Abstract
Chinese quince seed proteins were sequentially extracted based on the modified Osborne method. Investigations showed that albumin and glutelin were the major fractions. The physicochemical and functional properties of these two fractions were determined. The results showed that both albumin and glutelin posed appropriate essential amino acid composition and met the minimum recommendation (World Health Organization/Food and Agriculture Organization) for adult diet, except for methionine. The hydrophobicity of albumin and glutelin were 1063.56 and 1170.21, respectively. According to differential scanning calorimeter analysis, the denaturation temperature of albumin and glutelin was 101.44 °C and 108.36 °C respectively, and the glutelin fraction had a better thermal stability. The solubility and apparent viscosity of albumin and glutelin were presented to be greatly influenced by pH values. The water holding capacity and oil adsorption capacity of glutelin were 5.44 g/g and 8.15 g/g, higher than those of albumin which were 3.76 g/g and 3.71 g/g, respectively. Circular dichroism determination revealed albumin and glutelin were mainly composed by α-helix and random coil structures. Albumin and glutelin presented the potential as favorable nutrition and functional additive in food industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteins are necessary for human as they provide us with essential nutrition and functions. In general, animal-derived proteins are the first choice as they pose more appropriate essential amino acid composition and are easier to be adsorbed by human. Whereas, with the ever-increasing consumption for proteins and the increasing worries about the safety of animal-based proteins. Generally, the sources of animal-based protein were fish, shrimp, poultry meat, egg, and milk. However, with the booming population, natural cultured way could not meet the explosive demand of animal-based protein. The large scale of artificial culture therefore emerged. Whereas, the bad breeding environment, feed safety problems and abuse of antibiotic caused consumers worries about the safety of animal-based proteins. Therefore, the exploration of new source of plant proteins had drawn growing interests in recent years.
Plant proteins are abundant and renewable in nature, therefore they can be a better alternative to replace or partially replace the expensive animal-derived proteins in food industries. Besides being directly served as foods, plant proteins are also widely used as food additives, such as thickeners, stabilizers, emulsifiers, gelling agents, as well as packaging films. The extensive applications of plant proteins in food products are mainly due to their special functional properties. There are considerable amounts of reports investigating the functional characteristics of plant proteins from different species, while many of them are undesirable, such as poor solubility of Pisum sativum L. seed protein isolate (below 30%) (Adebiyi and Aluko 2011), low thermal stability of field pea protein isolate (mean denaturation temperature was 83.8 °C) (Shevkani et al. 2015), and weak oil adsorption capacity of Gingerbread plum proteins (1.06–2.06 g/g) (Amza et al. 2011). These disadvantages greatly limit their application in food industry, and therefore the studies on new plant proteins are urgent.
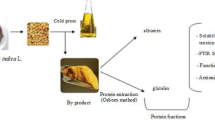
Chinese quince (Chaenomeles speciose (Sweet) Nakai) belongs to the Rosaceae family, and is mainly planted in China. Previous study had reported the pulps of Chinese quince posed many outstanding bioactivities, such as antitumor, antioxidant and immunoregulatory properties. Thus, Chinese quince fruits are widely served as food and health products. Chinese quince seed, the main by-product after pulps processing, is underutilized. Chinese quince and its plant seeds were presented in Fig. 1. In our previous study, a high protein content (about 28%) was detected in Chinese quince seed (dry base) indicating it is a great source of proteins. However, no references refer to the usage of Chinese quince seed protein as food or food additive, and this may due to little research had been carried out about its physicochemical and functional properties. Plant seeds generally contained different types of proteins, and their nutrition and functional properties could be different. These would affect their applications in food industry. The explorations of Chinese quince seed proteins nutrition and functionalities could make it to be applied as food additive in food industries.
Hence, this study aims to investigated proteins extracted from Chinese quince seed. According to Osborne procedure, proteins can be divided into 4 fractions: albumin, globulin, prolamin, and glutelin. Lots of works has been conducted to investigate the various source of plant protein fractions, such as Akebia trifoliata var. australis seed protein fractions (Du et al. 2012), rice bran protein fractions (Adebiyi et al. 2010), and grape seed endosperm protein fractions (Gazzola et al. 2014). Meanwhile, the content of each fractions in different plant protein varies greatly. Cowpea was rich in albumin (71.4%) (Ragab et al. 2004), while 61% globulin was detected in tomato seed (Chen et al. 2018). Prolamin was the major fraction in corn, however glutelin was the dominant fraction in yellow field pea seed protein isolate (Adebiyi and Aluko 2011). Moreover, the physicochemical and functional characteristics among diverse fractions are also different. The albumin of African yam bean seed was almost soluble in water, while the solubility of globulin was below 50% (Ajibola et al. 2016). The thermal property of albumin and glutelin in chia seeds were significantly different, as the denaturation temperature of albumin was 103.6 °C, whereas was only 91.3 °C of that in glutelin (Sandovaloliveros and Paredeslópez 2013). These different characters greatly influence their applications in food industries. Whereas, no information related to Chinese quince seed protein fractions, especially physicochemical and functional properties can be referred to. Given the convenient availability, high quality and little studies on Chinese quince seed proteins (CSP), explorations on the CSP are essential.
