Abstract
Wines produced from Sangiovese (sg) grapes, the most cultivated red grape variety in Italy and widely grown across the world, is often subjected to loss of clarity due to the formation of a deposit constituted by fine needle-shaped crystals. In this work, a qualitative study by 1H-NMR and 13C-NMR analysis of the deposit obtained by filtering cloudy sg wines showed that it was constituted by crystals of quercetin (Q). The analysis of hydro-alcoholic solutions (12% ethanol and pH 3.2.) and red wines added with increasing amounts of Q showed that, above 3 mgL−1 of Q, a deposit can be detected and, the time necessary for its formation depends on the medium. The comparison among sg and other 11 monovarietal wines showed that sg was the richest in Q and quercetin glycosides (GQ). Both Q and GQ decreased in the analyzed solutions over time and the decrease was faster for Q than for GQ. The controlled exposure to oxygen determined a decrease of Q higher than the 50% of the initial values. Data obtained in this study suggested that practices as micro-oxygenation and wood aging could help to decrease the amount of Q in sg wines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sangiovese (sg) is the most cultivated red grape variety in Italy (53,865 ha representing 7.9% of the national vineyard in 2017) and constitutes the basis of prestigious wines such as Chianti, Brunello di Montalcino, and Nobile di Montepulciano (OIV 2017). Sg is also certified for the production of several other important Italian wine denominations as DOCG (Denominazione di Origine Controllata e Garantita), DOC (Denominazione di Origine Controllata) and IGT (Indicazione Geografica Tipica).
Over the last years, some lots of commercial sg wine have become cloudy over time due to the formation of a precipitate. As this precipitate may continue to form during bottle aging, often, wines, even if clear when bottled, result in commercial products with poor appearance and decreased acceptability. Several studies report the formation of these deposits in sg wines owing to an excess of quercetin (Waterhouse et al. 2016; Lanati et al. 2014) but an unambiguous characterization of the deposit has not been achieved yet.
Quercetin, as other flavonols, is extracted from grape skins during maceration. Typical flavonols of Vitis vinifera red grape cultivars are glycosylated forms (glucosides, galactosides, rhamnosides, rutinosides and glucuronides) of the six flavonoid structures, namely kaempferol, quercetin, isorhamnetin, myricetin, laricitrin, and syringetin. The most abundant flavonols of Vitis vinifera grape cultivars occur as 3-O- glycosylated. In almost all grape varieties quercetin and its glycosides are the main flavonols (Mattivi et al. 2006). Given the differences in terms of flavonol patterns among cultivars, these compounds, together with anthocyanins, have been investigated for chemo-taxonomical purposes (Mattivi et al. 2006). Flavonols act in grape as UV- and photo-protectors (Price et al. 1995) and their biosynthesis in plant tissues is greatly influenced by exposure to sunlight (Spayd et al. 2002) so that quercetin 3-glucoside concentrations have been found to be four to eight times higher in skins from sun-exposed clusters than in those from shaded clusters (Spayd et al. 2002). Other factors including temperature seem to be less influent (Spayd et al. 2002). The quercetin concentration in wines depends on the vinification procedure and on the degree of hydrolysis undergone by flavonol glycosides during winemaking and wine aging. Flavonols play important roles in wine. One such function is acting as copigments that enhance color response of anthocyanins. The large extended π electron conjugation of these compounds facilitates the π–π interactions with anthocyanins and determines the formation of stable copigmentation complexes in red wine (Waterhouse 2016).
While the glycosides are quite soluble in wines, the aglycone quercetin is particularly insoluble in aqueous solutions, at room temperature and at pH 3 the solubility is of 0.4 µg mL−1 (Li et al. 2013).
