Abstract
Roasting is an important step in the production of edible argan oils. The effect of argan kernel roasting temperature (ranging from 150 to 200 °C) and time (from 10 to 50 min), on oil yield, contents in total phenolic compounds, α- and γ-tocopherol, and oxidative stability, was researched using response surface methodology. Increases in roasting temperature and time have a significant effect on all the responses. This study showed that the optimum roasting conditions of argan kernel (indirect heat by convection) for the production of edible argan oils were 150 °C and 50 min, which allowed reaching a maximum oil yield of 32.45%. Edible argan oil, obtained under these conditions, had a content of total phenolic compounds of 78.01 mg/kg, α- and γ-tocopherol of 30.28 and 495.03 mg/kg, respectively, and an oxidative stability of 37.58 h. Furthermore, it presented olfactory notes of ‘almond, dried fruits, hazelnut and waffle’, with ‘sweet’ and ‘fruity’ as positive attributes, without any defect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Argan oil extracted from the fruits of Argania spinosa L. is commonly produced in the south-west of Morocco. It is recognized for its nutritional value, constituting a good source of essential fatty acids and minor compounds like tocopherols (Harhar et al. 2011). The extraction processes (traditional and press) of edible argan oil are based on a previous thermal treatment (direct heat) of argan kernel before extraction, while the cosmetic oil is obtained from unroasted kernels. Roasting is usually carried out in clay containers over wood fire or in rotating oven applying gas burners (Charrouf and Guillaume 2014). Indeed, roasting is a unit operation very important for the extraction of edible argan oils, as it increases oil yield and allows the development of its specific organoleptic properties such as flavours, aromas and colour (Matthäus et al. 2010).
Although the effect of argan kernel roasting-time, at a constant temperature of 110 °C, on edible virgin argan oil composition and stability was previously studied (Harhar et al. 2011), scarce information is available concerning the effect of roasting temperature and time on the oil extraction yield and physico-chemical characteristics of edible argan oil (Belcadi-Haloui et al. 2018; Demnati et al. 2018).
Temperature and time are the main factors controlled during roasting since the treatment of argan kernels and processing have significant influence on the sensory quality and oxidative state of edible argan oil (Matthäus et al. 2010). Besides, roasting influences its composition, oxidative stability, colour, flavour, and commercial quality (Hilali et al. 2005; Matthäus et al. 2010; Marfil et al. 2011).
Response surface methodology (RSM) has been widely used for optimizing roasting seed process, to define the relationships between the responses and independent variables. Roasting conditions (temperature and time) of Arabica coffee bean have been optimized, using RSM, in order to produce superior quality of roasted coffee product (Ku Madihah et al. 2013). Vujasinović et al. (2012) have applied RSM to optimize hull-less pumpkin seed roasting conditions, maximizing the biochemical composition and antioxidant capacity of virgin pumpkin oils. In the same way, the effect of roasting temperature and duration on oil yield and quality of melon seed has been studied using RSM (Akinoso and Oni 2012). The response surface methodology has also been used to determine the optimal roasting temperature and time for preparing a coffee-like beverage from maize kernels (Youn and Chung 2012). This suggests that the same is possible for roasting conditions (temperature and time) for argan kernel, with the aim to produce high-quality argan oils, improving oil extraction yield at the same time.
The objective of this research was studying the effect of kernel roasting temperature and time, using indirect heat by convection in a rotating roaster, on the physico-chemical and sensory characteristics of edible argan oils, establishing the optimal roasting conditions in order to obtain high-quality product with maximum oil yield. Indeed, energy transfer by convection avoids the direct heat of the flame on the argan kernel and lead to homogeneous roasting over the kernel surface. Roasting temperature and time should be then quantitatively controlled to obtain a desired quality of edible argan oils.
Materials and methods
Materials
Dry argan fruit was purchased from Essaouira region, in the south-west of Morocco. Skin and pulp were manually removed and argan nuts were cracked, collecting kernels which were stored in plastic bags in dark at 4 °C until use. Previously, its moisture (3.49%) and total fat content (54.34% in dry basis) were determined.
