Abstract
Starch is the main carbohydrate consumed by humans, obtained from many vegetable sources and known for its non-toxicity, applications and low cost. The aim of this work was the extraction and study of characteristics of non-conventional starch arising from inhambu (Dioscorea trifida L.). Chemical, physicochemical, thermal, morphological, structural and technological properties were evaluated. The starch yield percentage obtained from the extraction, starch and amylose contents of inhambu starch were considerably high, 22.76%, 84.56% and 36.82% respectively. Native starch presented high purity due the low ash, protein and fat content. The thermogravimetric analysis shown 69% weight loss in a 293.12–476.59 °C temperature range and the endothermic peak was at 100.0 °C. The starch granule morphology shown spherical shapes and smooth surfaces and size ranging from 5.06 to 14.59 µm. Considering the unique starch characteristics, its application in different industrial sectors can be foreseen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is one of most abundant polysaccharides in nature, probably the most promising material because of its easy availability, low cost, biodegradable and for being renewable (Zhang et al. 2015). It is the main source of carbohydrates, providing the basic energy needed for humans. The main starch reserves are found in cereals (40–90%), roots (30–70%), tubers (65–85%), legumes (25–50%) and immature or unripe fruits (40–70%) (Santana and Meireles, 2014). The starch granule amount is exclusive to each botanical species, varying according to shape (round, ovoid, or polyhedral), particle size (0.5–100 μm), structure and composition (Smith 2001).
Made up of glucosyl polymers, starch shows a complex hierarchical structure developed in higher plants plastids, synthesized in leaves and consists of amylose and amylopectin, besides other minor components such as protein and lipids (Tanackovic et al. 2014). Amylose, which makes up 25–30% of the starch granule, contains α-1.4 linkage structure and can be partially branched through the α-1,6 linkage. Amylopectin, makes up about 70–75% of the starch granule and contains α-1.6 grouped linkages (Tanackovic et al. 2014; Larson et al. 2016). These parameters are determined by having important effects on the physico-chemical, technological, morphological and thermal properties of the starch. The relative proportion of amylose and amylopectin, organization and chain length distribution within the starch granules depend on the botanical sources.
Native starches have been used for a very long time to meet the needs of different industrial segments, such as food, paper, textiles, pharmaceuticals, cosmetics, adhesives, and animal feed (Oliveira et al. 2014). They act as thickening agents and, texture stabilizers and provide volume, swelling power and solubility. Therefore it is necessary to understand the characteristics that determine their behavior. On the other hand, unconventional starch sources have been gaining recognition and are being constantly studied, because of growing demand. The use of these starches, like those from roots and tubers, can provide options to expand the spectrum of the desired and required properties for food industry processes.
Many studies have been conducted with new sources of starch and different methods for its extraction, as for example cultivars of Dioscorea spp. Jiang et al. (2013), starches extracted from D. Jiujinhuang, D. Chenji, D. Ximão, D. Tiegun, D. Baiyu, D. Huai, D. Ruichang using the citric acid solution pretreatment methodology. Otegbayo et al. (2014) performed five pellet (starch) washes with the species D. alata, D. rotundata, D. bulbifera, D. dumetorum, D. cayenensis; Vashisht et al. (2015) worked with D. Da1 and D. Da2 varieties and the obtained starch was dried at room temperature for 48 h; Hornung et al. (2017) worked with the species D. sp. and D. piperifolia and the method was performed with the suspension left to stand for 12 h; Sharlina et al. (2017) with D. pyrifolia made four starch washes. However, there are no reports in the literature with white inhambu starch (Dioscorea trifida) and its extraction without the use of chemical reagents.
The inhambu (Dioscorea trifida L.). Dioscorea is the largest and most important genus of the Dioscoreaceae family with approximately six hundred known species. However, only ten are considered relevant in human nutrition (Ramos-Escudero et al. 2010; Zhang et al. 2016). They are widely cultivated in tropical and subtropical regions of the world, contain high carbohydrate content and medicinal value, besides the wide diversity of shapes and colors (Ramos-Escudero et al. 2010; Zhang et al. 2016). Although they present great potential for food industry are generally ignored. In particular, there is no information in the literature on inhambu of the white cultivar regarding its characteristics and uses. As such it is necessary to use techniques that increase the productivity and the quality of this raw material, as for example in the extraction of starch.