Waterhouse and colleagues (2016) hypothesized that some sg wines can throw a precipitate of quercetin upon aging from the hydrolysis of glycosides resulting in aglycon supersaturation. The supersaturation was explained considering that flavonols, particularly quercetin, provide a strong co‐pigmentation effect with anthocyanins, thus justifying the solubility of quercetin in wine even at supersaturated levels. Although the commercial relevance of this issue and the possible impact that the global warming can exercise on the risk of excessive Q in wines, no scientific study on the characterization of sg deposits is available in literature. Poor is also the knowledge related to the content of Q and GQ in sg wines. To the aim of furnishing winemakers with appropriate instruments to prevent the formation of deposits in sg wines, in this study a trial to evaluate the saturation limit for Q in hydro-alcoholic solution and sg wines was carried out. The content of Q and GQ in numerous monovarietal Italian red wines including sg and the effect of factors such as the vintage, the aging and the controlled exposure to oxygen was also evaluated.
Materials and methods
Wines containing precipitates
Five bottled Sg commercial samples and 8 samples of bulk wine belonging to three Tuscan wineries, all containing a deposit, were filtered and the precipitate was collected as described below.
Separation of the precipitates
The collection of the particulate from Sg wines showing a precipitate was carried out as follow: 750 mL of wine poured into a funnel passed through a filter (Albet 400, peso 80 g m−2), which retained the particulate, and the filtrate was collected into a filter flask, via a vacuum pumping.
Solubility of quercetin in hydro-alcoholic solution and real wine
A hydro-alcoholic solution (12% ethanol, 5 g L−1 tartaric acid adjusted at pH 3.2 with NaOH 1 N) and a sg wine (year 2016) containing 0.006 mgL−1 of Q and 0.049 mgL−1 of GQ were added with pure quercetin at increasing concentrations of 3, 5, 7, 10, 20, 25, 30, 35, 40, 50, 60 mgL−1. Samples were stored at 20 °C for 15 days in glass vials (7 mL capacity) and the visual and optical microscopic analysis (400×) were carried out daily on the precipitate (when it was present on the bottom of the vial).
Monovarietal red wines
22 sg wines (8 of 2016, 6 of 2017, 5 of 2018, 3 of 2015), 28 Aglianico (a) wines (24 of 2017, 4 of 2018), 2 Cabernet Sauvignon (cab) (1 of 2017 and 1 of 2018), 2 Nerello Mascalese (ner) (1 of 2018 and 1 of 2017), 1 Primitivo (p) (2017), 1 Nero di Troia (nt) (2018), 6 Pallagrello 2012 (pg), 6 Casavecchia (cs), 1 Magliocco 2017 (mag), 1 Syrah 2018 (s), 1 Merlot 2018 (mer), 2 Nero d’avola 2018 (na).
Aging effect
Six sg wines produced in 2016 were stored in stainless steel tank aging (2 tanks each of 15 L capacity) just after the end of the malolactic fermentation (November 2016). Samples were withdrawn for analysis after 6, 9 and 23 months from the end of the malolactic fermentation.
Vintage effect
Sg wines produced in 2016 (n = 8), 2017 (n = 6) and 2018 (n = 5) vintages were compared on the basis of their Q and GQ contents. All wines were produced from grapes grown in the Chianti DOC area (Tuscany region, Italy) and analyzed 9 months after the fermentation. Wines belonging to the same vineyard showed the same code followed by the year of production (wine 1, wine 2, wine 3, wine 7 and wine 8).
Oxidation effect
The oxidation experiment consisted of four consecutive air saturation cycles applied to 6 sg wines (wine a–wine f) showing different initial content of Q and GQ. The air saturations were performed as previously reported (Gambuti et al. 2018). Briefly, two 1 L bottles of each wine containing PSt3 oxygen sensors (Nomacorc SA, Thimister-Clermont, Belgium) were saturated with air by adding a gentle flow of oxygen through a mini-compressor for 15 min until the oxygen level of the wine reached 6.6 mgL−1. At the end of each saturation, the bottles were filled up completely with saturated wines, and were carefully closed under nitrogen to avoid any headspace. Wines were stored in an incubator in the dark at 25 °C and dissolved oxygen level was monitored at least once a day with a Nomasense oxygen analyzer from Nomacorc S.A. (Thimister-Clermont, Belgium). The oxidation cycle was considered finished once O2 levels dropped to 10% of the initial concentration. Then the bottles were opened and a sample for intermediate analysis was taken. The remaining volume of wine was subjected to a new saturation cycle. The total oxygen consumption was 26.4 mgL−1 (6.6 mgL−1 for each saturation cycle). The saturation experiments were performed in duplicate for each wine.