Before being applied in certain food system, it is necessary to characterize the physicochemical and functional properties of CSP. Therefore, in this study, CSP will be sequentially extracted according to Osborne method. Physicochemical and functional characteristics of major protein fractions will be evaluated. These may contribute to the innovative utilization of Chinese quince seed, still underutilized, as new protein source to be served as functional ingredients in food industries.
Materials and methods
Materials
The Chinese quince (Chaenomeles speciose (Sweet) Nakai; family Rosaceae) seeds were produced in Sichuan, China. Sodium 8-anilino-1-naphthalenesulfonate (ANS) was bought from TCI Co., Ltd. (Shanghai, China); all other reagents and chemicals were of analytical grade.
Protein extraction and fraction
Prior to extraction, the hull of Chinese quince seed was removed, and then they were grinded into flour. Next, the dehulled seed was defatted in a Soxhlet unit by petroleum ether at 45 °C for 12 h and was dried until the solvent was completely removed.
The extraction procedure referred to a modified Osborne method (Deng et al. 2011) with some modifications. Briefly, defatted seed flour was mixed with distilled water (ratio 1:10, w/v) firstly and the suspension was stirred for 2 h at room temperature, then the suspension was centrifuged at 8000g for 10 min. The supernatant was collected and designated as crude albumin faction. The pellet was re-dispersed and extracted with 0.5 M NaCl, after centrifuging the resulting supernatant was denoted as globulin fraction. The residue was then dispersed in 70% ethanol and extracted under constant stirring, and this slurry was then centrifuged and the supernatant was referred as prolamin fraction. The glutelin fraction was extracted from the residue by 0.1 M NaOH. Supernatant containing albumin, globulin and glutelin was precipitated using 1 M HCl or NaOH and placed at 4 °C for 1 h. After centrifuging at 6000g for 10 min, the precipitate was washed with distilled water twice and neutralized. After that, the dispersion was dialyzed against distilled water for 48 h, and each fraction was freeze-dried. To obtain prolamin, the supernatant was dialyzed against distilled water for 48 h and freeze-dried.
Physicochemical properties
Chemical composition
The moisture, ash, lipid and protein (%N × 6.25) contents were determined using Association of Official Analytical Chemists procedures 925.1, 923.03, 920.85 and 920.87 [AOAC (2000). Official methods of analysis of AOAC international (19th ed.)]. To exam the moisture, a certain amount of sample was placed in a drying oven at 105 °C for 3 h and the moisture could be obtained by calculating the loss of weight after drying. To determine the content of ash, pre-weighted sample was burned in a muffle at 600 °C for 4 h. The mass of the burned residue divided the mass of pre-weighted sample was the ash content. The protein content was analyzed using micro-kjeldahl method. The total carbohydrate content was analyzed according to the phenol–sulphuric acid method (Dubois et al. 1980). Briefly, a gradient of glucose solution was used to build a standard curve and the total carbohydrate content was determined using spectrophotometry method.
Amino acid composition
The determination of amino acid composition of Chinese quince seed protein fractions was carried out according to previous report by Rezig et al. (2013) with slight modifications. First, 20 mg protein samples were accurately weighted and placed in dry tubes, then 4 mL of 6 M HCl was added into each tube. Next, the tested tubes were air-exhausted and the protein samples were hydrolyzed at 110 °C for 24 h. After that, the hydrolyzed samples were analyzed using an automatic amino acid analyser L-8800 (Hitachi, Tokyo, Japan). In general, cystine was unstable in direct hydrochloric acid hydrolysis and this could lead to comparatively low value. Therefore, protein samples need to be oxidized to retain stability before hydrochloric acid hydrolysis. In order to determine the content of cystine, 20 mg of protein sample was incubated with 2 mL of performic acid solution [containing formic acid/30% hydrogen peroxide (19:1, v/v)] for 16 h at room temperature. Subsequently, 0.3 mL HBr was transferred to the mixture to end the reaction and the mixture was dried using rotary evaporation method. The resultant sample was redissolved in 1 mL distilled water, and then it was evaporated. This procedure was repeated for three times to remove the residual performic acid. Finally, the obtained sample was processed with hydrochloric acid hydrolysis as mentioned above. Tryptophan could be destroyed during the acid hydrolysis, and therefore sodium hydroxide was used instead of HCl to hydrolyze the sample. The content of tryptophan was determined according to a previous report (Spies 1967). The amino acid contents were expressed as g amino acid/100 g protein.