Experimental design and statistical analysis
The roasting temperatures and times were selected according to a central composite design (CCD). The independent variables, temperature (X1) and time (X2), varied from 150 to 200 °C and 10 to 50 min, respectively. Each independent variable had three levels: − 1, 0 and + 1, and eleven combinations were randomly chosen, including three times the central point, 175 °C, during 30 min (Fig. 1).
The dependent variables (responses, Y) were: oil yield (OY, %), content in total phenolic compounds (TPC, mg/kg), α-, γ-tocopherol contents (α-T and γ-T, mg/kg), and oxidative stability calculated as induction time (IT, h). The responses were related to the independent variables by a second-degree polynomial, Eq. (1):
where, β0 was a constant, β1 and β2 linear coefficients, β12 interaction coefficient, and β11, β22 quadratic coefficients.
Two-way analysis of variance (ANOVA) and differences among the means of triplicate samples were determined setting the significant level at p = 0.01, by means of Statgraphics Centurion program XVI.II, version 16.2.04.
The optimal conditions were calculated at finding the levels of roasting temperature and time which could maximize oil yield, producing at the same time high-quality edible argan oils.
Roasting and extraction processes
Argan kernels were roasted at temperatures and times shown in Fig. 1, using indirect heat by convection, in an electric air roaster (Gene bean roaster, CBR-101 model, France) equipped with removable rotating drum of 200 g capacity and a controller of temperature and time. Once the choosing roasting time had elapsed, the roaster automatically began the cooling cycle till 60 °C.
The low and high limits of temperature and time were chosen according to the results of preliminary experiments. Temperatures higher than 200 °C and time over 50 min led to production of ‘burnt’ flavour and dark colour in the extracted argan oils. Kernels did not allow oil extraction at roasting temperatures lower than 150 °C and time less than 10 min.
After each roasting run, 400 g of roasted kernels were crushed in a hammer mill and carried to extraction stage using an Abencor® system (MC2, model 100). The grinding paste was then mixed for 45 min at 50 °C, adding 35% (w/w) of ultrapure hot water at 40 °C. Malaxing time and temperature, as well as percentage and temperature of water added to the grinding paste, were chosen according to preliminary tests carried out to select the optimum mixing conditions that could provide the best oil yields. Each sample was centrifuged for 10 min at 7500 rpm (Hettich, Universal 32 model), and oil was then filtrated (Filter-lab, PL 1300100 model) before using for analysis. Roasting and extracting runs were conducted in triplicate in order to extract the maximum amount of edible argan oil for the analysis.
The oil yield (OY), expressed as a percentage, was calculated dividing argan oil volume obtained, by the weight of grinding paste and then multiplying by argan oil density of 0.915 kg/m3 (IMANOR 2003).
Characterization of extracted oils
In this section, total phenolic compounds, tocopherol content, oxidative stability and sensory profile were evaluated.
Total phenolic compounds
TPC were determined according to the Folin–Ciocalteau method (Vázquez Roncero et al. 1973); 10 g of oil was dissolved in 50 cm3 of n-hexane, followed by liquid–liquid extraction in triplicate, using 20 cm3 of methanol/water (60/40, v/v) mixture. 5 cm3 of aqueous extract of the oil was reacted with the Folin–Ciocalteau reagent in NaOH solution and kept in the dark for 45 min. Absorbance was measured at 725 nm using ultrapure water as reference, in UV–visible spectrophotometer, Thermo, “Helλos,” Gamma model. Calibration curve was performed and the results were expressed as mg caffeic acid/kg argan oil.
Determination of tocopherols
Tocopherol contents were calculated following the IUPAC standard method (IUPAC 1992). 2 ± 0.01 g of oil were dissolved in 25 cm3 of n-hexane and transferred in injection vials for analysis in HPLC equipped with a C18 column (Varian, LiChrosphereSil 60A, particle size 5 μm, and 250 × 4 mm I.D.). Detection was carried out in a fluorescence detector (Shimatzu RF-20A) programmed with excitation wavelength of 290 nm and emission wavelength of 330 nm. Mobile phase was 0.5% propan-2-ol in n-hexane (v/v), with a flow rate of 0.5 cm3/min.
