Abstract
The physicochemical properties of pressurized hot-water extract (PHE) from abalone muscle and the antioxidant activities of its digestive products were investigated in this study. The PHE contained protein of 49.58% and carbohydrate of 41.95%. After ethanol graded precipitation, most of carbohydrate and protein in PHE were successively remained in 40% ethanol precipitate (EP40) and 80% ethanol precipitate (EP80), respectively. High proportions of Glu and Gly were found in the PHE, EP40 and EP80, but the proportion of Ala in ethanol soluble extract (ESE) reached up to 46.00%. Both PHE and EP40 were rich in glucose, while galactose and glucose were main monosaccharides in the EP80 and ESE. Based on the results of SDS-PAGE and HPLC, high molecular weight components from PHE were precipitated in the EP40, but oligopeptides and free amino acids were fractionated in EP80 and ESE. Among the PHE and the digestive products, the highest antioxidant ability was found to be EP80 hydrolysate, the IC50 values of which for scavenging activity on hydroxyl radical, DMPD radical and ABTS radical were 1.05 mg/mL, 1.40 mg/mL and 0.56 mg/mL, respectively. It is concluded that carbohydrate of abalone muscle was dissolved more easily into hot water than protein, and protein hydrolysate of PHE might play an important role in antioxidant activity of gastrointestinal digestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abalone is one of the most valuable seafood in East Asia since it contains high quality of protein, carbohydrate and minerals (Li et al. 2013). Especially in China and Japan, abalone has been used as a health functional food, medicine and tonics, and it had certain physiological benefits in terms of fatigue recovery and detoxification (Lee et al. 2008). In traditional Chinese cooking, nutritious soup is usually prepared by boiling, stewing and simmering. Among them, stewed soup is the most nutritious since nutrients can be easily dissolved into soup by stewing. The hot water extract of leather carp (Cyprinus carpio nudus) could improve physical exercise performance and prevent oxidative stress caused by exhaustive workouts, and thus could be used as a nourishing tonic soup and as an aid for recovery from physical fatigue (Lee et al. 2015). Based on the result of animal experiments, it was suggested that the hot water extract of freshwater clam (Corbicula fluminea) was a potential immunomodulating agent and ameliorates acute liver injury (Peng et al. 2008). In a previous study, effect of extracting time on component of boiling water extracts from abalone muscle was investigated, it was found that glycoprotein from abalone muscle was easily dissolved in the boiling water. However, the functional properties of pressurized hot-water extract (PHE) from abalone muscle have not been reported.
On the other hand, oxygen free radicals like reactive oxygen species are usually produced in various physiological processes and cellular metabolism. The excessive free radicals would be implicated in a number of human diseases, including diabetes, cardiovascular disease and neurological disorders. The oxidative stress could be regulated by exogenous antioxidants except for antioxidant defense systems in the body. The protein/polypeptide ingested is absorbed in the form of oligopeptides or amino acids after digesting in the gastrointestinal tract (Siemensma et al. 1993). The functional properties of extracts or protein/polypeptide with antioxidant activity are mainly reflected by the digest in the gastrointestinal tract, which is a major site of pro-oxidant and antioxidant action (Halliwell et al. 2000). Therefore, studies on the biological activity of shrimp peptides and loach protein hydrolysates were explored by in vitro simulated digestion methods (Jensen et al. 2009; You et al. 2010).
Pressurized hot-water extraction is regarded as a green technique, and it has been used extensively for the extraction of bioactive substances such as phenolics and essential oil (Çam et al. 2019). Furthermore, production of nutraceuticals has to go through macroscopic pretreatment, macro- and micro-molecules separation, among of which alcohol precipitation is able to remove macro-molecules including polysaccharides (Galanakis 2013). In this study, the physicochemical properties including proximate composition, molecular weight distribution, amino acid composition and monosaccharide composition of PHE from abalone muscle and its ethanol graded components were investigated. Furthermore, the antioxidant properties of them after in vitro simulated digestion were also evaluated by measuring various radical scavenging abilities.