Considering the importance of unconventional starch use and valorization of botanical sources of tubers, the objective of this study was the starch extraction from inhambu of the white Dioscorea cultivar, yield and the study of its chemical composition, physical–chemical, thermal, morphological, structural and technological properties, in order to verify its possible aplication in the food industry.
Materials and methods
Material
The raw material used in this study to obtain the starch was the inhambu (Dioscorea trifida L.), collected at plantations of the Instituto Federal Baiano, Uruçuca campus, Brasil (14° 35′ 35″ S latitude, 39° 17′ 04″ W longitude). After harvest it was used for starch extraction. The other materials were: chilled water (5 °C), distilled water, sodium bisulfite (NaHSO3) P.A., absolute ethyl alcohol, anthrone, sulfuric acid (H2SO4), D-glucosyl, iodine, acetic acid (C2H4O2), sodium hydroxide (NaOH) P.A., pure potato amylose, potassium iodide, hexane, methyl orange indicator, bromocresol green indicator, phenolphthalein indicator and boric acid. All chemical reagentes used in the analyzes were of analytical grade and manufactured by the Synth Company. The experiments were conducted in the laboratories of the State University of Southwest Bahia (UESB), Itapetinga campus—Bahia and the Federal Institute of Baiano (IFBAIANO), Uruçuca campus.
Starch extraction
Inhambu (Dioscorea trifida L.) starch was obtained using the method describe by Melo Neto et al. (2016), considering the insolubility principle. Initially, the tubers were separated from contaminants and impurities, selected and sanitized in chlorinated water (100 ppm) for 15 min, washed with potable water, peeled, and ground in an industrial blender. The suspended pulp was sieved through of 0.149 mm and 0.079 mm meshes, the residue was discarded. The sediment was then washed 4 times with chilled water and purified with absolute alcohol, filtered and dried at 35 °C for 12 h in an air circulation kiln. The dry starch obtained was then milled (48 mesh) and packaged in polyethylene bags for further analysis. The extraction yield was analyzed by the ratio between the mass of the extracted starch and the mass of raw material studied, being expressed as a percentage.
Chemical and physical–chemical composition
Starch content was quantified according to the anthrone method (Moraes and Chaves 1988). Amylose content of the defatted starch was determined according to the methodology proposed by Martínez et al. (1989) and amylopectin found by difference. The following parameters were determined according to Association of Official Analytical Chemists—AOAC (2005): moisture content in kiln at 105 °C until constant weight; ash content in muffle chamber at 550 °C; crude protein content by the Kjeldahl method using a conversion factor of 6.25. Fat in Soxhlet extractor based on the weight loss of the material extracted with ethyl ether, for 8 h; pH by potentiometric process by pH meter; Total acidity by titration with NaOH 0,01 Mol/L standardized; water activity was measured with an Aqua-Lab digital instrument, model CX-2, Decagon Devices Inc., EUA.54vv.
Color analysis
The color was evaluated through direct analysis in a digital colorimeter, HunterLab ColorQuest XE (Sunset Hills Road, Reston, VA, USA). The instrument was equipped with illuminant D65/8° with a specular. The L*-axis variation represents brightness changes in the range of 0 (black) to 100 (white). The a* parameter expresses green/red axis variation (− a*/a*) and parameter b* shows blue/yellow axis variation (− b*/b*) according to international color scale specifications (CIE—The International Commission on Illumination).
Thermogravimetric analysis of starch
Thermal stability of inhambu starch was evaluated using the Simultaneous Thermal Analyzer (STA) 6000 (Perkin Elmer, USA), assisted by Pyris Series software. The thermogravimetric (TGA) and differential thermal analyses (DTG) of the starch (5.0 mg) were obtained in the temperature range from 30 to 800 °C with a heating rate of 10 °C/min. The analyses were conducted under nitrogen atmosphere with a 20 mL/min flow rate.
Thermal properties of inhambu starch were observed using a differential scanning calorimeter (DSC), model DSC-60 (Shimadzu, Japão). Starch samples (5.0 mg) were weighed into an aluminum cap. The cap was then hermetically sealed at room temperature for 1 h. An empty aluminum cap was used as reference. The analysis was performed at a temperature range between 10 and 225 °C, with a heating rate of 10 °C/min. The transitions were characterized by the onset temperature (To), peak temperature (Tp), final temperature (Tf) and the enthalpy change (ΔH) associated with starch gelatinization process.