α-Tocopherol (α-T) and γ-tocopherol (γ-T), known as important antioxidants, were identified comparing their retention times with those of standard tocopherols, and quantified through respective calibration curves, expressed in mg/kg argan oil.
Oxidative stability
Induction time (IT) at 98 °C was determined to study the oxidative stability of oils, following the bibliographical methodology (ISO 2016), in a Rancimat equipment (Metrohm, 743 model, Switzerland), with an air flow of 10 dm3/h; 3 g of oil were weighed into vessel, and the induction time was defined as the required time to reach the inflection point of the ionic conductivity curve, expressed in hours (IT, h).
Sensory evaluation
In the designations of virgin argan oils, the quantitative sensory evaluation is not taken into account by the Moroccan standard, as in the case of virgin olive oils (IMANOR 2003).
Therefore, to establish a sensory profile of the extracted argan oils, tests were conducted using a sensory panel constituted of 10 expert tasters, from the University of Jaen; seen that the minimum number of tasters recommended is eight, more de panel leader. The samples were served as 14 g in coded transparent cups, at 28 ± 2 °C, following the International Olive Council standard (IOC 2018). The panel had to determine sensory characteristics such as ‘fruity’ and ‘sweet’ (positive attributes) and olfatory notes, as well as eventual defects (negative attributes) detected, such as ‘rancid’, ‘burnt’ and ‘musty’, in a profile sheet inspired by that applied for virgin olive oils, using a 10 cm scale to score the samples. The results were then expressed as medians (IOC 2018).
Results and discussion
The experimental values of oil yield, content of total phenolic compounds, α- and γ-tocopherol contents and induction time were reported, Table 1.
Oil yield
The oil yield was observed to vary in the range of 16.56% to 32.45% (Table 1). It increased with roasting temperature and time, which could be attributed, during roasting, to changes in the structure of seed material, with disruption oil-bearing cells (Matthäus et al. 2010). Furthermore, by increasing the roasting temperature, the absolute viscosity of the oil could decrease and at the same time, the effective diffusion coefficients of each of the components constituting the extracted oil increased. This fact justifies the increase in fat yields when the temperature increases. The best result, OY = 32.45%, was obtained at roasting conditions of 150 °C and 50 min, result coinciding with those obtained by Charrouf and Guillaume (2014) in the traditional extraction technique. At highest temperatures of 175 °C, during 30 and 50 min, and 200 °C, during 10, 30 and 50 min, the oil yield did not change too much, with values close to 30%. In fact, under the extraction conditions (35% of water added), the grinding paste was drier at these temperatures and times, with a lower moisture content, which did not improve the oil yield. Lower temperatures and times did not allow total disruption of cell walls, with oil yield below 30%.
The analysis of the variance highlighted the significant effect of roasting temperature and time (p ≤ 0.01) on oil yield, with a determination coefficient, R2 of 0.983. Furthermore, linear terms (X1, X2) have high importance on the oil yield (Eq. 2). Thus, temperature and time of roasting kernels, prior to extraction significantly affected oil yield (Akinoso and Oni 2012; Youn and Chung 2012).
Antioxidant components
TPC content varied between 59.52 mg/kg, for the lowest kernel roasting conditions, and 231.66 mg/kg for the hardest ones (Table 1), which was higher than that collected in the literature (Cayuela et al. 2008; Marfil et al. 2011). Roasting facilitated the transfer of these compounds from the grinding paste to the oil; the higher the roasting temperature and time, the higher the total phenolic compounds (Matthäus et al. 2010; Marfil et al. 2011; Vujasinović et al. 2012; Youn and Chung 2012; Demnati et al., 2018). However, only a small amount of phenolic compounds is transferred to the oil, the main concentration remaining in the press cake which presents a 37 times higher TPC than an edible oil. This fact can be related to the higher affinity of TPC for water rather than for oil (Cayuela et al. 2008; Matthäus et al. 2010). Furthermore, variation in the kernel roasting temperature and time had a critical role on the total phenolic compounds, with a determination coefficient, R2 of 0.993.
