Abstract
This study aimed to optimize the co-production of conjugated linoleic acid (CLA), exopolysaccharides (EPSs) and bacteriocins (BACs) by Lactobacillus acidophilus LA-5 in dairy food-grade by-product. The factorial design revealed that the significant factors were temperature, time, and yeast extract. Then the response surface methodology was used for optimization. At the optimal conditions the viable cell number, CLA, EPSs, and inhibition activity were 2.62 ± 0.49 × 108 CFU/mL, 51.46 ± 1.50 μg/mL, 348.24 ± 5.61 mg/mL and 12.46 ± 0.80 mm, respectively. FTIR, GC, TLC, and SDS page analysis revealed the functional groups of pharmabiotics. The FTIR, GC, TLC, and SDS page analysis showed that both CLA isomers (c-9, t-11, and t-10, c-12) produced. The FTIR, GC, TLC, and SDS page analysis indicated that produced EPSs were composed of glucose, mannose, galactose, xylose, and fructose. FTIR, GC, TLC, and SDS page used to report BACs molecular weight, which showed two fractions by molecular mass 35 and 63 kDa. Previously the ability of different probiotic bacteria investigated and optimized the production of CLA, EPSs, and BACs, but, there was no report on the co-producing capacity of these bioactive metabolites by probiotics. The present work was investigated to optimize the co-production of pharmabiotic metabolites by L. acidophilus LA-5, in supplemented cheese whey as a cultivation medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumer’s demand and interest in bio-functional foods with health-associated bio-actives are increasing (Amiri et al. 2020a; Adnan et al. 2017a). Probiotics such as Lactobacilli, which is also the flora of gut microbiota, received more attention to producing functional foods in recent years. A new opening for the advance bio-functional foods is the potential of probiotics to synthesize biologically active molecules with health-promoting benefits, which called “Pharmabiotics,” such as conjugated linoleic acid (CLA), exopolysaccharides (EPSs) and bacteriocins (BACs). Lactobacilli were used to the biosynthesis of pharmabiotics in much research because of two reasons: (1) Almost all species of this genus have the ability to produce CLA, EPSs, and BACs and (2) all species of the family are GRAS (Generally Recognized as Safe) (Amiri et al. 2019, 2020b; Adnan et al. 2017b; Ünlü et al. 2015).
The term “CLA” is defined as a set of bioactive isomers of linoleic acid (LA; C18:2), which contain conjugated bonds. Ruminal microorganisms biosynthesized these bioactive lipids during the exponential phase by the bio-hydrogenation of dietary polyunsaturated fatty acids in the rumen. The main isomers of CLA are cis-9, trans-11 and trans-10, cis-12, due to the having health-beneficial effects. The CLA has some health-promote effects, for example, anti-cancer, anti-oxidative, anti-atherosclerosis, anti-obesity, anti-diabetes, and anti-inflammatory activity as well as reducing body fat, increasing immune functions, and enhancing bone mass (Terán et al. 2015). EPSs is a term to define polysaccharides molecules which are primary metabolites and secrete by some bacteria into the culture media and serve as soluble fibers to improve digestion and lower blood sugar. However, this type of polysaccharides can promote the microbial communities that colonize the gastrointestinal tract to improve health, and prevent or treat disease (Holscher 2017). In recent years, EPSs received increasing attention and used as additives in food and pharmaceutical industry due to beneficial effects on human health. Anti-oxidant, anti-cancer, and anti-inflammatory, as well as immune-related effects are examples of EPSs health-promoting activities (Amiri et al. 2019). BACs is a generic name for small bacterial peptides which bio-synthesized ribosomally and have antagonistic activity against closely related BACs producer species as well as some food-borne pathogens and spoilage bacteria. Recently, BACs have got extra attractive due to health-related activities such as anti-cancer, anti-viral, and spermicidal activities (Alshammari et al. 2019; Drider et al. 2016).
Individual production of pharmabiotics by probiotics is typical and their creation, extraction, and purification are time-consuming and costly. Hence, a practical approach will be utilizing a cheap food-grade medium to in situ co-production of these bioactive metabolites. Due to, no report about co-production of CLA, EPSs, and BACs as pharmabiotics by Lactobacilli species. The purposes of this study were: (1) screening of critically independent variables that improve co-production of pharmabiotics (conjugated linoleic acids, EPSs, and BACs), (2) optimizing of pharmabiotics co-production by response surface methodology and obtain a quadratic model to each pharmabiotics bio-synthesize in fermentation bioprocess, and (3) characterizing produced pharmabiotics by Fourier-transform infrared, gas chromatography, Thin-layer chromatography, and SDS page analysis.
Materials and methods
Probiotic bacterium and cultivation condition
Lactobacillus acidophilus LA-5 (Chr. Hansen, Hørsholm, Denmark) achieved and used, according to the recommendation of the company. After that, it was cultured in 0.1% tween 80 (AppliChem, Darmstadt, Germany) added MRS (de Man, Rogosa, and Sharpe) broth (Merck, Germany) for 18 h at 37 °C. The cultivation medium was centrifuged at 2360 × g for 15 min and washed two times by sterile normal saline. Finally, the pellet was re-suspended in the sterile normal saline to achieve approximately 1 × 109 CFU/mL active L. acidophilus LA-5 (Amiri et al. 2019).