HPLC–DAD Analysis of Flavonols
HPLC separation, identification, and quantification of flavonols were performed on an HPLC Shimadzu LC10 ADVP apparatus (Shimadzu Italy, Milan) equipped with a SCL-10AVP system controller, two LC-10ADVP pumps, a SPD-M 10 AVP detector, and an injection system full Rheodyne model 7725 (Rheodyne, Cotati, CA). The method described by Castillo-Muñoz et al. (2007) was used with slight modification. A Waters Spherisorb column (250 × 4.6 mm, 4 μm particles diameter) with pre-column and a 50 µL loop were used. 50 µL of either calibration standards or wine were injected into the column. The absorbance signals at 360 nm were detected. All the samples were filtered through 0.45 mm Durapore membrane filters (Millipore–Ireland) into glass vials and immediately injected into the HPLC system. The HPLC solvents were: solvent A: water ultrapure (Sigma Aldrich)/formic acid (Sigma Aldrich ≥ 95%)/acetonitrile (Sigma Aldrich ≥ 99.9%) (87:10:3) v/v/v; solvent B: water/formic acid/acetonitrile (40:10:50) v/v/v. Quantification was carried out by using the DAD chromatograms obtained at 360 nm; for calibration the external standard method was used; the calibration curve was plotted for quercetin (Sigma-Aldrich, Merck, Milan, Italy) and quercetin 3-glucosides (Sigma-Aldrich, Merck, Milan, Italy). The amount of GQ was determined as the sum of peak areas relative to quercetin 3-galactosides, rutin, quercetin 3-glucuronides and quercetin 3-glucosides and expressed as quercetin 3-glucosides. Calibration solutions (Q and quercetin 3-glucosides) were obtained by preparing a stock solution in methanol and diluting it in hydro-alcoholic solutions (ethanol 12%, pH 3.2). Four analytical replicates (two experimental samples x two HPLC analysis for each replicate) were carried out for each wine.
NMR experiments
Extraction procedure. Filters containing the precipitate were extracted with 30 mL of H2O:EtOH 2:8 (v/v) overnight. The obtained extracts were concentrated to remove all the ethanol, brought to 20 mL with distilled water and then partitioned twice against ethyl acetate (99.8% purity; from Sigma-Aldrich, Merck, Milan, Italy) (20 mL × 2). Both the aqueous and lipophilic extracts were separately dried and finally solubilized in 600 μL of D2O (99.96% purity; from Sigma-Aldrich, Merck, Milan, Italy) and CD3OD (99.8% purity; from Sigma-Aldrich, Merck, Milan, Italy), respectively, for NMR analyses. NMR experiments were run on a Varian Unity Inova 700 spectrometer equipped with a 13C Enhanced HCN Cold Probe and by using a Shigemi 5 mm NMR tubes. CD3OD (δH 3.31; δC 49.0 ppm) and D2O (δH 4.79 ppm) were selected as deuterated solvents. 1H-NMR, HSQC and HMBC standard Varian pulse sequences were employed. One bond 1H–13C connectivities were determined by gradient 2D HSQC experiments, while two and three bond 1H–13C connectivities were determined by gradient 2D HMBC experiments optimized for a 2,3 J of 8 Hz. The time domain data matrix for the 2D NMR spectra was set at 2048 (F2) by 512 (F1) with 40 transients obtained per increment. Spectra were processed by zero-filling and Fourier transform to 2 K by 1 K data points after employing a sine filter multiplication.
Microscopic analysis
One ml of wine, taken from the bottom of the bottles containing a precipitate (ref. 2.1), was centrifuged at 14,000 rpm for 5 min. The pellet was resuspended in 100 μL of sterile water and 25 μL of suspension was subjected to microscopic analysis (Motic model BA310, VWR international SRL, Milan, Italy) at 400 magnifications. The images were captured with Visicam 10.0 (VWR international SRL, Milan, Italy) and exported as a “.TIF” file.
Data analysis
One‐way ANOVA analysis, Fisher’s least significant differences procedure were used to discriminate among the means of the variables for Q and GQ analyses when true the assumption of variance homogeneity. Elaborations were carried out by means of XLSTAT software (Addinsoft, XLSTAT 2017).