Regarding to α- and γ-tocopherol contents, they ranged between 29.38 and 42.07 mg/kg, and 430.93 and 515.94 mg/kg, respectively. The contents obtained are in the same range as those indicated by Cayuela et al. (2008) and Harhar et al. (2011). The lowest contents corresponded to the highest roasting conditions (200 °C, 50 min), and the highest ones to the lower roasting conditions (150 °C, 10 min), showing a decrease in each tocopherol content of argan oils, with the increase of kernel roasting conditions. This could be interpreted as an effect of thermal degradation of these components induced by the kernel roasting, which agreed with the higher thermosensitivity of tocopherols (Hilali et al. 2005; Cayuela et al. 2008; Youn and Chung 2012). Vujasinović et al. (2012) reported that roasting process of hull-less pumpkin caused an increase of more than 50% in the content of α-tocopherol in the extracted pumpkin oils. Harhar et al. (2011) found that prolonged kernel roasting, up to 45 min, at 110 °C, did not influence tocopherol content of argan oils.
As it could be noted, roasting conditions have an important role on α- and γ-tocopherol contents, with determination coefficients of 0.995 and 0.994, respectively.
Oxidative stability
The induction time fluctuated between 31.40 h, for oil obtained from kernel roasted at 150 °C during 10 min, and 54.06 h, for oil extracted from kernel roasted at 200 °C during 50 min. Harhar et al. (2011) indicate an induction time from 29 to 38 h for oils obtained from roasted kernels at 110 °C, for 15 and 30 min, respectively. Therefore, the increase in roasting temperature and time of argan kernels, improved the oxidative stability of extracted oils (Matthäus et al. 2010; Demnati et al. 2018). Roasting conditions have an important impact on argan oil oxidative stability, with a determination coefficient, R2 of 0.982, and roasting temperature as a significant model term, Eq. (3).
The high stability of edible argan oil, prepared from roasted kernels, could be in part explained by its high tocopherol content and a better extractability of antioxidant compounds (Matthäus et al. 2010).
Sensory analysis
Oil extracted with a maximum oil yield of 32.45%, at kernel roasting conditions of 150 °C during 50 min, had no defects, with ‘sweet’ and ‘fruity’ median of 6.0 and 6.75, respectively, presenting olfactory notes of ‘almond (7.0), dry fruits (6.0), hazelnut (5.0), and waffle (5.0)’, Fig. 2. Therewith, it presented a typical golden colour of edible argan oils. According to Matthäus et al. (2010), a typical ‘nutty’ and ‘roasty’ attributes were found in fresh argan oils obtained by traditional and mechanical extractions, without any negative attribute. However, they used a sensory evaluation technique quite different from IOC one, with a panel of four trained tasters, instead of eight or ten.
Extracted oils from kernels roasted at high conditions, have a very dark colour, with a smell and taste of burning (Demnati et al. 2018).
Optimization of the roasting process of argan kernel
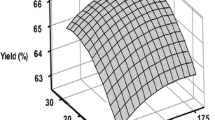
According to the RSM analysis, roasting conditions of 150 °C and 50 min were considered to maximize oil yield, with a predicted value of 32.13%, while the experimental value was 32.45% (Fig. 3 ). At these roasting conditions, TPC, α- and γ-tocopherol, predicted contents were respectively 81.33, 30.68, and 492.70 mg/kg, and predicted oxidative stability, expressed as induction time was 37.97 h, whereas experimental values were 78.01, 30.28 and 495.03 mg/kg, respectively, and 37.58 h, with a percentage of relative error between 0.5 and 4.1%. According to Harhar et al. (2011), a roasting time up to 30 min, at 110 ± 5 °C, using gas burners (direct heat), appears to be optimum to preserve argan oil properties. Belcadi-Haloui et al. (2018) recommended roasting temperature between 125 and 150 °C, during 10 min, using electric oven under continuous aeration.
