Abstract
Mineral content, total phenolic compounds (TPC), and antioxidant capacity were determined in three samples of purple-açaí (coarse-PAC, medium-PAM, and fine-PAF), and one of white-açaí (coarse-WAC) and their respective bioaccessible fractions. TPC content differed in all samples, with PAC (583.79 mgAGE/100 g) having the highest content; however, PAM showed higher bioaccessibility (32.27%). PAC presented higher antioxidant capacity in the FRAP tests (74.34 μM FeSO4/g) and ABTS (55.05 μM Trolox/g). However, no differences were found in DPPH between PAC (1986.66 EC50) and PAM (2408.88 EC50) samples. Antioxidant capacity was decreased in all samples after digestion. Potassium was in the highest proportion (7121.90 mg/100 g-PAC), followed by Ca (349.92 mg/100 g-PAM), and Mg (169.41 mg/100 g-PAM), in all the samples. However, Ca presented the highest bioaccessible fraction, followed by Mg and Mn, with the highest percentages observed in WAC samples (90.30, 74.30, and 64.52%, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The search for a food, adundant in nature and minimally processed, led to an increase in the consumption of fruits, mainly tropical, due to their nutritional values, contents of secondary compounds of phenolic nature, and pleasant sensorial characteristics. Among the many tropical fruit trees, the açaízeiro (Euterpe oleracea Mart.), an atypical palm, native to the Brazilian Amazon, is found mainly in lowland and flooded forest lands of the Amazon River estuary (Neri-Numa et al. 2018).
The açaízeiro belongs to the family Arecaceae, and presents clusters of globular drupe or lightly fast, rounded, and fibrous with 1–2 cm in diameter and weighing between 0.8 and 2.3 g. The epicarp is characterized by a hard and thin layer that can, when ripe, present a green (white-açaí) or purple (purple-açaí) colour, according to the botanical variety of the species. In contrast, the mesocarp has a thickness of 1–2 mm, is pulpy and is of variable colour, which may originate purplish pulps when originating from the purple-açaí or cream/greenish when coming from the white-açaí; the endocarp corresponds to 85–95% of the fruit, exhibiting a hard, brownish yellow seed (Dall’Acqua et al. 2015; Neri-Numa et al. 2018).
The fruits of Eutepe oleracea, when ripe, are used commercially for the production of the juice of the açai tree, popularly known as “açaí” or “açaí drink”. In Brazil, the production of açaí pulps is regulated by the Ministry of Agriculture, Livestock and Supply, which defines as açaí the product obtained from the extraction with water of the edible part of the mature fruit of the E. oleracea and Euterpe precatoria vegetable species (Brasil 2018); and classify them into: coarse açaí pulp which is the pulp that presents total solids percentages above 14%; medium açaí pulp that presents contents between 11 and 14% and fine açaí pulp with percentages between 8 and 11% of total solids (Brasil 2000).
Microscopically, E. oleracea pulps can be identified by the existence of stony cells dispersed below the epicarp and aggregated cells in the mesocarp (Atui et al. 2012). Thus, macroscopically and/or microscopically, the absence of foreign matter in açaí pulps is recommended by the National Sanitary Surveillance Agency (Brasil 2014).
In addition to socioeconomic factors, purple-açaí is a product of high nutritional benefit, due to the high energy value that is directly related to its high lipid and protein contents (Schauss et al. 2006). In the present study, it was found that the minerals such as potassium, calcium, and magnesium (Carvalho et al. 2017; Gordon et al. 2012); and phenolic compounds such as anthocyanins, flavonoids and phenolic acids (Dantas et al. 2019) are present in this fruit. Due to the large presence of bioactive compounds, studies suggest that purple-açaí may exert protective effects, due to its antioxidant activity (Garzón et al. 2017). In contrast, studies involving the composition of white-açaí are scarce.
However, many of these studies that involve the composition of foods have neglected physicochemical changes that occur during the digestive process in the body, making it important to assess the bioaccessibility of these metabolites, since many of these components may be unavailable for absorption (Celep et al. 2017). According to literature, bioaccessibility can be defined as the fraction of the food matrix released in the gastrointestinal tract that is available for absorption (Alminger et al. 2014).
The evaluation of these parameters can be performed by means of in vivo or in vitro studies. As in vivo studies, usually performed in humans and animals are time consuming, expensive, and require evaluation for ethics; in vitro studies, which simulate the physiological conditions of the human digestive tract have been developed, to investigate the effects of digestion on the nutrients and bioactive compounds. Such studies make it possible to examine the release of substances from the food matrix, to study bioaccessibility, and to assess changes in profiles of substances before absorption (Minekus et al. 2014).
Thus, the objective of this study was to determine the bioaccessibility of minerals, total phenolic compounds and antioxidant capacity of commercial pulps of purple and white açaí. Considering that few studies refer to the different types of açaí commercial pulps, especially with regard to white açaí pulp, this is the first study, at least to our knowledge, which evaluates the bioaccessibility of bioactive compounds and minerals in these matrices.
Materials and methods
Chemicals and reagents
Folin-Ciocalteu phenol, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and bovine bile were purchased from Sigma-Aldrich. Ferric chloride hexahydrate, sodium bicarbonate was obtained from Merck; gallic acid from Neon Comercial and the ferrous sulphate heptahydrate from Química Moderna. Pancreatin from MP Biomedicals, pepsin from Dinâmica. Standard solutions of minerals; sodium, potassium, calcium, magnesium, copper and manganese were purchased from Inorganic Ventures.