Results and discussion
The deposit obtained by filtering the wine under analysis appeared as a yellow–grey–brown sludge (Fig. 1 a). In Fig. 1b the microscopic appearance of the crystals obtained by analyzing the precipitate taken from the bottom of the container is shown. Apart from bitartrate crystals and some microorganisms, fine (approximately 50 micrometers long) needle-shaped crystals could be distinguished. The morphology of these precipitates corresponds to the typical needle-like crystals of quercetin obtained on recrystallisation from organic solvent (Patel et al. 2012). The crystal structure of Q has been previously described by Rossi et al. (1986) and Jin et al. (1990). Q crystallizes in the space group P1 with two molecules in the unit cell. The crystal shape derives from the fact that quercetin participates in several intra- and inter-molecular hydrogen bonds due to the oxygen atoms involved in five hydroxyl and one carbonyl groups, respectively. However, unambiguous evidence that the deposit was constituted by quercetin crystals was achieved by NMR 1H and 13C analysis.
NMR—based identification of the major metabolites contained in wine residues
Wine residues were extracted from filters by a hydro-ethanolic solution and, after removal of ethanol by vacuum evaporation, the residual aqueous layers were partitioned against ethyl acetate, as detailed in materials and methods. Eventually, for each wine sample both an aqueous and a lipophilic extracts were obtained and subjected to NMR analysis for metabolite identification. By comparison with NMR data available in literature, in the aqueous layers we could unambiguously identify glycerol, carbohydrates, among which glucose and fructose were the most abundant, aminoacids including valine, isoleucine, leucine, alanine and proline, and organic acids, such as tartrate, succinate, acetate and lactate.
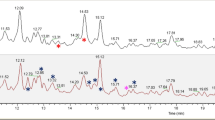
In the 1H-NMR spectrum of the ethyl acetate extracts quercetin was identified on the basis of its 1H (Fig. 2) and 13C NMR signals that overlapped those of an authentic sample (Supplementary Table 1). Only in one sample low amounts of kaempferol were detected, by comparison of its 1H–NMR data with those of an authentic sample. 13C NMR resonances for kaempferol could not be obtained due to the small amount of compound.
Formation of the precipitate of quercetin in hydro-alcoholic solution and wine
The water solubility of quercetin at pH 3 is 0.4 µg mL−1 (Li et al. 2013); no reports about the solubility of Q in hydro-alcoholic solutions in the typical wine pH range are available. The range of Q levels in wines free of deposits reported in literature was instead very wide (from 0 to 40 mgL−1). Therefore, to the aim of individuating the concentration of Q at which a precipitate is to be expected in wine, 11 hydro-alcoholic solutions (pH 3.2 and 12% ethanol) and 11 red wines were spiked with increasing concentrations of quercetin, from 3 to 60 mgL−1. The experimental solutions and wines were stored in the dark at 20 °C and the formation of precipitates was evaluated by visual and microscopic analyses. As expected, the higher the Q concentration, the faster the formation of precipitate. In hydro-alcoholic solutions the formation of precipitates was immediate in solutions containing at least 7 mgL−1 of Q while, after 10 days, all of the hydro-alcoholic solutions added with Q showed a precipitate. In wines, after 1 week a precipitate was observed in the wines added with at least 7 mgL−1 of Q, after 12 days a precipitate was detected in those added with 5 mgL−1 of Q and, after 15 days a precipitate was detected in wines spiked with 3 gmL−1 of Q. Reasonably, a clear dependence on the concentration of Q and on the medium was observed. Our data are in agreement with previous results showing that the aqueous solubility of quercetin at 20 °C is lower than 0.00263 mg mL−1 (Chebil et al. 2007; Srinivas et al. 2010). Although Abraham et al. (2014) showed that an increasing percentage of ethanol in water causes an increase in terms of Q solubility, the solubility in wine is not very different from that in water. The dependence on time of the formation of precipitate that we observed is also consistent with a previous study (Chebil et al. 2007). In regards to the effect of the medium on the formation of Q precipitates, it is clear that wine compounds, other than tartaric acid and ethanol, act as limiting factors for precipitation. This is due to the fact that wine is a colloidal solution and numerous compounds (organic acids, condensed flavanols, polysaccharides, among others) could act as protector colloids as usually observed in the case of the formation of crystals of potassium bitartrate (Waterhouse et al. 2016). The intermolecular bonds involved in the co-pigmentation phenomenon can also play an important role in the stabilization of the system and could limit the Q precipitation (Waterhouse et al. 2016).