RSM procedure could be used to predict biochemical content of edible argan oils, just as for pumpkin oils (Vujasinović et al. 2012). This also revealed that the quality of edible argan oils could be improved by optimizing the roasting process, as for coffee beverages (Ku Madihah et al. 2013).
To obtain edible argan oils with a maximum total phenolic compounds value, argan kernels have to be roasted at 200 °C during 50 min, and TPC content was 237.71 mg/kg (Fig. 4a). However, at these roasting conditions, extracted argan oils presented a defect of ‘burnt’ and olfactory note of ‘burned almond’.
Response surface diagrams for total phenolic compounds content (TPC, mg/kg) of extracted argan oils as affected by roasting temperature and time (a), and oxidative stability, expressed as induction time (IT, h), of extracted argan oils as a function of roasting temperature and time (b), from central composite design (CCD)
Harhar et al. (2011) found that a prolonged roasting time to 45 min, at 110 °C, in a homemade gas roaster with direct heat, caused ‘burnt’ taste in the argan oils. Belcadi-Haloui et al. (2018) advised to avoid high temperatures in order to preserve nutritional properties and minimize tocopherols destruction of argan oil.
In the same way, the maximum contents of α- and γ-tocopherol, in the extracted oils, were obtained at roasting conditions of 150 °C during 10 min: 42.15 and 516.15 mg/kg, respectively (Fig. 5a, b). Nevertheless, in these roasting conditions, the oil yield was very low, 16.56%, since all the cell walls were not destructed (Table 1).
Finally, the best value of oxidative stability was obtained for argan oils extracted from kernels roasted at 195.8 °C during 50 min, with an induction time of 61.36 h (Fig. 4b). However, at these roasting conditions, argan oils presented a defect of ‘burned’ and olfactory note of ‘burned almond’.
Conclusion
With the Abencor® system, adding 35% ultrapure hot water at 40 °C to the roasted kernel grinding paste, kneading it at 50 °C for 45 min, a maximum oil yield of 32.5% was obtained, result that coincided with those obtained by Charrouf and Guillaume (2014). This result corresponded to kernel roasting conditions of 150 °C and 50 min, and similar one was obtained, using RSM, with an oil yield of 32.1%, under the same roasting conditions.
The roasting process contributed to a greater transfer of phenolic compounds from the solid phase to oil. However, contents in α- and γ-tocopherol decreased markedly, with the increase of roasting temperature and time, due to its thermodegradation. On the other hand, roasting had a beneficial effect on the oxidative stability of extracted argan oils, because this stability depended on different parameters, such as content in total phenolic compounds and tocopherols.
In the experimental conditions tested, it has been determined that kernel roasting temperature of 150 °C during 50 min, allowed to reach a maximum oil yield. Argan oils, obtained under these conditions, have a total phenolic compounds content of 78.0 mg/kg, α- and γ-tocopherol of 30.3 and 495.0 mg/kg, respectively, with an oxidative stability of 37.6 h (evaluated at 98 °C), values that coincided with those determined by applying the RSM procedure. In relation to the sensory parameters, these edible argan oils did not present any defect and showed typical positive attributes (medium–high fruity) and olfactory notes (almond and dry fruits) of high-quality oils.