Structural analysis of starch
The Fourier Transform Infrared Spectroscopy was generated using the medium Infrared Cary 630 FTIR equipment (Agilent Technologies Inc., Santa Clara, CA, USA), equipped with attenuated total reflectance cell (ATR) and deuterated triglycerin sulfate detector (DTGS). Agilent MicroLab PC software was used to process the results. The starch sample was then placed in the accessory compartment where the infrared rays reflect (diamond crystal). Spectra were obtained in transmittance mode.
Samples were evaluated in the spectral region with a wave-length from 4000 to 650 cm−1, and triangular apodization and 64 scans for each spectrum. All the spectra were recorded at a resolution of 4 cm−1 at room temperature.
Morphological analysis
The starch diffraction patterns were obtained using an X-ray diffractometer Model Bruker D2 Phaser diffractometer (Bruker AXS, Karlsruhe, Germany) with operating voltage 30 kV and 10 mA. The assay was performed at room temperature 25 °C with a 2θ angle between 5° and 50°.
The scanning electron microscopy (SEM) was performed with a Quanta 400 model from the FEI Company, with a maximum operating voltage and a nominal resolution of 1.2 nm, in a high vacuum at a voltage of 20 kV. The images were obtained with a secondary electron detector. The starch was fixed on sample dish and covered with a gold layer. The sizes of the granules were determined by measuring the mean diameter of the starch granules by means of an ocular micrometer attached to the microscope lenses.
Technological properties of starch
The methodology used to quantify the starch swelling power and solubility index as a temperature function was that described by Leach et al. (1959) with some modifications, in which the starch suspensions were homogenized and heated in a water bath at 50 °C, 60 °C, 70 °C, 80 °C and 90 °C for 30 min. The suspensions were the cooled and centrifuged at 2120g for 15 min. For determination of the samples solubility index (%) the supernatant was placed in crucibles which were previously weighed and the volumes dried in an air circulation kiln at 105 °C until constant mass. The swelling power (g water/g dry sample) was determined throught the precipitate remaining at the bottom of the centrifuge tubes. The calculations were obtained from Eqs. (1) and (2):
Results and discussion
The extraction of inhambu starch resulted in a yield of 22.76% (dry base) with a characteristic aspect: white, insipid and odorless. This result can be explained mainly by the methodology used for obtaining the starch, botanical variety and source. The inhambu starch yield can be considered satisfactory when compared to research in the literature. Lower values were verified with different extraction methods and yam starches, Liporacci et al. (2005) found 12% and 13% when using 0.1% (w/v) sodium metabisufit solution; Amoo et al. (2014) verified 12.61%, 14.23%, 15.63% and 20.89% after cloth sieving and drying of starches in solar dryers. Zhang and Wang (2017) reported variation from 12.88 to 15.91% with application of ultrasound-assisted extraction and deep eutectic solvent.
Chemical and physical–chemical composition
The inhambu starch parameters evaluated are shown in Table 1. There is a high starch amount in the inhambu and a high amylose/amylopectin ratio. This criterion is associated with factors like cultivar, genotype, harvest place, season, temperature and storage time, as well the method and proceedings used in the extraction (Zhang and Wang 2017).
The amylose content was higher than that specified by Mali et al. (2010) in Dioscorea alata species, presenting 30%, and in the main commercial starch sources: corn (25%), potato (23%), rice (15%), wheat (20%), manioc (16%) and oat grain (16%). Hornung et al. (2017) reports that botanical sources of yam starches contain approximately 15–30% amylose. These components play an important role in the starch internal structure and its digestibility. The different amylose proportions in the starch composition have a direct influence on its structure, properties and consequently on its application in foods (Mali et al. 2010). The high amylose content in the inhambu starch provides some unique characteristics due its configuration and linear nature, such as the ability to form biodegradable plastic films, (Mali et al. 2010) the production of foods which require crunchiness and resistance as in the manufacturing of fried or baked, savory chip-like snacks.
Although there are no quality and identity parameters for non-conventional source starch it is possible to verify the low moisture content present in the inhambu starch shown in Table 1. This factor depends on the extraction procedure and the drying process, together with the humidity of the surrounding atmosphere (Reddy et al. 2014). Besides the amylose and amylopectin molecules, the starch granules have non-starch constituents such as ash, protein and fat. In this study, the starch presented low contents of these elements which were lower than in the study of Reddy et al. (2014), who analyzed isolated yam starch and found an ash content of 2.3%, protein 0.9% and 0.2% fat.The results found for the inhambu starch are considered desirable since they will not have a negative influence on its other characteristics. In addition, the results indicate a higher quality and extraction efficiency, that is, a high degree of purity.
