Abstract
The aim of this study was to determine the proximate chemical composition, dietary fiber functional properties, viscosity, total phenolic content, antioxidant capacity and color of purple cactus pear waste (PCPW) in comparison with commercial fibers. PCPW had similar moisture, ash and functional properties as the other fibers, also low protein, fat and total dietary fiber content but showed higher fat absorption capacity (3.38 g g−1). All fibers had a non-Newtonian pseudoplastic behavior and had high total phenolic content except the commercial fiber of Plantagum psylium which formed a gel and had the lowest total phenolic content. PCPW had the highest antioxidant activity measured by ABTS with 1485.14 µmol TE 100 g−1 dry basis, high content of digestible carbohydrates (73%) and an intense red color in comparison to the commercial samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The industry of nutritional supplements in Mexico generates 892 million dollars thanks to consumers that expect a positive impact on their health [1]. Several forms of dietary fiber such as guar gum, plantago psyllium and others, have been used as supplements to improve gastrointestinal health, increase glucose tolerance and insulin response, lower the risk of certain cancers, and modify lipid digestion contributing to bodyweight control [2]. Nonetheless, an abusive consumption of these supplements could adversely affect the normal functioning of the gastrointestinal tract [3].
The industry of fruit juice produces significant amounts of by-products that may cause pollution and disposal problems. This waste could have high amounts of dietary fiber that can be used to develop new natural ingredients for the food [4] and nutritional supplements industry. According to Lousada-Júnior et al. [5] estimate that during fruit pulp production, 65–70% of the raw material becomes by-products or waste that may represent a serious environmental problem.
In 2014, Mexico exported 10,000 tons of cactus pear, but during high production seasons up to 60% is lost due to difficult market access [6, 7]. Cactus pear fruit is occasionally consumed in Mexico (5.87 kg per capita) [8, 9] as compared to fruits such as banana (19.7 kg per capita) [10]. In México, cactus pear fruit is mainly consumed fresh, but also has the potential to be processed into other products. The edible part of the fruit comprises the fleshy pulp that has seeds which includes many dispersed seeds dispersed in a mucilaginous matter; during fruit processing (peeling, clarification, etc.) peel and seeds that may be recovered are discarded and considered waste [11]. These fruits and cladodes (Opuntia ficus indica) are an interesting source of bioactive compounds, minerals and phytochemicals, such as phenolics and carotenoids with high antioxidant activity, fiber and other pigments [12, 13]. Around 45% of the fresh weight of cactus pear fruit is attractive by-products for use as functional food ingredients. Such an application for the cactus pear could have economic and health benefits since these are by-products with legitimate food ingredients [12]. The aim of this study was to evaluate the chemical composition, dietary fiber functional properties, viscosity, phenolic content, antioxidant activity and color of purple cactus pear waste in comparison with commercial fibers.
Materials and Methods
Sample and Treatments
Purple cactus pear (Opuntia ficus indica) fruit was provided by a Mexican association CoMeNTuna (Consejo Mexicano del Nopal y la Tuna, Hidalgo, Mexico) in the spring of 2015. Only fruits without external injuries were selected, washed and manually peeled. Juice extraction was performed stirring the pulp with an industrial blender (38BL52 LBC10, Waring Commercial®, USA) and passing it through a conventional strainer to remove seeds. The purple cactus pear waste (PCPW) was obtained after clarifying the juice by centrifugation at 15,300×g (Allegra 25™, Beckman Coulter Inc., California, USA) during 25 min at 4 °C. The resulting pellet was frozen at − 32 °C and then dried in a lyophilizer (LABCONCO VW 26671-581, Kansas, USA). The commercial fibers analyzed were: Opuntia ficus (OF), Plantago psyllium (PP), Aloe vera (AV), Triticum aestivum (TA) and Salvia hispanica, Opuntia ficus, Linum usitatissium and Ananas sativus (SOLA). All samples were milled and sieved through 500 µm and stored at − 32 °C in a deep freeze until analysis.
Chemical Composition Analysis
The chemical composition of the PCPW and the commercial fibers was determined using the official AOAC methods [14]. Moisture was determined by drying samples at 105 °C until a constant weight was achieved. Protein content was calculated by multiplying nitrogen content obtained after distillation systems (Vapodest 50, Gerhardt®, Germany) by 6.25. Fat content was quantified gravimetrically after hexane extraction using a Soxhlet apparatus (Extraction System B-811, Buchi®, Switzerland). Digestible carbohydrates (CHOd) were calculated by difference of the proximate parameters and total dietary fiber as it is shown in the following equation:
Finally, ash was obtained by incinerating the sample in a muffle furnace (FD1535M Bornstead Thermolyne®, USA) at 525 °C for 5 h and then the content was determined gravimetrically. All results were reported in percentage.