Samples
Four samples of açaí pulp, three of purple-açaí, marketed as coarse açaí-PAC, medium açaí-PAM and fine açaí-PAF; and a sample of white-açaí, marketed as coarse açaí-WAC were acquired from the Central Market of Belo Horizonte, Minas Gerais, Brazil (SisGen registration number-A0287A7). These samples were stored at − 27 °C until analysis. All samples were from the same brand and from the same supplier; according to information presented on the label, all pulps were produced in the state of Pará-Brazil.
However, it should be noted that the samples differ by the total solids content (coarse, medium and fine), by the botanical varieties (purple/white) and by the production lot. According to information obtained from the manufacturer, pulp production follows an annual industry calendar, in which pulps classified as coarse (PAC-August and WAC-September), followed by the medium pulps (PAM-October) and fine (PAF-December).
Histological analysis and research of foreign matters
To verify the conformity of the açaí pulps with the parameters of identity and quality required by the Brazilian legislation (Brasil 2000, 2018), the histological identification of the pulps is described in the standard plant anatomy techniques (Kraus and Arduin 1997).
We used these techniques for this study. Initially, 2 g of the samples were added to 10% cold sodium hypochlorite individually, to lighten the material, for later filtration using a qualitative filter paper. After preparation of the samples, the slides were assembled and observed under an optical microscope (Olympus-CX41), coupled with a digital camera (Olympus-QColor5), with polarized light and under 200× magnification.
From the identification of the characteristic histological elements of the açaí, a comparison was made with the reference material corresponding to the macerated açaí fruit, through the image bank of the Service of Microscopy of Products-SMCP belonging to the Laboratory of Food Microscopy of the Fundação Ezequiel Dias-Funed, from the SMCP laminaria-Ali113.
For analysis of foreign material in the açaí pulps, a trap bottle flotation method is described by Atui et al. (2012). Using this method, the obtained material was evaluated under a stereoscopic microscope (ZeissStemi2000) with 10× magnification, the foreign matter was observed under an optical microscope with an increase from 40× to 57× magnification, for taking photographs.
In vitro gastrointestinal digestion (IVGD) procedure
To perform IVGD, the protocol developed by Minekus et al. (2014), a method that simulates the oral, gastric and small intestine phases during digestion was used. Because the samples of açaí pulps were in a liquid state and not have a high content of starch, they were not exposed to the in vitro oral phase of digestion, that is, the process of digestion was initiated directly in the gastric phase. All steps were performed in triplicate.
Gastric phase
First, the enzymatic pepsin solution (2000 U/mL) was prepared by dissolving 0.45 g of pepsin in 60 mL of simulated gastric fluid (pH3.0), prepared by adding 0.514 g KCl; 0.122 g KH2PO4; 2.10 g NaHCO3; 2.761 g NaCl; 0.020 g MgCl2(H2O)6; 0.075 g of CH3NO3 and 0.022 g of CaCl2(H2O)2 in 1,000 mL of distilled water.
To simulate the gastric phase, 4 g of each sample were weighed and added to 50 mL falcon tubes and 5 ml of the pepsin enzyme solution was added, adjusting pH to 3.0 ± 0.1; the samples were then incubated for 2 h at 37 ± 1.0 °C under agitation on an orbital shaker (Kasvi-K40-3020) at 20,9625×g in a greenhouse (Nova Ética-403/3 N), adjusting pH again after 1 h of incubation.
Simulation of phase of the small intestine
The enzymatic solution of bile (100 U/mL) was prepared by dissolving 1.93 g of bovine bile in 60 mL of simulated intestinal fluid (pH7.0), prepared by the addition of: 0.507 g KCl; 0.109 g KH2PO4; 7.14 g NaHCO3; 2.246 g NaCl; 0.067 g MgCl2(H2O)6; and 0.088 g of CaCl2(H2O)2, in 1000 mL of distilled water. Additionally, the enzymatic solution of pancreatin (3.6 mmol/mL) was prepared by dissolving 6.81 g of pancreatin in 60 mL of simulated intestinal fluid.
To simulate the small intestine phase, 5 mL of bile enzyme solution was added to the samples, followed by the addition of 5 mL of the pancreatin enzyme solution. At this stage, pH was adjusted to 7.0 ± 0.1 and the samples were incubated for further 2 h under the same conditions as in the previous step, by adjusting pH again to 7.0 ± 0.1 after 1 h of incubation.
To terminate the IVGD process the falcon tubes were centrifuged for 30 min at 5000×g (Centrilab-cod8011154V); the supernatants were collected and stored at − 27 °C until further analysis.
Bioaccessibility
The percentage of bioavailable fraction (%BF) was defined as the proportion of the compound released in the IVGD process compared to the compound content in the sample, according to the formula below (Leufroy et al. 2012).
