moisture content in the amorphous region of starch associated is represented by the peaks at 1630–1660 cm−1. Similar results were reported for horse grams by (Chavan et al. 2010). Other peaks also noticed in finger millet starch are at 1417 cm−1 and 1001 cm−1, which may be associated with C-H bending and C–O stretching. The peaks between 860 and 770 cm−1 represent glycosidic links of starch. Similar discussions were reiterated by (Karwasra et al. 2017). No apparent differences were noticed between the FTIR spectrums of starches from three different finger millet varieties. Further peaks at 450–950 cm−1 were assigned to skeletal vibrations of the pyranoid ring.
Thermal Analysis
Thermo gravimetric analysis (TGA) is the most common technique used to examine the thermal stability of the material. It indicates the maximum processing temperature of the material. The TGA curves of three different finger millet starch are demonstrated in Fig. 1b. From the results, it seems the starch was decomposed into two stages. The temperature profile starts at a temperature of 35 °C. When temperature escalated from 75 to 120 °C, unbounded moisture present in the extracted finger millet starch was lost. From Fig. 1b, it notices that the concentration of unbounded moisture in VL-347 starch was lower than that of VL-315 and VL-324. As temperature further enhanced from 120 to 270 °C, small starch structure and bounded moisture was noticed. Initial weight loss in VL-347 was lower compared to VL-315 and VL-324 in the temperature of 80–270 °C. By further increasing the temperature from 270 to 350 °C, starch pyrolysis took place and released various products such as water, carbon dioxide, carbon monoxide, acetaldehyde, furan, and 2-methyl furan (Villalobos et al. 2017). It is the most important step associated with the decomposition mechanism of starch. Further, the decomposition of main starch components such as amylose and amylopectin occurred in the same temperature range. Similar outcomes regarding the thermal stability of sweet potato starch have been documented by (Liu et al. 2019). Further, a rise in the temperature from 350 to 600 °C, carbon black formed (Liu et al. 2013).
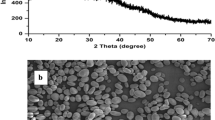
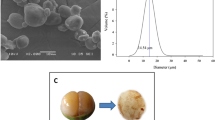
SEM analysis
SEM images of extracted starch from the finger millets are presented in Fig. 2. These images were used to examine the shape and structure of extracted starch granules. Finger millet starch granules obtained from three different varieties such as VL-315, VL-324, and VL-347 shows polygon shapes with smooth surfaces. The results of the present work are in agreement with a reported work (Sodhi et al. 2003). In addition, all starch granules without any pore or holes in the surface exhibited the presence of unbroken starch granules. Similar outcomes regarding the morphology of proso millets have been documented by (Wen et al. 2014). Smooth surfaces without cracks in extracted finger millets starch granules indicate the purity in starch isolation, but granules morphologies can be diverse and depend on many parameters such as plant genetics, environmental conditions, and many other parameters have been assumed to influence the size and shape of starches granules (De Castro et al. 2018).
XRD analysis
The XRD diffractogram was used to identify the degree of crystallinity of extracted finger millets starch granules. Based on the obtained XRD pattern, the crystal pattern of starch is divided into three categories- A, B, and C. Categories A, B, and C are cereals, tubers, and legumes, respectively. Category A starch granules exhibited a sharper XRD pattern compared with the other two categories. The present study shows all finger millet starch in category A of crystalline pattern with four main diffraction peaks at 2θ of 15°, 17°, 18°, and 23° (Fig. 3). All XRD patterns of extracted starch exhibited sharp peaks. This suggests that the extracted starch contains fine granules, which enhance the structural stability of the material (Matignon and Tecante 2017). Similarly, Londoño-Restrepo et al. (2018) also reported that the sharp peaks obtained in the XRD pattern of isolated starch are related to a nanocrystalline network of amylose or amylopectin. Also, a greater number of counts in the XRD pattern demonstrate excellent structural stability. All finger millets starch (VL-315, VL-324, and VL-347) exhibited a varying range of crystallinity from 14.5% -19.3%. Variation in the crystallinity of isolated starch associated with crystallite size and orientations of double helices within the crystalline region (Kumar et al. 2019b).
Film thickness and moisture content
Homogeneous, continuous without cracks and translucent films from extracted starch have been successfully fabricated. The results of MC and film thickness of extracted starch-based film are indicated in Table 3. The film thickness and MC of all prepared film from finger millet starches are varied from 0.03 mm to 0.04 mm and 10.32 ± 0.65% to 12.56 ± 074%, respectively. Variation in the thickness and MC is related with amylose content and crystallinity of extracted starch from the finger millets (Chandla et al. 2017).