Total Dietary Fiber (TDF)
Total dietary fiber was determined using an assay kit (Sigma TDF-100A, Missouri, USA) based on the official method of AOAC [14]. Samples were incubated with amylase at ~ 95 °C and then at 60 °C with protease and amyloglucosidase, followed by treatment with ethanol in order to precipitate soluble fiber and remove depolymerized protein and glucose from starch. The residue was filtered, washed with 78% ethanol, 95% ethanol, and acetone, dried and weighed. One duplicate was analyzed for protein and the other was incinerated at 525 °C to determine ash content. The TDF was calculated as the weight of the filtered and dried residue minus the weight of the protein and ash. TDF was determined in duplicate and results were expressed in g 100 g−1 db.
Functional Properties of Fiber
Water Retention Capacity (WRC)
A weighted dry sample (250 mg) was placed in a test tube and 10 mL of distilled water was added. The mixture was allowed to stand for 19 h at room temperature, and then centrifuged (Centrifuge Compact, VanGuard V6500, Hamilton Bell®, USA) at 1160×g for 30 min. The supernatant was decanted and measured to determine the amount of water that was not absorbed. The pellet was placed in aluminum trays and dried in an oven for 2 h at 105 °C. The WRC was expressed as the absorbed water per dry weight of pellet (g g−1) [15].
Swelling Capacity (SWC)
The volume occupied by 500 mg of dry sample in a 25 mL graduated cylinder was measured (V0) (mL) prior to the addition of 10 ml of distilled water. The mixture was stirred for 5 min manually and allowed to stand for 24 h at room temperature. Subsequently, the final volume of the sample was measured (V1) (mL) [16] and swelling capacity (mL g−1) was calculated by the following equation:
Emulsifying Capacity (EC)
The emulsifying capacity was assessed as described by Temelli [17]. Dry sample (0.5 g) was added to 5 mL of distilled water and incubated in a water bath at 35 °C for 20 min (Water bath IH6L, Lumistell®). Then 7.5 mL of sunflower oil was added and the mixture was stirred with a homogenizer (HG-15D, WiseTis®, Korea) at 11,500 rpm for 1 min. The samples were transferred to 15 mL centrifuge graduate tubes and centrifuged at 1160×g for 25 min at room temperature (Centrifuge Compact, Van Guard V6500, Hamilton Bell®, USA). Subsequently, the final volume of emulsion was measured. The emulsion capacity was expressed as the percentage of the difference between the initial volume and the final oil volume (%).
Fat Adsorption Capacity (FAC)
For this analysis, 0.5 g of sample was added to 10 mL of sunflower oil and stirred manually for 10 s. The content was allowed to stand 24 h, and then the tubes were centrifuged at 1160×g for 10 min (Centrifuge Compact, VanGuard V6500, Hamilton Bell®, USA). The free oil was decanted and the absorbed oil was then determined by difference. The FAC was expressed as g oil per gram of sample (g g−1) [18].
Glucose Adsorption Capacity (GAC)
This parameter was carried out following the method described by Ou et al. [19] and Chau et al. [20]. Briefly, 10 mL of glucose solution (concentrated to 2 g of glucose in 100 mL of water) were added to 250 mg of fiber and stirred. The mixture was held in a shaking water bath (LSB-015S, LabTech®, Korea) at 37 °C for 6 h and centrifuged at 4000×g for 20 min. Then, glucose content in the supernatant was determined by the DNS (3,5-dinitrosalicylic acid reagent) colorimetric method [21].
Viscosity
Sample solutions at 1 and 5% were prepared by slowly adding an appropriate amount of each fiber to distilled water (0.35 g to 34.65 mL−1 and 1.75 g to 33.25 mL−1 respectively) and mixing it for 1 min. The solutions were allowed to stand for 24 h to let entrapped air be released before performing viscosity measurements at room temperature. A calibrated Brookfield Viscometer (DV-III, Stoughton, Mass, USA) with a precision cylindrical spindle (LV#61) was used to measure the viscosity of the solutions at 0.3, 0.6, 1.5, 3, 6, 12, 30, 50 and 60 rpm and was expressed as cP (centipoise) [22].