Extraction of antioxidants
To determine the total phenolic compounds (TPC) content and antioxidant capacity, samples of açaí pulps, and the bioaccessible fractions of all samples obtained after IVGD, were weighed in centrifuge tubes and extracted with 4 mL methanol:water (50:50v/v) for 1 h at room temperature. After a resting period, the tubes were centrifuged for 15 min (Fanem-204RN) at 25,407×g and the supernatant was recovered in a 10 mL volumetric flask. From the residues from the first extraction, 4 mL of acetone:water (70:30v/v) were added to the tubes, homogenized and allowed to stand for 1 h at room temperature. Subsequently, the tubes were centrifuged again for 15 min at 25.407×g, the supernatants were collected, added to the methanolic extract and the volume of the flasks were diluted with distilled water (Rufino et al. 2010).
Total phenolic compounds (TPC)
The determination of TPC was performed according to the methodology described by Singleton et al. (1998). A volume of 1000μL of the extracts diluted in 70% acetone were added to 5 mL of the 10% Folin-Ciocalteu solution, maintained for 1–8 min, followed by the addition of 4 mL of 7.5% sodium carbonate solution and incubation for 2 h at room temperature in the absence of light. After the incubation period, readings were obtained at 760 nm in a UV–visible absorption spectrophotometer (Micronal-AJX1900) using 70% acetone as blank. The whole analysis was performed in triplicates, and the results were expressed in gallic acid equivalent [mgGAE/100 g-F.W. (fresh weight)] calculated from a standard curve of gallic acid.
Determination of total antioxidant activity
All analyses of antioxidant capacity were carried out in triplicate and protected from light, according to the methodologies described by Rufino et al. (2010).
Iron reduction method (FRAP)
Initially, the FRAP reagent solution was prepared by combining 0.30 M sodium acetate buffer (pH 3.6), 10 nM TPTZ solution and 20 nM aqueous ferric chloride solution in the ratio of 10:1:1 respectively. Subsequently, three dilutions were prepared at different concentrations of the extracts. A 90 μL aliquot of each dilution was added to 270 μL of distilled water and 2.70 mL of FRAP reagent, homogenized and incubated for 30 min at 37 °C in a Maria (Fanem-M100) bath.
After the incubation period the reading was carried out at 595 nm in a UV–visible absorption spectrophotometer using only the FRAP reagent as blank. The results were expressed in μM FeSO4/g-F.W., calculated from a standard ferrous sulphate curve.
Free radical capture method ABTS·+
Initially, the ABTS·+ radical was prepared by from the reaction of 5 mL stock solution of 7 nM ABTS with 88μL of 140 nM potassium persulfate solution in dark and at room temperature for 16 h. Subsequently the radical ABTS·+ was diluted in ethyl alcohol until it reached an absorbance of 0.70 nm ± 0.05 nm at 734 nm. Later, three dilutions were prepared at different concentrations of the extracts. Thirty μL of each dilution was added to test tubes, together with 3 mL of the solution containing the ABTS·+ radical, homogenized and after 6 min readings were measured in a UV–visible absorption spectrophotometer at 734 nm, using ethyl alcohol as blank. The results were expressed in μM Trolox/g-F.W., calculated from the standard Trolox curve.
Free radical capture method DPPH
Three dilutions were initially prepared at different concentrations of extracts; subsequently, a 0.10 mL aliquot of each dilution was added with 3.90 mL of the 0.06 mM DPPH solution and homogenized. The UV–visible absorption spectrophotometer readings were then measured every minute, at 515 nm, using methyl alcohol as blank; observing the reduction of the absorbance until its stabilization. The antioxidant capacity was evaluated as the antioxidant concentration required to reduce the initial amount of free radicals by 50% (EC50), with the final result expressed in g/gDPPH-F.W.
Analysis of minerals by ICP-OES
Acid digestion
To determine the content of the minerals, such as Na, K, Mg, Cu, and Mn in the açaí pulp samples and in the respective bioaccessible fractions, acid digestion of the samples was first performed according to the methodology described by the Instituto Adolfo Lutz (2008). One grams of each sample was weighed and oven-dried at 105 °C (Marconi-MA035) to reduce the moisture content. Subsequently, the samples were carbonised and placed in at Muffle chamber at 550 °C (Vulcan-3-1750), where they remained for 8 h. Later, the samples were removed from the flask, cooled, added to 1 mL of 37% nitric acid and placed in the flask again. This procedure was repeated until the samples were completely mineralized. The ashes were then dissolved in 1 mL of hydrochloric acid and quantitatively transferred with MilliQ water (Millipore Water-Rivers Purifier and gradient) to a 25 mL volumetric flask. The whole experiment was performed in triplicate using a blank sample.
The same procedure described above was performed in duplicate with a reference sample with known content of the following minerals: Na (32.80 mg/L); K (28.20 mg/L); Ca (34.10 mg/L); Cu (24.10 mg/L); Mn (23.50 mg/L). The reference sample is normally used as a re-test sample at the Funed Metallic Contaminants Laboratory to evaluate the reproducibility of the mineral determination method.
Determination of mineral content
The mineral content in the açaí samples and in the respective bioaccessible fractions was estimated using the inductively coupled argon plasma optical emission spectrometer (ICP-OES) (Perkin Elmer, Optima2000DV Sampler-As90plus). The ICP-OES parameters used were as follows: radio frequency power of 1,330watts; plasma argon flux rate of 15 L/min; auxiliary argon flow rate of 0.20 L/min; nebulizing gas flow rate of 0.60 L/min; orientation of the plasma relative to the axial optical path; sample flow rate 1.50 mL/min. Replica 1 and monitored spectral lines at: 589,592 nm (Na); 766.490 nm (K); 422.673 nm (Ca); 280.271 nm (Mg); 327.393 nm (Cu), and 257.610 nm (Mn).