Materials and methods
Materials and chemicals
Abalone (Haliotis discus hannai Ino) was obtained from Xiamen Dongyueyu Marine Food Co., Ltd. (Xiamen, China) in January. Amino acid standards and o-Phthalaldehyde (OPA) were purchased from Agilent Technologies Inc. N,N-Dimethyl-p-phenylen-e-diamine dihydrochloride (DMPD), 9-Fluorenylmethoxycarbonyl chloride (FMOC), 3-Methyl-1-phenyl-2-pyrazolin-5-one (PMP), and 2,2′-azino-bis(3-ethylbenzothi azoline-6-sulfonic acid) (ABTS) were purchased from Sigma Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Preparation of abalone muscle extract
The abalone muscle (100 g) was minced with 500 ml distilled water using a Hand Blender (Braun, Frankfurt, Germany), and cooked at 120 °C for 30 min using a stainless-steel pressure cooker (100 kPa maximum pressure). The cooking solution was cooled in ice water immediately and filtered with a N-1100 vacuum pump (Ankeyq, Shanghai, China). The obtained filtrate was freeze-dried, and the dry matter as PHE was stored at − 20 °C until use. The PHE was precipitated by adding ethanol to a final concentration of 40% (w/v). After standing at 4ºC for 12 h, the precipitation was collected by centrifuging at 4000 rpm for 10 min and freeze-dried as 40% ethanol precipitate (EP40). The obtained supernatant was precipitated by subjecting to ethanol fractional precipitation up to 80% (v/v) ethanol. The obtained precipitate was freeze-dried as 80% ethanol precipitate (EP80), and the supernatant was evaporated at 60 °C to remove ethanol and freeze-dried as ethanol soluble extract (ESE). The obtained PHE, EP40, EP80 and ESE were stored at − 20 °C until analysis.
The extraction yields of PHE, EP40, EP80 and ESE were calculated as follows: Extraction yield (%) = 100 W/W0, where W is the dry weight of each fraction and W0 is the weight of fresh abalone muscle used for extraction.
Proximate analysis
The protein (AOAC 992.15) and ash (AOAC 920.153) contents were determined according to the AOAC methods (2005), and carbohydrate content was measured at 490 nm by phenol–sulfuric acid method using D-glucose as a reference (Dubois et al. 1956). Three replications were carried out in every sample.
SDS-PAGE
SDS-PAGE was carried out according to the method of Laemmli (1970) using 4% stacking gel and 12% separating gel. After electrophoresis, some gels were stained with 0.025% Coomassie Brilliant Blue R-250 (Merck, Darmstadt, Germany) containing 5% methanol and 10% acetic acid (v/v), then destained in 30% methanol and 10% acetic acid (v/v). The standard protein mixtures (Fermentas Life Sciences, Hanover, MD, USA), ranging in molecular mass from 10 to 200 kDa, were used.
Determination of molecular weight
Molecular weight distribution was determined by Agilent 1200 high performance liquid chromatography (HPLC) system (Agilent Technologies, Palo Alto, USA) equipped with an ultraviolet (UV) detector using a TSK gel G2000 SWXL column (7.8 × 300 mm; Tosoh, Tokyo, Japan) at 214 nm. The mobile phase (isocratic elution) was 45% acetonitrile containing 0.1% trifluoroacetic acid, at a flow rate of 0.5 ml/min. Cytochrome C (12,500 Da), aprotinin (6500 Da), oxidized glutathione (613 Da), Gly-Gly-Gly (189 Da) and Gly (75 Da) were used as standards.
Amino acid composition
The sample was hydrolyzed in 6 M HCl at 110 °C for 22 h in presence of 0.1% phenol and HCl was removed by evaporation. The amino acid composition of the hydrolyzate was analyzed by Agilent 1200 HPLC equipped with an UV detector using Zorbax Eclipse AAA column (4.6 × 150 mm, 5 μm; Agilent Technologies, Palo Alto, CA, USA) at 40 °C with OPA and FMOC.