Phenolic Content and Antioxidant Activity
Antioxidants Extraction
Antioxidants were extracted by placing 250 mg of sample in a centrifuge tube containing 10 mL of an acidic methanol/water solution (50:50, v v−1). The mixture was shaken at room temperature for 30 min at 116 g using a shaking incubator (LSI-3016A, LabTech®, Korea), and then centrifuged (Centrifuge Compact, VanGuard V6500 Hamilton Bell®, USA) at 1310×g for 10 min. The supernatant was recovered, and 10 mL of acetone/water (70:30, v v−1) were added to the residue, and shaking and centrifugation were repeated. Both, methanolic and acetonic supernatants were combined and adjusted to 25 mL with a 1:1 of methanol/water and acetone/water solution [23].
Analysis of Total Phenolic Content
The Folin–Ciocalteu method was used to determine total phenols as follows. An aliquot (100 µL) of the sample extract was mixed with 500 µL of 1:10 diluted Folin–Ciocalteu reagent (Sigma-Aldrich, Steinheim, Germany). Then, 400 µL (7.5%) of sodium carbonate were added and the mixture was incubated for 30 min at room temperature. The absorbance of the mixture was measured at 765 nm in a microplate reader (Power Wave XS UV-Biotek, software KC Junior, USA) using gallic acid as a reference standard. The results were expressed as milligrams of Gallic Acid Equivalent/100 g of dry basis (mg GAE 100 g−1 db) [24].
Antioxidant Activity
Antioxidant activity by ABTS∙+ and DPPH were measured. The radical cation ABTS∙+ was produced by reacting 7 mmol L−1 of ABTS∙ + stock solution with 2.45 mmol L−1 potassium persulfate in the dark at room temperature for 16 h before being used. The ABTS.+ solution was diluted with deionized water to an absorbance of 0.70 ± 0.10 at 754 nm. An aliquot of 20 µL of sample was added to 980 µL of the diluted ABTS∙+ solution, and absorbance readings were taken after 7 min of incubation at room temperature. The absorbance of the mixture was measured at 754 nm in the microplate reader (Power Wave XS UV-Biotek, software KC Junior, USA), and antioxidant capacity was expressed as mg Trolox equivalent antioxidant capacity per 100 g on a dry basis (µmol TE 100 g−1 db) [25].
Antiradical activity was measured using DPPH (1,1-diphenyl-2-picrylhydrazyl) [26]. An ethanolic solution (7.4 mg 100 mL−1) of the stable DPPH∙ radical was prepared and added (500 µL) to 100 µL of extract placed in vials. After the mixture was left to sit at room temperature for 1 h, the absorbance at 520 nm was measured in the microplate reader, and µmol TE 100 g−1 db was obtained.
Color
Color in dried PCPW and commercial fibers (OF, PP, AV, TA and SOLA) were measured using a Hunter Lab colorimeter (MiniScan XE™, Hunter associates Laboratory Inc., Reston, Virginia, USA) using the D65 illuminant with an angle of observation of 10°. Measures were performed in triplicate for all samples. Color was recorded using the CIE—L*a*b* values, where L* indicates lightness (L* = 0 or 100 indicate black and white respectively); a* the axis of chromaticity between green (−) to red (+), and b* the axis between blue (−) to yellow (+). Numerical values of L*, a* and b* were used to obtain the chroma (C) and ° hue (°h) according to the following equations [27]:
Statistical Analysis
All values were obtained from three independent experiments and samples were analyzed in triplicate (n = 9), except for total dietary fiber and the proximate analysis values that were performed in duplicate. Results were expressed as means ± standard deviation (SD), and the one-way analysis of variance (ANOVA) test was used to analyze the data. Differences among means were compared with a Duncan test at a level of significance of p < 0.05, the correlation coefficient was obtained with a level of significance of p < 0.01 using IBM SPSS Advanced Statistics for Windows, Version 15.0 (SPSS Inc, Chicago, IL, USA).
Results and Discussion
Chemical Composition and TDF
General composition of the samples and TDF are described in the Table 1. The PCPW and commercial fibers exhibited low moisture content in the range of 7–11% with the exception of SOLA with 17%. Low moisture is desirable in these samples so a longer shelf life can be achieved. The content of protein, lipids and ash was low (< 5%), except to SOLA which was higher. The content of digestible carbohydrates was between 31 and 73%, the PCPW, OF, AV and TA had the higher values (> 49%). SOLA had significantly (p < 0.05) high values of moisture, protein and lipids compared to the other samples, while for CHOd, ash and TDF were PCPW, OF and PP samples. According with Ramirez-Moreno et al. [13], the sugars that had been identified in the edible part as pulp in cactus pear are sucrose, fructose and glucose in a greater proportion. Since the evaluated waste constitutes a part of the edible pulp also had a high proportion of these sugars [28]. Sugars content in waste may be desirable because it could contribute to flavor and reduces the necessity of adding flavoring agents when used as an additive in the food industry [11].