The determination of the mineral content by ICP-OES was performed using a calibration curve prepared in the following concentrations: Na (5–50 mg/L); K, Ca and Mg (0.50–5 mg/L); Cu and Mn (0.05–0.50 mg/L). The limits of detection and quantification were: Na (5 mg/L); K, Ca and Mg (0.50 mg/L); Cu and Mn (0.05 mg/L).
The results were presented in mg/100 g of samples expressed as fresh weight (F.W.) and dry weight (D.W.). For conversion of the D.W results, the samples were submitted for moisture analysis at 105 °C (Instituto Adolfo Lutz 2008).
Statistical analysis
All results regarding TCF content, antioxidant capacity, and minerals content are presented as mean ± SD for the four samples, and in the respective bioaccessible fractions. The results were subjected to analysis of variance (ANOVA) and Tukey test to 5% of significance through the statistical program Sisvar, version 5.6.
Results and discussion
Histological analysis and research of foreign matters
Fragments of açaí fruit pulp formed by the thick-walled cell structures and stone cells can be observed in all the samples (Fig. 1). The stone cells were checked under polarized light.
The results found in the microscopic evaluation of the açaí pulps are in agreement with the studies described in literature (Paula 1975; Atui et al. 2012) as well as with the reference material used in this research.
Therefore, the four açaí pulps have shown compliance with the current legislation, which establishes the identity and minimum quality characteristics of vegetables, fruit products and edible mushrooms (Brasil 2005); and also with Normative Instruction No. 37 of October 01, 2018, which establishes identity and quality standards for pulps (Brasil 2018).
As for the presence of foreign matter in the pulps, only one PAM sample, with an insect fragment and a unidentified fragment; and one WAC sample, with four insect fragments (Fig. 2) presented nonconformity with the current legislation; which recommends the absence of soils as fragments of insects and rodent hair in 100 g of sample (Brasil 2014). The presence of insect fragments specific to the açaí crop and the storage site considered as foreign matter, although indicative of failures in good manufacturing practices, is without risk to human health.
Total phenolic compounds before and after in vitro digestion
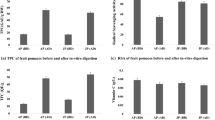
The content of TPC of the açaí pulps and the respective bioaccessible fractions, as well as the bioaccessible percentage of the samples are presented in Table 1. Examining the data presented, it is verified that all the samples differed with respect to the TPC content before IVGD, the PAC sample (583.79 mgGAE/100 g-F.W.) being the one with the highest content of TPC and the WAC sample (180.55 mgGAE/100 g-F.W.) with the lowest content. Results similar to the one found for the PAM sample (400.33 mgGAE/100 g-F.W.) were reported by Rufino et al. (2010), when evaluating purple-açaí pulp (454 mgGAE/100 g-F.W.).
As for the TPC content of the white-açaí pulp-WAC, a lower result than the present study was presented by Silveira et al. (2017) who, when evaluating a sample of lyophilized white-açaí juice, obtained an average of 11.20 mgGAE/100 g. However, the inferiority of the WAC sample in relation to the other purple-açaí samples (PAC, PAM and PAF) is emphasized. This fact may be related to the absence of anthocyanin compounds in white-açaí (Silveira et al. 2017), since these pigments are one of the main phenolic compounds present in purple-açaí (Carvalho et al. 2017; Gordon et al. 2012). Nevertheless, the observed differences between the samples may also be related to the processing of the pulps, since the samples are commercially distinct as to the total solids content.
After digestion, only the PAC (133.65 mgGAE/100 g-F.W.) and PAM (129.12 mgGAE/100 g-F.W.) samples did not differ statistically. However, it is possible that the TPC content reduced in all the samples after being processed by IVGD. This reduction can be justified by the chemodiversity of the phenolic compounds, since during the digestion process, these compounds are constantly exposed to different physical as well chemical and biochemical conditions, which may promote structural and chemical changes, resulting in variations in biological activity and consequently affecting the bioaccessibility and bioactivity of the compounds (Celep et al. 2015).
The bioaccessibility of TPC ranged from 22.89 to 32.27%. The pulp with the highest bioaccessible fraction was PAM with 32.27%, differing statistically from the others (Table 1). Schulz et al. (2017) also verified that in Euterpe edulis of 79.98 mg of phenolics/100 g−1 of dry matter to was reduced to 19.71 mg of phenolics/100 g−1 of dry matter using LC–ESI–MS/MS before and after IVGD. Following the same reasoning used in this work to calculate the %BF, only 24.64% of the phenolics quantified by Schulz et al. were available after the digestion process, a result consistent with that found in our research, confirming that gastrointestinal conditions directly affect sample composition.
On the other hand, Chen et al. (2014) observed an increase in the TPC content of 25 fruits, by performing literature search for 33 fruits before and after IVGD. The fruits that showed the highest increase in TPC content were pear (Pyrus serrulata), increasing from 15.20 to 127.23 mgGAE/100 g after IVGD, followed by melon (Cucumis melo) from 56.27 for 106.64 mgGAE/100 g, and apple (Malus pumila) from 56.89 to 106.64 mgGAE/100 g.