Dairy by-products, including milk permeate and cheese whey achieved from cheese-making plants in Urmia, Iran. The pH of milk permeate and cheese whey was adjusted to 4.5 by 5N HCl, then were heated (121 °C, 15 min) and the precipitates were separated by centrifugation (2360 × g, 5 min). After that, the pH was adjusted rendering to the statistical design and sterilized at 121 °C for 15 min. Next, LA (99% purity linoleic acid; Sigma-Aldrich, USA) in tween 80 (2% W/V), and yeast extract (Sigma-Aldrich, USA) were added according to the experimental design using membrane filter (cellulose acetate, by 0.45 μm pore size). The fermentation process was done in 100 mL flasks with 50 mL medium, which was inoculated with 1 × 107 CFU/mL L. acidophilus LA-5. Finally, according to the experiment, they were incubated in different temperature and time conditions (Amiri et al. 2019).
Quantitative determination of CLA
Produced CLA concentration was determined by the method, which is based on spectrophotometric detection of CLA, described by Amiri et al. (2020a, b) with some modifications. Briefly, to extract CLA from culture media, 10 mL of cultivation media were centrifuged at 6800 × g for 5 min at 4 °C. Then, 6 mL of isopropanol were added to 3 mL of the supernatant and vortexed for 1 min. After that, 5 mL of hexane were added and vortexed for 1 min and finally, centrifuged at 448 × g for 5 min at 4 °C. Total CLA measurements were carried out in triplicate for 2 mL of the CLA extract in quartz cuvettes by hexane as a blank at 233 nm, using a UV–Vis spectrophotometer (80-2088-64, Pharmacia LKB Biochrom, Cambridge, UK). The concentration of CLA was calculated using an equation obtained by the standard curve. The calibration curve was created for 0–30 (mg/mL, in 2% tween 80) concentration of CLA (99% purity; Sigma-Aldrich, USA) at 233 nm, obtained equation was y = 0.5391x + 0.1164 (R2 = 0.9935).
Quantitative determination of EPSs
The phenol–sulfuric acid method used for the measurement of EPSs concentration. Briefly, to isolate EPSs from culture media, 5 mL of cultivation broth were centrifuged at 2800 × g for 30 min at 4 °C. To deactivate degrading enzymes, 5 mL of trichloroacetic acid added to the supernatant. Cold ethanol was added to the supernatant for the precipitation of the polysaccharides. After that, EPSs were dissolved in 10 mL distilled water and dialyzed. Measurements of total EPSs were carried out in triplicate, and a standard curve calculated the EPSs content. The calibration curve was built for glucose (0–150 mg/L, Merck, Germany) at 500 nm (Amiri et al. 2019).
Determination of BACs’ inhibition activity
Determination of produced BACs’ inhibition activity was carried out following the method described by Ünlü et al. (2015), which based on inhibitory zone diameter of indicator pathogen strain, with some modifications. Briefly, to purify the BACs, 1 mL of culture medium was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was filtered through syringe filters with 0.45 μm pore size (Supor® membrane, Paul Co. Ltd., Ann Arbor, MI). Then, the activity of BACs was estimated using the agar well diffusion method as described below. First, Brain heart infusion agar (BHI agar) (Merck, Darmstadt, Germany) was cooled to 47 °C and inoculated with 1 mL overnight culture containing 1 × 107 CFU/mL of Listeria monocytogenes PTCC 1297, quality control strain (Persian Type Culture Collection, isolated from mammal (brain sheep circling disease)) as an indicator strain. Then, inoculated BHI agar was poured into a sterile plate at room temperature. The wells (6 mm in diameter) were cut after solidification and were filled with 50 μL of produced BACs suspension (after extraction from the model medium and partial purification). The plates were kept in the refrigerator (4 °C) for 2 h to diffuse supernatant and the inhibition zone diameters were determined after incubation at 37 °C for 24 h.
Enumeration of L. acidophilus LA-5
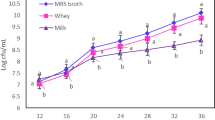
For this propose, viable-cell count procedure used to determine the counts of L. acidophilus LA-5 in samples. At the end of incubation time (according to the excremental design), samples were homogenized by vortex (Genius 3, IKA WERKE GMBH, Germany) and 1 mL of each fermented media added to 9 mL of sterile peptone water (1 mg/100 g) and serially diluted (up to 10−10) using the same diluent. Subsequently, a 1 mL of each dilution dispensed into MRS agar (Merck, Germany) using the pour-plate method and the plates incubated at 37 °C for 72 h. The counts were expressed as log CFU/mL (the log of the colony-forming units per milliliter of fermentation media) (Moghanjougi et al. 2020).