Sulfhydryl group (SH) and disulfide bond (SS) contents determination
The sulfhydryl group and disulfide bond were determined referred to Beveridge et al. (2010) with some modifications. To determine the free SH, 30 mg protein sample was dissolved in 10 mL Tris-Gly buffer (0.086 M Tris, 0.09 Gly and 4 mM EDTA, pH 8, denoted as Tris-Gly) containing 8 M urea. Then, 50 μL of Ellman’s reagent (5,5′-dithio-bis-2-nitrobenzoic acid in Tris-Gly buffer, 4 mg/mL) was added and dispersion was incubated at 25 °C for 60 min. After centrifuging, the supernatant was collected and its absorbance at 412 nm was measured, meanwhile the Tris-Gly buffer containing Ellman’s reagent was used as a blank. To determine total SH, 15 mg protein sample was suspended in 10 mL Tris-Gly buffer containing 8 M urea, and 100 μL of 2-mercaptoethanol was subsequently added. This dispersion was allowed to incubated for 60 min at 25 °C. After incubation, 10 mL 12% trichloroacetic acid was added and followed by centrifuging at 8000g for 10 min. The precipitate was twice washed with 12% trichloroacetic acid to remove 2-mercaptoethanol. The precipitated proteins were redissolved in 10 mL Tris-Gly, and 50 μL Ellman’s reagent was added for color development. The content of SH and SS was calculated as Eqs. (1) and (2),
where 73.53 is a coefficient derived from 106/(1.36 × 104); 1.36 × 104 was the molar absorptivity and 106 was for conversions from the molar basis to the μmol/mL basis and from mg protein to g protein; A412 was the absorbance of protein dispersion at 412 nm; D was the dilution factor; C was the protein concentration (mg/mL).
Determination of surface hydrophobicity (S0)
The surface hydrophobicity was determined based on the method from Kato and Nakai (1980) using sodium 8-anilino-1-naphthalenesulfonate (ANS) as the hydrophobic fluorescent probe. Briefly, protein samples were dissolved in distilled water and stirred for 30 min at room temperature. After centrifuging, the protein concentration of supernatant was determined according to Bradford method (Bradford 1976), then each supernatant was serially diluted to obtain protein concentration ranging from 50 to 250 μg/mL. Next, 50 μL of ANS (8.0 mM) was added to 4 mL solution. The fluorescence intensity (FI) of the sample was determined at 380 nm (excitation) and 470 nm (emission) using a LS 55 fluorescence spectrophotometer (PerkinElmer, USA). The FI of diluted solutions without probe and ANS solutions were also measured as controls. S0 was the initial slope of FI values plot against protein concentrations plot as calculated linear regression.
Thermal properties
The thermal characteristics of protein samples were evaluated by a differential scanning calorimeter (DSC 8500, PerkinElmer, USA). First, 15% protein sample dispersion was prepared in distilled water and allowed to hydrate over night before analysis. Next, the dispersion was accurately weighted and hermetically sealed in an aluminium pan. The sealed pan was scanned by the calorimeter from 30 to 140 °C at 5 °C/min. An empty pan was used as a reference.
Functional properties
Protein solubility (PS)
The solubility of Chinse quince seed protein factions were determined according to Cepeda et al. (1998) with few modifications. Briefly, 1% (w/v) protein dispersions were prepared in distilled water; 10 mL dispersion were taken and adjusted to desired pH using 1 M HCl or 1 M NaOH. The dispersion was then centrifuged at 6000g for 10 min and the protein content was measured using the Bradford (1976) method, meanwhile the bovine serum albumin was used as a standard. The Combustion Nitrogen Analysis was used to determine the total protein content of samples. The protein solubility was calculated according to Eq. (3).
Determination of water holding capacity (WHC)
The method to evaluate the WHC was according to Shchekoldina and Aider (2014) with some modifications. Briefly, 3 g protein sample was taken and mixed with 25 mL of distilled water in a pre-weighted centrifuge tube. The tube was vortexed thoroughly for 1 min and stand for 30 min at room temperature. After that, the dispersion was centrifuged at 6000g for 10 min, and the supernatant was decanted carefully. The weight of precipitate and centrifuge tube were determined. WHC of the sample was calculated as Eq. (4),
where W2 (g) was the weight of centrifuge tube and precipitated protein after absorbing water; W1 (g) was the weight of centrifuge tube and protein sample; W0 (g) was the weight of protein.