The pH value close to neutrality and low acidity of the inhambu starch were within the expected. The water activity value found ensures product stability, preventing the development of deteriorating and pathogenic microorganisms and starch deterioration, which would limit its potential applications (Gutkoski et al. 2007).
The color of food ingredients is a fundamental attribute for the food industry, for determining food quality and also for consumer acceptance (Wu and Sun 2013). The inhambu starch presented result of 97.03 ± 0.03 for black/white (L*) aspect having a positive influence on luminosity. The parameters for green/red (a*) and blue/yellow (b*) intensity obtained an average of 0.28 ± 0.01 and 2.73 ± 0.04 respectively. Hornung et al. (2017) studied the same parameters in different species and verified higher clarity in Dioscorea sp., starch which was similar to inhambu starch. Therefore, the studied starch was considered white and this effect may be associated to washing with water at low temperature during extraction, avoiding darkening.
Thermogravimetry (TGA), differential thermal analysis (DTG) and differential scanning calorimetry (DSC)
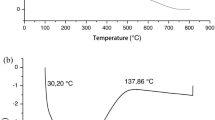
The inhambu starch thermograms TGA and DTG (differential thermal analysis), which show two relevant thermal phenomena, are shown in Fig. 1a, b. The differential scanning calorimeter (DSC) curve of the inhambu starch is shown in Fig. 1c.
In Fig. 1, the first mass loss occurrence as a function of temperature is represented by a small peak, with 11% mass lost from the start of the analysis to 130 °C, and then reaching stability. The second mass loss event is shown by a larger peak, referring to a 69% mass loss in the temperature range from 293.12 to 476.59 °C and the third event there was a 15% loss up to the temperature of 800 °C. According to Zhang et al. (2011) and Melo Neto et al. (2016) the smaller peak corresponds to volatile components, mainly water present in the starch granules and the larger peak probably represents the degradation of the glucose ring in the starch polymers, amylose and/or amylopectin, classified as the main stage of decomposition of the material with intense weight loss.
According Vashisht et al. (2015) and Melo Neto et al. (2015), this analysis has fundamental importance so that the polymers can be used successfully in various industrial applications. Analyzing the TGA and DTG results, the starch under study obtained a distinct characteristic of Dioscorea opposita Thunb starch, in which the maximum decomposition peak was at the 273 °C (Zhang et al. 2011), such as reported by Hornung et al. (2017) for the varieties D. sp. (112 °C) and D. piperifolia (109 °C). However both presented the same gravimetric profile. Thus, inhambu starch has higher thermal stability than other starches in the literature and can be processed at a maximum temperature of 293.12 °C.
The onset temperature (To), peak temperature (Tp) and endothermic final temperature (Te) in gelatinization were 30 °C; 70 °C and 137 °C, respectively (Fig. 1c). The gelatinization interval is represented by the temperature range Te–T0, which was 107 °C and the enthalpy was 323.82 J/g. This analysis confirms the TGA evaluation since the starch shows weight loss within this temperature range.
Results bellow the temperature range of gelatinization and enthalpy of inhambu (Dioscorea trifida L.) starch were found in studies on different varieties of Dioscorea starch: D. opposite Thunb (68.10–92.03 °C), D. alata Linn (71.26–82.11 °C), D. nipponica Makino (70.09–85.18 °C), D. bulbifera Linn (71.63–81.23 °C), D. septemloba Thunb (75.06–84.74 °C) (Jiang et al. 2012). The inhambu starch DSC profile showed a prolonged temperature range compared to the literature, indicating that the process required more energy to break the bond and gelatinize. As such, the starch granules showed to be less predisposed to break up with prolonged heating, because of their resistant glycosidic bonds (Melo Neto et al. 2016).
The gelatinization process is an essential property for starch application in the industrial segment. According to Melo Neto et al. (2016) the peak obtained represents the endothermic gelatinization reaction, that is the, starch granule transition phase from an ordered molecules configuration to one of disorder which occurs when it is heated in the presence of excess water in a temperature range characteristic of the starch source.