Monosaccharide analysis
Monosaccharide composition was analyzed according to the reported method (Dai et al. 2010) using reversed phase high performance liquid chromatography. Briefly, each sample (2 mg) was hydrolyzed with 1.0 mL of 2.0 M trifluoroacetic acid at 110 ºC for 6 h. The resulting hydrolyzate was derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP). Then, the PMP-labeled carbohydrates were analyzed using HPLC system (Agilent Technologies) equipped with an Atlantis® dC18 column (5 μm, 4.6 mm × 250 mm) with detection at UV 245 nm and a flow rate of 0.5 mL/min. The mobile phase was 0.1 mol/L phosphate buffer (pH 7.0) containing 18% acetonitrile. Mannose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, arabinose and fucose were used as monosaccharide standards.
In vitro digestion
Simulated gastrointestinal digestion using pepsin and pancreatin was carried out according to Cinq-Mars et al. (Cinq-Mars et al. 2008) with a slight modification. The lyophilized samples (0.5 g) were suspended in flasks containing 50 ml 0.02 M HCl (pH 2.0) in a 3/100 (w/w) enzyme/substrate ratio. After incubation at 37 °C for 2 h, the pepsin-digested mixture was adjusted to pH 7.5 with 0.5 M NaOH to stop the enzymatic reactions. The neutralized pepsin-digested mixture was further digested at 37 °C for 2 h by adding pancreatin in a 4/100 (w/w) enzyme/substrate ratio. After digestion, the digested samples were boiled to inactivate enzymes for 10 min at 100 °C. The obtained digested samples were cooled by ice water immediately and measured antioxidant activities.
Antioxidant activity
Hydroxyl radical scavenging ability
The hydroxyl radical scavenging ability was determined according to the method of Halliwell et al (1987) with slight modifications. The sample (1 mL) was mixed with 0.3 mL of 8 mM FeSO4 solution, 0.25 mL of 20 mM 30% H2O2 and 1 mL of 3 mM salicylic acid. The mixture was incaubated at 37 °C for 30 min and then cooled by ice water immediately. The obtained reaction mixture was added with 0.45 mL of distilled water and centrifuged at 5000 × g for 10 min. The absorbance of the supernatant was recorded at 517 nm and the scavenging ability was calculated using the following equation:
where A is the absorbance of the blank solution using distilled water instead of sample; AS is the absorbance of the sample; and A0 is the absorbance of distilled water instead of salicylic acid.
DMPD radical scavenging ability
DMPD radical scavenging ability was measured according to the method of Peksel et al. (2013). DMPD+ reagent contained 1 mL of 100 mM DMPD solution, 0.2 mL of 50 mM FeCl3 and 100 mL of 100 mM acetate buffer (pH 5.3). The sample (0.5 mL) was mixed with 1 mL of DMPD+ reagent, and incubated at 25 °C for 10 min in the dark and the absorbance was read at 505 nm. The DMPD radical scavenging activity was calculated according to the Eq. (1).
ABTS radical scavenging ability
The ABTS assay was conducted as describing by Rufiánhenares and Delgadoandrade (2009) with slight modifications. The ABTS working solution was prepared by mixing 10 mL of 2 mM ABTS solution and 10 mL of 2.45 mM potassium persulfate solution and placing in the dark for 12 h, and then diluted in 95% of ethanol to obtain the ABTS+ reagent with absorbance at 734 nm of about 0.7. The sample (0.3 mL) was mixed with 1.2 mL of ABTS+ working solution and then incubated for 6 min in the dark. The absorbance was recorded at 734 nm and the ABTS radical scavenging activity was calculated according to the Eq. (1).
Statistical analysis
The obtained data were subjected to analysis of variance (ANOVA). Differences among the mean values were measured by Duncan’s multiple range test (P < 0.05).
Results and discussion
Extraction yields
The extraction yield of PHE from abalone muscle and the ethanol graded precipitation components is shown in Fig. 1. The solid content of abalone muscle was 26.03%, and the extraction yield of PHE from abalone muscle was 11.00%. Similar extraction yield was also reported by Tsai et al (2008) on the hot water extract from hard clam (10.4%), but high extraction yield was reported on the hot water extract from leather carp (16.3%) (Lee et al. 2015). Low extraction yield of shellfish muscle could be due to the high content of insoluble protein like stroma protein. When the PHE was separated by graded alcohol precipitation, the extraction yield of the obtained EP40, EP80 and ESE were 6.53%, 1.12% and 2.98% respectively, suggesting that the components in the PHE were mainly precipitated by low concentration of ethanol.