Fourier-transform infrared (FTIR) analysis
The functional groups of purified EPSs, BACs, and CLA investigated by Bruker TENSOR 27, FTIR spectrometer (Bruker Optik, Ettlingen, Germany). For this purpose, the freeze-dried EPSs and BACs samples (5 mg) and methyl ester of CLA (1 mL) were added to KBr powder (200 mg) and pressed into tablets. The samples were scanned in the range of 4000–400 cm−1 for EPSs and BACs, as well as 3500–500 cm−1 for CLA (Amiri et al. 2019; Kadamne et al. 2011).
Gas chromatography analysis of CLA isomers
First, 3 mL of 1N Methanolic HCl solution were added to a test tube with 3 mL sample, and vortexed for the 30 s. After that, the test tube held in a water bath at 55 °C for 5 min and then cooled to room temperature. CLA methyl esters were extracted with 3 mL n-hexane by vortex for 1 min. The n-hexane extract washed with 3 mL NaOH (1.0N)–ethanol (50%) solution and 3 mL distilled water. The sample dried over anhydrous sodium sulfate for GC analysis. A gas chromatography instrument (Agilent 7890A, Wilmington, USA) with flame ionization detector (FID) equipped by silica capillary column (30 m) used for the analysis of CLA isomers. The carrier gas was N2, the oven temperature was increased from 180 to 200 °C at 2 °C/min and kept for 30 min. The injection volume was 1 mL and the temperature of injector and detector was 240 °C and 260 °C, respectively (Gurovic et al. 2014).
Thin-layer chromatography (TLC) analysis of EPSs composition
TLC was used to analysis the monosaccharide compositions of the purified EPSs. For this purpose, 10 mg of the EPSs dissolved in 2 mL of 2 M trifluoroacetic acid (TFA) at 100 °C for 4 h. Then, the samples dried by nitrogen, and 6 mL methanol was added to eliminate TFA. After that, hydrolyzed EPSs samples dissolved in double distilled water for further study. The silica gel plates (20 cm × 20 cm) in n-butanol: ethyl acetate:pyridine:acetic acid:distilled water (at 4:4:1:5:1 v/v/v/v/v ratio) were used to investigate the EPSs hydrolysates. Additionally, the mixture of seven different monosaccharides including glucose, fructose, mannose, rhamnose, galactose, xylose, and arabinose (5 mg/mL) used as standards. EPSs spots were detected after spraying urea-sulphuric acid at 105 °C for 5 min (Zhou et al. 2016).
SDS Page analysis of BACs fractions
Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE) (Mini-PROTEAN® Tetra Cell, Bio-Rad Laboratories, USA) used to estimate the molecular weight of partial purification BACs at 120 V for 3 h. Then the gel was stained by the Coomassie brilliant blue R-250 (Sigma Aldrich, USA) (Sarikhani et al. 2018). The molecular weight of the BACs measured by comparison with the protein molecular size marker 11–180 kDa (Cina Clon, Tehran, Iran) using gel documentation system BIOMATE.
Statistical design and analysis
ANOVA statistical analysis was applied to evaluate the impact of the effect of the studied factors and their interactions (α = 0.05). Design-Expert Version 11 (Stat-Ease, Int. Co., Minneapolis, USA) was used to do statistical analysis of experimental data and draw plots.
Factorial design
A fractional factorial design selected most important variables responsible to the co-production of pharmabiotics. For this purpose, six independent variables including initial pH, temperature, incubation time, free linoleic acid (FLA) and yeast extract and types of cultivation media investigated to choose the most effective independent variables to co-produce CLA, EPSs, and BACs.
Box–Behnken design
Response surface methodology was applied to optimize and achieve a statistical model for the co-production of CLA, EPSs, and BACs using selected effective variables. Temperature (°C), fermentation time (h) and yeast extract (%) at three levels subjected to Box–Behnken design by 16 experiments. Accordingly, results fitted to the quadratic polynomial equation (1) for each response (Y).
where Y, β0, βi, βii, and βij were predicted response, a constant, linear coefficient, squared coefficient, and interaction coefficient, respectively.
Numerical optimization
For this purpose, the desirability function was performed to determine the optimal fermentation condition of pharmabiotics co-production. This technique converts each response (Yi) into an individual desirability function (di) which varies from 0 to 1, then selects the independent variables to maximize the total desirability of response as the follow equation:
where m, di = 1, and di = 0 were the number of responses, shows that Yi is at its target value and displays that it is not in an acceptable state.
Results and discussion
In the initial phase of this study, the factorial design was performed to screen the most effective independent variables which were responsible to co-produce CLA, EPSs, and BACs by L. acidophilus LA-5. This statistical design revealed that temperature, time, and yeast extract were the most important factors to co-produce CLA, EPSs, and BACs by L. acidophilus LA-5. Incubation temperature is an effective factor in fermentation bioprocess, which has a significant effect on bacterial growth and metabolite bio-production. Incubation time is another important parameter in fermentation, which is effective to control the metabolite bio-production (Ye et al. 2013). It established that the production of metabolites in a fermentation bioprocess influenced by the growing ability of a bacterium and adding of growth-promoting nutritional supplements. Yeast extract is a favorable nitrogen source to facilitate probiotics growth and improve metabolites production, due to the high content of amino acids, peptides, and other nutritional factors such as vitamins (Amiri et al. 2019). Besides, most suitable medium, initial pH and FLA concentration were cheese whey, 5 μL and 100 μL, respectively.