Fiber was in the range of 14–52% of the sample dry matter. PP had the higher TDF content (52%) while the PCPW had the lowest (14%). In the other samples the content was between 23 and 34%. It is recognized that dietary fiber from fruits and vegetables has a positive effect on health. Many diseases of public health concern such as obesity, cardiovascular disease and type 2 diabetes can be prevented and treated through the intake of fiber-containing foods [29]. The American Dietetic Association recommends a fiber intake of 25–30 g per day or 10–13 g/1000 kcal for adults [29]. The commercial products TA and SOLA suggests an intake of 30–60 g per day, which exceeds the established recommendations and may cause flatulence, abdominal distension, bloating and abdominal pain, or complications as intestinal obstruction and formation of phytobezoars when ingestion of high doses of non-fermentable fiber is accompanied by insufficient water intake [30]. Fiber intake should not exceed what is recommended and should be part of an adequate diet considering that around 44% of dietary fiber comes from bread and cereals, 13% from fruit and 28% from vegetables [31].
Functional Properties
Functional properties of dietary fiber such water, fat and sugars retention capacity within the matrix [4] determine if it can be successfully incorporated in fiber enriched foods [18]. Sources of dietary fiber are often by-products from the food industry i.e. juice extraction [30], that could be incorporated into food products depending on their rheological properties and water retention capacity in addition to provide some health related benefits [32]. Fibers are considered food thickeners, stabilizers and emulsifiers, and high WRC may accelerate intestinal transit and increase of fecal volume which prevents constipation problems [33].
The functional properties of the PCPW and commercial samples are presented in Table 2. Results were similar to other vegetable foods, as grapefruit, lemon, orange, and apple, artichokes and pineapple cores [4, 34, 35]. Although, PCPW had less TDF content (Table 1) functional properties were similar to PP in terms of WRC, SWC and EC, it also had the higher levels for FAC (p < 0.05). These results suggest that PCPW could have the potential to retard fat absorption into the blood through the intestine, reducing cholesterol levels and modifying low density lipoprotein (LDL) composition [36]. The GAC results showed that all fibers can absorb at least 70 g 100 g−1 of glucose, but SOLA was the one with higher capacity.
A good GAC of fibers may keep low glucose concentrations in the small intestine [19]. When these types of fibers are consumed, the rate of glucose appearance in the blood is slower, and insulin secretion decreases. These beneficial effects are most evident for individuals with diabetes mellitus because rapid insulin secretion in these patients accelerates glucose delivery and increase its concentration in blood [37]. Nevertheless, excessive consumption of fiber supplements may damage the normal function of the intestine and is suggested that a daily recommendation of fiber should be complemented with a correct diet [3].
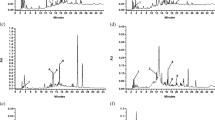
Viscosity
Viscous dietary fibers induce thickening when mixed with liquids, this property depends on factors such as structure, chemical composition, concentration and molecular weight [38]. Figure 1 shows the viscosity of the PCPW and commercial fibers at 1 and 5% (Fig. 1a, b, respectively). All samples exhibited a non-Newtonian pseudoplastic behavior so that viscosity decreased as the speed increased [33]. At 1%, the TA fiber had the highest viscosity and PCPW and OF presented a similar behavior (Fig. 1a), this could be attributed to the presence of other polysaccharides associated with soluble dietary fiber such as pectins, gums and β-glucans related with a high water retention capacity [33]. Viscous dietary fibers aid reduce hunger feeling and food intake by absorbing large quantities of water which increase stomach distension and thus the sense of intestinal fullness [39]. The PCPW (5%) had higher viscosity than PP, and in general all mixes at 5% exhibited higher values than at 1% (Fig. 1b).