Molecular weight distribution
Figure 2a depicts the SDS-PAGE patterns of PHE from abalone muscle and the ethanol graded components. It was found that the proteins of PHE contained high molecular weight fraction (HMWF1 and HMWF2), the protein of 75 kDa, actin and tropomyosin. Comparing with the PHE, no significant change was found in the protein bands of EP40. The protein pattern of EP80 appeared as a smear, with a few discrete weak bands above 80 kDa. However, no protein bands were observed in the ESE, suggesting that peptides and amino acids might be mostly presented in ethanol aqueous solution.
Since SDS-PAGE could only detect proteins with molecular weight above 10 kDa, the low molecular weight distribution of PHE and its ethanol graded components were analyzed by HPLC (Fig. 2b). The molecular weight of oligopeptides in the PHE was mainly distributed in three regions including 25000–13000 Da, 13000–8000 Da and 1000–200 Da, besides a small amount of amino acids and minerals below 200 Da (Fig. 2b). After ethanol graded precipitation, the peptide fractions with molecular weight above 8000 Da were found in EP40 and EP80, while the components with molecular weight below 1000 Da were predominantly found in ESE. These results showed again that high molecular weight fractions were precipited by ethanol of 40% or more, while peptides, amino acids and minerals were mostly presented in ethanol aqueous solution.
Proximate composition
The proximate compositions of PHE and the ethanol graded components were determined and shown in Table 1. As can be seen, a ratio of protein to carbohydrate in the PHE was 1.2, which was less than that in the abalone muscle (3.4), indicating that carbohydrate of abalone muscle was dissolved more easily into hot water than protein. In the abalone muscle, the myofibrillar and stroma proteins were accounted for more than 60% of the total protein content (Porturas et al. 1993), but just a part of them were dissolved in the boiling water (Zhu et al. 2011; Hatae et al. 1996).
After 40% ethanol precipitation, the contents of protein and carbohydrate in the obtained EP40 were 21.91% and 76.09%, respectively. On the other hand, protein was found to be a major component in both EP80 and ESE. These suggested that most of carbohydrate in the PHE was precipitated by 40% of ethanol. It was reported that when tea flower extract solution was dialyzed by dialysis membrane of 3500 Da, the amount of sediment by 30% (v/v) of ethanol was close to that by 70% (v/v) of ethanol (Wang et al. 2012). As ethanol concentration increased, the solution polar decreased in favor of the aggregation of protein molecules (Boulet et al. 2001). Therefore, carbohydrate and protein in the PHE were successively precipitated by 40% and 80% of ethanol. Besides protein and carbohydrate, ash content in ESE was up to 28.52%, which was much higher than EP40 and EP80, suggesting that the minerals in hot water extract from abalone muscle were dissolved mainly in ethanol aqueous solution.
Amino acid composition
The complete amino acid (CAA) and free amino acid (FAA) compositions of PHE and the ethanol graded components were summarized in Table 2. In the case of CAA, the total amino acid content of PHE was 39.89 g/100 g, and the most abundant amino acids were found to be Ala, Glu, Gly and Arg, accounting for 57.38% of the total amino acids. This is consistent with the previous findings on the abalone meat extract by cooking at 100 °C for 30 min (Hatae et al. 1996). After fractionation by ethanol graded precipitation, the tendency in the amino acid content was quite similar to the protein contents, which were 20.78% in EP40, 86.87% in EP80 and 58.87% in ESE, respecyively (Table 1). High proportions of Glu and Gly were found in the both EP40 and EP80, but the proportions of Ala and Arg were much lower than those in PHE. It was obvious to note that the proportion of Ala reached up to 46.00% in the ESE, followed by Arg.