Measurement of fat absorption capacity (FAC)
The FAC was measured as reported by Beuchat (1977) with minor modifications. First of all, 1 g protein sample was taken and mixed with 20 mL commercial corn oil in a pre-weight centrifuge tube. The mixture was vortexed thoroughly and stand for 1 h, and then it was centrifuged at 6000g for 10 min. The supernatant oil was discarded, and the weight of sediment and centrifuge tube were determined. The FAC was calculated as Eq. (5),
where G2 (g) was the weight of centrifuge tube and precipitated protein after absorbing oil; G1 (g) was the weight of centrifuge tube and protein sample; G0 (g) was the weight of protein.
Heat coagulability
The method to evaluate the heat coagulability was referred to Myers et al. (1994). In brief, 5% protein dispersion was prepared in 0.1 M phosphate buffer (pH 7.0) with stirring for 1 h, then the dispersion was centrifuged at 8000g for 20 min. After centrifugation, the protein content of supernatant was measured using the Biuret method, and meanwhile 15 mL of the supernatant was taken and transferred into a test tube. The tube contained protein solution was boiled in a water bath for 30 min, cooled to ambient temperature and then centrifuged at 8000g for 20 min. The protein content of the supernatant was determined and the loss of protein solubility after heating was denoted as heat coagulability.
Viscosity measurement
The apparent viscosity of protein samples at different shear rate was determined according to Gao et al. (2018) with minor modifications. Briefly, 1.5 g protein sample was dispersed in 10 mL distilled water and pH was adjusted to 7.0. The dispersion was stirred for 1 h and scored at 4 °C for overnight. Before analysis the dispersion was balanced at 25 °C for 2 min, and the viscosity of sample dispersion was determined by a rotational viscometer (Haake Mars, Thermo Scientific, USA).
Circular dichroism (CD) spectra determination
CD spectroscopy was conducted by using an instrument J-1500 (Elementar) and a quartz cell with 0.1 mm optical path length at 25 °C. For determination, protein sample was dissolved in 0.01 M phosphate buffer to provide 0.2 mg/mL solution. The second structural composition of sample was measured using the CD spectra from 185 to 245 nm. Each measurement for the samples was repeated three times. The structural parameters (α-helix, β-Sheet, β-turn and random coil) were automatically calculated by the system software.
Statistical analysis
All experiments were conducted at least in triplicate. The data analyses were performed using Microsoft Excel 2016. The results were expressed as the mean ± standard deviation.
Results and discussions
Protein fractions
Chinese quince seed protein fractions were extracted referred to modified Osborne procedure as described above. Results showed that albumin (water soluble) and glutelin (NaOH soluble) were the major fractions in Chinese quince seed, accounting for 27.31% and 69.53% respectively. Whereas, only 2.69% globulin (salt soluble) and 0.47% prolamin (ethanol soluble) were detected. This indicated the major storage proteins of Chinese quince seed were albumin and glutelin fraction. Therefore, in the following study, we will focus on the study of physicochemical and functional properties of albumin and glutelin in the aim of evaluating their potential application in food industry.
Physicochemical properties
Chemical composition of Chinese quince seed, albumin and glutelin
The proximate compositions of whole Chinese quince seed, Chinese quince seed albumin and Chinese quince seed glutelin were determined and shown in Table 1. As observed, the moisture, ash, lipid, total carbohydrate and protein in whole Chinese quince seed was 6.85%, 2.29%, 29.83, 17.62% and 28.9%, respectively. Except for the lipid, protein was the major composition of this plant seed. After husking and defatting, the seed protein content reached 55.3%, the defatted seed meal was extracted according to Osborne procedure. Two major protein fractions, albumin and glutelin, were obtained. The moisture, ash, lipid, total carbohydrate and protein in albumin was 5.12%, 1.83%, 1.13%, 1.76% and 88.1%, respectively. They were found to be 4.43%, 1.51%, 0.81%, 2.89% and 85.20% in glutelin. The high protein content of both albumin and glutelin indicated the protein could be easily obtained for industry. It could be implied that Chinese quince seed might be a potential new source of plant protein since it has high contents of proteins.
Surface hydrophobicity (S0) and sulfhydryl and disulfide bond contents
S0 mainly depends on the amounts of exposed hydrophobic regions on the protein surface. Meanwhile, the hydrophobic amino acids and pH also influence S0. In this study, S0 of albumin and glutelin at neutral condition was evaluated as 1063.56 and 1170.21 respectively. The S0 of albumin and glutelin was much higher than that of A. trifoliata var. australis seed protein isolate (319.4) (Du et al. 2012). This could be due to their high amounts of hydrophobic amino acids. The markedly high S0 values of albumin and glutelin indicated a substantial existence of unaggregated proteins in each protein fraction which could diffuse quickly at the air/liquid interface, and enhance the foaming capacity. Additionally, high S0 imparts proteins with better surface activity and can promote their interactions with oil target, and therefore albumin and glutelin could be used as emulsifying agent.