Content of insoluble fiber with more water retention capacity which in turn delays gastric emptying and also may have a prebiotic effect [40]. During viscosity measurements of PP at 5%, values of 0 were obtained because a gel was readily formed when water was added (Fig. 1b). According to Zhao et al. [41] higher concentrations than 5% could tend to be associated to form a gel networks by electrostatic attractions, hydrogen bonds, Var der Waals forces and others. This behavior is attributable to the capacity of insoluble fiber to include water in the fiber matrix causing swelling and gel formation [4], which agrees with the PP high swelling capacity (Table 2).
Total Phenolic Content
Polyphenols are a group of natural compounds with high scientific and therapeutic interest [42]. The total phenolic contents are shown in Fig. 2. The PCPW had similar phenolic content to the other commercial fibers being all extracted from plants. OF and AV exhibited the higher values (p < 0.05) in the range of 900–1000 mg GAE 100 g−1 db, followed by TA and PCPW between 600 and 800 mg GAE 100 g−1 db. The rest of fibers had values below 300 mg GAE 100 g−1 db being PP, the more accepted commercially, the one with lowest total phenolic content. The results are lower compared to those reported by Bensadón et al. [12], for red cactus pear by-products (1.54 g 100 g−1 db).
Total phenolic contents (mg Galic Acid Equivalent 100 g−1 dry basis) of cactus pear waste and comercial fibers. Different letters indicate statistical difference (p < 0.05). PCPW purple cactus pear waste; OF: Opuntia ficus; PP: Plantago psyllum; AV: Aloe vera; TA: Triticum aestivum; SOLA: Salvia hispánica, Opuntia ficus, Linum usitatissium and Ananas sativus
The differences could be explained because they included the pericarp of the fruit while we only utilized the waste obtained after processing the fruit. A portion of the fruit phenols could have remained associated to the insoluble fiber fraction as tannins, and/or to the soluble fraction as phenolic acids. These compounds joined to the fiber, could be used as a therapeutic agent in the treatment and prevention of non-communicable chronic degenerative diseases [43].
Antioxidant Activity by ABTS and DPPH
The ABTS test monitors the decay of the radical-cation ABTS + produced by the oxidation of 2,2′-azinobis(3-ethylbenzothiaziline-6-sulfonate) by H-donors as well as DPPH which is based on the capability to stabilize free radical 2,2-diphenyl-1-picrylhydrazyl [44]. The antioxidant capacity by ABTS and DPPH is shown in Fig. 3. PCPW presented the highest antioxidant capacity by ABTS (1485.14 µmol TE 100 g−1 db), and similar antioxidant capacity by DPPH to TA while PP had the lowest ABTS and DPPH values (Figs. 2, 3).
Antioxidant capacity of cactus pear waste and comercial fibers by a ABTS (µmol Trolox Equivalent 100 g−1 dry basis), and b DPPH (µmol Trolox Equivalent 100 g−1 dry basis). Different letters indicate statistical difference (p < 0.05). PCPW purple cactus pear waste; OF: Opuntia ficus; PP: Plantago psyllum; AV: Aloe vera; TA: Triticum aestivum; SOLA: Salvia hispánica, Opuntia ficus, Linum usitatissium and Ananas sativus
ABTS is frequently used by the food industry and agricultural research field to measure the antioxidant activity of foods. It measures the ability of pure substances or crude extracts to trap free radicals by donating hydrogen atoms or electrons [45]. The difference between ABTS and DPPH observed in PCPW could be because ABTS activity includes hydrophilic and lipophilic compounds, while DPPH measures only lipophilic compounds [46]. Furthermore, the correlation between phenols and antioxidant activity by ABTS (r = 0.8 p < 0.01) is stronger than with antioxidant activity by DPPH (r = 0.6 p < 0.01). These results obtained are relevant because the antioxidants play a role in the prevention of oxidative stress-related diseases and in the reduction of total mortality associated to these conditions [47].
Color
The color of food is the first quality parameter evaluated by consumers and it is critical to product acceptance. Food appearance is determined mostly by color because is the first sensation that the consumer perceives and may determine acceptance or rejection of the product [48]. Color parameters (L*, a*, b*, h°, C*) are presented in Table 3 and in Fig. 4. All samples were situated in the red space, however, the PCPW had the higher a* values (19.31) and an attractive redness color in comparison with the other samples. The betanins are the most abundant pigments in red–purple cactus pear and betaxanthins in yellow–orange pigments [24, 49]. The PCPW also had the higher C* (saturation level) respect to the other fibers which reveals that it has a more intense color [50]. The PCPW had the closest value to 0° compared to the other samples, which means a purer color [51]. For its color characteristic PCPW could be used as an attractive coloring additive for foods or nutraceutical supplements.