On the other hand, the FAA content accounted for 37.90% of CAA content in the PHE, indicating that there were a large number of free amino acids in the PHE from abalone muscle. This result was lower than the hot-water extract of yellowtail muscle (46.50%) (Kubota et al. 2002). The free amino acid contents in the fraction of EP40 and EP80 were 1.68 g/100 g and 4.75 g/100 g, the proportions of which in CAA were 9.48% and 6.25%, respectively. This result suggested that the obtained EP40 and EP80 were mainly composed of glycopeptides or peptides. Respective of EP40 or EP80, it was found that the amino acid contents of Asp, Glu, Gly, Arg, Hyp and Pro in the CAA were much richer than those in the FAA. And, these amino acid ratios in the CAA of EP40 and EP 80 were accounted for 60.46% and 64.62%, respectively. This suggested that Asp, Glu, Gly, Arg, Hyp and Pro were main constituent amino acids of peptides in the EP40 and EP80. It was also reported that the glycoprotein obtained from the hot water extract from abalone had a large amount of Asp and Gly (Uchida et al. 1987). The free amino acid contents were 34.32 g/100 g in the ESE, which were accounted for 71.09% of complete amino acids, suggesting that the most of free amino acids from PHE were in the ESE. Based on the amino acid content of CAA and FAA, it was found that some peptides were also existed in ESE, which could be mainly composed of Ala, Glu and Gly.
Monosaccharide composition
There was rich carbohydrate in the PHE and the ethanol graded components (Table 1), then the monosaccharide compositions were determined as PMP derivatives using high-performance liquid chromatography (Table 3). As can be seen, both PHE and EP40 were rich in glucose, which was similar to that in abalone muscle (data not shown). However, galactose, glucose and glucuronic acid were the main monosaccharides in the EP80, with the molar ratio of more than 70%. ESE was mainly composed of galactose and glucose, accounting for 76.59%. It was reported that oligosaccharides could be precipitated by high ethanol concentration (Sen et al. 2011). The results of Table 3 suggested that the polysaccharides in the PHE was mainly composed of glucose, while other monosaccharides in EP80 like galactose might form oligosaccharides and bind protein hydrolysate. It has been reported that the main monosaccharides from abalone pleopods were galactose, glucuronic acid, glucosamine and fucose (Wang et al. 2014), probably due to the different breeding conditions.
Antioxidant activity
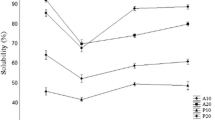
The antioxidant capacity was often reflected by IC50 value, which represented the concentration of the sample that caused a 50% inhibition of radicals (Schmidt et al. 2006).The lower IC50 value represents the stronger antioxidant ability. The radical scavenging abilities of simulated digestion products including hydroxyl, DMPD and ABTS were determined and the IC50 values were calculated (Fig. 3).
Hydroxyl radicals are highly reactive compared with other ROS, and the hydroxyl radical system was also frequently selected to investigate potential antioxidative activity (Wiriyaphan et al. 2015). As illustrated in Fig. 3a, the IC50 value of EP80 hydrolysate for hydroxyl radical scavenging ability was the lowest, and was close to the GSH, a potent antioxidant (Fig. 3a). This suggested that the hydroxyl radical scavenging activity of EP80 hydrolysate was the strongest among the obtained PHE and three ethanol graded components. A similar trend was observed in the DMPD radical scavenging ability in the simulated digestion products (Fig. 3b), but the IC50 value of EP80 hydrolysate was higher than GSH. Furthermore, the IC50 value of ESE hydrolysate for ABTS radical scavenging was lower than the others from abalone muscle (Fig. 3c), but was higher than GSH.
During the simulated digestion, protein was hydrolyzed by pepsin and pancreatin, whereas polysaccharide was not digested (Ding et al. 2017). Moreover, it was found that the antioxidant activities of PHE and the ethanol graded components after simulated digestion were higher than that of before (data not shown). These results indicated that protein might be the primary antioxidant component of the obtained hot-water extract from abalone muscle. Jung et al (2007) also reported that the antioxidant activity of mussel protein became stronger after in vitro gastrointestinal digestion due to the release of low-molecular-weight peptide.
Although various synthetic antioxidants show superior efficacy and high stability in the lipid peroxidation inhibition, there is an increasing concern about their safety. Thus, dietary antioxidants derived from food processing by-products have much attracted attention recently (Galanaki 2018). Application of natural antioxidants in processed meat products and sponge cake will improve their quality and safety, leading to design more sustainable and healthier products (Galanaki 2018; Prokopov et al. 2015). Pressurized hot-water extraction has been reported to be useful for recovering polar and nonpolar antioxidants, and the technique may be suitable in industrial applications (Kovačević et al. 2018). During the natural antioxidant purification, ethanol concentration is very significant in obtaining high antioxidants (Wong et al. 2015). The present study also found that ethanol concentration affected the physicochemical properties of PHE and antioxidant activities of PHE digests. In the processing of dried abalone and steamed abalone, it generates a lot of hot-water extracts, which could be used as raw materials to prepare natural antioxidants.