Content of quercetin and quercetin glycosides in monovarietal Italian wines
As reported (Makris et al. 2006), there are wide variations in terms of Q and GQ contents in the individual red wines (Fig. 3). Several reasons can explain such variability. Quercetin and its glycosides accumulate in the skins of red grapes (Price et al. 1995), and the variety is one of the main factors influencing their level (Mc Donald et al. 1998). Mc Donald (1998) suggested that wines containing higher concentrations of flavonols are mainly derived from “thick-skinned” grapes, such as Cabernet Sauvignon, which are characterized by a high skin:volume ratio, rather than “thinner-skinned” varieties such as Grenache with a low skin:volume ratio. Given that sg is characterized by an elevated morphological variability owing to its high reactivity to cultivation pedoclimatic conditions (Bergamini et al. 2013), for this grape cultivar it is not possible to correlate the skin thickness of berries with the flavonol content. There is also a trend toward higher flavonol levels in wines produced from grapes grown in warmer and sunnier climates, such as Chile, rather than cooler regions, such as northern Italy or northern France. This is in agreement with a study showing that pinot noir wines obtained from sun-exposed grape clusters contain quercetin glycosides concentrations that are over sevenfold higher than those detected in wines made from shaded berries (Price et al. 1995). Viticultural (Romboli et al. 2017) and enological (Castellari et al. 2001) practices also affect flavonol contents. In the red wines analyzed in the present study we have never detected more than 8.6 mgL−1 for Q (minimum value 0.01 mgL−1) and 33.9 mgL−1 for GQ (minimum value 2.02 mgL−1). These results are in agreement with those reported for numerous wines produced from international grape cultivars such as Gamay noir, Merlot, Cabernet Sauvignon, Cabernet Franc, and Pinot noir (Soleas et al. 1997; Goldberg et al. 1998; Vuorinen et al. 2000; McDonald et al. 1998; Buiarelli et al. 2018; Parpinello et al. 2019) and numerous Italian grape cultivars (Pellegrini et al. 2000).
Content of Q and GQ of commercial wines. Numbers among parenthesis are the number of samples analyzed for each monovarietal wines. The codes represent the kind of monovarietal wine: sg, Sangiovese; pg, Pallagrello; cs, Casavecchia; ag, Aglianico; cab, Cabernet sauvignon, Mag, magliocco; ner, Nerello Mascalese; pri, Primitivo; s, Syrah; nt, Nero di Troia; mer, Merlot; na, Nero d’Avola
Sg is the grape variety showing the higher concentration of Q (from 0.4 to 8.6 mgL−1) and GQ (from 3.1 to 33.9 mgL−1). Pallagrello nero (pg) is the second one in terms of Q (from 5.6 to 8.1 mgL−1) and GQ (from 11.8 to 12.4 mgL−1) abundances. For all of the monovarietal wines analyzed, the GQ levels were always higher than Q levels. A similar behavior for sg wines has already been reported (Castillo-Munoz et al. 2007) following a comparative study according to which concentrations higher than those detected in the present study were shown. Also, Viñas et al. (2000), in nine samples from various red cultivars, including Cabernet Sauvignon, Syrah, Merlot and Tempranillo, detected higher quercetin content (until 43.1 mgL−1). Differences in the range of concentrations detected between our results and some studies available in literature could be due to the different method used for the HPLC analysis and the solvent employed for the building of the calibration curve as already observed by Buiarelli et al. (2018) that showed that the matrix effect can greatly affect the reproducibility and accuracy of the method.