Conclusions
The trend towards natural ingredients and products promoting health is increasing. In this sense, the results showed that PCPW has potential as a functional ingredient with good source of fiber with functional and antioxidant properties with natural sugars and colorants that can be used by the food and nutraceutical industry. Nonetheless, further studies are needed to determine if the purple cactus pear waste can be used in specific applications, e.g. as an additive in foods or as fiber supplement.
References
Cofepris. Suplementos Alimenticios. http://www.cofepris.gob.mx/Paginas/Suplementos%20Alimenticios/Suplementos-Alimenticios.aspx (2016). Accessed 16 Oct 2016)
Ayala-Zavala, J., Vega-Vega, V., Rosas-Dominguez, C., Palafox-Carlos, H., Villa-Rodríguez, J.A., Siddiqui, W.M., Dávila-Aviñaa, J.E., González-Aguilar, G.A.: Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 44, 1866–1874 (2011). https://doi.org/10.1016/j.foodres.2011.02.021
Papathanasopoulos, A., Camilleri, M.: Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 138, 65–72 (2010)
Figuerola, F., Hurtado, M.L., Estevez, A.M., Chiffelle, I., Asenjo, F.: Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 91, 395–401 (2005). https://doi.org/10.1016/j.foodchem.2004.04.036
Lousada-Júnior, J.E., Correia da-Costa, J.M., Miranda-Neiva, J.N., Rodríguez, N.M.: Physical-chemical characterization of tropical fruit by-products for use in animal feed. Rev. Ciênc. Agron. 37, 70–76 (2006)
SAGARPA. Exportan productores nopal y tuna xoconostle a Estados Unidos. http://www.gob.mx/sagarpa/articulos/exportan-productores-nopal-y-tuna-xoconostle-a-estados-unidos (2016). Accessed 20 Jan 2016
SAGARPA. Inicia exportación de nopal, tuna xoconostle y penca de maguey del Estado de México a Estados Unidos. http://www.gob.mx/sagarpa/prensa/inicia-exportacion-de-nopal-tuna-xoconostle-y-penca-de-maguey-del-estado-de-mexico-a-estados-unidos (2015). Accessed 14 Jan 2016
Cassano, A., Conidi, C., Drioli, E.: Physico-chemical parameters of cactus pear (Opuntia ficus-indica) juice clarified by microfiltration and ultrafiltration processes. Desalination 250, 1101–1104 (2010). https://doi.org/10.1016/j.desal.2009.09.117
SAGARPA.: Nopal y tuna, una mirada a su realidad actual. Claridades Agropecuarias 213, 3–12 (2011)
García-Mata, R., González-Machorro, M.F., García-Sánchez, R.C., Mora-Flores, J.S., González-Estrada, A., Martínez-Damian, M.A.: Banana (Musa paradisiaca) market in México 1971–2017. Agrociencia 47, 399–410 (2013)
Saenz, C.: Processing technologies: an alternative for cactus pear (Opuntia spp.) fruits and cladodes. J. Arid Environ. 46, 209–225 (2000). https://doi.org/10.1006/jare.2000.0676
Bensadón, S., Hervert-Hernández, D., Sáyago-Ayerdi, S.G., Goñi, I.: By-products of Opuntia ficus indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 65, 210–216 (2010). https://doi.org/10.1007/s11130-010-0176-2
Ramírez-Moreno, E., Cordoba-Díaz, D., Sánchez-Mata, M.D., Díez-Marqués, C., Goñi, I.: Effect of boiling on nutritional, antioxidant and physicochemical characteristics in cladodes (Opuntia ficus indica). LWT-J. Food. Sci. Technol. 51, 296–302 (2013). https://doi.org/10.1016/j.lwt.2012.10.005
Horwitz, W., Latimer, G. (eds.): Official Methods of Analysis of AOAC International, 18th edn. AOAC International, Gaithersburg (2005)
Symons, L.J., Brennan, C.S.: The effect of barley β-glucan fiber fractions on starch gelatinization and pasting characteristics. J. Food Sci. 69, 257–261 (2004). https://doi.org/10.1111/j.1365-2621.2004.tb06325.x
Raghavendra, S.N., Ramachandra-Swamy, S.R., Rastogi, N.K., Raghavarao, K.S.M.S., Kumar, S., Tharanathan, R.N.: Grinding characteristics and hydration properties of coconut residue: a source of dietary fiber. J. Food Eng. 72, 281–286 (2006). https://doi.org/10.1016/j.jfoodeng.2004.12.008