The optical density of L. acidophilus LA-5
The results showed that incubation time and yeast extract concentrations had a significant quadratic effect on the optical density of L. acidophilus LA-5 (p < 0.05). But, the optical density of L. acidophilus LA-5 was not significantly affected by the incubation temperature (p > 0.05). Increasing yeast extract concentration from 2% to 6% caused an increase in the optical density of L. acidophilus LA-5 (Fig. 1). This figure shows that the optical density of L. acidophilus LA-5 increased by increasing incubation time from 12 to 60 h and adding yeast extract. The following model sum of squares showed that the quadratic model was significant (p < 0.05), and the R2 (coefficient of determination) and the adj-R2 (Adjusted coefficient of determination) of the quadratic polynomial model (Eq. 3) were 0.95 and 0.91, respectively.
Previous studies reported that the different strains had diverse tolerance to cell growth inhibition effect of free linoleic acid and common probiotic strains are able to grow in low concentrations of FLA (Amiri et al. 2020b). The optimal temperature for the growth of L. acidophilus is 37 °C (Drider et al. 2016). Lactobacillus strains are fastidious bacteria, and their growth can enhance with the addition of nitrogen sources such as peptone, yeast extract, and beef extracts, as well as tween 80, sodium acetate and magnesium salts to the culture medium (Amiri et al. 2020b). Previous studies reported that the nitrogen source improves biological changes in the fermentation bioprocess and an increase in its content in the cultivation medium directed to increase the biomass of bacteria (Amiri et al. 2019). In similar study, Alonso et al. (2003), investigated the effect of yeast extract concentration on the growth of L. acidophilus and Lactobacillus casei in the industrial cheese whey and reported that cell density was found to be higher in compared with non-supplemented cheese whey by yeast extract. Amiri et al. (2019), suggested that yeast extract was the most effective nitrogen source to increase biomass, because of including amino acids and vitamins, besides of high nitrogen content. So it is the best nitrogen source for increasing the biomass of probiotic bacteria in compare with the various complex sources of nitrogen (Van Nieuwenhove et al. 2007).
CLA biosynthesize
The results showed that the temperature, incubation time and yeast extract concentrations, as well as the interaction of temperature with yeast extract concentrations, and the interaction of incubation time with yeast extract concentrations had a statistically significant effect on the CLA biosynthesize (p < 0.05). Although CLA biosynthesize increased by increasing the temperature, its biosynthesize decreased by increasing yeast extract concentrations. In the better word, CLA biosynthesizes at 34 °C increased by increasing of yeast extract concertation from 2% and 6%, but at temperatures above 37 °C, its biosynthesize decreased by increasing yeast extract supplementation (Fig. 2a). It seems that we are facing a crab tree effect phenomenon in optimal conditions. Although the study does not seek to confirm this claim, however, the highest CLA output at 42 °C could be a reason for this claim. Where the metabolism pathway completely shifted to CLA production. Maximum CLA achieved at 42 °C by supplementing 2% yeast extract. Figure 2b illustrates that increasing incubation time from 12 to 60 h, CLA biosynthesizes increased, but its biosynthesize decreased by increasing yeast extract concentration from 2% to 6%. Maximum CLA achieved at 60 h by supplementing 2% yeast extract. The R2 and the adj-R2 values for the achieved model (Eq. 4) were 0.93 and 0.89, respectively.
The determination coefficient value of 0.93 provides 93% variability in the biosynthesis of CLA, and only 7% total variation cannot explain by Eq. (4).