The wide variability in Q and GQ levels in sg wines is not surprising because numerous viticultural (Romboli et al. 2017) and enological practices (Castellari et al. 2001) can affect Q and GQ content in wines produced from sg grapes grown in Tuscany indicating a great plasticity of this grape cultivar and the determinant role of grape growers and winemakers in managing the risk of the formation of the deposits.
Effect of aging in stainless steel tank
During aging in stainless steel tanks, significant changes in the contents of these Q and GQ flavonoids were observed (Table 1). The loss of Q was faster over the first 9 months of storage than over the successive 15 months of aging reaching values below 3 mgL−1 already 9 months after the end of the fermentation. A previous study also showed that from the third to the ninth month from the end of malolactic fermentation the content of total flavonols decreased quickly (Gil-Muñoz et al. 1999). It is known that flavonols, particularly when they occur in their deglycosylated form, are labile molecules and may be degraded upon exposure to heat (Makris and Rossiter 2000), enzymes and oxidative chemical species, such as free radicals (Makris and Rossiter 2002). The involvement of Q in reactions of formation of high-molecular-mass polymeric polyphenols (Suo et al. 2019) can also explain the loss of Q.
The hydrolysis of GQ was instead slower (Fig. 4). A similar decline in flavonol glucoside (25%), probably ascribed to hydrolysis, over a period of 7 months was previously shown (Zafrilla et al. 2003). Only for SG-4 wine the decrease of GQ was close to 75% of the initial amount after 23 months from the end of fermentation. For all the other wines the loss after 23 months was hovering around 50% of the initial value. It is known that the rate of hydrolysis of glycosides depends on several factors such as pH, temperature, solvent, glycoside concentration (Dou et al. 2015). In our study the pH of wine SG4 did not differ significantly from those of wines SG-3, SG-5 and SG-6 and the storage conditions (time and temperature) were the same for all wines. Therefore, it seems that there is no direct factor that could explain the differences observed in the hydrolysis rate. Further studies are necessary to investigate the multiple factors affecting the hydrolytic cleavage of the quercetin glycosides during wine aging.
Vintage effect
ANOVA analysis showed that sg wines produced in different years differed by the Q content according to the following order year 2017 > year 2018 > year 2016 (p < 0.0001). No significant differences in GQ were instead observed. As expected, given the higher solar radiation exposure and temperature (http://www.osservatoriochianti.it) which grapes were exposed to in 2017, higher values of Q were detected in wines produced in that year. It is in fact known that grapes highly exposed to daylight increase the flavonol biosynthesis and that berries (V. vinifera cv. Merlot) belonging to sun exposed clusters might contain an amount of flavonol ten times as high as that found in samples obtained from shaded clusters (Spayd et al. 2002). However, other studies highlighted that flavonol biosynthesis can be also induced by environmental conditions other than UV-B radiation (Koyama et al. 2012). Most of these factors can be easily regulated by applying the appropriate viticultural practices (Romboli et al. 2017) and/or limiting the extraction of flavonols from berry skin during wine production.
Effect of oxidation
The effect of four successive air saturations, each one suppling wine with almost 6 mgL−1 of oxygen, is shown in Fig. 5. The highest decrease of Q and GQ (− 65% and − 16% respectively) was recorded during the first saturation cycle in wine-a and, for Q in wine-e (− 80%). A slight decrease of Q was even observed in wines b, c, d and f during the first saturation while, after consuming the first 6 mgL−1 of oxygen, a slight decrease of GQ occurred (less than 9%) in wine-b, wine-c and wine-d. In agreement with our results, Castellari et al. (2000) showed that supplementation with oxygen during storage decreased quercetin levels by more than 50% over a period of 6 months (Castellari et al. 2000). Also, the supply of oxygen naturally passing through the wood determined a loss of both glycosides and aglycones depending on the kind of wood container used (De Simón et al. 2003). This finding highlighted the impact of the oxidative reactions and suggested that practices such as the micro-oxygenation and the wood aging could help decreasing the risk of Q precipitation in finished wines. The slower and often no significant decrease of GQ is instead coherent with the slow release of Q by hydrolysis of its glycosides.