Temelli, F.: Extraction and functional properties of barley β-glucan as affected by temperature and pH. J. Food Sci. 62, 1194–1201 (1997). https://doi.org/10.1111/j.1365-2621.1997.tb12242.x
Femenia, A., Lefebvre, A.C., Thebaudin, J.Y., Robertson, J.A., Bourgeois, C.M.: Physical and sensory properties of model foods supplemented with cauliflower fiber. J. Food Sci. 62, 635–639 (1981). https://doi.org/10.1111/j.1365-2621.1997.tb15426.x
Ou, S., Kwok, K., Li, Y., Fu, L.: In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 49, 1026–1029 (2001). https://doi.org/10.1021/jf000574n
Chau, C.F., Huang, Y.L., Lee, M.H.: In vitro hypoglycemic effects of different insoluble fiber-rich fractions prepared from the peel of Citrus sinensis L. cv. Liucheng. J. Agric. Food Chem. 51, 6623–6626 (2003). https://doi.org/10.1021/jf034449y
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Frost, J., Hegedus, E.F., Glicksman, M.: Objective characterization of hydrocolloid organoleptic properties. Food Technol. 38, 118–122 (1984)
Pérez-Jiménez, J., Arranz, S., Tabernero, M., Díaz-Rubio, M.E., Serrano, J., Goñi, I., Saura-Calixto, F.: Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res. Int. 41, 74–85 (2008). https://doi.org/10.1016/j.foodres.2007.12.004
Stintzing, F.C., Herbach, K.M., Mosshammer, M.R., Carle, R., Yi, W., Sellappan, S., Akoh, C.C., Bunch, R., Felker, P.: Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 53, 442–451 (2005). https://doi.org/10.1021/jf048751y
Kuskoski, E.M., Asuero, A.G., Troncoso, A.M., Mancini-Filho, J., Fett, R.: Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 25, 726–732 (2005). https://doi.org/10.1590/S0101-20612005000400016
Morales, F.J., Jiménez-Pérez, S.: Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 72, 119–125 (2001). https://doi.org/10.1016/S0308-8146(00)00239-9
Francis, F.J.: Color quality evaluation of horticultural crops. HortScience 15, 58–59 (1980)
Ramírez-Moreno, E., Hervert-Hernández, D., Sánchez-Mata, M.C., Díez-Marqués, C., Goñi, I.: Intestinal bioaccessibility of polyphenols and antioxidant capacity of pulp and seeds of cactus pear. Int J. Food Sci. Nutr. 62, 839–843 (2011). https://doi.org/10.3109/09637486.2011.580731
Slavin, J.L.: Impact of the proposed definition of dietary fiber on nutrient databases. J. Food Compos. Anal. 16, 287–291 (2003). https://doi.org/10.1016/S0889-1575(03)00053-X
Álvarez, E.E., Sánchez, P.G.: La fibra dietética. Nutr. Hosp. 21, 61–72 (2006)
Russell, D.G., Parnell, W.R., Wilson, N.C., Faed, J., Ferguson, E., Herbison, P., Horwath, C., Nye, T., Reid, P., Walker, R., Wilson, B., Tukuitonga, C.: NZ Food: NZ People: Key Results of the 1997 National Nutrition Survey. Mon Bull Ministry of Health Public Health Laboratory Service (G. B.), Wellington (1999)
Waldron, K.W., Parker, M.L., Smith, A.C.: Plant cell walls and food quality. Compr. Rev. Food Sci. Food Saf. 2, 128–146 (2003). https://doi.org/10.1111/j.1541-4337.2003.tb00019.x
Goñi, I., Valdivieso, L., Gudiel-Urbano, M.: Capacity of edible seaweeds to modify in vitro starch digestibility of wheat bread. Nahrung 46, 18–20 (2002). https://doi.org/10.1002/1521-3803(20020101)
López, G., Ros, G., Rincón, F., Periago, M.J., Martínez, M.C., Ortuño, J.: Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J. Agric. Food Chem. 44, 2773–2778 (1996). https://doi.org/10.1021/jf9507699
Prakongpan, T., Nitithamyong, A., Luangpituksa, P.: Extraction and application of dietary fiber and cellulose from pineapple cores. J. Food Sci. 67, 1308–1313 (2002). https://doi.org/10.1111/j.1365-2621.2002.tb10279.x
Stintzing, F.C., Schieber, A., Carle, R.: Phytochemical and nutritional significance of cactus pear. Eur. Food Res. Technol. 212, 396–407 (2001). https://doi.org/10.1007/s002170000219
Slavin, J.L.: Dietary fiber and body weight. Nutrition 21, 411–418 (2005). https://doi.org/10.1016/j.nut.2004.08.018