Conclusion
The PHE from abalone muscle was rich in proteins and carbohydrates. The major amino acids in the protein of pressurized hot water extract were Ala, Glu, Gly and Arg, while Ala was mainly present in a free form. On the other hand, the main monosaccharide in the carbohydrates was glucose. After ethanol graded precipitation, the polysaccharide in the hot water extract was precipitated firstly, followed by protein, while peptides, amino acids and minerals were dissolved in the ethanol aqueous solution. Based on the antioxidant abilities of simulated digestion products, stronger radical scavenging ability was found to contain higher protein content, suggesting that protein hydrolysate might play an important role in antioxidant activity.
References
AOAC (2005) Official methods of analysis of International, 18th edn. Association of Official Analytical Chemists International, Gaithersburg
Boulet M, Britten M, Lamarche F (2001) Dispersion of food proteins in water-alcohol mixed dispersants. Food Chem 74:69–74
Çam M, Yüksel E, Alaşalvar H, Başyiğit B, Şen H, Yılmaztekin M, Ahhmed A, Sağdıç O (2019) Simultaneous extraction of phenolics and essential oil from peppermint by pressurized hot water extraction. J Food Sci Technol 56:200–207
Cinq-Mars CD, Hu C, Kitts DD, Li-Chan EC (2008) Investigations into inhibitor type and mode, simulated gastrointestinal digestion, and cell transport of the angiotensin I-converting enzyme–inhibitory peptides in Pacific hake (Merluccius productus) fillet hydrolysate. J Agric Food Chem 56:410–419
Dai J, Wu Y, Chen SW, Zhu S, Yin HP, Wang M, Tang J (2010) Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr Polym 82:629–635
Ding Q, Nie S, Hu J, Zong X, Li Q, Xie M (2017) Invitro and invivo, gastrointestinal digestion and fermentation of the polysaccharide from ganoderma atrum. Food Hydrocoll 63:646–655
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Galanakis CM (2013) Emerging technologies for the production of nutraceuticals from agricultural by-products: as viewpoint of opportunities and challenges. Food Bioprod Process 91:575–579
Galanaki CM (2018) Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci Technol 79:98–105
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal biochem 165:215–219
Halliwell B, Zhao KC, Whiteman M (2000) The gastrointestinal tract: a major site of antioxidant action? Free Radic Res 33:819–830
Hatae K, Nakai H, Tanaka C, Shimada A, Watabe S (1996) Taste and texture of abalone meat after extended cooking. Fish Sci 62:643–647
Jensen IJ, Abrahamsen H, Maehre HK, Elvevoll EO (2009) Changes in antioxidative capacity of saithe (Pollachius virens) and shrimp (Pandalus borealis) during in vitro digestion. J Agric Food Chem 57:10928–10932
Jung WK, Qian ZJ, Lee SH, Choi SY, Sung NJ, Byun HG, Kim SK (2007) Free radical scavenging activity of a novel antioxidative peptide isolated from in vitro gastrointestinal digests of mytilus coruscus. J Med Food 10:197–202
Kovačević DB, Barba FJ, Granato D, Galanakis CM, Herceg Z, Dragović-Uzelac V, Putnik P (2018) Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem 254:150–157
Kubota S, Itoh K, Niizeki N, Song XA, Okimoto K, Ando M, Murata M, Sakaguchi M (2002) Organic taste-active components in the hot-water extract of yellowtail muscle. Food Sci Technol Res 8:45–49
Laemmli UK (1970) Physicochemical properties and film-forming ability of fish skin collagen extracted from different freshwater species. Nature 227:680–685
Lee KA, Shin ES, Lee HK, Kim MJ, Kim KBWR, Byun MW, Lee JW, Kim JH, Ahn DH, Lyu ES (2008) Quality characteristics of abalone porridge with viscera. J Korean Soc Food Sci Nutr 37:103–108