The biosynthesis process of CLA was dependent on the FLA concentration in cultivation medium, which is in agreement with the result of this study (Van Nieuwenhove et al. 2007). Van Nieuwenhove et al. (2007), demonstrated that FLA concentration has not significantly effect on produced cell growth. Some researchers proposed that the main reason of FLA bioconversion to CLA by bacteria could be due to the inhibitory effect of FLA and detoxification mechanism of bacteria for growth (Khosravi et al. 2015). Previous researchers reported that some compounds of cultivation media, such as proteins, could neutralize the negative effects of FLA on probiotics growth. So, growth of probiotics was not affected by adding of FLA into the milk-based media, like cheese whey. The result of this study is similar to the findings of earlier studies which reported the CLA production in the milk-based media (Terán et al. 2015). Terán et al. (2015), reported that adding FLA concentrations lower than 500 μg/mL led to highest bioconversion percentages of FLA to CLA; nevertheless, higher concentrations of FLA caused to decrease these percentages. According to the result of Alonso et al. (2003), L. acidophilus strains produced the highest amount of CLA by adding 200 μL of FLA to cultivation medium, and its amount decreased by increasing free FLA to 500 μL. According to the result of Ye et al. (2013), the CLA bio-synthesis increased from 54.17 μg/mL at pH 4.5 to 110.70 μg/mL at pH 6.5 in skim milk. Khosravi-Darani et al. (2014), demonstrated that parallel with an increase in temperature, the biosynthesis of CLA was enhanced. According to the results of Ye et al. (2013), the total amount of CLA production was significantly affected by temperature. Soto (2013), showed that 37 °C was the best temperature for FLA conversion to CLA by L. acidophilus. Previous studies showed that the highest CLA biosynthesis by Lactobacillus strains, is done in the first 24 h of incubation time, in exponential phase and near stationary phase, which is in good agreement with the results of this study (Khosravi-Darani et al. 2014). Khosravi et al. (2015), illustrated that the CLA productions were highly affected by yeast extract concentration. The high buffering capacity of yeast extract with complex nutritional factors, consisting of free amino acids, small peptides, nucleotides, some carbohydrates, trace elements, and Group-B vitamins, it is the most-frequently-used nitrogen source for microbial fermentation. Some researchers reported on the linear effect of yeast extract concentration on CLA production. In similar study Khosravi-Darani et al. (2014), reported that CLA biosynthesis increased by the addition of 4% whey powder to cultivation medium, which could be due to the role of proteins in the oxidation of FLA and formation of its radical.
EPSs bio-production
Figure 3a shows that the EPSs bioproduction was increased by increasing incubation temperature and its production decreased with increasing fermentation time. As shown in Fig. 3b, maximum EPSs were biosynthesized after 12 h and supplementing with 2% yeast extract. The EPSs bioproduction increased by increasing fermentation time and the concentration of yeast extract. Figure 3c shows that increasing temperature and concentration of yeast extract led to decrease in EPSs production. The R2 and the adj-R2 values for the model (Eq. 5) were 0.96 and 0.92, respectively.
The R2 = 0.96 for Eq. (5) illustrated that the statistical model could explained 96% of the total variation for EPSs production.
Haj-Mustafa et al. (2015), illustrated that the highest EPSs production achieved about pH 5.8, which is near to the result of this study. In contrast with the results of this study, Deepak et al. (2016), demonstrated that the effect of incubation temperature on the EPSs production was not significant. According to Deepak et al. (2016), by increasing incubation time, the EPSs secretion was increased, which is in line of our study. They presented that, the EPSs bioproduction increased by increasing the yeast extract concentration. Furthermore, our results showed that the yeast extract effect on EPSs bioproduction is not statistically significant. Macedo et al. (2002) established that supplementation of whey permeate-based medium by nitrogen sources caused to increase EPSs bio-production using Lactobacillus rhamnosus, which was in agreement with our results. The effect of fermentation variables on EPSs bioproduction by L. rhamnosus in skimmed milk was investigated by Haj-Mustafa et al. (2015). They reported that the effect of pH and yeast extract on the EPSs bioproduction was significant.
Inhibition activity of BACs
According to the results, the incubation time and yeast extract concentration, as well as their interaction had statistically significant effects on BACs activity (p < 0.05). Furthermore, the temperature had no significant effect on BACs activity (p > 0.05). As shown in Fig. 4, inhibition zone increased by increasing incubation time and yeast extract concentration. The R2 and the adj-R2 of the quadratic polynomial model (Eq. 6) were 0.52 and 0.40, respectively.
The R2 of the model (Eq. 6) was 0.52, which implied that the 52% variation for BACs inhibition was attributed to the variables and about 48% of the total variance could not be explained by the model.
Previous studies showed that pH had a direct effect on both cell growth and BACs production and lower pH had a negative impact on them, due to the accumulation of lactic acid in medium (Zamfir et al. 2000). Similar studies established differences between optimal cultivation temperature for bacterial growth and BACs production (Abo-Amer 2011). Kumar et al. (2012), reported, the optimal temperature for BACs production was 35 °C using L. casei LA-1 and Micrococcus sp. GO5 which is close to the optimum temperature obtained in this study. Previous studies demonstrated that the optimal temperature for maximum bacterial growth of most of Lactobacilli was obtained at 37 °C while the optimal temperature for the BACs production by them was lower than 37 °C (Abo-Amer 2011). BACs are primary metabolites and produce during the exponential phase. Previous studies showed that BACs are reached to the maximum amount at the exponential phase or at the beginning of the stationary phase. Therefore, the maximum activity of BACs can be usually observed at the first 24 h of fermentation (Zamfir et al. 2000). Investigation on the effect of different nitrogen sources on BACs production by L. acidophilus AA11 showed that the highest BACs’ activity (12,246 AU/mL) seen in bacterial cells grown in M17 broth supplemented with 1.0% yeast extract; which was twofold higher than that observed in other nitrogen sources (Abo-Amer 2011). They demonstrated that yeast extract provides a large proportion of free amino acids and short peptides and enhances bacterial growth. Kumar et al. (2012), described that the concentration of yeast extract is one of the critical factors for BACs production and high level of yeast extract is favorable for the production of BACs. According to the previous study, which used cheese whey as an alternative substrate for BACs production BACs produced in a medium only after supplementing it with yeast extract (Schirru et al. 2014). According to Schirru et al. (2014), cheese whey concentration influenced BACs production. Kumar et al. (2012), illustrated that adding adequate levels of cheese whey in cultivation medium can enhance bacterial growth and BACs production by L. casei LA-1, due to the high amount of protein and carbohydrate in cheese whey. According to their results, BACs’ activity was increased in whey-based medium and indicated the potential of industrial cheese whey as a cultivation medium to produce BACs.