Amylose content plays an important role in the gelatinization of starch, since the increase of mobility is perceived first in the amorphous regions, which, physically, have a vitreous nature, thus, the higher the amylose content in a starch, the greater the enthalpy required for the gelatinization to occur and to decrease the starch crystallinity (Hornung et al. 2017). The high enthalpy value can be explained by the amylose and amylopectin ratio, granular architecture and higher linear chain concentration, which limited water accessibility at lower to high temperatures in inhambu starch.
X-ray diffraction (XRD)
The crystal patterns of inhambu starch are represented in Fig. 2. The X-ray diffractogram of the starch showed well-defined peaks at 6.45°, 15°, 17° and 23°, which made it possible to classify this starch as having a type C diffraction pattern, an intermediate crystalline form constituted by the mixture of type A and B starch (Qin et al. 2016). A-Type (15, 17, 18, 20 and 22)° results in formation of the structure in double helices made by amylose and amylopectin compressed, often found in cereals; B-Type (5, 6, 14, 17, 18, 19 and 23)° consists of a clearly defined structure, being highly hydrated with two double helices arranged into a hexagon arrangement, typical of starches of tubers and roots. However, the inhambu starch was considered an exception because it did not present a type B diffraction pattern, with similarity to the results of other different tubers like potato and of the same Dioscorea species (Hornung et al. 2017; Jiang et al. 2013, Sharlina et al. 2017). Generally starches with types B and C crystallinity tend to be more resistant to enzymatic digestion (STARCHES 2015).
Morphological profile of starch granules
Figure 3a, b [Micrograph obtained by scanning electron microscopy (SEM)] present the morphology of inhambu starch granules such as size and shape. The results of the microscopic examination showed predominantly spherical granules, smooth surfaces, regular particles and few cracks, in which a few particles showed spherical deformation. These factors depend on the particular inhambu biochemistry, as well as plant physiology and botanical origin and amylose content (Mesquita et al. 2016). The inhambu starch showed similar characteristics in the study of Otegbayo et al. (2014) on starch granules of differents species of yam such as D. rotundata, D. alata and D. cayenensis, but different from the species D. dumetorum and D. bulbifera, in which hexagonal or polyhedral and triangular, shapes, respectively, were reported.
According to Lindeboom et al. (2004), the size of starch granules can be classified as large granules (> 25 µm), medium (10–25 µm), small (5–10 µm) and very small (< 5 µm). In this study, inhambu starch showed a diameter ranging from 5.06 to 15.44 μm and a mean diameter of 12.95 μm placing it in the category with small and medium starch granules. Higher values were presented by Peroni et al. (2006) in arrowroot and yam starches, with mean diameters of 21.1 µm and 25.3 µm respectively, while manioc, sweet potato and ginger starches showed mean values close to those of inhambu starch, 15.9 µm, 13.9 µm and 15.8 µm, respectively.
The shape of the starch granules has no influence on its technological properties, but can be used to identify the starch source, whereas the size plays an important role for extraction facility and starch sedimentation, important for food and industrial applications, as well as starch paste composition properties, crystallinity, swelling and solubility power and gelatinization temperature. However, several other factors are also influential, including amylose/amylopectin ratio, molecular weight and granule fine structure (Lindeboom et al. 2004).
For food products, granule diameter is an important parameter related to the particle interactions, mixture and homogeneity of formulations (Falade and Christopher 2015). Since inhambu starch has small and medium granules, it will probably have a better digestibility after cooking, since the surface area is larger than the volume and may be important for applications, such as encapsulation and water retention (Madrigal-Aldana et al. 2011). In addition, starch composed of small granules increases the gelatinization temperatures Tp and Te, as found in the present study (Hornung et al. 2017). Another parameter revealed in the inhambu starch granules was the low variability among the diameters, a desirable characteristic for application in biodegradable plastic films (Leonel 2007).
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of inhambu starch are shown in Fig. 4. Because the starch is made up of amylose, amylopectin and water, the spectral bands are attributed to the bonds between the atoms of these molecules. There are peaks in the region of the inhambu starch wave-length at 3283 cm−1 with a broad base and a rounded peak due to characteristcs of the symmetrical and asymmetrical O–H bonded stretching, indicating important water molecules contribution (Andrade-Mahecha et al. 2012); The bands at 2935 cm−1 (found in native starches) corresponds to the asymmetrical C–H bonded stretching; and the band at 1644 cm−1 with H–O–H bonded stretching vibrations, was attributed to adsorbed water (Melo Neto et al. 2016; Sukhija et al. 2016).