These observed differences among the studies may be the result of variations of methods used to simulate IVGD and quantifications of the content of phenolic compounds in different food matrices. However, it is believed that such differences may also be related to the physicochemical and biological properties of each food studied, since it is probable that a certain proportion of phenolic compounds will have different structures with different chemical characteristics during the digestive process. These properties, therefore, would increase the amount of these compounds of certain matrices in some cases, while reducing them in others (Celep et al. 2017).
Antioxidant activity before and after in vitro gastrointestinal digestion
Table 2 shows the antioxidant capacity of pulps and their respective bioaccessible fractions, according to the FRAP, ABTS and DPPH methods. Observed that the PAC sample presented higher antioxidant capacity in the FRAP and ABTS tests. Only in the DPPH test, its antioxidant capacity did not differ from the PAM sample, either before or after IVGD. The WAC sample presented lower antioxidant capacity in the FRAP and DPPH tests and did not differ from the PAF sample both before and after the IVGD in the ABTS test.
All the samples had different antioxidant capacities regarding the FRAP test before digestion, but after the IVGD the PAC (14.48 μM FeSO4/g-F.W.) and PAM (12.00 μM FeSO4/g-F.W.) did not differ, which was also observed for the PAF samples (7.63 μM FeSO4/g-F.W.) and WAC (6.14 μM FeSO4/g-F.W.).
In the ABTS test, the PAF and WAC samples did not differ statistically either before or after IVGD, however the PAC sample after digestion (11.94 μM Trolox/g-F.W.) did not differ from PAF samples (11.49 μM Trolox/g-F.W.) and WAC (13.69 μM Trolox/g-F.W.) prior to IVGD. Similarly, the PAM after digestion (9.14 μM Trolox/g-F.W.) did not differ from the PAC sample and the PAF before IVGD.
Rufino et al. (2010), evaluated the antioxidant capacity of a sample of purple-açaí by the FRAP method, and obtained a lower value (32.10 μM FeSO4/g-F.W.) than in our study for the PAC sample (73.34 μM FeSO4/g-F.W.) and PAM (45.80 μM FeSO4/g-F.W.) but higher for PAF pulps (24.69 μM FeSO4/g-F.W.) and WAC (19.74 μM FeSO4/g-F.W.).
Silva et al. (2017), when evaluating the antioxidant capacity by the ABTS method, obtained a mean of 17.15 μM Trolox/g-F.W., lower than the values obtained in this study for the PAC samples (55.05 μM Trolox/g-F.W.) and PAM (48.68 μM Trolox/g-F.W.) prior to IVGD. Despite this, the results of Silva et al. were similar to those that we obtained for PAF (11.49 μM Trolox/g-F.W.) and WAC (13.69 μM Trolox/g-F.W.) samples. However, the antioxidant capacity by the ABTS method presented by the pulps prior to IVGD in this study was superior to that found by Garzón et al. (2017) which, when evaluating the purple-açaí pulp produced in Colombia, obtained a mean of 3.10 μM Trolox/100 g-F.W.
Rufino et al. (2010) also evaluated processed purple-açaí by the DPPH method, and obtained a lower result (4,265 g/gDPPH-F.W.) than the present study when comparing PAC (1986.66 g/gDPPH-F.W.), PAM (2,408.88 g/gDPPH-F.W.) and PAF (3167.14 g/gDPPH-F.W.), but obtained a very similar result to that for WAC sample (4673.15 g/gDPPH-F.W.). With respect to the DPPH test, it is emphasized that the lower the EC50 average presented by a sample, the greater the antioxidant stability of the sample.
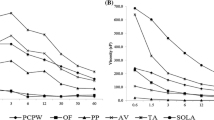
After IVGD, a decrease in the antioxidant capacity of all pulps and in all the tests was observed. Regarding the FRAP method, the highest reduction was found in the PAC sample, followed by PAM, PAF and WAC. In the ABTS method, the highest reduction in antioxidant capacity was observed in the PAM sample, followed by the PAC, PAF and WAC. As for the DPPH method, the PAM sample presented the lowest percentage of increase in the value of the EC50, followed by the PAC, PAF, and WAC samples as shown in Fig. 3.
Celep et al. (2015) found a reduction of 47% in antioxidant capacity of the digested sample of blackberry wine compared to the sample digested using by the DPPH method. Schulz et al. (2017), evaluating the antioxidant capacity before and after the IVGD of E. edulis at different stages of maturation, observed a decrease of 64–78% in DPPH elimination capacity and 55–67% in antioxidant capacity by the FRAP method. Dantas et al. (2019) observed a reduction of 15.72% in the FRAP test and an increase in the value of EC50 of 90,166.66% when evaluating a pulp of purple-açaí, higher values than those obtained in this study for FRAP and lower than that for DPPH.
In addition to the reduction in the content of TPC, factors such as changes in pH and interferences of other dietary constituents released during the digestion process, such as iron, minerals, fibres or dietary proteins can promote changes in the antioxidant capacity of food. In addition, neutral conditions and the presence of oxygen in most models of IVGD can also promote non-enzymatic degradation of some phenolic compounds; and consequently affect their bioaccessibility, leading to a reduction of antioxidant capacity (Alminger et al. 2014).