The structural stability and rigidity of proteins are generally corelated to the sulfhydryl groups and disulfide bonds (Hu et al. 2010). In this study, the free sulfhydryl groups, total sulfhydryl groups and disulfide bonds contents of albumin and glutelin were determined, and the results were shown in Table 1. The free SH content of glutelin was 6.08 μM/g, significantly higher (p < 0.05) than that of albumin (3.76 μM/g). This was probably due to the alkali extraction solvent (0.1 M NaOH) unfolded the proteins and more SH were exposed, while albumin was extracted by distilled water and therefore showed lower SH. The SS content of albumin and glutelin was 9.09 and 16.57 μM/g of protein respectively. According to Du et al. (2012), the proteins in folded conformation can be stabilized by SS and the conformational entropy will also be reduced, thereby, promoting the thermal stability. This hinted that Chinese quince seed proteins might have good thermal performance due to its higher content of SS in glutelin.
Amino acid composition
The amino acid composition greatly influenced the proteins biological properties. The amino acid composition of albumin and glutelin was showed in Table 2. It can be seen that albumin and glutelin had very similar amino acid patterns. Glutamic acid in albumin and glutelin were 25.80 g/100 g and 24.2 g/100 g respectively, and it was the dominant amino acid. According to Sandovaloliveros and Paredeslópez (2013), the protein contained high levels of glutamic acid exhibited the ability to stimulate the immune and central nervous systems of humans. In addition, glutamic acid was credited with good antioxidant capacity as the existence of excess electrons could interact with the free radicals. Albumin and glutelin also presented high amounts of aspartic acid, i.e., 11.0 g/100 g and 11.3 g/100 g respectively. Though aspartic acid was non-essential amino acid, it could be helpful to regulate the hormonal levels and facilitate the nervous system running properly. Additionally, higher levels of arginine (8.98–9.48 g/100 g) and lower lysine (1.78–1.90 g/100 g) in albumin and glutelin could affect the cells uptake of arginine, which might be helpful in treating cardiovascular disease (Wells et al. 2005). The characteristic amino acid patterns of albumin and glutelin were also summarized in Table 2. Both albumin and glutelin showed high amounts of acidic amino acid, and it was similar to the result of Chinese quince seed protein isolate (Deng et al. 2019). This was a common phenomenon in seed storage proteins. Moreover, the contents of hydrophobic acids found in albumin (30.14 g/100 g) and glutelin (31.13 g/100 g) were much higher than that of tree peony seed protein (20.87%) (Gao et al. 2018), and this would impart albumin and glutelin with more stable structures by forming compact interior core. The steady interior core reduced the exposure of hydrophobic regions and this could avoid the aggregation of proteins (Siow and Gan 2014).
In order to better understand the nutritional benefits, the essential amino acids of albumin and glutelin were compared with those of World Health Organization/Food and Agriculture Organization (WHO/FAO) recommendations for adult diet and the result was presented in Table 2. The unbalanced essential amino acid composition was the major reason which limited the application of plant proteins in food industries. As observed, both albumin and glutelin showed desired essential amino acid composition and the contents were basically exceeded WHO/FAO recommendations for adults except for methionine. Though albumin and glutelin showed low contents of methionine, they were still higher than that of Paeonia suffruticosa Andr. seed protein (0.48%) (Zhong et al. 2014). Considering the abundant essential amino acids though less lever of methionine, albumin and glutelin in Chinese quince seed still can be important sources of essential amino acids in food industry, especially for adult diet.
Thermal properties
Heating is a common way during food processing, and can cause thermal disruptions of proteins. DSC can be used to supply essential information about thermal properties which can guide the protein processing in industries. The thermal characteristics of albumin and glutelin were evaluated. The peak denaturation temperature (Td) of albumin and glutelin was 101.44 °C and 108.36 °C respectively, and was much higher than that of many plant proteins, such as albumin (Td= 72.96 °C) and glutelin (Td= 69.92 °C) protein fractions from mulberry leaf (Sun et al. 2016). This suggests that both albumin and glutelin in Chinese quince seed have good thermal stability. This could be owed to their high amounts of hydrophobic amino acid in albumin and glutelin fraction as strong hydrophobic interactions generally result in high Td which is consistent with our result that albumin and glutelin contains considerable hydrophobic amino acid (Chen and Paredes-Lopez 2010). Moreover, glutelin displayed higher Td than albumin in this study and was consistent with the conclusion that the polar albumin had lower Td as compared with less polar glutelin (Angel et al. 2003). Overall, high Td of proteins ensured less loss of natural bioactivity in heating processing. As denaturation takes place, the internal hydrophobic regions will be exposed and decrease protein solubility, influencing the application in industries, and this should be prevented. The ∆H is the energy needed for denaturation and can reflect the extent of undenatured proteins. The ∆H of albumin and glutelin was determined as 96.07 J/g and 207.20 J/g respectively which was superior to other reported plant proteins, such as, the albumin (∆H = 12.60 J/g) and glutelin (∆H = 6.2 J/g) from chia seed protein fractions (Sandovaloliveros and Paredeslópez 2013). Our results suggested that the natural characteristics of albumin and glutelin were almost preserved as less denaturation were generated during extraction and freeze-drying procedure. Considering the thermostability of albumin and glutelin found in Chinese quince seeds, they are possible to be applied in food industries undergoing high-temperature processing, like cake batters.