Solubility and swelling index
Table 2 represents the results obtained for S and SI of extracted starch based thin and flexible films. S and SI of prepared films are considered as an important parameter when fabricated finger millet starch films use as a packaging material. Furthermore, water behaves as a plasticizer; therefore, the traces of water molecules in developed films can alter their functional properties. The SI and SI of the prepared films were varied from 11.89 ± 1.2% to 15.62 ± 1.1% and 25.61 ± 0.97% to 30.14 ± 1.5%, respectively. Both functional properties of finger millet starch film were found higher compared to corn starch film (Dai et al. 2015).
Water vapor permeability
The primary objective of the food packaging materials is to prevent the transport of moisture from the surrounding atmosphere to the food materials. WVP values of finger millet starch films are shown in Table 3. WVP of finger millet starch film was observed in the range of 1.1 ± 0.08 × 1010 gm–1 s–1 Pa–1 to 2.09 ± 0.04 × 10–10gm–1 s–1 Pa–1. The results illustrated that WVP of finger millet starch films was similar to WVP values reported for mango kernel starch film (0.98 ± 0.04 × 10–10gm–1 s–1 Pa–1) (Nawab et al. 2016), and amaranth starch (2.76 ± 0.05 × 10–10gm–1 s–1 Pa–1) (Chandla et al. 2017). Various factors such as crystallinity, environmental conditions, film thickness, and amount and nature of plasticizers influenced the WVP of the film made with isolated finger millet starches (Thakur et al. 2019).
Conclusion
In the present work, starch was extracted from three finger millet varieties (VL-315 VL-324, and VL-347) using an alkali soaking approach and subsequently studied its variation in thermal, morphological, and physicochemical properties. Physicochemical properties, including moisture content, swelling, solubility index, ash content, and amylose content of the extracted starch found to be superior to other cereals such as wheat, rice, and maize. Characterization techniques called XRD, SEM, and FTIR showed that the starch granules were polygonal, angular, and tightly packed structure. The evaluation of the physicochemical properties of finger millet starch provides knowledge for the prepared food and non-food materials. In current work, the results of this work were taken in the fabrication of flexible and clear thin films. From the results, it seems that flexible and clear thin film made with finger millet starch can eliminate excessive petroleum-based packaging and provide good quality to food materials in one or another way. Isolated finger millet starch can develop different varieties of composite material that may be comprehended as an approbative consumer benefit shortly.
References
Abdel-Rahman ESA, El-Fishawy FA, EL-Geddawy MA, Kurz T, EI-Rify MN (2008) Isolation and physico chemical characterization of mung bean starches. Int J Food Eng. https://doi.org/10.2202/1556-3758.1184
Balasubramanian S, Sharma R, Kaur J, Bhardwaj N (2011) Isolation, modification and characterization of finger millet ( Eleucine coracana ) starch. J Food Sci Eng 1:339–347
Chandla NK, Saxena DC, Singh S (2017) Amaranth (Amaranthus spp.) starch isolation, characterization, and utilization in development of clear edible films. J Food Process Preserv. https://doi.org/10.1111/jfpp.13217
Chandra D, Chandra S, Pallavi SAK (2016) Review of Finger millet (Eleusinecoracana (L.) Gaertn: A power house of health benefiting nutrients. Food sci human wellness 5:149–155
Chavan UD, Shinde BG, Kadam SS, Amarowicz R (2010) Isolation and characterization of starch from horse gram. African J Food Sci Technol 1:64–67
Dai L, Qiu C, Xiong L, Sun Q (2015) Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem 174:82–88
De Castro DS, dos Santos MI, de Melo Silva LM, Lima JP, Silva WPD, Gompes JP, Figuiersdo RMFD (2018) Isolation and characterization of starch from pitomba endocarp. Food Res Int 124:181–187
Hoover R, Li YX, Hynes G, Senanayake N (1997) Physicochemical characterization of mung bean starch. Food Hydrocoll 11:401–408
Hoover R, Smith C, Zhou Y, Ratnayake RMWS (2003) Physicochemical properties of Canadian oat starches. Carbohydr Polym 52:253–261
Karwasra BL, Gill BS, Kaur M (2017) Rheological and structural properties of starches from different Indian wheat cultivars and their relationships. Int J Food Prop 20:S1093–S1106
Kumar R, Ghoshal G, Goyal M (2019a) Synthesis and functional properties of gelatin/CA–starch composite film: excellent food packaging material. J Food Sci Technol 56:1954–1965
Kumar R, Ghoshal G, Goyal M (2019b) Moth bean starch (Vigna aconitifolia): isolation, characterization, and development of edible/biodegradable films. J Food Sci Technol 56:4891–4900