Conclusion
Qualitative analysis of the deposits in sg wines confirmed that they are constituted by crystals of Q. Thanks to a trial aimed at evaluating the possible saturation limit for Q in hydro-alcoholic solutions and in red wines, for the first time, a concentration of 3 mgL−1 for the risk of appearance of deposits of Q was determined. Among the 12 monovarietal wines analyzed, only sg and pg overcame a content of Q higher than 3 mgL−1 while GQ was higher than 10 mgL−1. This highlights that the grape variety is the main factor affecting the “precipitation risk”. In regards to the hydrolysis of glycosides, the present results showed that at wine pH the release is very slow and, only in one case, after 23 months more than the 70% of the initial content of GQ was degraded. Further studies are needed to investigate the role of typical wine compounds in the rate of the glycoside hydrolysis. The most interesting result obtained in this study is related to the effect of oxidation: 6.6 mgL−1 of oxygen turned out to be enough to decrease by more than 50% the initial amount of Q. Because sg is a red wine that often undergoes micro-oxygenation or storage in wood container through which the oxygen gets in contact with the wine, these data suggest that the application of these practices, together with a quantitative analysis of the “precipitation risk” of finished wines, constitute a first useful tool to avoid the formation of the deposit after bottling.
References
Abraham MH, Acree WE Jr (2014) On the solubility of quercetin. J Mol Liq 197:157–159
Bergamini C, Caputo AR, Gasparro M, Perniola R, Cardone MF, Antonacci D (2013) Evidences for an alternative genealogy of ‘Sangiovese’. Mol Biotechnol 53(3):278–288
Buiarelli F, Bernardini F, Di Filippo P, Riccardi C, Pomata D, Simonetti G, Risoluti R (2018) Extraction, purification, and determination by HPLC of Quercetin in some Italian wines. Food Anal Methods 11(12):3558–3562
Castellari M, Matricardi L, Arfelli G, Galassi S, Amati A (2000) Level of single bioactive phenolics in red wine as a function of the oxygen supplied during storage. Food Chem 69(1):61–67
Castellari M, Piermattei B, Arfelli G, Amati A (2001) Influence of aging conditions on the quality of red Sangiovese wine. J Agric Food Chem 49(8):3672–3676
Castillo-Muñoz N, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I (2007) Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J Agric Food Chem 55(3):992–1002
Chebil L, Humeau C, Anthoni J, Dehez F, Engasser JM, Ghoul M (2007) Solubility of flavonoids in organic solvents. J Chem Eng Data 52(5):1552–1556
De Simón BF, Hernández T, Cadahía E, Dueñas M, Estrella I (2003) Phenolic compounds in a Spanish red wine aged in barrels made of Spanish, French and American oak wood. Eur Food Res Technol 216(2):150–156
Dou M, He XH, Sun Y, Peng F, Liu JY, Hao LL, Yang SL (2015) Controlled acid hydrolysis and kinetics of flavone C-glycosides from trollflowers. Chin Chem Lett 26(2):255–258
Gambuti A, Picariello L, Rinald A, Moio L (2018) Evolution of Sangiovese wines with varied tannin and anthocyanin ratios during oxidative aging. Front Chem 6:63
Gil-Muñoz R, Gómez-Plaza E, Martınez A, López-Roca JM (1999) Evolution of phenolic compounds during wine fermentation and post-fermentation: influence of grape temperature. J Food Compos Anal 12(4):259–272
Goldberg DM, Tsang E, Karumanchiri A, Soleas GJ (1998) Quercetin and p-coumaric acid concentrations in commercial wines. Am J Enol Vitic 49(2):142–151
Jin GZ, Yamagata Y, Tomita K (1990) Structure of quercetin dihydrate. Acta Crystallogr C 46(2):310–313
Koyama K, Ikeda H, Poudel PR, Goto-Yamamoto N (2012) Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 78:54–64
Lanati D, Marchi D, Cascio P (2014) Precipitati di Quercetina nei vini. In: 37th World Congress of Vine and Wine and 12th General Assembly of the OIV, 06007
Li B, Konecke S, Harich K, Wegiel L, Taylor LS, Edgar KJ (2013) Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr Polym 92(2):2033–2040