Functional properties
Protein solubility (PS)
Solubility was a vital characteristic for proteins as it greatly affected those functional properties, such as water holding ability, emulsifying properties and gelling capacity. So, they will influence their applications. The PS of albumin and glutelin affected by pH were investigated and the results were displayed in Fig. 2a.
Functional properties of albumin and glutelin: a solubility of albumin and glutelin affected by pH values; b the apparent viscosity of albumin and glutelin at different shear rate. Results are expressed as the mean ± standard deviation (n = 3). the same index marked with different letter means significantly different (p < 0.05)
As observed, pH values greatly affected the PS of albumin and glutelin. As pH increased from 2 to 4, the PS of albumin and glutelin decreased sharply, and it reached minimum values at pH 4.0. This indicated the isoelectric point of albumin and glutelin was near pH 4.0. The poor PS around isoelectric point was contributed by the low net charge of protein molecules which weaken the electrostatic repulsion among the protein molecules and resulted in aggregation and precipitation. However, as pH increased from 5 to 12, the PS of albumin and glutelin increased gradually and they were most soluble at pH 12.0. Two major reasons might result in the growing PS, i.e., one being the increasing net charge of protein molecules enhanced the electrostatic repulsion, and another being the interactions between water and proteins were strengthened as pH increased. Furthermore, albumin showed better PS than glutelin among the tested pH values. This could be explained by the higher content of hydrophobic amino acid in glutelin, meanwhile our result also suggested the higher hydrophobicity of glutelin. Solubility and hydrophobicity were opposite characteristics, as solubility was the tendency of protein to interact with water, while hydrophobicity was the tendency of protein to interact with protein molecules becoming insoluble. The PS of albumin was 46.5 at pH 7.0 which was lower than that of Torreya grandis seed protein isolate (over 80%), whereas it was still comparatively to that of freeze-dried soy protein isolate (40.8%) (Yu et al. 2017; Hu et al. 2010). The high PS of albumin at neutral condition was important for industrial applications, like beverage.
Water holding capacity (WHC) and oil adsorption capacity (OAC)
WHC is an important functional property of proteins as it greatly influences the mouthfeel and viscosity of foods, like soups. It was found that WHC of albumin and glutelin was 3.76 g/g and 5.44 g/g respectively. Glutelin showed significantly (p < 0.05) higher WHC than albumin. The solubility was a possible reason which led to this result. Eladawy (2000) reported the more soluble protein presented poorer WHC. The WHC of albumin from Africa yam bean seed was not reported as it was completely soluble (Ajibola et al. 2016). The WHC of albumin and glutelin is unremarkable when compare with that of hemp seed protein isolate (12.05 g/g) (Malomo and Aluko 2015). But it was higher than safflower protein isolate (2.22 g/g) and freeze-dried chia seed protein isolate (2.9 g/g) (Ulloa et al. 2011; Timilsena et al. 2016). According to the recommendation from Aletor et al. (2002), the WHC of proteins ranges from 1.49 to 4.72 g/g can be used in viscous food. Thus, albumin of Chinese quince seed was also showed to be possibly used in food industry.
OAC reflects the ability of nonpolar side chains in proteins to bind fat. OAC is an important parameter as it influences shelf life and flavor retention. OAC can be affected by protein type, charge, hydrophobicity and oil applied. The OAC of albumin and glutelin were evaluated and the OAC of glutelin was 8.15 g/g, while only 3.71 g/g of that was found in albumin. This was probably due to the higher content of hydrophobic side chains in glutelin resulting in stronger protein-lipid interactions. Similar result was reported by Sun et al. (2016) that the glutelin from mulberry leaf presented higher OAC than albumin. Moreover, the OAC of glutelin was higher than previous reported proteins, such as lablab bean protein (4.9 g/g) and T. grandis seed protein isolate (3.02–3.03 g/g) (Lawal et al. 2005; Yu et al. 2017). The remarkable OAC of glutelin in Chinese quince seed imparts its probable application in food industries where high OAC is desired, such as sausages.