Numerical optimization
The maximization co-production of CLA, EPSs, and inhibition zone production was the target of numerical optimization. The optimal fermentation condition for pharmabiotics co-production by L. acidophilus LA-5 was found to be temperature 42 °C, time 12 h and yeast extract 2%, by the total desirability equal to 0.897. In this optimal condition, the predicted value of viable cell number, CLA, EPSs, and inhibition zone were 2.62 × 108 CFU/mL, 51.46 μg/mL, 348.24 mg/mL, 12.46 mm, respectively. Validate value of viable cell number, CLA, EPSs, and inhibition zone were 2.62 ± 0.49 × 108 CFU/mL, 51.46 ± 1.50 μg/mL, 348.24 ± 5.61 mg/mL, and 12.46 ± 0.80 mm, respectively. Contour plot and bar graph of optimal condition for pharmabiotics co-production by L. acidophilus LA-5 during fermentation bioprocess in cheese whey.
FTIR spectroscopy
FTIR analysis has been reported to diagnose the shape of cis and trans bonds in oils. The FTIR analysis result for the produced CLA was showed in Fig. 5a. The produced CLA had an absorption peak of 2920 cm−1 caused to the hydrocarbon chain asymmetric fatty acids −CH2 (Roach et al. 2002). The peak at 1790 cm−1, which is due to the carbonyl ester (Roach et al. 2002). Produced CLA showed a peak at 1115 cm−1, which is due to the strength of the C–O (Roach et al. 2002). Kadamne et al. (2011) reported that the absorption 1100–1300 cm−1 was due to the extension of the C=O and C=C. The peak at 1027 cm−1, showed the binary cis and trans bond separated by more than one methylene group (Roach et al. 2002). The peak at 949 cm−1, created by cis and trans isomers, have separated by one or more methylene groups (Kadamne et al. 2011). Produced CLA showed a weak peak at 776 cm−1, which was produced by adsorption of methylene vibrations and is characterized by high chain fatty acids (Roach et al. 2002).
Figure 5b shows the FT-IR spectrum of the EPSs, which was displays the properties of a polysaccharide. The broad stretched peak at around 3450 cm−1, was related to the hydroxyl groups (O–H). A weak peak at 2930 cm−1 shows C–H vibration in the sugar ring, that indicates the aliphatic methyl group of EPSs. The sharp peak in 1627 cm−1 is due to the C–C group. The peak in 1700–1550 cm−1 is a characteristic of EPSs which is belong to C–C stretching. Amiri et al. (2019) demonstrated that the absorbance peaks below than 1500 cm−1 are the fingerprint region of EPSs. A peak at 1397 cm−1, which is due to the carboxyl (COO−) groups. The peaks at 1252, 1060 cm−1, showed the existence of C–O bonds. The peaks at 1300–1000 cm−1 are the typical peaks of polysaccharides and are due to the C–O bonds. The peak at 833 cm−1, identified the existence of carbohydrates because the 1200–800 cm−1 range is the fingerprint area of polysaccharides. The absorption area 1200–1000 cm−1 representing the sugars and assigned by the C–O and C–O–C bands (Amiri et al. 2019).
The FTIR spectrum of the isolated BACs is showed in Fig. 5c verified a characteristic of a peptide. The peaks at 3500 and 3400 cm−1, were related to the N–H stretching (Perumal and Venkatesan 2017). Perumal and Venkatesan (2017), stated that a broad, intense peak at 3000–3600 cm−1 reveals NH group. Furthermore, they commented that peaks between 3200 and 3500 cm−1 shows the presence of amide group. BACs had two peaks at 1740 and 1630 cm−1, which were due to carbonyl stretching of in the amide I. A peak at 1534 cm−1 that attributed to amide II (Feliatra et al. 2018).
Figure 5d shows the FTIR spectrum of the cheese whey. The peaks at 882 and 1015 cm−1 were due to the asymmetric tensile vibrations of C–C and C–O–C bands of glycosidic bonds between glucose and galactose in the lactose. A weak peak in the 1635 cm−1 was related to flexural vibrations of O–H. The peaks in the range of 2900–3200 cm−1 were related to the tensile vibration of the O–H (Amiri et al. 2019). The peak in 590 cm−1 was due to the disulfide bonds (S–S). The peaks in 1250–1400 cm−1 were due to the tensile bond of the C–N and the flexure bond of the N–H. Amide II and amide I had adsorption peaks in 1540 cm−1 and 1650 cm−1, respectively, which were due to the N–H and C–N groups, and showed the secondary structure of proteins (Feliatra et al. 2018).