Mineral content before and after digestion gastrointestinal in vitro
The mineral content of açaí pulp samples, as well as the bioaccessible fraction of minerals, except for Na and K, because they are present in large concentrations in the fluids used to simulate IVGD, are presented in Table 3.
Of the six minerals evaluated in this study, K is the mineral present in the highest proportion, followed by Ca and Mg in all samples. However, the K content in the PAC sample differs from PAF and WAC, whereas the PAM sample shows no significant difference compared to WAC. Regarding the Ca content, only the PAC and PAF samples did not differ, whereas all samples differed in the Mg content.
In addition to our findings, Menezes et al. (2008) evaluated 25 minerals in lyophilized purple-açaí pulp. The highest mineral content was K (900 mg/100 g), followed by Ca (330 mg/100 g), and Mg (124.40 mg/100 g). The results were similar to those reported by Bichara and Rogez (2011), wherein the levels of K (119 mg/100 g), Ca (37 mg/100 g) and Mg (21.50 mg/100 g) were found in purple-açaí juice. However, both results are lower than those demonstrated in our study.
As observed in Table 3, it is possible to verify that the Na content present in the PAC sample is not statistically different from the PAM, as the PAF sample is statistically similar to the WAC; the same is observed for the Mn content between the PAF and WAC samples. However, the Cu content did not present statistical difference between the samples.
The results were lower than that of K (108 mg/100 mL), Ca (23 mg/100 mL), Mg (8 mg/100 mL), Cu (0.01 mg/100 mL) and Mn (0.40 mg/100 mL) than those reported by Llorent-Martínez et al. (2013) when evaluating four purple-açaí juices from different brands marketed in Spain. When comparing the results of this research with those reported in the Brazilian Table of Food Composition (TACO 2011), it is possible to verify that the levels of Na (5 mg/100 g-F.W.), K (124 mg/100 g-F.W.), Ca (35 mg/100 g-F.W.) and Mg (17 mg/100 g-F.W.) obtained in these studies are superior to those described, whereas Cu content (0.18 mg/100 g-F.W.) were lower (Table 3).
To date, only one stud (Smith et al. 2012) referring to the mineral content of different purple-açaí pulps when evaluating the content of K, Ca, Mg and P in lyophilized thick, medium and fine açaí pulps, obtained results lower than those obtained in our study. The contents of K ranged from 277 to 271 mg/100 g; those of Ca around 195 mg/100 g; with respect to Mg, the contents varied between 1321 to 1,197 mg/100 g and the P between 162 to 153 mg/100 g.
To date, there are still no studies in literature evaluating the content of minerals as well as the bioaccessibility of the white-açaí pulp. The data obtained in our research, with those reported in the literature can also be related to the sampling of the analysed pulps. Menezes et al. (2008), for example, used only a sample of açaí-purple pulp found in the local market of Belém-Brazil. Another important point is that, with the increase in the demand for açai, it has started being cultivated instead of exclusively extracted in Brazil. As this system is based on good agricultural practices related to the management, regarding fertilization, irrigation, and use of genetically improved plants with high productivity; the fruits of açaí palms can present higher nutritional value and, consequently, so can their derivatives (Nogueira and Santana, 2016).
The findings of the present study reinforce that the evaluation of the bioaccessibility of minerals can help to predict the possible nutritional effects related to the consumption of açaí pulps. However, the number of studies on the bioaccessibility assessment of açaí pulp has been limited to some minerals such as Cu; for example. Ruzik and Wojcieszek (2016) evaluated the bioaccessible fraction in purple-açaí and reported that 100% of Cu present is bioaccessible, concluding that açaí can be treated as a natural source of copper, a result superior to that of our study.
Among the minerals evaluated for bioaccessibility it is possible to verify in Table 3 that Ca is the mineral with the highest bioaccessible fraction, followed by Mg and Mn. The highest percentages of these were observed in the WAC sample (90.30; 74.30; 64.52%) and the lowest percentage in the PAM sample (14.66; 25.42; 14.47%).
Schulz et al. (2017), when evaluating E. edulis pulp, obtained higher values than those obtained in this study regarding %BF of Ca (65.50%) and Mn (35.10%) with respect to PAC and PAM, and lower for the PAF and WAC samples. Regarding Mg, they presented lower BF% (44.90%) than that obtained in this study for the PAC, PAF and WAC samples.
A value similar to that obtained for the WAC sample as %BF of Ca was presented by Biluca et al. (2017) when evaluating thirteen samples of honey bee multiflorosa (Meliponinae), when they obtained a mean of 90.10%. Pereira et al. (2018) when evaluating the bioaccessibility of minerals in blackberry. In raspberry, blueberry and strawberry; %BF was 36, 37, 10, and 52% respectively for Mn, results close to those obtained in this study.
Although the our study presents new data on the effect of IVGD on the total phenolic compounds, antioxidant capacity and mineral content of the different commercial açaí pulps; the work has some limitations. Analysis of the phenolic profile, as well as a study on the effect of IVGD on phenols, would complement the data. Another point would be a more comprehensive analysis of other minerals, such as the heavy metals class, since the minerals analysed in this study demonstrated high values.