According to Warren et al. (2016) larger adsorption bands arising from the starch can be observed in the 1200–1000 cm−1 region, assigned to CO, CC and COH bond stretching and curved COH bond, as can be seen in the inhambu starch at 1150 cm−1 referring to glycosidic CO bond stretching and 1075 cm−1 assigned mainly to starch C–O–H stretch (Melo Neto et al. 2016; Sukhija et al. 2016).
Swelling power and Solubility index as a function of temperature
Figure 5 represents the solubility index and swelling power of inhambu starch granules with the respective temperatures analyzed. The starch absorbed more water and swelled as the temperature increased. The increasing process for solubility and swelling power in inhambu starch promoted the weakening of granule internal bonds from 50 °C, this behavior was confirmed in DSC, since the onset gelatinization temperature was lower.
Figure 5a, b shows higher effect at temperature of 90 °C, with solubility of 32.87% and swelling power of 20.52 g/g, since most of the granules were gelatinized or swollen. This is because in the gelatinization process there is swelling of the starch granules, which then break with consequent amylose leaching. With the rupture of these granules and an increase in solubility, there is a reduction in swelling power (Singh et al. 2017). Peroni et al. (2006) studied starch sources in roots and tubers and found high swelling power values at 90 °C in sweet potato starch, arrowroot and manioc, which were between 40 and 80 g/g and low results for yam and ginger starches, which were below 20 g/g. When compared with inhambu starch, there is indication of limited swelling power, i.e., low water absorption capacity.
The difference in starch solubility and swelling power has been attributed to variation in amylose content and molecular weight of amylose and amylopectin molecules. It can be seen that starch from inhambu (Dioscorea trifida L.) is composed of small and medium granules, with high amylose content and wide temperature range, more energy being necessary to swell and gelatinize the granules, since they are comprised of stronger bonds (Jiang et al. 2013).
Conclusion
Inhambu starch presented high starch content, with high starch yield and satisfactory characteristics, being clear, odorless and insipid and showing high purity and quality in the extraction process. Considering all the unique characteristics of the extracted starch, it is concluded that its application in many industrial segments is technologically viable.
For presenting starch granules with high amylose content, stronger and less susceptible to breakage under prolonged warming, it can be used in food applications which require high temperatures, crunchy texture, as well in film production, coatings and biodegradable packages. Therefore, adding value to an unconventional source of starch, which is little explored in order to evaluate its potentiality and possible applications, is of great importance as an alternative in the area of food and other industrial segments.
References
Amoo ARN, Dufie WMF, Ibok O (2014) Physicochemical and pasting properties of starch extracted from four yam varieties. J Food Nutr Sci 2(6):262–269
Andrade-Mahecha MM, Tapia-Blácido DR, Menegalli FC (2012) Physical–chemical, thermal, and functional properties of achira (Canna indica L.) flour and starch from different geographical origin. Starch/Stärke 64:348–358. https://doi.org/10.1002/star.201100149
Association of Official Analytical Chemists—AOAC (2005) Official methods of analysis of association of official chemists. 13ª ed. AOAC, Washington
Falade KO, Christopher AS (2015) Physical functional, pasting and thermalproperties of flours and starches of six Nigerian rice cultivars. Food Hydrocoll 44:478–490. https://doi.org/10.1016/j.foodhyd.2014.10.005
Gutkoski LC, Bonamigo JMA, Teixeira DMF, Pedó I (2007) Development of oat based cereal bars with high dietary fiber content. Sci Technol Food 27(2):355–363. https://doi.org/10.1590/S0101-20612007000200025