Isomers of CLA
The conventional identification of the CLA structure produced during in vitro incubation is based on methyl ester of fatty acid by GC analysis (Devillard et al. 2009). Figure 6A shows produced isomers of CLA by LA-5 pure culture. The results showed that both CLA isomers include CLA 1: c-9, t-11 C18:2 methyl ester; CLA 2: t-10, c-12 C18:2 methyl ester were produced by this probiotic, and the quantities of different isomers were consistent with the results of previous studies (Gurovic et al. 2014). Gurovic et al. (2014), reported producing a mixture of both CLA isomers by L. acidophilus species.
Monosaccharides composition of EPSs
The results of TLC analysis indicated that purified EPSs from L. acidophilus LA-5 were mainly composed of glucose (Glu), mannose (Man), galactose (Gal), xylose (Xyl), and fructose (Fru) (Fig. 6B) which is in agreement with the previous study of Amiri et al. 2019.
The molecular weight of BACs
Figure 6C revealed the presence of two bands with estimated molecular mass 35 and 63 kDa in partially purified BACs loaded on SDS-Page analysis of partially purified BACs produced by L. acidophilus LA-5
Conclusion
First, the effects of six independent variables on co-production of CLA, EPSs, and BACs investigated by factorial design. The results of the screening design showed that the significant factors were temperature, time, and yeast extract. As well as, best medium, initial pH and FLA concentration to co-production of CLA, EPSs, and BACs were cheese whey, 5 μL and 100 μL, respectively. Then to find the optimal condition, the effects of different levels of significant variables were evaluated with Box–Behnken design in constant values of initial pH and FLA concentration by L. acidophilus LA-5 in cheese whey. The optimal condition for pharmabiotics co-production by L. acidophilus LA-5 was established to be temperature 42 °C, fermentation time 12 h, and concentration of yeast extract 2%. In optimal condition, viable cell number, CLA, EPSs, inhibition zone were 2.62 × 108 CFU/mL, 51.46 μg/mL, 348.24 mg/mL, 12.46 mm, respectively. The FTIR test confirmed the production of all three pharmabiotic metabolites, and they characterized by instrumental analysis. Co-production potential of CLA, EPSs, and BACs by LA-5 in cheese whey optimized successfully in this work.
References
Abo-Amer AE (2011) Optimization of bacteriocin production by Lactobacillus acidophilus AA11, a strain isolated from Egyptian cheese. Ann Microbiol 61:445–452
Adnan M, Ashraf SA, Khan S, Alshammari E, Awadelkareem AM (2017a) Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA J Food 15:617–621
Adnan M, Patel M, Hadi S (2017b) Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 5:e3085
Alonso L, Cuesta EP, Gilliland SE (2003) Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin1. J Dairy Sci 86:1941–1946
Alshammari E, Patel M, Sachidanandan M, Kumar P, Adnan M (2019) Potential evaluation and health fostering intrinsic traits of novel probiotic strain Enterococcus durans F3 isolated from the gut of fresh water fish Catla catla. Food Sci Anim Resour 39:844
Amiri S, Mokarram RR, Khiabani MS, Bari MR, Khaledabad MA (2019) Exopolysaccharides production by Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. lactis BB12: optimization of fermentation variables and characterization of structure and bioactivities. Int J Biol Macromol 123:752–765
Amiri S, Aghamirzaei M, Mostashari P, Sarbazi M, Tizchang S, Madahi H (2020a) The impact of biotechnology on dairy industry. In: Microbial biotechnology in food and health. Academic, Cambridge, pp 53–79
Amiri S, Mokarram RR, Khiabani MS, Bari MR, Khaledabad MA (2020b) In situ production of conjugated linoleic acid by Bifidobacterium lactis BB12 and Lactobacillus acidophilus LA5 in milk model medium. LWT 132:109933
Deepak V, Ram Kumar Pandian S, Sivasubramaniam SD, Nellaiah H, Sundar K (2016) Optimization of anticancer exopolysaccharide production from probiotic Lactobacillus acidophilus by response surface methodology. Prep Biochem Biotechnol 46:288–297
Devillard E, McIntosh FM, Paillard D, Thomas NA, Shingfield KJ, Wallace RJ (2009) Differences between human subjects in the composition of the faecal bacterial community and fecal metabolism of linoleic acid. Microbiology 155:513–520
Drider D, Bendali F, Naghmouchi K, Chikindas ML (2016) Bacteriocins: not only antibacterial agents. Probiot Antimicrobial Proteins 8:177–182