Dikeman, C.L., Fahey, G.C.: Viscosity as related to dietary fiber. Crit. Rev. Food Sci. Nutr. 46, 649–663 (2006). https://doi.org/10.1080/10408390500511862
De Graaf, C., Blom, W.A., Smeets, P.A., Stafleu, A., Hendriks, H.F.: Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 79, 946–961 (2004)
Marciani, L., Gowland, P.A., Spiller, R.C., Manoj, P., Moore, R.J., Young, P., Al-Sahab, S., Bush, D., Wright, J., Fillery-Travis, A.J.: Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J. Nutr. 130, 122–127 (2000)
Zhao, Y., Cao, Y., Yang, Y., Wu, C.: Rheological study of the sol-gel transition of hybrid gels. Macromolecules 36, 855–859 (2003). https://doi.org/10.1021/ma020919y
Yeddes, N., Chérif, J.K., Guyot, S., Baron, A., Trabelsi-Ayali, M.: Phenolic profile of Tunisian Opuntia ficus indica thornless form flowers via chromatographic and spectral analysis by reversed phase-high performance liquid chromatography-UV-photodiode array and electrospray ionization-mass spectrometer. Int. J. Food Prop. 17, 741–751 (2014). https://doi.org/10.1080/10942912.2012.665404
D’ Archivio, M., Filesi, C., Di Benedetto, R., Gargiulo, R., Giovannini, C., Masella, R.: Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 43, 348 (2007)
Roginsky, V., Lissi, E.A.: Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 92, 235–254 (2005). https://doi.org/10.1016/j.foodchem.2004.08.004
Coria-Cayupán, Y.S., Ochoa, M.J., Nazareno, M.A.: Health-promoting substances and antioxidant properties of Opuntia sp. fruits: changes in bioactive-compound contents during ripening process. Food Chem. 126, 514–519 (2011). https://doi.org/10.1016/j.foodchem.2010.11.033
Arnao, M.B.: Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci. Technol. 11, 419–421 (2000). https://doi.org/10.1016/S0924-2244(01)00027-9
Brighenti, F., Valtueña, S., Pellegrini, N., Ardigo, D., Del Rio, D., Salvatore, S., Piatti, P.M., Serafini, M., Zavaroni, I.: Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br. J. Nutr. 93, 619–625 (2005). https://doi.org/10.1079/BJN20051400
Leon, K., Mery, D., Pedreschi, F., Leon, J.: Color measurement in L* a* b* units from RGB digital images. Food Res. Int. 39, 1084–1091 (2006). https://doi.org/10.1016/j.foodres.2006.03.006
Castellanos-Santiago, E., Yahia, E.M.: Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 56, 5758–5764 (2008). https://doi.org/10.1021/jf800362t
Aguedo, M., Kohnen, S., Rabetafika, N., Bossche, S.V., Sterckx, J., Blecker, C., Beauve, C., Paquot, M.: Composition of by-products from cooked fruit processing and potential use in food products. J. Food Compos. Anal. 27, 61–69 (2012). https://doi.org/10.1016/j.jfca.2012.04.005
Schieber, A., Hilt, P., Streker, P., Endre, H.U., Rentschler, C., Carle, R.: A new process for the combined recovery of pectins and phenolic compounds from apple pomace. Innov. Food Sci. Emerg. Technol. 4, 99–107 (2003). https://doi.org/10.1016/S1466-8564(02)00087-5
Acknowledgements
This study was possible thanks to the financial support from the Programa de Fortalecimiento de la Calidad en Instituciones Educativas (PROFOCIE 2015–2016). The authors acknowledge to the Mexican association CoMeNTuna A. C. (Comisión Mexicana del Nopal y la Tuna A.C.), Hidalgo, México, for providing the plant materials. The first and second author developed this study during their Bachelor of Nutrition studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Monter-Arciniega, A., Hernández-Falcón, T.A., Cruz-Cansino, N.d.S. et al. Functional Properties, Total Phenolic Content and Antioxidant Activity of Purple Cactus Pear (Opuntia ficus-indica) Waste: Comparison with Commercial Fibers. Waste Biomass Valor 10, 2897–2906 (2019). https://doi.org/10.1007/s12649-018-0320-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0320-z