Conclusion
According to the histological analyses, it can be concluded that the samples used in the present study are actually açaí. Microscopic analysis of extraneous matter alerts us to the need for more effective monitoring of good manufacturing practices in the açaí production chain, since açaí pulps are increasingly consumed. As for the phenolic compounds content and the antioxidant capacity of the different açaí pulps, the results of this study demonstrate that they were statistically lower after IVGD. In addition, the results indicate that the PAC and PAM samples are relatively superior to the PAF and WAC sample regarding the phenolic compounds content and antioxidant capacity. Among the evaluated minerals, the content of K, Ca and Mg in all the samples were increased. As for bioaccessibility, Ca was the mineral that presented the highest bioaccessible fraction. To date, according to literature, this is the first study to evaluate the effects of IVGD on total phenolic compounds, antioxidant capacity, and mineral content in commercial açaí pulps; especially, in relation to the pulp of white-açaí, thus elucidating that despite having different colours, the compounds evaluated did not differ significantly from each other. Future studies, especially using clinical trials, will be relevant to assess the bioaccessibility and biological activity of phenolic compounds and minerals. Therefore, although in vitro static models are much simpler and do not reproduce all the dynamic aspects of the gastrointestinal tract, they are increasingly useful in estimate in vivo digestion. Thus, they offer food and health professionals with robust, ethics-free and relatively high tools for investigation the digestibility of food and understanding the effect of acting conditions on the digestive fate of certain food constituents.
References
Alminger M, Aura AM, Bohn T, Dufour C, El SN, Gomes A, Karakaya S, Martínez-Cuesta MC, Mcdougall GJ, Requena T, Santos CN (2014) In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr Rev Food Sci Food Saf 13(4):413–436. https://doi.org/10.1111/1541-4337.12081
Atui MB, Nogueira MD, Silva AM, Marciano MAM, Fioravanti MIA, Chansin LB, Franco VPA, Silva LA, Oliveira MML, Cardoso-Gustason P, Hayashi AH (2012) Manual de análise microscópica em polpas de frutas: açai (Euterpe oleracea), goiaba (Psidium guajava), manga (Mangifera sp), morango (Fragaria vesca), tomate (lycopersicum sp). Instituto Adolfo Lutz, São Paulo
Bichara CMG, Rogez H (2011) Açai (Euterpe oleracea Martius). In: Yahia EM (ed) Postharvest biology and technology of tropical and subtropical fruits. Woodhead Publishing Limitedk, Sawston. https://doi.org/10.1533/9780857092762.1
Biluca FC, Gois JS, Schulz M, Braghini F, Gonzaga LV, Maltez HF, Rodrigues E, Vitali L, Micke GA, Borges DLG, Costa ACO, Fett R (2017) Phenolic compounds, antioxidant capacity and bioaccessibility of minerals of stingless bee honey (Meliponinae). J Food Compos Anal 63:89–97. https://doi.org/10.1016/j.jfca.2017.07.039
Brasil (2000). Intrução Normativa No01, de 7 de Janeiro de 2000-Ministério da Agricultura Pecuária e Abasteceimento. Diário Oficial da União, 54
Brasil (2005) Resolução RDC No272, de 22 de setembro de 2005-Agência Nacional de Vigilância Sanitária. Diário Oficial da União, 1–6
Brasil (2014) Resolução N°14, de 28 de março de 2014-Agência Nacional de Vigilância Sanitária. Diário Oficial da União, 58
Brasil (2018) Intrução Normativa No37, de 01 de outubro de 2018-Ministério da Agricultura, Pecuária e Abastecimento. Diário Oficial da União, 23-28
Carvalho AV, Silveira TFF, Mattietto RA, Oliveira MSP, Godoy HT (2017) Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J Sci Food Agric 97(5):1467–1474. https://doi.org/10.1002/jsfa.7886
Celep E, Charehsaz M, Akyüz S, Acar ET, Yesilada E (2015) Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res Int 78:209–215. https://doi.org/10.1016/j.foodres.2015.10.009
Celep E, İnan Y, Akyüz S, Yesilada E (2017) The bioaccessible phenolic profile and antioxidant potential of Hypericum perfoliatum L. after simulated human digestion. Ind Crops Prod 109:717–723. https://doi.org/10.1016/j.indcrop.2017.09.032
Chen GL, Chen SG, Zhao YY, Luo CX, Li J, Gao YQ (2014) Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind Crops Prod 57:150–157. https://doi.org/10.1016/j.indcrop.2014.03.018
Dall’Acqua YG, Cunha Júnior LC, Nardini V, Lopes VG, Pessoa JDC, Almeida GHT (2015) Discrimination of Euterpe oleracea Mart. (Açaí) and Euterpe edulis Mart. (Juçara) intact fruit using near-infrared (NIR) spectroscopy and linear discriminant analysis. J Food Process Preserv 39(6):2856–2865. https://doi.org/10.1111/jfpp.12536
Dantas AM, Mafaldo IM, Oliveira PML, Lima MS, Magnani M, Borges GSC (2019) Bioaccessibility of phenolic compounds in native and exotic frozen pulps explored in Brazil using a digestion model coupled with a simulated intestinal barrier. Food Chem 274:202–214. https://doi.org/10.1016/j.foodchem.2018.08.099