Feliatra F, Muchlisin ZA, Teruna HY, Utamy WR, Nursyirwani N, Dahliaty A (2018) Potential of bacteriocins produced by probiotic bacteria isolated from tiger shrimp and prawns as antibacterial to Vibrio, Pseudomonas, and Aeromonas species on fish. F1000Research 7:415–425
Gurovic MSV, Gentili AR, Olivera NL, Rodríguez MS (2014) Lactic acid bacteria isolated from fish gut produce conjugated linoleic acid without the addition of exogenous substrate. Process Biochem 49:1071–1077
Haj-Mustafa M, Abdi R, Sheikh-Zeinoddin M, Soleimanian-Zad S (2015) Statistical study on fermentation conditions in the optimization of exopolysaccharide production by Lactobacillus rhamnosus 519 in skimmed milk base media. Biocatal Agric Biotechnol 4:521–527
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184
Kadamne JV, Castrodale CL, Proctor A (2011) Measurement of conjugated linoleic acid (CLA) in CLA-rich potato chips by ATR-FTIR spectroscopy. J Agric Food Chem 59:2190–2196
Khosravi A, Safari M, Khodaiyan F, Gharibzahedi SMT (2015) Bioconversion enhancement of conjugated linoleic acid by Lactobacillus plantarum using the culture media manipulation and numerical optimization. J Food Sci Technol 52:5781–5789
Khosravi-Darani K, Reihani FS, Feili R (2014) Bioproduction of conjugated linoleic acid in yogurt by probiotic bacteria. Int J Biotechnol Wellness Industries 3:62–68
Kumar M, Jain AK, Ghosh M, Ganguli A (2012) Industrial whey utilization as a medium supplement for biphasic growth and bacteriocin production by probiotic Lactobacillus casei LA-1. Probiot Antimicrobial Proteins 4:198–207
Macedo MG, Lacroix C, Champagne CP (2002) Combined effects of temperature and medium composition on exopolysaccharide production by Lactobacillus rhamnosus RW-9595M in a whey permeate based medium. Biotechnol Prog 18:167–173
Moghanjougi ZM, Bari MR, Khaledabad MA, Almasi H, Amiri S (2020) Bio-preservation of white brined cheese (Feta) by using probiotic bacteria immobilized in bacterial cellulose: optimization by response surface method and characterization. LWT 117:108603
Perumal V, Venkatesan A (2017) Antimicrobial, cytotoxic effect and purification of bacteriocin from vancomycin susceptible Enterococcus faecalis and its safety evaluation for probiotization. LWT Food Sci Technol 78:303–310
Roach JA, Mossoba MM, Yurawecz MP, Kramer JK (2002) Chromatographic separation and identification of conjugated linoleic acid isomers. Anal Chim Acta 465:207–226
Sarikhani M, Kermanshahi RK, Ghadam P, Gharavi S (2018) The role of probiotic Lactobacillus acidophilus ATCC 4356 bacteriocin on effect of HBsu on planktonic cells and biofilm formation of Bacillus subtilis. Int J Biol Macromol 115:762–766
Schirru S, Favaro L, Mangia NP, Basaglia M, Casella S, Comunian R, Todorov SD (2014) Comparison of bacteriocins production from Enterococcus faecium strains in cheese whey and optimised commercial MRS medium. Ann Microbiol 64:321–331
Soto C (2013) Lactobacillus plantarum as source of conjugated linoleic acid: effect of pH, incubation temperature and inulin incorporation. J Biochem Technol 5:649–653
Terán V, Pizarro PL, Zacarías MF, Vinderola G, Medina R, Van Nieuwenhove C (2015) Production of conjugated dienoic and trienoic fatty acids by lactic acid bacteria and bifidobacteria. J Funct Foods 19:417–425
Ünlü G, Nielsen B, Ionita C (2015) Production of antilisterial bacteriocins from lactic acid bacteria in dairy-based media: a comparative study. Probiot Antimicrobial Proteins 7:259–274
Van Nieuwenhove CP, Oliszewski R, González SN, Perez Chaia AB (2007) Conjugated linoleic acid conversion by dairy bacteria cultured in MRS broth and buffalo milk. Lett Appl Microbiol 44:467–474
Ye S, Yu T, Yang H, Li L, Wang H, Xiao S, Wang J (2013) Optimal culture conditions for producing conjugated linoleic acid in skim-milk by co-culture of different Lactobacillus strains. Ann Microbiol 63:707–717
Zamfir M, Callewaert R, Cornea PC, Vuyst L (2000) Production kinetics of acidophilin 801, a bacteriocin produced by Lactobacillus acidophilus IBB 801. FEMS Microbiol Lett 190:305–308
Zhou K, Zeng Y, Yang M, Chen S, He L, Ao X, Zou L, Liu S (2016) Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr Polym 144:205–214
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Data S1
(DOCX 255 kb)
Rights and permissions
About this article
Cite this article
Amiri, S., Mokarram, R.R., Khiabani, M.S. et al. Optimization of food-grade medium for co-production of bioactive substances by Lactobacillus acidophilus LA-5 for explaining pharmabiotic mechanisms of probiotic. J Food Sci Technol 58, 1–12 (2021). https://doi.org/10.1007/s13197-020-04894-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04894-5