Kumar R, Ghoshal G, Goyal M (2020) Development and characterization of corn starch based nanocomposite film with AgNPs and plant extract. Mater Sci Energy Technol 3:672–678
Lawal OS, Adebowale KO, Ogunsanwo BM, Barba LL, Ilo NS (2005) Oxidized and acid thinned starch derivatives of hybrid maize: Functional characteristics, wide-angle X-ray diffractometry and thermal properties. Int J Biol Macromol 35:71–79
Li G, Zhu F (2018) Quinoa starch: Structure, properties, and applications. Carbohydr Polym 181:851–861
Liu C, Wang S, Copeland L, Wang S (2015) Physicochemical properties and in vitro digestibility of starches from field peas grown in China. LWT - Food Sci Technol 64:829–836
Liu X, Wang Y, Yu L, Tong Z, Chen L, Liu H, Li X (2013) Thermal degradation and stability of starch under different processing conditions. Starch/Starke 65:48–60
Liu Y, Yang L, Ma C, Zhang Y (2019) Thermal behavior of sweet Potato starch by non isothermal thermo gravimetric analysis. Molecules 12:699
Londoño-Restrepo SM, Rincón-Londoño N, Contreras-Padilla M, Millan-Malo BM, Garcia MER (2018) Morphological, structural, thermal, compositional, vibrational, and pasting characterization of white, yellow, and purple Arracacha Lego-like starches and flours (Arracacia xanthorrhiza). Int J Biol Macromol 113:1188–1197
Lu Y, Zhang X, Yang Y, Qi Y, Hao W, Wang L, Liu Q, Ling Y, Zhang C (2019) Relationship between structure and physicochemical properties of ginkgo starches from seven cultivars. Food Chem 314:125082
Matignon A, Tecante A (2017) Starch retrogradation: from starch components to cereal products. Food Hydrocoll 68:43–52
Nawab A, Alam F, Haq MA, Hasnain A (2016) Biodegradable film from mango kernel starch: Effect of plasticizers on physical, barrier, and mechanical properties. Starch/Staerke 68:919–928
Pérez-Pacheco E, Moo-Huchin VM, Estrada-León RJ, Fernandez AQ, Hernandez LHM, Soberanis CRR, Ancona DB (2014) Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr Polym 101:920–927
Punia R, Sharma MM, Kalita D, Mukharjee J, Nayak T, Singh H (2017) Physicochemical, morphological, thermal and pasting characteristics of starches from moth bean (Vigna aconitifolia) cultivars grown in India: an underutilized crop. J Food Sci Technol 54:4484–4492
Rathore T, Singh R, Kamble DB (2019) Upadhyay A (2019) Review on finger millet: processing and value addition. Pharma Innov J 8:283–291
Shah U, Naqash F, Gani A, Masoodi FA (2016) Art and science behind modified starch edible films and coatings: a review. Compr Rev Food Sci Food Saf 15:568–580
Sodhi NS, Singh N (2003) Morphological, thermal and rheological properties of starchesseparated from rice cultivars grown in India. Food Chem 80:99–108
Simsek S, Tulbek MC, Yao Y, Schatz B (2009) Starch characteristics of dry peas (Pisum sativum L.) grown in the USA. Food Chem 115:832–838
Suma PF, Urooj A (2015) Isolation and characterization of starch from pearl millet (Pennisetum typhoidium) flours. Int J Food Prop 18:2675–2687
Tejavathi DH, Sujatha BS, Karigar CS (2020) Physicochemical properties of starch obtained from Curcuma karnatakensis - A new botanical source for high amylose content. Heliyon 429(6):e03169
Thakur R, Pristijono P, Scarlett CJ, Bowyer M, Singh SP, Vuong QV (2019) Starch-based films: Major factors affecting their properties. Int J Biol Macromol 132:1079–1089
Vieira MGA, Da Silva MA, Dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: A review. Eur Polym J 47:254–263
Villalobos K, Rojas H, González-paz R (2017) Production of Starch Films Using Propolis Nanoparticles as Novel Bioplasticizer. J Renew Mater 5:189–198
Vithu P, Dash SK, Rayaguru K, Panda NK, Neduncheziyan M (2020) Optimization of starch isolation process for sweet potato and characterization of the prepared starch. J Food Meas Charact 14:1520–1532
Wen Y, Liu J, Meng X (2014) Characterization of proso millet starches from different geographical origins of China. Food Sci Biotechnol 23:1371–1377
Zhang Z, Saleh ASM, Wu H (2019) Effect of starch isolation method on structural and physicochemical properties of acorn kernel starch. Starch/starke 72:1900122
Acknowledgements
This research work was supported by Dr. B.R. Ambedkar National Institute of Technology (NIT), Jalandhar, Punjab, Inda with the help of Ministry of Human Resource Development (MHRD), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest related to this research article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gautam, N., Garg, S. & Yadav, S. Underutilized finger millet crop for starch extraction, characterization, and utilization in the development of flexible thin film. J Food Sci Technol 58, 4411–4419 (2021). https://doi.org/10.1007/s13197-020-04926-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04926-0