Makris DP, Rossiter JT (2000) Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J Agric Food Chem 48(9):3830–3838
Makris DP, Rossiter JT (2002) An investigation on structural aspects influencing product formation in enzymic and chemical oxidation of quercetin and related flavonols. Food Chem 77(2):177–185
Makris DP, Kallithraka S, Kefalas P (2006) Flavonols in grapes, grape products and wines: burden, profile and influential parameters. J Food Compos Anal 19(5):396–404
Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R (2006) Metabolite profiling of grape: flavonols and anthocyanins. J Agric Food Chem 54(20):7692–7702
McDonald MS, Hughes M, Burns J, Lean ME, Matthews D, Crozier A (1998) Survey of the free and conjugated myricetin and quercetin content of red wines of different geographical origins. J Agric Food Chem 46(2):368–375
OIV Focus 27 (2017) Distribution of the world’s grapevine varieties. (http://www.oiv.int/en/oiv-life/the-distribution-of-the-worlds-grapevine-varieties-new-oivstudy-available). Accessed 9 Aug 2019
Parpinello GP, Ricci A, Rombolà AD, Nigro G, Versari A (2019) Comparison of Sangiovese wines obtained from stabilized organic and biodynamic vineyard management systems. Food Chem 283:499–507
Patel AR, Heussen PC, Hazekamp J, Drost E, Velikov KP (2012) Quercetin loaded biopolymeric colloidal particles prepared by simultaneous precipitation of quercetin with hydrophobic protein in aqueous medium. Food Chem 133(2):423–429
Pellegrini N, Simonetti P, Gardana C, Brenna O, Brighenti F, Pietta P (2000) Polyphenol content and total antioxidant activity of vini novelli (young red wines). J Agric Food Chem 48(3):732–735
Price SF, Breen PJ, Valladao M, Watson BT (1995) Cluster sun exposure and quercetin in Pinot noir grapes and wine. Am J Enol Vitic 46(2):187–194
Romboli Y, Di Gennaro SF, Mangani S, Buscioni G, Matese A, Genesio L, Vincenzini M (2017) Vine vigour modulates bunch microclimate and affects the composition of grape and wine flavonoids: an unmanned aerial vehicle approach in a Sangiovese vineyard in Tuscany. Aust J Grape Wine Res 23(3):368–377
Rossi M, Rickles LF, Halpin WA (1986) The crystal and molecular structure of quercetin: a biologically active and naturally occurring flavonoid. Bioorg Chem 14(1):55–69
Soleas GJ, Dam J, Carey M, Goldberg DM (1997) Toward the fingerprinting of wines: cultivar-related patterns of polyphenolic constituents in Ontario wines. J Agric Food Chem 45(10):3871–3880
Spayd SE, Tarara JM, Mee DL, Ferguson JC (2002) Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am J Enol Vitic 53(3):171–182
Srinivas K, King JW, Howard LR, Monrad JK (2010) Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J Food Eng 100(2):208–218
Suo H, Tian R, Li J, Zhang S, Cui Y, Li L, Sun B (2019) Compositional characterization study on high-molecular-mass polymeric polyphenols in red wines by chemical degradation. Food Res Int 123:440–449
Vinas P, Lopez-Erroz C, Marın-Hernandez JJ, Hernandez-Cordoba M (2000) Determination of phenols in wines by liquid chromatography with photodiode array and fluorescence detection. J Chromatogr A 871(1–2):85–93
Vuorinen H, Määttä K, Törrönen R (2000) Content of the flavonols myricetin, quercetin, and kaempferol in Finnish berry wines. J Agric Food Chem 48(7):2675–2680
Waterhouse AL, Sacks GL, Jeffery DW (2016) Understanding wine chemistry. Wiley, Hoboken
Zafrilla P, Morillas J, Mulero J, Cayuela JM, Martínez-Cachá A, Pardo F, López Nicolás JM (2003) Changes during storage in conventional and ecological wine: phenolic content and antioxidant activity. J Agric Food Chem 51(16):4694–4700
Acknowledgements
All authors have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gambuti, A., Picariello, L., Rinaldi, A. et al. New insights into the formation of precipitates of quercetin in Sangiovese wines. J Food Sci Technol 57, 2602–2611 (2020). https://doi.org/10.1007/s13197-020-04296-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04296-7