References
Akinoso R, Oni PO (2012) Optimization of solvent extracted melon seed oil using RSM. Eur J Lipid Sci Technol 114(5):607–611. https://doi.org/10.1002/ejlt.201100231
Belcadi-Haloui R, Zekhnini A, El-Alem Y, Hatimi A (2018) Effects of roasting temperature and time on the chemical composition of argan oil. Int J Food Sci. https://doi.org/10.1155/2018/7683041
Cayuela JA, Rada M, Pérez-Camino MC, Benaissa M, Elamrani A, Guida A (2008) Characterization of artisanally and semiautomatically extracted argan oils from Morocco. Eur J Lipid Sci Technol 110(12):1159–1166. https://doi.org/10.1002/ejlt.200800146
Charrouf Z, Guillaume D (2014) Argan oil, the 35-years-of-research product. Eur J Lipid Sci Technol 116(10):1–6. https://doi.org/10.1002/ejlt.201400261
Demnati D, Pacheco R, Martínez L, Sánchez S (2018) Effect of roasting temperature and time on the chemical composition and oxidative stability of argan (Argania spinosa L.) oils. Eur J Lipid Sci Technol 120(7):1–6. https://doi.org/10.1002/ejlt.201700136
Harhar H, Gharby S, Kartah B, El Monfalouti H, Guillaume D, Charrouf Z (2011) Influence of argan kernel roasting-time on virgin argan oil composition and oxidative stability. Plant Foods Hum Nutr 66:163–168. https://doi.org/10.1007/s11130-011-0220-x
Hilali M, Charrouf Z, Soulhi A, Hachimi L, Guillaume D (2005) Influence of origin and extraction method on argan oil physico-chemical characteristics and composition. J Agric Food Chem 53:2081–2087. https://doi.org/10.1021/jf040290t
IMANOR (2003) Huiles d’argane. Spécifications. Norme marocaine NM 08.5.090, Rabat (Morocco). http://www.imanor.gov.ma/?keyword-type=course_id&s=08.5.090
IOC (2018) Sensory analysis of olive oil. Method for the organoleptic assessment of virgin olive oil. COI/T.20/Doc. No 15/Rev. 10. http://www.internationaloliveoil.org/estaticos/view/224-testing-methods
ISO (2016) Animal and vegetable fats and oils: determination of oxidative stability (accelerated oxidation test). ISO, Geneva
IUPAC (1992) Standard methods for the analysis of oils, fats and derivatives, vol 7. Blackwell, Oxford
Ku Madihah KY, Zaibunnisa AH, Norashikin S, Rozita O, Misnawi J (2013) Optimization of roasting conditions for high-quality Arabica coffee. Int. Food Res. J. 20(4):1623–1627. https://doi.org/10.1016/j.apcbee.2012.11.035
Marfil R, Giménez R, Martínez O, Bouzas PR, Rufián-Henares JA, Mesías M, Cabrera-Vique C (2011) Determination of polyphenols, tocopherols, and antioxidant capacity in virgin argan oil (Argania spinosa, Skeels). Eur J Lipid Sci Technol 113(7):886–893. https://doi.org/10.1002/ejlt.201000503
Matthäus B, Guillaume D, Gharby S, Haddad A, Harhar H, Charrouf Z (2010) Effect of processing on the quality of edible argan oil. Food Chem 120:426–432. https://doi.org/10.1016/j.foodchem.2009.10.023
Vázquez Roncero A, Janer Del Valle C, Janer Del Valle ML (1973) Determinación de los polifenoles totales del aceite de oliva. Grasas Aceites 24:350–357
Vujasinović V, Radočaj O, Dimić E (2012) Optimization of hull-less pumpkin seed roasting conditions using response surface methodology. J Food Sci 77(5):532–538. https://doi.org/10.1111/j.1750-3841.2012.02675.x
Youn KS, Chung HS (2012) Optimization of the roasting temperature and time for preparation of coffee-like maize beverage using the response surface methodology. LWT Food Sci Technol 46:305–310. https://doi.org/10.1016/j.lwt.2011.09.014
Acknowledgements
The authors are grateful to the “Spanish International Development Cooperation Agency” (AECID) for its financial support of this work carried out by the Research Group “Bioprocesses TEP-138” (‘Junta de Andalucía’, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demnati, D., Pacheco, R., Martínez, L. et al. Optimum roasting conditions of argan kernels (Argania spinosa L.) for the production of high-quality edible argan oil. J Food Sci Technol 57, 840–847 (2020). https://doi.org/10.1007/s13197-019-04115-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04115-8