Lee GH, Harwanto D, Park SM, Choi JS, Kim MR, Hong YK (2015) Hot water extract of leather carp (Cyprinus carpio nudus) improves exercise performance in mice. Prev Nutr Food Sci 20:246–252
Li J, Kim BS, Kang SG (2013) Analysis and comparison of general compositions, amino acids, fatty acids and collagen of abalone harvested in three different regions in Korea. Korean J Food Preserv 20:441–450
Peng TC, Subeq YM, Lee CJ, Lee CC, Tsai CJ, Chang FM, Lee RP (2008) Freshwater clam extract ameliorates acute liver injury induced by hemorrhage in rats. Am J Chin Med 36:1121–1133
Peksel A, Arisan I, Yanardag R (2013) Radical scavenging and anti-acetylcholinesterase activities of aqueous extract of wild pistachio (Pistacia atlantica Desf.) leaves. Food Sci Biotechnol 22:515–522
Porturas RO, Ushio H, Watabe S, Takada K, Hatae K (1993) Toughness and collagen content of abalone muscles. Biosci biotechnol biochem 57:6–11
Prokopov T, Goranova Z, Baeva M, Slavov A, Galanakis CM (2015) Effects of powder from white cabbage outer leaves on sponge cake quality. Int Agrophys 29:493–500
Rufiánhenares JA, Delgadoandrade C (2009) Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res Int 42:394–400
Schmidt E, Jirovetz L, Buchbauer G, Eller GA, Stoilova I, Krastanov A, Stoyanova A, Geissler M (2006) Composition and antioxidant activities of the essential oil of cinnamon (cinnamomum zeylanicum blume) leaves from sri lanka. J Essent Oil Bear Pl 9:170–182
Sen D, Gosling A, Stevens GW, Bhattacharya PK, Barber AR, Kentish SE, Bhattacharjee C, Gras SL (2011) Galactosyl oligosaccharide purification by ethanol precipitation. Food Chem 128:773–777
Siemensma AD, Weijer WJ, Bak HJ (1993) The importance of peptide lengths in hypoallergenic infant formulae. Trends Food Sci Technol 4:16–21
Tsai JS, Chen JL, Pan BS (2008) ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem 43:743–747
Uchida H, Sasaki T, Uchida NA, Takasuka N, Endo Y, Kamiya H (1987) Oncostatic and immunomodulatory effects of a glycoprotein fraction from water extract of abalone, Haliotis discus hannai. Cancer Immunol Immunother 24:207–212
Wang Y, Yu L, Wei X (2012) Monosaccharide composition and bioactivity of tea flower polysaccharides obtained by ethanol fractional precipitation and stepwise precipitation. CYTA-J Food 10:1–4
Wang YM, Wu FJ, Du L, Li GY, Takahashi K, Xue Y, Xue CH (2014) Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. Int J Biol Macromol 66:354–361
Wiriyaphan C, Xiao H, Decker EA, Yongsawatdigul J (2015) Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem 167:7–15
Wong WH, Lee WX, Ramanan RN, Tee LH, Kong KW, Galanakis CM, Sun J, Prasad KN (2015) Two level half factorial design for the extraction of phenolics, flavonoids and antioxidants recovery from palm kernel by-product. Ind Crop Prod 63:238–248
You L, Zhao M, Regenstein JM, Ren J (2010) Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem 120:810–816
Zhu BW, Dong XP, Sun LM, Xiao GH, Chen XJ, Murata Y, Yu CX (2011) Effect of thermal treatment on the texture and microstructure of abalone muscle (Haliotis discus). Food Sci Biotechnol 20:1467–1473
Acknowledgements
This work is sponsored by National Natural Science Fund (31571835), Fujian Key Project of Natural Science Fund (2019J02013), Special Scientific Research Fund of Marine Public Welfare (201405016) and Xiamen Science and Technology Project (3502Z20173032).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, P., Weng, W. Characteristics of pressurized hot water extract from abalone muscle and the antioxidant ability during simulated digestion in vitro. J Food Sci Technol 57, 4076–4083 (2020). https://doi.org/10.1007/s13197-020-04441-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04441-2