Heat coagulability
Heat coagulability evaluates the susceptibility of proteins to heating, and reflects the solubility reduction of proteins under heating treatment. High temperature processing is a common procedure in industries, and it can induce the unfolded protein aggregation which greatly influenced the functional properties of proteins. Many factors affected protein’s heat coagulability, such as the variety of proteins, pH, concentration, and ironic strength. Results showed that glutelin presented lower (25.39%) loss of solubility after heating treatment. And this also suggested it had better thermal stability. In contrast, albumin showed higher heat coagulability, and about 33.65% of its proteins turned to be insoluble suggesting that albumin was more heat-labile. This was consistent with our previous result as discussed in DSC analysis that glutelin posed higher denaturation temperature and ∆H. Though albumin exhibited a poor capacity to resist heating in this study, it was better than the albumin from tepary seeds which lost 49.58% solubility after heating process (Idouraine et al. 2010). To summary, glutelin in Chinese quince seed would be more suitable to be served in certain food systems where high temperature is inevitable, such as soup and bakery products.
Viscosity analysis
Viscosity can influence the mouthfeel of these foods with addition of proteins, especially for beverages. The apparent viscosity of albumin and glutelin at different shear rate was evaluated and the result was shown in Fig. 2b.
It was seen that both albumin and glutelin solution were shear-dependent fluids and exhibited shear-thinning behavior. The possible reason is that the apparent viscosity decreased as shear rate increased. The viscosity decreased as the shear rate increased possibly due to the disentanglement of macromolecular chains and the alignment of microstructure in the shear flow direction. This resulted in less interaction between adjacent polymer chains. The apparent viscosity of glutelin solution decreased more sharply than that of albumin solution. The different viscosity characteristics of various protein solutions could be attributed to their composition, surface charge, and molecular size, etc. In this study, the significantly apparent viscosity reduction was observed in glutelin compared with that in albumin. This might be due to the higher hydrophobic amino acid content imparted glutelin molecules with more compact conformations. On the other hand, as the shear rate increased, protein molecules deform and disentangle consequently.
Circular dichroism (CD) spectroscopy
CD spectra is generally used to determine proteins secondary structures. In this study, the secondary structure composition of albumin and glutelin was tested. The albumin was composed of 36.6% α-helix, 14.8% β-sheet, 12.2% β-turn and 36.4% random coil. The CD spectra revealed that glutelin was composed of 32.0% α-helix, 9.4% β-sheet, 17.9% β-turn and 40.6% random coil. Overall, α-helix and random coil were the major compositions for both albumin and glutelin. However, a remarkable decrease of α-helix and a significant increase of random coil was observed in glutelin as compared with albumin. This was probably due to the extreme alkali conditions during glutelin preparation which led to an increase of unordered structures, and a similar result was reported by Du et al. (2012). Moreover, according to Gao et al. (2018), the protein with higher content of random coil will be more easily to be digested in human body. Thus, glutelin in Chinese quince seed may be more appropriate to be used as a food additive.
Conclusion
In this study, two major protein fractions, albumin and glutelin, were obtained from Chinese quince seed. Both albumin and glutelin showed high nutritional value as they contained high amount of EAA. They could be candidate for new source of protein for adult diet. The higher denaturation temperature (108.36 °C) and better heat coagulability (25.39%) were observed in glutelin. Therefore, glutelin might be preferable for use in food systems where high temperature treatment was necessary. Glutelin also exhibited satisfactory water holding capacity (5.44 g/g) and oil holding capacity (8.15 g/g). These imparted glutelin the potential to be applied as food additive, such as sausages, cakes and breads. In addition, both albumin and glutelin exhibited high hydrophobicity. Proteins with high hydrophobicity could enhance their interaction with lipid target of target organs. The possibility of albumin or glutelin to be applied in controlled/targeted delivery system would be conducted in our following work. Meanwhile, the bioactivities of glutelin and albumin would be conducted in the next step.