Hornung PS, Cordoba LP, Lazzarotto SRS, Schnitzler E, Lazzarotto M, Ribani RH (2017) Brazilian Dioscoreaceas starches thermal: structural and rheological properties compared to commercial starches. J Therm Anal Calorim 127:1869–1877. https://doi.org/10.1007/s10973-016-5747-5
Jiang Q, Gao W, Li X, Xia Y, Wang H, Wu S et al (2012) Characterizations of starches isolated from five different Dioscorea L species. Food hydrocoll 29(1):35–41. https://doi.org/10.1016/j.foodhyd.2012.01.011
Jiang Q, Gao W, Shi Y, Li X, Wang H, Huang L et al (2013) Physicochemical properties and in vitro digestion of starches from different Dioscorea plants. Food hydrocoll 32:432–439. https://doi.org/10.1016/j.foodhyd.2013.02.001
Larson ME, Falconer DJ, Myers AM, Barb AW (2016) Direct characterization of the maize starch synthase IIa product shows maltodextrin elongation occurs at the non-reducing end. J Biol Chem 291(48):24951–24960. https://doi.org/10.1074/jbc.M116.754705
Leach HW, McCowen LD, Schoch TJ (1959) Structure of starch granule. I. Swelling and solubility patterns of various starches. Cereal Chem 36(6):534–544
Leonel M (2007) Analysis of the shape and size of starch grains from different botanical species. Sci Technol Food 27(3):579–588. https://doi.org/10.1590/S0101-20612007000300024
Lindeboom N, Chang PR, Tyler RT (2004) Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch/Stärke 56:89–99. https://doi.org/10.1002/star.200300218
Liporacci JSN, Mali S, Grossmann MVE (2005) Effects of extraction method on chemical composition and functional properties of yam starch (Dioscorea alata). Sci Agrar 26(3):345–352. https://doi.org/10.5433/1679-0359.2005v26n3p345
Madrigal-Aldana DL, Tovar-Gómez B, Oca MMM, Sáyago-Ayerdi SG, Gutierrez-Meraz F, Bello-Pérez LA (2011) Isolation and characterization of Mexican jackfruit (Artocarpus Heterophyllus L.) seeds starch in two mature stages. Starch/Stärke 63:364–372. https://doi.org/10.1002/star.201100008
Mali S, Grossmann MVEG, Yamashita F (2010) Starch films: production, properties and potential of utilization. Semina Sci Agrar 31(1):137–156. https://doi.org/10.5433/1679-0359.2010v31n1p137
Martínez C, Cuevas F, Medina LM (1989) Evaluación de la calidad culinaria y molineradel arroz. 3ª ed. International Center for Tropical Agriculture, Cali, p. 73. https://books.google.com.br/books?id=wC9yMXQwAbwC&printsec=frontcover&redir_esc=y#v=onepage&q&f=false
Melo Neto BA, Barbosa AA, Santos Leite CX, Almeida PF, Bonomo RCF, Pontes KV (2015) Chemical composition and functional properties of starch extracted from the pejibaye fruit (Bactris gasepaes Kunth.). Acta Sci Technol 37(1):105–110. https://doi.org/10.4025/actascitechnol.v37i1.20740
Melo Neto BA, Fernandes BS, Fornari Junior CCM, Franco M, Bonomo RCF, Almeida PF et al (2016) Thermal-morphological characterisation of starch from peach-palm (Bactris Gasipaes kunth) fruit (Pejibaye). Int J Food Prop 20:1007–1015. https://doi.org/10.1080/10942912.2016.1192645
Mesquita CB, Leonel M, Franco CML, Leonela S, Garcia EL, Santos TPR (2016) Characterization of banana starches obtained from cultivars grown in Brazil. Int J Biol Macromol 89:632–639. https://doi.org/10.1016/j.ijbiomac.2016.05.040
Moraes OMG, Chaves MB (1988) Spectrophotometric method for the determination of starch in meat products. In: National meeting of food analysts. 4th, Belo Horizonte, p 281
Oliveira CS, Andrade MMP, Colman TAD, Costa FJOG, Schnitzler E (2014) Thermal, structural and rheological behaviour of native and modified waxy corn starch with hydrochloric acid at different temperatures. J Therm Analy Calorim 115(1):13–18. https://doi.org/10.1007/s10973-013-3307-9
Otegbayo B, Oguniyan D, Akinwumi O (2014) Physicochemical and functional characterization of yam starch for potential industrial applications. Starch/Stärke 66:235–250. https://doi.org/10.1002/star.201300056
Peroni FHG, Rocha TS, Franco CML (2006) Some structural and physicochemical characteristics of tuber and root starches. Food Sci Technol Int 12(6):505–513. https://doi.org/10.1177/1082013206073045