Garzón GA, Narváez-Cuenca CE, Vincken JP, Gruppen H (2017) Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem 217:364–372. https://doi.org/10.1016/j.foodchem.2016.08.107
Gordon A, Cruz APG, Cabral LMC, Freitas SC, Taxi CMAD, Donangelo CM, Mattietto RA, Friedrich M, Matta VM, Marx F (2012) Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem 133(2):256–263. https://doi.org/10.1016/j.foodchem.2011.11.150
Instituto Adolfo Lutz (2008) Métodos físico-químicos para análise de alimentos. Instituto Adolfo Lutz, São Paulo
Kraus EJ, Arduin M (1997) Manual básico de métodos em morfologia vegetal. UFRRJ, Rio de Janeiro
Leufroy A, Noël L, Beauchemin D, Guérin T (2012) Use of a continuous leaching method to assess the oral bioaccessibility of trace elements in seafood. Food Chem 135(2):623–633. https://doi.org/10.1016/j.foodchem.2012.03.119
Llorent-Martínez EJ, Córdova MLF, Ortega-Barrales P, Ruiz-Medina A (2013) Characterization and comparison of the chemical composition of exotic superfoods. Microchem J 110:444–451. https://doi.org/10.1016/j.microc.2013.05.016
Menezes EMS, Torres AT, Srur AUS (2008) Valor nutricional da polpa de açaí (Euterpe oleracea Mart) liofilizada. Acta Amaz 38(2):311–316. https://doi.org/10.1590/S0044-59672008000200014
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le SF, Lesmes U, Macierzanka A, Mackie A, Marze S, Mcclements DJ, Ménard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Brodkorb A (2014) A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct 5(6):1113–1124. https://doi.org/10.1039/c3fo60702j
Neri-Numa IA, Sancho RAS, Pereira APA, Pastore GM (2018) Small Brazilian wild fruits: nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res Int 103:345–360. https://doi.org/10.1016/j.foodres.2017.10.053
Nogueira AKM, Santana AC (2016) Socioeconomic benefits from the new technologies in the açaí cultivation in Pará State. Rev Ceres 63(1):1–7. https://doi.org/10.1590/0034-737X201663010001
Paula JE (1975) Anatomia de Euterpe oleracea Mart. (Palmae da Amazônia). Acta Amaz 5(3):265–278. https://doi.org/10.1590/1809-43921975053265
Pereira CC, Silva EM, Souza AO, Vieira MA, Ribeiro AS, Cadore S (2018) Evaluation of the bioaccessibility of minerals from blackberries, raspberries, blueberries and strawberries. J Food Compos Anal 68:73–78. https://doi.org/10.1016/j.jfca.2016.12.001
Rufino MSM, Alves RE, Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J (2010) Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem 121(4):996–1002. https://doi.org/10.1016/j.foodchem.2010.01.037
Ruzik L, Wojcieszek J (2016) In vitro digestion method for estimation of copper bioaccessibility in Açaí berry. Monatsh Chem 147(8):1429–1438. https://doi.org/10.1007/s00706-016-1798-3
Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, Kababick JP (2006) Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (Acai). J Agric Food Chem 54(22):8598–8603. https://doi.org/10.1021/jf060976g
Schulz M, Biluca FC, Gonzaga LV, Borges GSC, Vitali L, Micke GA, Gois JS, Almeida TS, Borges DLG, Miller PRM, Costa ACO, Fett R (2017) Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion. Food Chem 228:447–454. https://doi.org/10.1016/j.foodchem.2017.02.038
Silva AKN, Beckman JC, Rodrigues AMC, Silva LHM (2017) Composição nutricional e capacidade antioxidante da polpa de açaí (Euterpe oleracea M.). R Bras Tecnol Agroindustr 11(1):2205–2216. https://doi.org/10.3895/rbta.v11n1.2829
Silveira TFF, Souza TL, Carvalho AV, Ribeiro AB, Kuhnle GGC, Godoy HT (2017) White açaí juice (Euterpe oleracea): phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J Funct Foods 36:215–223. https://doi.org/10.1016/j.jff.2017.07.001
Singleton VL, Orthofer R, Lamuela-Raventós RM (1998) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Smith ER, Eaker J, Tran K, Smith C, Monroe DM, Menezes EMS, Sabaa-Srur AUA, Luo R, Wycoff W, Fales WH (2012) Proposed Benchmark Methods for Analyzing Acai (Euterpe oleraceae Mart.). Nat Prod J 2(2):76–85. https://doi.org/10.2174/2210315511202020076
TACO (2011) Tabela Brasileira de Composição de Alimentos. Campinas, NEPA-UNICAMP
Acknowledgements
The authors thank the Fundação Ezequiel Dias for the partnership granted; the Research Dean of the Universidade Federal de Minas Gerais and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais for providing scholarship to the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minighin, E.C., de Souza, K.F., Valenzuela, V.d.C.T. et al. Effect of in vitro gastrointestinal digestion on the mineral content, phenolic compounds, and antioxidant capacity of commercial pulps of purple and white açaí (Euterpe oleracea Mart.). J Food Sci Technol 57, 1740–1752 (2020). https://doi.org/10.1007/s13197-019-04207-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04207-5