References
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (pisum sativum l.) seed protein isolate. Food Chem 128:902–908
Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K (2010) Preparation and characterization of high-quality rice bran proteins. J Sci Food Agric 87:1219–1227
Ajibola CF, Malomo SA, Fagbemi TN, Aluko RE (2016) Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocoll 56:189–200
Aletor O, Oshodi AA, Ipinmoroti K (2002) Chemical composition of common leafy vegetables and functional properties of their leaf protein concentrates. Food Chem 78:63–68
Amza T, Amadou I, Zhu K, Zhou H (2011) Effect of extraction and isolation on physicochemical and functional properties of an underutilized seed protein: gingerbread plum (neocarya macrophylla). Food Res Int 44:2843–2850
Angel SSD, Martínez EM, López MAV (2003) Study of denaturation of corn proteins during storage using differential scanning calorimetry. Food Chem 83:531–540
Beuchat LR (1977) Functional and electrophoretic characteristics of succinylated peanut flour protein. J Agric Food Chem 25:258–261
Beveridge T, Toma SJ, Nakai S (2010) Determination of SH-and SS- groups in some food proteins using Ellman’s reagent. J Food Sci 39:49–51
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cepeda E, Villaran MC, Aranguiz N (1998) Functional properties of faba bean (Vicia faba) protein flour dried by spray drying and freeze drying. J Food Eng 36:303–310
Chen S, Paredes-Lopez O (2010) Isolation and characterization of the 11 s globulin from amaranth seeds. J Food Biochem 21:53–65
Chen J, Mu T, Zhang M et al (2018) Structure, physicochemical and functional properties of protein isolates and major fractions from cumin (Cuminum cyminum) seeds. Int J Food Prop 21:685–701
Deng Q, Wang L, Wei F et al (2011) Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem 124:1458–1465
Deng Y, Huang L, Zhang C, Xie P, Cheng J, Wang X, Li S (2019) Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem 283:539–548
Du Y, Jiang Y, Zhu X et al (2012) Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem 133:923–929
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1980) Colorimetric method for determination of sugars and related substances. Anal Chem 89:449–454
Eladawy TA (2000) Functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate. Food Chem 70:83–91
Gao LL, Li YQ, Wang ZS et al (2018) Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem 240:980–988
Gazzola D, Vincenzi S, Gastaldon L et al (2014) The proteins of the grape (Vitis vinifera l.) seed endosperm: fractionation and identification of the major components. Food Chem 155:132–139
Hu XZ, Cheng YQ, Fan JF et al (2010) Effects of drying method on physicochemical and functional properties of soy protein isolates. J. Food Process Pres 34:520–540
Idouraine A, Yensen SB, Weber CW (2010) Tepary bean flour, albumin and globulin fractions functional properties compared with soy protein isolate. J Food Sci 56:1316–1318
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta 624:13–20
Lawal OS, Adebowale KO, Ogunsanwo BM, Sosanwo OA, Bankole SA (2005) On the functional properties of globulin and albumin protein fractions and flours of African locust bean (Parkia biglobossa). Food Chem 92:681–691
Malomo SA, Aluko RE (2015) Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innov Food Sci Emerg 31:151–159
Myers DJ, Hojilla-Evangelista MP, Johnson LA (1994) Functional properties of protein extracted from flaked, defatted, whole corn by ethanol/alkali during sequential extraction processing. J Am Oil Chem Soc 71:1201–1204
Ragab DM, Babiker EE, Eltinay AH (2004) Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by ph and/or salt concentration. Food Chem 84:207–212
Rezig L, Chibani F, Chouaibi M, Dalgalarrondo M, Hessini K, Hamdi S (2013) Pumpkin (Cucurbita maxima) seed proteins: sequential extraction processing and fraction characterization. J Agric Food Chem 61:7715–7721
Sandovaloliveros MR, Paredeslópez O (2013) Isolation and characterization of proteins from chia seeds (Salvia hispanica l.). J Agric Food Chem 61:193–201
Shchekoldina T, Aider M (2014) Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J Food Sci Technol 51:2331–2343
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689
Siow HL, Gan CY (2014) Functional protein from cumin seed (Cuminum cyminum): optimization and characterization studies. Food Hydrocoll 41:178–187
Spies JR (1967) Determination of tryptophan in protein. Anal Chem 39:1412–1416
Sun C, Wu W, Ma Y, Min T, Lai F, Wu H (2016) Physicochemical, functional properties and antioxidant activities of protein fractions obtained from mulberry (Morus atropurpurea Roxb.) Leaf. Int J Food Prop 20:s3311–s3325
Timilsena YP, Adhikari R, Barrow CJ, Adhikari B (2016) Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem 212:648–656
Ulloa JA, Rosas-Ulloa P, Ulloa-Rangel BE (2011) Physicochemical and functional properties of a protein isolate produced from safflower (Carthamus tinctorius L.) meal by ultrafiltration. J Sci Food Agric 91:572–577
Wells BJ, Rd MA, Everett CJ (2005) Association between dietary arginine and C-reactive protein. Nutrition 21:125–130
Yu M, Zeng M, Qin F, He Z, Chen J (2017) Physicochemical and functional properties of protein extracts from Torreya grandis seeds. Food Chem 227:453–460
Zhong C, Sun Z, Zhou Z, Jin MJ, Tan ZL, Jia SR (2014) Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. J Agric Food Chem 62:3217–3222
Funding
This study was founded by the Jiangsu provincial natural science foundation of China [grant number BK20181124].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., Huang, L., Zhang, C. et al. Chinese quince seed proteins: sequential extraction processing and fraction characterization. J Food Sci Technol 57, 764–774 (2020). https://doi.org/10.1007/s13197-019-04109-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04109-6