Qin Y, Liu C, Jiang S, Xiong L, Sun Q (2016) Characterization of starch nanoparticles prepared by nanoprecipitation: influence of amylose content and starch type. Ind Crops Prod 87:182–190. https://doi.org/10.1016/j.indcrop.2016.04.038
Ramos-Escudero F, Santos-Buelga C, Pérez-Alonso JJ, Yáñez JÁ, Dueñas M (2010) HPLC-DAD-ESI/MS identification of anthocyanins in Dioscorea trifida L. yam tubers (purple sachapapa). Eur Food Res Technol 230:745–752. https://doi.org/10.1007/s00217-010-1219-5
Reddy CK, Haripriya S, Mohamed NA, Suriya M (2014) Preparation and characterization of resistant starch III from elephant foot yam (Amorphophallus paeonifolius) starch. Food Chem 155:38–44. https://doi.org/10.1016/j.foodchem.2014.01.023
Santana AL, Meireles MAA (2014) New starches are the trend for industry applications: a review. Food Publ Health 4(5):229–241. https://doi.org/10.5923/j.fph.20140405.04
Sharlina EMS, Yaacob WA, Lazim AM, Fazry S, Lim SJ, Abdullah S et al (2017) Physicochemical Properties of Starch from Dioscorea pyrifolia tubers. Food Chem 220:225–232. https://doi.org/10.1016/j.foodchem.2016.09.196
Singh A, Geveke DJ, Yadav MP (2017) Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT - Food Sci Technol 80:155–162. https://doi.org/10.1016/j.lwt.2016.07.059
Smith AM (2001) The biosynthesis of starch granules. Biomacromol 2(2):335–341. https://doi.org/10.1021/bm000133c
STARCHES (2015) Food ingredients Brazil Nº 35 – 2015. 31-56. Available in: http://www.revista-fi.com/materias/499.pdf
Sukhija S, Singh S, Riar CS (2016) Effect of oxidation, cross-linking and dual modification on physicochemical, crystallinity, morphological, pasting and thermal characteristics of elephant foot yam (Amorphophallus paeoniifolius) starch. Food hydrocoll 55:56–64. https://doi.org/10.1016/j.foodhyd.2015.11.003
Tanackovic V, Svensson JT, Jensen SL, Buléon A, Blennow A (2014) The deposition and characterization of starch in Brachypodium distachyon Vanja. J Exp Bot 65(18):5179–5192. https://doi.org/10.1093/jxb/eru276
Vashisht D, Pandey A, Kumar J (2015) Physicochemical and release properties of carboxymethylated starches of Dioscorea from Jharkhand. Int J Biol Macromol 74:523–529. https://doi.org/10.1016/j.ijbiomac.2014.11.039
Warren FJ, Gidley MJ, Flanagan BM (2016) Infrared spectroscopy as a tool to characterise starch ordered structure- a joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr Polym 139:35–42. https://doi.org/10.1016/j.carbpol.2015.11.066
Wu D, Sun DW (2013) Colour measurements by computer vision for food quality control—a review. Trends Food Sci Technol 29(1):5–20. https://doi.org/10.1016/j.tifs.2012.08.004
Zhang L, Wang M (2017) Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int J Biol Macromol 95:675–681. https://doi.org/10.1016/j.ijbiomac.2016.11.096
Zhang L, Liu P, Wang Y, Gao W (2011) Study on physico-chemical properties of dialdehyde yam starch with diferente aldehy de group contentes. Thermochim Acta 512:196–201. https://doi.org/10.1016/j.tca.2010.10.006
Zhang XU, Zhang Y, Liao J, Yu T, Hu R, Wu Z, Wu Q (2015) Preparation and properties of compatible starch-polycaprolactone composites: effects of molecular weight of soft segments in polyurethane compatilizer. J Appl Polym Sci 132(32):1–10. https://doi.org/10.1002/app.42381
Zhang Y, Yu H-Y, Chao L-P, Qu L, Ruan J-Y, Liu Y-X et al (2016) Anti-inflammatory steroids from the rhizomes of Dioscorea septemloba Thunb. Steroids 112:95–102. https://doi.org/10.1016/j.steroids.2016.05.007
Acknowledgments
We would like to thank Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES) for the financial support and research grants provided.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, G.M.d., Veloso, C.M., Santos, L.S. et al. Extraction and characterization of native starch obtained from the inhambu tuber. J Food Sci Technol 57, 1830–1839 (2020). https://doi.org/10.1007/s13197-019-04216-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04216-4